Abstract
Purpose
We will review the literature on adjuvant therapies for patients with high-risk localized kidney cancer following surgical treatment. Two recently published prospective trials with conflicting results will be reconciled within the context of their respective designs. Finally, we will spotlight upcoming trials that use novel immunotherapy-based checkpoint inhibitors and have the potential to establish a new standard of care.
Materials and Methods
A PubMed search was performed for English language articles using the keywords “renal cell carcinoma,” “kidney cancer,” “immunotherapy,” “targeted therapy,” and “adjuvant therapy” published through January 2017. Clinicaltrials.gov was queried for ongoing studies. Relevant data recently presented at the major urology and medical oncology meetings was also included.
Results
Adjuvant therapies for high-risk localized kidney cancer can be grouped into four categories: 1) traditional immunotherapy; 2) inhibitors of the VEGF and mTOR pathways; 3) vaccines and antibody-dependent cytotoxic agents; and 4) immune checkpoint inhibitors. Several trials of traditional immunotherapy, such as IFN-α and high-dose IL-2, failed to show benefit as adjuvant treatments and were associated with significant adverse events. VEGF and mTOR inhibitors have less severe toxicities in metastatic disease and therefore are natural considerations for adjuvant trials However, current data is conflicting: the ASSURE trial found no RFS benefit to sorafenib or sunitinib over placebo, while the S-TRAC trial found that one year of sunitinib improved RFS by 1.2 years. Vaccine-based treatments and antibody-dependent cytotoxic agents have had mixed results. New trials evaluating immune checkpoint inhibitors are planned given impressive efficacy and tolerability as second line agents in metastatic disease. Future adjuvant trials are likely to be guided by molecular signatures to treat patients most likely to benefit.
Conclusions
Based on the available data, there appears to be no role for traditional immunotherapy as adjuvant treatment in patients with high-risk localized kidney cancer following surgical resection. The S-TRAC trial provides evidence that one year of adjuvant sunitinib in patients with higher-risk locoregional disease increases the median time to recurrence; however, the data on overall survival are immature, and adverse effects were common. Results from trials investigating immune checkpoint inhibitors are highly anticipated.
Keywords (MeSH Terms): Adjuvant, Renal cell carcinoma, Kidney cancer, ASSURE, S-TRAC
INTRODUCTION
In the current era of incidental, imaging-based diagnoses of small kidney tumors, complete surgical resection often results in cure.1 The 5-year cancer specific survival (CSS) for patients with stage I disease is >90%.1,2 However, late relapse in the form of local and even distant disease is not uncommon. In a study of nearly 1,500 patients undergoing radical nephrectomy with a median follow up of 10 years, 5% and 15% experienced local and distant metastatic recurrence, respectively.3 In patients harboring high-risk features, disease recurrence may occur in as many as 35–40% of patients. Recurrence rates in lymph node positive patients can be as high as 80%.4 Once the disease progresses to a metastatic state, only high-dose IL-2 has been shown to produce durable complete responses.5 As such, there is interest in the development of effective adjuvant therapies to prevent or delay relapse in high-risk patients.
Trials aimed at developing an effective adjuvant therapy for surgically resected kidney cancer date back several decades (Table 1). A number of negative trials evaluating a variety of treatment strategies have since dimmed the prospect of a successful adjuvant therapy. However, a recently published prospective study, the S-TRAC (Sunitinib as Adjuvant Treatment for Patients at High Risk of Recurrence of Renal Cell Carcinoma Following Nephrectomy) trial, demonstrated improvement in disease-free survival (DFS) in patients with advanced locoregional kidney cancer taking sunitinib for one year.6 These results, in combination with highly anticipated trials using immune checkpoint inhibitors, have reinvigorated hope for the use of adjuvant therapy in appropriately selected patients with a high risk of recurrence following surgical treatment.
Table 1.
Adjuvant trials in high-risk localized kidney cancer.
| Early Adjuvant Trials | |||||
|---|---|---|---|---|---|
| Author | Journal, year | Agent, control | N | Stage | Result |
| 25 Kjaer, et al. | Int J Radiat Oncol Biol Phys, 1987 | Int: Radiation Cntrl: Observation |
65 | pT3–4 N0–3 | Negative |
| 26 Pizzocaro, et al. | J Urol, 1987 | Int: Medroxyprogesterone Cntrl: Observation |
136 | pT1–4 N0–3 | Negative |
| Traditional Immunotherapy Trials | |||||
| Author | Journal, year | Agent, control | N | Stage | Result |
| 27 Pizzocaro, et al. | JCO, 2001 | Int: IFN-α Cntrl: Placebo |
247 | pT3–4a N0 pTany N0–3 |
Negative (DFS, OS) |
| 28 Messing, et al. | JCO, 2003 | Int: IFN-α Cntrl: Observation |
283 | pT3–4a N0 pTany N0–3 |
Negative (DFS, OS) |
| 29 Clark, et al. | JCO, 2003 | Int: HD IL-2 Cntrl: Observation |
44 M0, 25 M1 resected | pT3b–4 N0 Tany N1–3 M1 resected |
Negative (PFS) |
| 30 Atzpodien, et al. | Br J Cancer, 2005 | Int: HD IL-2, IFN-α2a/5-FU Cntrl: Observation |
203 | pT3–4 N0 Tany N1–3 M1 resected |
Negative (DFS, OS) |
| 31 Passalacqua, et al. | J Immunother, 2014 | Int: low-dose IL-2, IFN-α Cntrl: Observation |
310 | pT1–3b N0 pTany N1–3 |
Negative (RFS, OS) |
| Targeted Therapy Trials | |||||
| Author | Journal, year | Agent, control | N | Stage | Result |
| 40 Haas, et al. | Lancet, 2016 | Int: Sunitinib Cntrl: Placebo |
1,943 | ≥pT1b G3–4, all histology |
Negative (DFS, OS) |
| 6 Ravaud, et al. | NEJM, 2016 | Int: Sunitinib or Sorafenib Cntrl: Placebo |
615 | pT2 N0 (grades 3–4) pT3–4 N0 Tany N1 Clear-cell only |
Positive (DFS) |
| SORCE NCT00492258 |
TBD | Int: Sorafenib x1 yr, Sorafenib x3 yr Cntrl: Placebo |
1420 | Intermediate – High risk patients | TBD (DFS) |
| ATLAS NCT01599754 |
TBD | Int: Axitinib Cntrl: Placebo |
592 | T3–4, N0 Tany N+ Clear-cell predominant |
TBD (DFS) |
| EVEREST NCT01120249 |
TBD | Int: Everolimus Cntrl: Placebo |
1218 | pT1b (grades 3–4) pT2–4 N0 Tany N+ Clear-cell or papillary |
TBD (DFS) |
| PROTECT NCT01235962 |
TBD | Int: Pazopanib Cntrl: Placebo |
1500 | High-grade pT2 N0 Any pT3–4 N0 Any N+ |
Negative, pending presentation |
| Vaccines and Antibody Dependent Cytotoxic Agents Trials | |||||
| Author | Journal, year | Agent, control | N | Stage | Result |
| 44 Galligioni, et al. | Cancer, 1996 | Int: Tumor cells + BCG Cntrl: Observation |
120 | Stages I – III | Negative (DFS) |
| 45 Jocham, et al. | Lancet, 2004 | Int: Renal tumor cell vaccine Cntrl: Observation |
379 | pT2–3b N0 pTany N1–3 |
** Positive (PFS) |
| 46 Wood, et al. | Lancet, 2008 | Int: HSPPC-96 Cntrl: Observation |
819 | pT1b–T4 N0 Tany N1–2 |
Negative (DFS) |
| 47 Chamie, et al. | JAMA Oncol, 2016 | Int: anti-CAIX Cntrl: Placebo |
864 | pT1b–2 (Fuhrman ≥3) pT3–4 N0 pTany N+ Clear-cell only |
Negative (DFS) |
| Immune Checkpoint Inhibitor Trials | |||||
| Author | Journal, year | Agent, control | N | Stage | Result |
| PROSPER NCT03055013 |
TBD | Int: Neoadjuvant. nivolumab, nephrectomy, adjuvant nivolumab Cntrl: Nephrectomy alone |
TBD | pT2–4 N0 pTany N1–2 All grades |
TBD (DFS) |
| IMmotion010 NCT03024996 |
TBD | Int: Atezolizumab Cntrl: Placebo |
TBD | pT2 (grade 4) pT3a (grades 3–4) pT3b/c (any grade) pTany N+ Fully resected M1 |
TBD (RFS) |
IFN-α, interferon-alpha; DFS, disease-free survival; OS, overall-survival; HD IL-2, high dose interleukin-2; PFS, progression-free survival; 5-FU, 5-fluorouracil; BCG, bacillus Calmette-Guérin; TBD, to be determined; CAIX, carbonic anhydrase IX;
study results subject to methodological critiques, see discussion in text.
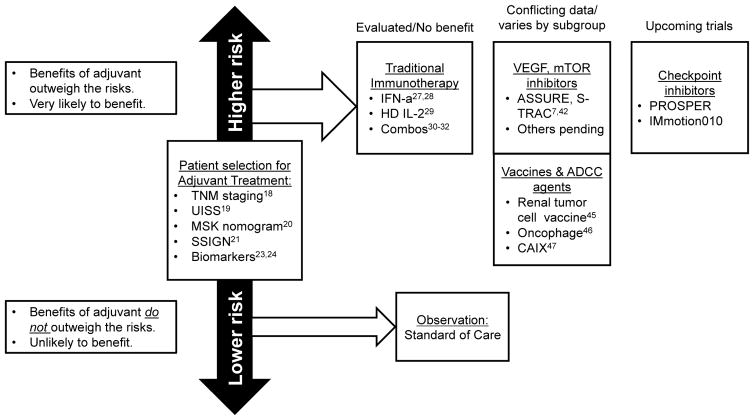
In this review, we provide context by describing the history and development of adjuvant therapy as it relates to other malignancies. We then review the natural history of high-risk kidney cancer following surgical resection, discuss the strategies used to identify patients who may require adjuvant treatment, and explore the evolution of adjuvant therapies (Figure 1). We categorize adjuvant therapies into four main groups: 1) traditional immunotherapy; 2) inhibitors of VEGF and mTOR pathways; 3) vaccines and antibody-dependent cytotoxic agents; and 4) immune checkpoint inhibitors. For each category, evidence of efficacy in the metastatic setting is reviewed as this generally provides rationale for subsequent adjuvant studies. We then analyze the results of prospective clinical trials testing adjuvant therapies in patients with high-risk localized kidney cancer. Finally, we discuss ongoing and upcoming studies, in particular the highly anticipated future trials using immune checkpoint inhibitors.
Figure 1.
Current landscape of risk stratification and adjuvant trials for high-risk localized kidney cancer. TNM, tumor node metastasis classification; UISS, University of California Los Angeles Integrated Staging System; MSK, Memorial Sloan Kettering; SSIGN, stage size grade necrosis; IFN-α, interferon-α; HD IL-2, high-dose interleukin-2; VEGF, vascular endothelial growth factor; mTOR, mammalian target of rapamycin; ADCC, antibody-dependent cellular cytotoxic; CAIX, carbonic anhydrase IX.
ADJUVANT THERAPY
History and Development of Adjuvant Therapy
The goal of adjuvant therapy is to reduce the risk of recurrence by treating unrecognized micro-metastatic residual disease. The concept of adjuvant therapy following tumor resection has been explored in numerous specialties, including genitourinary oncology. Adjuvant therapy first gained traction in the 1960s based on the “Cell Kill” hypothesis, which was proposed by the mathematical biologist Dr. Howard Skipper of the Southern Research Institute.7 This stated that systemic treatment killed a certain percentage of tumor cells proportional to the dose of chemotherapy rather than a constant number of cells. This resulted in a shift towards treatment with higher doses of chemotherapy, although this is clearly limited by the toxicity and side effects of the therapy. This led to the consideration that systemic treatment could be more efficacious in patients with lower tumor burdens—in those patients who undergo surgery to remove the primary tumor and are therefore left only with micro-metastatic disease. In the 1970s, Bernard Fisher et al., a breast cancer surgeon already challenging the field with minimally-invasive “lumpectomy,”8 and Gianni Bonadonna et al.9 published nearly concurrent prospective studies demonstrating the efficacy of adjuvant therapy in breast cancer, paving way for the concept of adjuvant therapy in other malignancies.
Adjuvant Agents and Strategies in Other Malignancies
Adjuvant therapy has been extensively studied in other malignancies, including colorectal, cervical, gastric, head and neck, pancreatic, lung, and ovarian cancers, among others.7 In some malignancies, such as in early stage colon cancer, the role of adjuvant therapy is controversial.10 However, in others, such as breast cancer, adjuvant therapy plays a vital role in management and is selected by tumor-specific factors and guided by patient preference. The magnitude of benefit varies by cancer and treatment type. Examples of successful adjuvant therapy in urologic oncology include radiation therapy in prostate cancer with adverse pathologic features following radical prostatectomy (hazard ratio of 0.48 for biochemical recurrence), single-dose carboplatin or radiotherapy in stage 1 seminoma (10.9% absolute risk reduction in recurrence), and platin-based chemotherapy in node-positive penile cancer (hazard ratio of 0.40 for overall survival).11–13
As will be discussed, the identification of kidney cancer specific targets has led to the development of biologic agents initially used in the metastatic setting that are now being evaluated as adjuvant treatments. However, one important consideration is how differences between metastatic and locally-resected disease states affects the mechanism of action of adjuvant agents. For example, inhibitors of the VEGF pathway in clear-cell kidney cancer limit blood supply to the tumor and result in regression in some patients, however this effect is often short-lived and progression, especially if the agent is discontinued, is common. As explained by the “angiogenic switch” theory and hypothesized in a review by Chism et al., micro-metastatic disease, as likely present following resection of high-risk disease, may not depend on angiogenesis for viability.14,15 As compared with cytotoxic chemotherapy, which demonstrates improved efficacy with lower tumor burdens, adjuvant therapy with a VEGF inhibitor may be unable to inhibit micro-metastatic disease and may actually lead to resistance. Furthermore, in the adjuvant setting, reduced tumor antigen is present and could have an effect on the benefit seen in patients treated with novel immune checkpoint inhibitors, which rely on host immune responses to tumor antigen. Therefore, adjuvant strategies may require combinations of agents guided by patient- and tumor-specific factors.
Identification of Patients Likely to Benefit from Adjuvant Therapy
Identifying those patients at the highest risk of recurrence and therefore most likely to benefit from adjuvant therapy is a primary consideration for whether the benefits of adjuvant therapy outweigh the harms following potentially curative surgery. Stage is the most important prognostic factor, with stage I, II, III, and IV tumors having 91–100%, 74–96%, 59–70%, and 16–32% 5-year CSS, respectively.16 Grade, performance status, and other tumor- and patient-related factors also influence prognosis. To help predict disease recurrence following surgery, multiple nomograms have been developed. The most commonly used nomograms are the University of California Los Angeles Integrated Staging System (UISS), the Memorial Sloan Kettering Cancer Center nomogram, and the Stage, Size, Grade, and Necrosis (SSIGN) score.17–19 Recent attempts to incorporate more advanced markers have been made.20,21 In an analysis of patients at our institution, a five biomarker panel was identified from 170 patients with localized clear-cell kidney cancer.20 This panel was independently associated with DFS and, when combined with clinicopathologic features, approached a concordance index of 0.91. More recently, Rini et al. developed a 16-gene biomarker assay, including genes associated with vasculogenesis, cell growth and division, immune response, and inflammation, from nearly 1,000 nephrectomy patients.21 The panel was predictive of CSS, DFS, and overall survival (OS), and could successfully identify high-risk stage I–II tumors and low-risk stage II–III tumors. The authors externally validated the recurrence score with a separate cohort of patients from France. However, the added cost of such biomarkers may limit widespread use. Finally, with the wealth of available options, molecular signatures may help guide specific adjuvant agent selection. Currently, however, there is no consensus on the optimal staging system for the purpose of clinical trial reporting. As a result, many of the adjuvant trials discussed in this review use different systems, which must be accounted for when interpreting and comparing results.
Risks of Adjuvant Therapy
Finally, the side effects of adjuvant therapies must not outweigh the benefits. While VEGF inhibitors have improved side effect profiles compared with traditional immunotherapies, considerable rates of adverse events are still reported in the adjuvant trials to date. Even immune checkpoint inhibitors, which generally have toxicities favorable to chemotherapy, can have significant adverse effects.22 While side effects may be tolerable in the setting of metastatic disease, these could be unacceptable in a patient receiving adjuvant treatment who may in fact have no residual disease. This is especially important as the duration of adjuvant therapy could potentially be lengthy or indefinite. Without improvements in patient selection allowing for reliable prediction of which patients are at the highest risk of recurrence, adjuvant therapies may carry an overly burdensome risk without sufficient potential benefit.
TRADITIONAL IMMUNOTHERAPY
For Metastatic Disease
Early treatments for advanced and metastatic kidney cancer, such as chemotherapy, hormone therapy, and radiation were disappointing.23–25 Realization of the immune-sensitivity of kidney cancer led to development of traditional immunotherapy, which initially consisted of interferon-alpha (IFN-α) and later included high-dose interleukin (HD IL)-2. Up to 5–10% of appropriately selected patients treated with HD IL-2 will experience a complete response, however, it comes at the cost of significant side effects, including capillary leak and cytokine release syndromes.5 Nevertheless, the low, yet complete response rates achieved with traditional immunotherapy for metastatic kidney cancer prompted its evaluation as adjuvant therapy for patients with high-risk localized disease following surgical resection.
In the Adjuvant Setting
Unfortunately, trials have demonstrated that traditional immunotherapy does not confer a survival benefit as an adjuvant therapy. A randomized study by Pizzocaro et al. found no difference in DFS or OS in 247 patients treated with IFN-α compared with controls.26 A randomized study by Messing et al. evaluating IFN-α versus observation also found no difference in DFS or OS.27 Clark et al. evaluated HD IL-2 in a randomized study that closed early after an interim analysis showed futility of meeting the primary endpoint.28 Studies combining traditional immunotherapies with other agents have similarly yielded negative results.29 The most recent combination trial, published in 2014, randomized patients to low-dose IL-2 and IFN-α or observation and found no difference in DFS or OS.30 Toxicities in these were significant, including more than half of patients requiring vasopressor support for hypotension in one study.28 Based on the prospective data available, traditional immunotherapy cannot be recommended as an adjuvant therapy in patients with completely resected high-risk kidney cancer.
VEGF AND MTOR INHIBITORS
Background and Treatment for Metastatic Disease
The discovery of the von Hippel Lindau (VHL) tumor suppressor gene and its role in the pathophysiology of clear-cell type kidney cancer established the foundation for the development of a revolutionary family of agents developed to specifically target the newly discovered aberrant signaling pathway. Sorafenib and sunitinib, approved for advanced renal cell carcinoma in 2005 and 2006, respectively, are small molecule tyrosine kinase inhibitors that bind and inhibit the activation of the vascular endothelial growth factor (VEGF) receptor and platelet derived growth factor receptor located on endothelial cells.31 VEGF inhibitors reduce the tumor-driven angiogenesis needed to support tumor growth. Sunitinib and sorafenib supplanted traditional immunotherapy as first line treatment for metastatic kidney cancer by improving response rates, PFS, and OS.32–34 The inhibitors of mammalian target of rapamycin (mTOR) are a different but related class of molecules that function by ultimately impeding cell cycle regulators such as HIF-1α, c-MYC, and cyclin-D1, and improve survival in VEGF-refractory cases.35 Novel VEGF and mTOR inhibitors including pazopanib, axitinib, everolimus, and cabozantinib, among others, are being studied as second- and third-line options.35–38
In the Adjuvant Setting
Because of the efficacy of VEGF pathway-targeted drugs in the metastatic space, their use as adjuvant treatments in patients at high risk of recurrence following surgical resection of localized kidney cancer has inspired ongoing interest. The first trial to produce results was the Adjuvant sunitinib or sorafenib for high-risk non-metastatic renal-cell carcinoma (ASSURE) trial by Haas et al. published in the Lancet in May of 2016,39 followed shortly by the S-TRAC trial by Ravaud et al. published in the New England Journal of Medicine.6 The ASSURE trial randomized 1,943 patients 1:1:1 to sunitinib, sorafenib, or placebo and showed no difference in the primary endpoint, PFS or OS. By contrast, the S-TRAC trial, which randomized 615 patients 1:1 to sunitinib or placebo, showed a significant improvement in the primary endpoint, DFS, from 5.6 years in the placebo group to 6.8 years in the sunitinib group. In a subgroup analysis of higher risk patients, the DFS benefit was longer at approximately 2.2 years. No OS benefit was demonstrated in the S-TRAC trial with approximately five years of follow-up.
The resultant discrepancy between the ASSURE and S-TRAC trials has resulted in some uncertainty regarding the benefit of adjuvant sunitinib, with advocates and skeptics focusing on methodological details as limitations in each trial (Table 2). The first key difference is the baseline risk of the study populations. ASSURE included patients with ≥pT1b high-grade (G3–4) disease, whereas S-TRAC included more advanced “locoregional” (≥pT3) disease. In ASSURE, more than one-third of patients had high-grade T1 or T2 disease and would not have met inclusion into S-TRAC. This difference is reflected in the median DFS of the placebo groups, which was one year longer in ASSURE. The higher risk population in S-TRAC more likely has micro-metastatic disease and, consequently, more to gain from adjuvant therapy. Additionally, approximately 20% of the ASSURE cohort had non-clear-cell histology, whereas S-TRAC only included clear-cell histology. As non-clear-cell histology kidney cancer is not driven by an aberrant VHL/HIF-1α pathway, VEGF inhibitors have demonstrated poorer response rates than in clear-cell kidney cancer.40 This discrepancy could have masked differences in responses to VEGF inhibitors and therefore biased the study to a null result. However, a planned subset analysis of patients with clear-cell histology only in the ASSURE trial failed to demonstrate a survival benefit. Secondly, dosing adjustments made to improve tolerability in each study differed slightly. In ASSURE, the dosages of sunitinib and sorafenib were decreased from 50 mg daily and 400 mg twice daily to 37.5 mg daily and 400 mg daily, respectively, in all patients starting in 2009. Dosages were increased as tolerated. In S-TRAC, dose reductions by 12.5 mg of sunitinib were allowed, but only one-third of patients required dose reduction. The result is decreased drug exposure in ASSURE patients, potentially reducing the observed efficacy compared with the control group. Both studies, however, had high rates of dropout secondary to toxicity (44% in ASSURE and 45% in S-TRAC), underscoring issues with tolerability of these agents. Third, while each trial had similar primary endpoints, ASSURE relied on blinded investigator-assessed endpoints, while S-TRAC endpoints were processed by an independent central review. Blinded central review reduces imaging reader bias. This is particularly important in studies with VEGF inhibitors as characteristic side effects that are not seen in the placebo arm (e.g. hand foot mouth syndrome) could influence the readers’ interpretation of the clinical endpoint. With the results of S-TRAC and these potential confounders in mind, Haas et al. recently reported a subgroup analysis of pT3, 4, and node-positive patients in the ASSURE cohort.41 No improvement in DFS or OS was found with sunitinib or sorafenib, even when stratified by dose quartiles. However, this analysis was not powered to detect differences in survival in this subgroup. Based on the currently available prospective data, patients most likely to derive a RFS benefit are those in the higher risk subgroups with clear-cell histology. The possible benefits of adjuvant sunitinib should be discussed in the context of these discordant results and potential for side effects.
Table 2.
Differences in clinicopathologic variables and outcomes between the ASSURE and S-TRAC trials.
| ASSURE | S-TRAC | Comment | |
|---|---|---|---|
| Sponsors | ECOG | Pfizer | - |
| Risk criteria & tumor stage | UISS intermediate-high risk pT1b–4, NX, M0, G1–3 pTany, N1–2, M0 Only grades 3–4 included Only ECOG 0 or 1 included |
UISS intermediate-high risk pT3, NX, M0 pT4, NX, M0 pTany, N1–2, M0 All grades included All ECOG PS included |
ASSURE included lower risk patients, whereas S-TRAC included higher risk patients |
| Histology | Included non-clear-cell histology (~20%) | Clear-cell histology only | ASSURE included patients without clear-cell histology |
| Lymph nodes | Included N+ disease if fully resected | Included N+ disease if fully resected | - |
| Interventions | Sunitinib or sorafenib or placebo x 1 year | Sunitinib or placebo x 1 year | - |
| Dosing | Initially: sunitinib 50 mg/day, sorafenib 400 mg twice daily *Revised: sunitinib 25–37.5 mg/day, sorafenib 400 mg/day *After ~1,300 patients, increased as tolerated. |
*Sunitinib 50 mg/day *Allowed reductions only to 37.5 mg/day, depending on toxicity and severity. |
ASSURE expanded dose reduction to all patients and allowed sunitinib dosing as low as 25 mg/day |
| Dosing outcomes | *Treatment completion: sunitinib 56%, sorafenib 55%, placebo 89% *Among those patients starting at full dose |
Treatment completion: sunitinib 55%, placebo 69.4% Dosing reduction: sunitinib 45.8%, placebo 4.9% |
Mid-study starting dose reduction improved treatment completion and dosing reduction rates in ASSURE; however, the effective dose was reduced. |
| Randomized | N=1,943 Stratified by: histology (clear- cell vs. non-clear-cell), surgery (lap vs. open), ECOG (0 vs. 1), risk category (int. high vs. high vs. very high risk) |
N=615 Stratified by: UISS risk group, ECOG PS (<2 vs. ≥2), Country |
- |
| Outcomes | Primary: DFS Secondary: OS, DFS for clear-cell histology, toxic effects by NCI CTCAE v3.0 |
Primary: DFS Secondary: OS, safety/toxicity, patient reported outcomes, biomarker analysis |
- |
| Evaluation | Imaging: q4.5 months within first year, q6 months in second year, q12 months thereafter | Imaging: q3 months in years 1–3, q6 months in years 4–5, q12 months thereafter | More frequent imaging evaluation in S-TRAC |
| Centralized review | Only blinded investigator radiology review | Blinded centralized and investigator radiology review | Only central review, but not investigator review, was significant in S-TRAC |
| Median disease-free survival | Sunitinib 5.8, sorafenib 6.1, placebo 6.6 years (sunitinib vs placebo: HR 1.02, 95% CI 0.85–1.23, p=0.80; sorafenib vs placebo: HR 0.97, 95% CI 0.80–1.17, p=0.72). | Sunitinib 6.8, placebo 5.6 years (HR 0.76, 95% CI 0.59–0.98, p=0.03) | ASSURE: negative study; S-TRAC: positive study |
| Overall survival | No difference between groups *Median OS not reached for any group. |
Not mature at publication | - |
| Adverse events | ≥Grade 3 events: sunitinib 63%, sorafenib 70%, placebo 24% | ≥Grade 3 events: sunitinib 60.5%, placebo 19.4% | Similar between studies |
| Accrual | 2006–2010 United States and Canada |
2007–2011 Multi-national (21 countries) |
Potentially more generalizable results from S- TRAC |
Several ongoing randomized trials evaluating other VEGF inhibitors are expected to yield results in the coming years. The SORCE (Sorafenib in Treating Patients at Risk of Relapse After Undergoing Surgery to Remove Kidney Cancer) trial is evaluating sorafenib in intermediate- to high-risk patients following surgery (NCT00492258). Patients are randomly assigned to placebo for 3 years, sorafenib for 1 year followed by placebo for 2 years, or sorafenib for 3 years. The primary outcome is DFS and results are pending. The ATLAS (Adjuvant Axitinib Therapy of Renal Cell Cancer in High Risk Patients) trial randomized patients with clear-cell kidney cancer with pathologic T3–4 or node positive disease to axitinib or placebo for three years (NCT01599754). The EVEREST (Everolimus in Treating Patients With Kidney Cancer Who Have Undergone Surgery S0931) trial in patients with clear-cell or papillary kidney cancer with high-grade T1b or T2–4 tumors randomized to everolimus or placebo for one year (NCT01120249). Finally, the PROTECT (A Study to Evaluate Pazopanib as Adjuvant Treatment for Localized Renal Cell Carcinoma) trial randomized high-risk patients (high-grade T2, any T3–4, or any N1 disease) with predominant clear-cell disease to pazopanib or placebo for one year (NCT01235962). Unfortunately, this trial did not meet its primary endpoint, based on information from the Novartis website, and presentation of the results are expected at an upcoming meeting. While these studies will help to provide context for the results of ASSURE and S-TRAC, the data generated thus far demonstrate that patient selection may be a more important factor than potentially small differences in specific VEGF inhibitors.
VACCINES AND ANTIBODY-DEPENDENT CYTOTOXIC AGENTS
Autologous Vaccine-based Treatments
Given the known sensitivity of kidney cancer to immune-based therapies, autologous vaccines have been studied extensively, most commonly in the metastatic setting but also as adjuvant treatment. There are several theoretical advantages of autologous whole-cell vaccines, including the formation of tumor-specific immunity with limited cross reactivity with normal tissue, limited pre-formed tolerance to tumor antigens, and the ability to generate an immune response to multiple tumor antigens, limiting resistance secondary to mutations.42
Based on positive data from a prospective trial of autologous tumor vaccines in colon cancer, Galligioni et al. randomized 120 patients to receive an autologous tumor cell vaccine admixed with bacillus Calmette-Guérin or observation.43 Although the treated patients demonstrated a delayed-type cutaneous hypersensitivity reaction at one month post-vaccination to tumor cells but not autologous normal renal cells, no improvement in DFS or OS was found in the treatment group compared with the observation group.
Jocham et al. also evaluated a post-nephrectomy renal tumor cell vaccine generated by inducing tumor cells in vitro with interferon-γ to increase antigenicity prior to subcutaneous injection.44 In this trial, patients were randomized prior to nephrectomy to receive either vaccine treatment or standard-of-care observation. DFS was significantly improved in the treatment group compared with the control group at five years (77.4% vs. 67.8%, p=0.0204). Despite claiming that the intention-to-treat cohort yielded positive results, this trial has significant methodological concerns. First, nearly one third of patients were excluded after randomization, secondary to pathologically benign disease, incorrect tumor stage, and inability to prepare vaccine. Consequently, the remaining cohort (called the intention-to-treat cohort by the authors) is a modified intention-to-treat cohort. Significant imbalances existed in this cohort. For example, the treatment group had higher rates of clear-cell histology, among other differences that may have influenced the results. An updated report in 2006 failed to demonstrate an OS benefit.42 The manufacturer of the vaccine, LipoNova (Hannover, Germany), filed for insolvency in 2008 and no further data on this strategy has been published.
Vitespen is a heat-shock protein (glycoprotein 96)-peptide complex (HSPPC-96) derived from a patient’s nephrectomy specimen and used as a renal tumor vaccine. In a phase III study 818 patients were randomized to receive adjuvant vitespen or standard of care observation following nephrectomy for a wide spectrum of disease.45 The vaccine consisted of an induction course of four weekly intradermal injections followed by maintenance. At a median follow-up of 1.9 years, there was no difference in recurrence between vitespen and the observation groups. A pre-defined subgroup analysis of patients with earlier stage disease yielded a trend towards improvement in RFS with adjuvant vitespen, however the result was not statistically significant, and subsequent studies evaluating efficacy in this group have not yet been reported.
Antibody-Dependent Cytotoxic Agents
The recently published ARISER trial (Adjuvant Rencarex Immunotherapy Phase 3 Trial to Study Efficacy in Non-metastatic RCC) was a placebo-controlled randomized study that treated patients with six months of girentuximab, a monoclonal antibody targeting CAIX, a cell surface receptor that is almost universally expressed on clear-cell kidney cancers and correlates with poor prognosis.46 Girentuximab generates an immune response, eliciting effector cells to destroy tumor cells, in a process called antibody-dependent cellular cytotoxicity. Unfortunately, investigators found no benefit of adjuvant girentuximab. An unplanned post hoc analysis did find an improvement in DFS in patients with the highest levels of CAIX expression, a finding which may become important as genetic and molecular patient selection criteria for adjuvant therapy gain traction. Further examining those patients with higher CAIX expression, a DFS improvement was seen in patients younger than 65 years, those with lower tumor grades (G1–2), and an ECOG of 0.
IMMUNE CHECKPOINT INHIBITORS
Another promising class of cancer therapies are the checkpoint inhibitors, such as anti-programmed cell death-1 (e.g. nivolumab) and anti-programmed cell death ligand-1 (e.g. atezolizumab), which function by releasing the immune system from tumor-driven T-cell inhibition.47 Checkpoint inhibitors are approved for a variety of malignancies and have rapidly expanding approvals and indications in genitourinary malignancies.
For Metastatic Disease
In kidney cancer, CheckMate025 is a recently published study comparing the checkpoint inhibitor nivolumab with the standard-of-care second-line everolimus in a group of highly pre-treated patients with metastatic clear-cell kidney cancer.48 Nivolumab was associated with an OS advantage of 5.4 months and subsequently gained approval as second-line therapy. Importantly, immunotherapy with checkpoint inhibitors is associated with significantly fewer side effects and improved quality of life compared with standard agents.49 As a result, several trials evaluating checkpoint inhibitors as adjuvant therapy for patients with high-risk localized kidney cancer are underway.
Future Adjuvant Trials Evaluating Immune Checkpoint Inhibitors
Two recent randomized adjuvant trials currently accruing patients are the PROSPER (Phase 3 Randomized Study Comparing Perioperative Nivolumab vs. Observation in Patients with Localized Renal Cell Carcinoma Undergoing Nephrectomy; NCT03055013) trial and the IMmotion010 (Phase 3 Study of Atezolizumab as Adjuvant Therapy in Participants with Renal Cell Carcinoma at High Risk of Developing Metastasis Following Nephrectomy; NCT03024996) trial (Table 3). Several critical trial design differences should be highlighted and will be important when analyzing and comparing results in the coming years. First, PROSPER is a randomized study comparing neoadjuvant nivolumab followed by surgical treatment and additional adjuvant nivolumab to standard of care surgical treatment followed by observation. Investigators hypothesize that neoadjuvant nivolumab may “prime” the immune system when higher levels of tumor antigen are present prior to nephrectomy, ultimately resulting in improved micro-metastatic tumor cell kill in the post-nephrectomy adjuvant setting. Critics of this approach will point to the non-placebo controlled comparator group, as well as logistical concerns with delays in treatment from neoadjuvant treatment compared with upfront surgery. In IMmotion010, investigators have employed a more traditional placebo-controlled randomized approach, using adjuvant atezolizumab, which may improve accrual in some practice settings when compared with PROSPER. However, the benefits of immunotherapy with checkpoint inhibition are debated in the adjuvant-only setting given a relative lack of tumor antigen for treatment priming. This argument can also be applied to PROSPER and questions whether a neoadjuvant-only arm is required to determine the added benefit of additional adjuvant immunotherapy with checkpoint inhibition. Finally, both studies should be commended for robust collection of adverse event, quality of life, and biologic marker expression data to better contextualize the results. While both studies have limitations, each will provide critical insight into the role of checkpoint inhibitors as adjuvant therapy in kidney cancer.
Table 3.
Comparison between the PROSPER and IMmotion010 immune checkpoint inhibitor trials.
| PROSPER | IMmotion010 | Comment | |
|---|---|---|---|
| Sponsor | ECOG | Genetech (in collaboration with the SUO Clinical Trials Consortium) | - |
| Risk criteria & tumor stage | pT2–4, NX, M0 pTany, N1–2, M0 All grades included Only ECOG 0 or 1 included *Metastasectomy excluded |
pT2, NX, M0, G4 pT3a, NX, M0, G3–4 pT3b/c, NX, M0, any grade pTany, N+, M0, any grade *Fully resected M1 disease *Selected patients |
PROSPER included lower risk patients |
| Histology | Includes up to 15% non- clear-cell component | Clear-cell, or non-clear- cell with sarcomatoid component | - |
| Lymph nodes | Included N+ disease if fully resected | Included N+ disease if fully resected | - |
| Interventions | Neoadjuvant nivolumab x 1 month → nephrectomy → adjuvant nivolumab x 9 months *No placebo group |
Adjuvant Atezolizumab or placebo x 12 months | Major differences include: no placebo arm and delay to surgery in PROSPER |
| Dosing | Nivolumab 3 mg/kg IV q2 weeks x 2 cycles Adjuvant: Nivolumab 3 mg/kg IV q2 weeks x 6 cycles then q4 weeks x 6 cycles |
Atezolizumab 1,200 mg IV q3 weeks x 16 cycles | - |
| Randomization | N=766 (estimated) Stratified by: size (cT2 vs. >cT2), node status (cN0 vs. cN+), histology *Requires pre-operative biopsy |
N=664 (estimated) Stratified by: disease stage (T2–3a vs. pT3b– 4/N+ vs. metastasectomy), PD-L1 status, Region |
Biopsy in PROSPER potentially leading to correlative data |
| Outcomes | Primary: DFS Secondary: toxicity, OS, RFS with clear-cell, predictive biomarkers |
Primary: DFS Secondary: OS, DFS, DSS, DMFS, toxicity, serum anti-therapeutic Abs, predictive biomarkers |
Both studies have robust secondary analyses including biomarkers and tissue based outcomes IMmotion010 DFS is investigator assessed |
CONCLUSIONS
Patients at high risk of recurrence following surgical resection of localized kidney cancer pose a challenging clinical scenario for urologists and medical oncologists. Results from ASSURE and S-TRACT using adjuvant VEGF inhibitors are conflicting and ultimately highlight the limitations of targeted therapies on disease pathophysiology, underscoring the importance of optimal patient selection. Based on current data, patients with high-risk locoregional clear-cell kidney cancer may be presented with the option of adjuvant sunitinib for one year following surgery in the context of known risk of side effects. We anxiously await the results of several adjuvant immune checkpoint inhibitor trials given their promising efficacy and relative tolerability in the advanced setting. Future trials should incorporate advanced molecular and genetic markers of recurrence to treat micro-metastatic disease early in those likely to benefit.
Abbreviations
- DFS
disease-free survival
- OS
overall survival
- VEGF
vascular endothelial growth factor
- mTOR
mammalian target of rapamycin
- UISS
University of California Los Angeles Integrated Staging System
- SSIGN
Stage Size Grade Necrosis
- IFN-α
interferon-α
- IL-2
interleukin-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hollingsworth JM, Miller DC, Daignault S, et al. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. 2007;109:1763. doi: 10.1002/cncr.22600. [DOI] [PubMed] [Google Scholar]
- 2.Tsui KH, Shvarts O, Smith RB, et al. Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. 2000;163:1090. doi: 10.1016/s0022-5347(05)67699-9. [DOI] [PubMed] [Google Scholar]
- 3.Kim SP, Weight CJ, Leibovich BC, et al. Outcomes and clinicopathologic variables associated with late recurrence after nephrectomy for localized renal cell carcinoma. Urology. 2011;78:1101. doi: 10.1016/j.urology.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Babaian KN, Kim DY, Kenney PA, et al. Preoperative predictors of pathological lymph node metastasis in patients with renal cell carcinoma undergoing retroperitoneal lymph node dissection. J Urol. 2015;193:1101. doi: 10.1016/j.juro.2014.10.096. [DOI] [PubMed] [Google Scholar]
- 5.Allard CB, Gelpi-Hammerschmidt F, Harshman LC, et al. Contemporary trends in high-dose interleukin-2 use for metastatic renal cell carcinoma in the United States. Urol Oncol. 2015;33:496e11. doi: 10.1016/j.urolonc.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375:2246. doi: 10.1056/NEJMoa1611406. [DOI] [PubMed] [Google Scholar]
- 7.DeVita VT, Jr, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68:8643. doi: 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Carbone P, Economou SG, et al. 1-Phenylalanine mustard (L-PAM) in the management of primary breast cancer. A report of early findings. N Engl J Med. 1975;292:117. doi: 10.1056/NEJM197501162920301. [DOI] [PubMed] [Google Scholar]
- 9.Bonadonna G, Brusamolino E, Valagussa P, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med. 1976;294:405. doi: 10.1056/NEJM197602192940801. [DOI] [PubMed] [Google Scholar]
- 10.Meyers BM, Cosby R, Quereshy F, et al. Adjuvant systemic chemotherapy for stages II and III colon cancer after complete resection: a clinical practice guideline. Curr Oncol. 2016;23:418. doi: 10.3747/co.23.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441. doi: 10.1016/j.juro.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Petrelli F, Coinu A, Cabiddu M, et al. Surveillance or adjuvant treatment with chemotherapy or radiotherapy in stage I seminoma: a systematic review and meta-analysis of 13 studies. Clin Genitourin Cancer. 2015;13:428. doi: 10.1016/j.clgc.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Djajadiningrat R, Zargar-Shoshtari K, et al. Adjuvant chemotherapy is associated with improved overall survival in pelvic node–positive penile cancer after lymph node dissection: a multi-institutional study. Urol Oncol. 2015;33:496e23. doi: 10.1016/j.urolonc.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 15.Chism DD, Rathmell WK. Kidney cancer: rest ASSUREd, much can be learned from adjuvant studies in renal cancer. Nat Rev Nephrol. 2016;12:317. doi: 10.1038/nrneph.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611. [PubMed] [Google Scholar]
- 17.Sorbellini M, Kattan MW, Snyder ME, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173:48. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- 18.Parker WP, Cheville JC, Frank I, et al. Application of the stage, size, grade, and necrosis (SSIGN) score for clear cell renal cell carcinoma in contemporary patients. Eur Urol. 2017;71:665. doi: 10.1016/j.eururo.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 20.Klatte T, Seligson DB, LaRochelle J, et al. Molecular signatures of localized clear cell renal cell carcinoma to predict disease-free survival after nephrectomy. Cancer Epidemiol Biomarkers Prev. 2009;18:894. doi: 10.1158/1055-9965.EPI-08-0786. [DOI] [PubMed] [Google Scholar]
- 21.Rini B, Goddard A, Knezevic D, et al. A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: development and validation studies. Lancet Oncol. 2015;16:676. doi: 10.1016/S1470-2045(15)70167-1. [DOI] [PubMed] [Google Scholar]
- 22.Marrone KA, Ying W, Naidoo J. Immune-related adverse events from immune checkpoint inhibitors. Clin Pharmacol Ther. 2016;100:242. doi: 10.1002/cpt.394. [DOI] [PubMed] [Google Scholar]
- 23.Drucker BJ. Renal cell carcinoma: current status and future prospects. Cancer Treat Rev. 2005;31:536. doi: 10.1016/j.ctrv.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Kjaer M, Frederiksen PL, Engelholm SA. Postoperative radiotherapy in stage II and III renal adenocarcinoma. A randomized trial by the Copenhagen Renal Cancer Study Group. Int J Radiat Oncol Biol Phys. 1987;13:665. doi: 10.1016/0360-3016(87)90283-5. [DOI] [PubMed] [Google Scholar]
- 25.Pizzocaro G, Piva L, Di Fronzo G, et al. Adjuvant medroxyprogesterone acetate to radical nephrectomy in renal cancer: 5-year results of a prospective randomized study. J Urol. 1987;138:1379. doi: 10.1016/s0022-5347(17)43647-0. [DOI] [PubMed] [Google Scholar]
- 26.Pizzocaro G, Piva L, Colavita M, et al. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: a multicentric randomized study. J Clin Oncol. 2001;19:425. doi: 10.1200/JCO.2001.19.2.425. [DOI] [PubMed] [Google Scholar]
- 27.Messing EM, Manola J, Wilding G, et al. Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: an Eastern Cooperative Oncology Group/Intergroup trial. J Clin Oncol. 2003;21:1214. doi: 10.1200/JCO.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Clark JI, Atkins MB, Urba WJ, et al. Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma: a cytokine working group randomized trial. J Clin Oncol. 2003;21:3133. doi: 10.1200/JCO.2003.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Atzpodien J, Schmitt E, Gertenbach U, et al. Adjuvant treatment with interleukin-2- and interferon-alpha2a-based chemoimmunotherapy in renal cell carcinoma post tumour nephrectomy: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN) Br J Cancer. 2005;92:843. doi: 10.1038/sj.bjc.6602443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passalacqua R, Caminiti C, Buti S, et al. Adjuvant low-dose interleukin-2 (IL-2) plus interferon-α (IFN-α) in operable renal cell carcinoma (RCC): a phase III, randomized, multicentre trial of the Italian Oncology Group for Clinical Research (GOIRC) J Immunother. 2014;37:440. doi: 10.1097/CJI.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 31.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 32.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 33.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 34.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 35.Buti S, Leonetti A, Dallatomasina A, et al. Everolimus in the management of metastatic renal cell carcinoma: an evidence-based review of its place in therapy. Core Evid. 2016;11:23. doi: 10.2147/CE.S98687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rini BI, Tomita Y, Melichar B, et al. Overall survival analysis from a randomized phase II study of axitinib with or without dose titration in first-line metastatic renal cell carcinoma. Clin Genitourin Cancer. 2016;14:499. doi: 10.1016/j.clgc.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer. 2013;49:1287. doi: 10.1016/j.ejca.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Yu SS, Quinn DI, Dorff TB. Clinical use of cabozantinib in the treatment of advanced kidney cancer: efficacy, safety, and patient selection. Onco Targets Ther. 2016;9:5825. doi: 10.2147/OTT.S97397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387:2008. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellmunt J, Dutcher J. Targeted therapies and the treatment of non-clear cell renal cell carcinoma. Ann Oncol. 2013;24:1730. doi: 10.1093/annonc/mdt152. [DOI] [PubMed] [Google Scholar]
- 41.Haas NB, Manola J, Dutcher JP, et al. Adjuvant treatment for high-risk clear cell renal cancer: updated results of a high-risk subset of the ASSURE randomized trial. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Poppel H, Joniau S, Van Gool SW. Vaccine therapy in patients with renal cell carcinoma. Eur Urol. 2009;55:1333. doi: 10.1016/j.eururo.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 43.Galligioni E, Quaia M, Merlo A, et al. Adjuvant immunotherapy treatment of renal carcinoma patients with autologous tumor cells and bacillus Calmette-Guèrin: five-year results of a prospective randomized study. Cancer. 1996;77:2560. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2560::AID-CNCR20>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 44.Jocham D, Richter A, Hoffmann L, et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet. 2004;363:594. doi: 10.1016/S0140-6736(04)15590-6. [DOI] [PubMed] [Google Scholar]
- 45.Wood C, Srivastava P, Bukowski R, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372:145. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 46.Chamie K, Donin NM, Klöpfer P, et al. Adjuvant weekly girentuximab following nephrectomy for high-risk renal cell carcinoma: the ARISER randomized clinical trial. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alme AKB, Karir BS, Faltas BM, et al. Blocking immune checkpoints in prostate, kidney, and urothelial cancer: an overview. Urol Oncol. 2016;34:171. doi: 10.1016/j.urolonc.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cella D, Grünwald V, Nathan P, et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:994. doi: 10.1016/S1470-2045(16)30125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]