Abstract
Cadaveric skin allograft is the current standard of treatment for temporary coverage of large burn wounds. Porcine xenografts are viable alternatives but undergo α-1,3-galactose (Gal)–mediated hyperacute rejection and are lost by post-operative day (POD) 3 because of naturally occurring antibodies to Gal in primate recipients. Using baboons, we previously demonstrated that xenografts from GalT-KO swine (lacking Gal) provided wound coverage comparable with allografts with systemic immunosuppression. In this study, we investigate topical immunosuppression as an alternative to prolong xenograft survival. Full-thickness wounds in baboons were created and covered with xenogeneic and allogeneic split-thickness skin grafts (STSGs). Animals were treated with slow-release (TyroSphere-encapsulated) topical formulations (cyclosporine-A [CSA] or Tacrolimus) applied 1) directly to the STSGs only, or 2) additionally to the wound bed before STSG and 1). Topical CSA did not improve either xenograft or allograft survival (median: treated grafts = 12.5 days, control = 14 days; P = 0.27) with similar results when topical Tacrolimus was used. Pretreatment of wound beds resulted in a significant reduction of xenograft survival compared with controls (10 vs 14 days; P = 0.0002), with comparable results observed in allografts. This observation was associated with marked reduction of inflammation on histology with Tacrolimus and not CSA. Prolongation of allograft and xenograft survival after application to full-thickness wound beds was not achieved with the current formulation of topical immunosuppressants. Modulation of inflammation within the wound bed was effective with Tacrolimus pretreatment before STSG application and may serve as a treatment strategy in related fields.
Much progress has been made in the treatment of acute burn injury with overall improved patient survival. A key component of this success derives from early excision and skin grafting of the burnt areas, which helps restore altered physiology including fluid dysregulation, metabolic disturbances, and systemic inflammatory response syndrome.1–8 While autografts are preferred for definitive closure of these wounds, the lack of sufficient donor skin may necessitate the use of cadaveric allografts, which remains the treatment of choice for temporary wound coverage.9 Application may be limited by cost and availability considerations however.
Alternative skin options include porcine xenografts consisting of dermis (EZ Derm, Brennan Medical, St. Paul, MN) or both epidermis and dermis (Mediskin, Brennan Medical, St. Paul, MN)9 that have been used, after chemical processing, in burn care patients clinically10 due to shared characteristics with human skin such as structural similarity of the rete ridges, papillary dermis, and epidermal thickness.11–15 However, typically by 72 hours after application, these xenografts often fail due to nonvascularization resulting from hyperacute rejection mediated by naturally occurring antibodies to α-1,3-galactose (Gal), a cell-surface antigen present on all porcine cells,15,16 that are found ubiquitously in primates.
Our laboratory has a genetically engineered herd of swine that lack the Gal moiety (GalT-KO).17 Organs and tissues from these GalT-KO pigs can thus be utilized, without the requirement for complement inhibition or antibody absorption, in xenotransplantation models of kidney, heart, liver, and skin12,18–20 without the development of hyperacute and acute humoral xenograft rejection. We have also recently demonstrated that GalT-KO skin grafts provided skin coverage comparable with allografts (median survival of xenografts = 14 days, allografts = 13 days) in baboon recipients.21 Prolongation of temporary burn wound coverage with alternative skin options would therefore be of considerable clinical benefit in allowing healing of autologous donor sites in the interim before reharvest for definitive wound closure.
Besides mitigation of immune responses through GalT-KO, systemic immunosuppression after burn injury has been demonstrated to have an association with prolonged allograft skin survival.22 Systemic immunosuppressive therapy may conceivably prolong both the survival of allogeneic and xenogeneic skin grafts but would, however, be undesirable in the context of severe burns in an already immunocompromised and critical patient. Topical delivery of immunosuppression would therefore represent an attractive alternative without the associated risks of systemic absorption. In the clinic, topical creams of Tacrolimus and steroids are currently available (e.g., Protopic, clobetasol) but frequent reapplication is required. Moreover, the highly lipophilic nature of these topical preparations and their poor absorption across the stratum corneum barrier23 may result in variable tissue dosages.24
To overcome the aforementioned limitations with topical immunosuppression, various skin penetration enhancers such as Azone, dimethyl sulfoxide, menthol, and both lipophilic and hydrophilic vehicles have been used to improve drug absorption,25 but these lipid-based colloidal systems have limited drug loading and low stability, thereby reducing their clinical use.26 Kohn et al have developed biodegradable nanoparticles made of tyrosine-derived tri-block copolymers (referred to herein as TyroSpheres). TyroSpheres are amphiphilic, self-assemble to form a hydrophobic core to house lipophilic molecules (e.g., cyclosporine-A [CSA],27 Paclitaxel28) and have an external hydrophilic shell that enhances aqueous stability.29 They have also been shown to provide controlled drug release,27,28 improve skin penetration of hydrophobic agents,30 and can be formulated into a gel dressing without easy “run-off.”27 Thus, the use of TyroSpheres can conceivably provide a convenient topical application schedule while maintaining continuous local release of the pharmaceutical26 and avoid systemic drug absorption and related complications.
By building on our previous work in which GalT-KO xenogeneic and allogeneic skin graft survival could not be prolonged despite additional systemic immunosuppression,21 the objectives of this study are: 1) to characterize the release of immunosuppressants from TyroSpheres in vitro, 2) evaluate the safety of TyroSpheres in a nonhuman primate (NHP) model, and 3) determine if topical immunosuppressant-loaded TyroSpheres alone could prolong the survival of GalT-KO xenogeneic and allogeneic skin grafts in an NHP model.
METHODS
Preparation of TyroSpheres Loaded With CSA or FK506
Polymeric micelles were prepared via a self-assembly technique. Preparation of the empty (vehicle only) and CSA-loaded TyroSpheres has been reported previously.27 For FK506-loaded Tyrospheres, polymer (60 mg/0.3 ml) and drug (60 mg/ml, equivalent to 30% wt/wt initial loading) were each dissolved in dimethyl formamide, mixed in equal amounts (0.3 ml each) and added drop-wise to phosphate-buffered saline (PBS; 14.4 ml) with constant stirring. The resulting suspension was passed through 0.22 μm polyvinylidene fluoride (PVDF) filters (Millipore) and ultra-centrifuged for 3 hours at 65,000 RPM and 18°C. The supernatant was then aspirated, polymeric micelles rinsed twice with PBS, resuspended in PBS, ultra-centrifuged again before resuspension in PBS overnight at 25 ± 2°C. FK506-loaded TyroSpheres with 10, 20, 40, 50, and 60 wt% theoretical drug loading were similarly prepared and characterized for loading efficiencies and actual (measured) loading content.
Determination of Drug Concentration Using HPLC
A Waters 2695 High-Performance Liquid Chromatography (HPLC) and Empower Pro software (Waters, Milford, CT) was used for validation of our FK506 method. Optimized chromatographic conditions include column—Waters-C18, 2.1 × 50 mm, 5 μm particle size; column temperature—50°C; injection volume—10 μL; detection wavelength—213 nm; mobile phase: A = HPLC water: B = acetonitrile (ACN; isocratic A:B [42.5:57.5]); flow rate—0.5 mL/min; run time—8 minutes; sample diluent—ACN.
Standards were prepared by making 1 mg/ml solution of FK506 in ACN before diluting to 500 µg/ml. Remaining standards were prepared by serial dilution to reach 7.8 µg/ml. A 100 µl aliquot of FK506-Tyrospheres was then lyophilized and extracted into 2 ml of ACN and filtered with 0.44 µm filters into HPLC vials. Samples were analyzed by the above parameters to determine the concentration of FK506 in TyroSpheres. HPLC quantification of CSA has been described.27
Loading Content and Loading Efficiency
Loading content and efficiency were determined by adding aliquots of drug-loaded (varying theoretical loading, 10–60 wt%, as described above) and empty TyroSpheres to PBS in preweighed vials. Samples were frozen on dry ice, lyophilized for ≥ 24 hours, and reweighed to determine the mass of salts, and empty and drug-loaded TyroSpheres. Two milliliters of ACN was added to loaded TyroSphere samples followed by 1 hour of vigorous vortexing to determine drug mass. Drug quantification was determined by HPLC and a standard curve. Equations used were
| (1) |
| (2) |
Release of FK506 From TyroSpheres
FK506 release was examined using 0.5 mL dialysis cassettes with a 10,000 molecular weight cut-off (MCWO) cellulose dialysis membrane (Slide-A-Lyzer, Fischer, Pittsburgh, PA). FK506-loaded TyroSpheres were diluted with PBS to a drug concentration of 400 µg/ml and added to the cassettes. Once loaded, cassettes were submerged in 200 mL of PBS and stored in a shaking (60 rpm) water bath at 37°C. Cassette content was then collected at predetermined time points for up to 168 hrs and added to the washed (rinsed 3 times with fresh PBS) compartment of these cassettes. FK506 concentration was determined from the donor compartment of cassettes via HPLC subsequent to lyophilization and extraction with ACN. The released FK506 within the receptor compartment was below detection limits by mass balance due to its large volume. CSA release from TyroSpheres has been described previously.27
Preparation of 30 wt% FK506-TyroSpheres-1% (wt/vol) HPMC Gel Formulation
Hydroxypropyl methylcellulose (HPMC, 150 mg) was suspended in 10 ml of 1× PBS and stirred at 400 rpm (to avoid bubble formation) for 48 hours at 25°C. Five milliliters of FK506-TyroSpheres (30 wt%) were then syringe filtered (0.22 μm, Millipore) and mixed with 1.5% (wt/vol) HPMC gel (10 ml) to create a suspension of 30 wt% FK506-TyroSpheres in 1% (wt/vol) HPMC gel (referred herein as FK506-TyroSpheres-gel), and stirred for 18 hours at room temperature. The suspension was then stored at 4°C until use.27 Vehicle-only controls were similarly prepared by replacing FK506-loaded Tyrospheres with empty TyroSpheres before mixing with 1.5% (wt/vol) HPMC gel as described above.
Animals
This study was approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee and the U.S. Army Medical Research and Materiel Command Animal Use and Review Office and performed in accordance with The Guide for the Care and Use of Laboratory Animals.31 Thirteen baboons (Papio hamadryas) were obtained from Mannheimer Foundation, Inc, Homestead, FL. All baboons were aged 2 to 5 years, weighed 7 to 12 kilograms each, and underwent routine pathogen screening and quarantine before commencement of experimental studies.
Genetically-engineered GalT-KO miniature swine were produced in our swine facility.18 The GalT-KO swine herd is monitored using a computerized system to ensure availability and quality control and housed in a purpose-built facility, which is fully integrated as part of the animal facilities in the laboratory with input and veterinary oversight from the Center for Comparative Medicine of the Massachusetts General Hospital.
Skin Graft Harvest
GalT-KO donor swine were anesthetized with I.M. Telazol 2 mg/kg, intubated, and had anesthesia maintained with 2% isoflurane and oxygen. Presurgery, the area of donor skin was disinfected with 2% chlorhexidine acetate (NolvasanR Surgical Scrub, Fort Dodge Animal Health, Fort Dodge, IA), 70% isopropyl rubbing alcohol (NolvasanR Surgical Scrub, Owens & Minor, Mechanicsville, VA), and 10% povidone-iodine (Betadine Solution, Purdue Products, L.P., Stamford, CT) before sterile draping. An air-driven Zimmer dermatome (Medfix Solution, Inc., Tucson, AZ) with a depth of 0.022 inches was used to harvest split-thickness skin grafts (STSGs) from the dorsum of the animal. Saline and epinephrine soaked gauze was applied to skin donor sites for hemostasis before dressing with Telfa™ (Covidien, Dublin, Ireland) and Tegaderm™ (3M, St. Paul, MN).
Baboon donors were sedated with I.M. atropine 0.1 mg/kg and I.M. ketamine 20 mg/kg, shaved, and transferred to the operating room for endotracheal intubation and general anesthesia with 2% isoflurane and oxygen similar to the above. Skin donor sites on the baboon’s back was prepped as described above for GalT-KO swine.
Wound Preparation and Skin Graft Placement
Baboon recipients were premedicated, shaved, anesthetized, and prepped as described above. Sharp excision of full-thickness skin down to muscle was performed to create defects measuring 4 × 5 cm. STSGs were fenestrated before application onto these defects. TyroSpheres-gel dressings were then applied as described below before pressure dressings were created with sterile dry gauze and secured with VetrapTM (3M, St. Paul, MN) bandaging tape to reinforce the underlying TelfaTM and TegadermTM wound dressings placed over the STSG.
Experimental Design
Thirteen baboons were used in 4 experiments. The first 3 experiments were designed for toxicity and dose titration studies (Table 1) while the final experiment investigated the clinical efficacy of CSA- and FK506-TyroSpheres-gel dressings (Table 2).
Table 1.
Toxicity and dose titration studies
| Experiment | N | Skin Grafts Per Animal | Treatment | Treatment Frequency | Study End Point |
|---|---|---|---|---|---|
| 1 (empty vehicle) | 2 | 3 autologous STSGs | • Control (BacitracinTM) • TyroSpheres-gel (from POD 0) • TyroSpheres-gel (from POD 2) |
Every other day up to POD 8 | POD 14 |
| 2 (toxicity and systemic uptake) | 2 | 1 autologous STSG | • 2.0 mL CSA-TyroSpheres-gel dressing (from POD 0) | Every other day | POD 14 |
| 3 (dose response) | 1 | 4 autologous STSGs | • CSA-TyroSpheres-gel dressing (from POD 0) • 0.2 ml • 0.5 ml • 1.0 ml • Control (BacitracinTM) |
Every other day | POD 14 |
CSA, cyclosporine-A; POD, postoperative day; STSG, split-thickness skin graft.
Table 2.
Design of experiment 4 to evaluate clinical efficacy of CSA- and FK506-TyroSpheres-gel dressings
| Group | N | Primary Grafts | Secondary Grafts | Treatment |
|---|---|---|---|---|
| 1A | 2 | GalT-KO xenogeneic STSG | Allogeneic STSG | CSA-TyroSpheres-gel dressings on alternate days |
| 1B | 2 | GalT-KO xenogeneic STSG | Allogeneic STSG | CSA-TyroSpheres-gel dressings to wound bed before STSG and on alternate days thereafter |
| 2A | 2 | GalT-KO xenogeneic STSG | Allogeneic STSG | FK506-TyroSpheres-gel dressings on alternate days |
| 2B | 2 | GalT-KO xenogeneic STSG | Allogeneic STSG | FK506-TyroSpheres-gel dressings to wound bed before STSG and on alternate days thereafter |
CSA, cyclosporine-A; FK506, Tacrolimus; GalT-KO, α-1,3-galactose (Gal) knock-out; POD, postoperative day; STSG, split-thickness skin graft.
In experiment 1, each animal received 3 autologous STSGs: 1) control (BacitracinTM only, no TyroSpheres application), 2) TyroSpheres-gel (vehicle only) dressing applied immediately postoperatively, and on alternate days thereafter, and 3) TyroSpheres-gel (vehicle only) dressing applied on alternate days from the second postoperative day. Previous experience with other topical immunosuppressants suggests that treatment in the immediate postoperative phase may impair vascularization of the STSG (data not published), hence the inclusion of a delayed treatment option. TyroSpheres-gel dressings were applied directly to the STSGs, with each application consisting of an empiric dose, sufficient to lightly coat the STSG with minimal excess as topical agents are most frequently applied in this manner in the clinic.
In experiment 2, animals that were previously sensitized to xenogeneic and allogeneic STSGs were chosen. Once the STSG was secured, a 2.0 ml dose of CSA-TyroSpheres-gel dressing (containing ~2.5 mg/ml CSA) was applied, in preference to the previously used empiric-dosing scheme in order to standardize the dose of CSA being administered and to facilitate interpretation of systemic uptake data, followed by standard dressings as before. STSGs were assessed, and CSA-TyroSpheres-gel dressings (2.0 ml) reapplied on alternate days. Systemic uptake of CSA was assessed by submission of peripheral blood samples for standardized assay.
In experiment 3, three autologous STSGs were each treated with a different volume (0.2, 0.5, or 1.0 ml) of CSA-TyroSpheres-gel dressing on alternate days. The fourth STSG served as an internal control (with BacitracinTM) and was not treated with CSA-TyroSpheres-gel dressings. Peripheral blood was drawn on alternate days for monitoring of CSA uptake.
In experiment 4, eight baboons received a series of STSGs as described previously.21,32 Briefly, each baboon received 4 STSGs on separate full-thickness wounds on the dorsum with GalT-KO xenografts placed first. Following rejection, the wound beds were debrided and allogeneic STSGs were placed. Recipient animals in Groups 1A and 2A were then treated with CSA- and FK506-TyroSpheres-gel dressings every other day respectively until rejection. In groups 1B and 2B, CSA- and FK506-TyroSpheres-gel dressings were applied at the time of surgery (before anchoring down of STSG) to assess whether immunosuppression bioavailability might be a confounding factor, followed by every other day application as described in groups 1A and 2A.
Clinical and Histological Assessment
Recipients underwent clinical evaluation every other day during which TyroSpheres-gel application was repeated and fresh dressings applied, up to post-operative day (POD) 14. Skin grafts were assessed for viability and integrity and determined to be rejected when less than 10% of viable graft tissue covered the wound.33 Median graft survivals were compared using a log-rank test in GraphPad Prism, version 5.04 for Windows, GraphPad Software (San Diego, CA). Six millimeter punch biopsies were taken from viable areas of the grafts at 4-day intervals postoperatively and H&E stained slides were evaluated for features of rejection by a pathologist blinded to specimen identity.
RESULTS
Drug Loading Content and Loading Efficiency
Figure 1 shows the drug loading content and loading efficiency of FK506 into TyroSpheres with the theoretical loading content ranging from 10% to 60% (wt/wt) following 2 ultracentrifugation steps, which are required to ensure proper purification of the TyroSpheres for subsequent evaluation in vivo. It was determined that the loading efficiency of FK506 into TyroSpheres was similar (40–50 wt%) regardless of theoretical loading content. The ~50% loss of drug occurs during the washing and filtration steps of the process. It is important to note that despite this loss of drug, the actual loading content nearly equals the theoretical loading content, suggesting that the actual (measured) loading content is very close to the theoretical loading content. Previous studies have shown that a theoretical loading content of 30 wt% was the maximum amount of CSA that could be loaded into TyroSpheres with a loading efficiency of nearly 50%.27 To compare the safety and efficacy of similar doses of drug, subsequent studies reported herein use 30 wt% CSA- or FK506-loaded TyroSpheres.
Figure 1.

Loading content and loading efficiency of FK506-loaded TyroSpheres with theoretical loading concentrations ranging from 10 to 60 wt%.
In Vitro Release of FK506 From TyroSpheres
Figure 2 shows the release profile of 30 wt% FK506 from TyroSpheres in PBS at 37°C under sink conditions. An initial burst was observed where 29% of the payload was released from the TyroSpheres within 8 hours of incubation, followed by significantly slower release kinetics throughout the remainder of the study (38% at 24 hours, 42% at 3 days, and 50% at 7 days). As previously reported, 30 wt% CSA-loaded TyroSpheres provided an initial burst release of 23% of the payload within 24 hours, followed by a sustained release of the remaining drug over the next 7 days (52% at 3 days and 75% at 7 days).27 The release of FK506 from TyroSpheres was significantly slower than CSA, probably due to the more hydrophobic nature of FK506 compared with CSA.
Figure 2.
Release profile of FK506 from dialysis cassettes in 1× PBS up to 7 days.
Toxicity and Dose Titration Studies
In experiment 1, application of TyroSpheres-gel (vehicle only) dressing on alternate days had no detectible negative effect on graft healing. No significant difference in outcome was detectable between grafts treated from POD 0 and those treated from POD 2 (Figure 3). These results suggest that the vehicle itself does not have an adverse effect on the vascularization of autologous STSGs. This remained true at all time points up to POD 8 and following cessation of treatment to the conclusion of follow-up at POD 14. Importantly, it was also observed that the skin surrounding the STSG also remained healthy. This experiment demonstrated that the topical application of TyroSpheres-gel dressing in an NHP model was nontoxic.
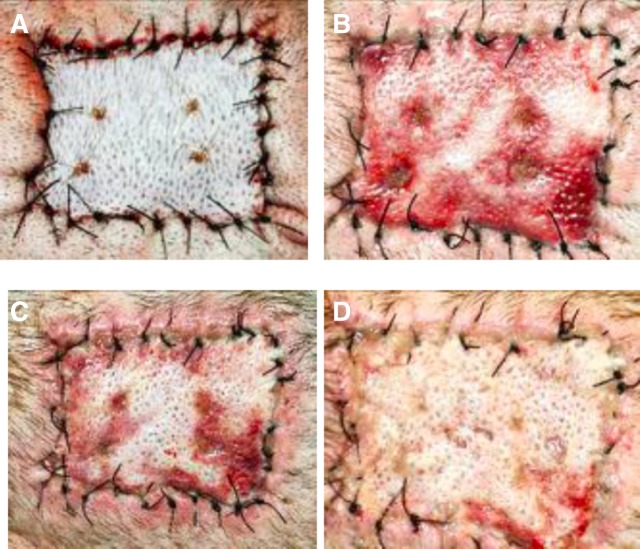
Figure 3.
Autografts were treated with empty TyroSpheres-gel to determine if skin graft healing would be affected by the vehicle. These photos are taken on POD 8. An untreated autograft was placed as a control (A). No detrimental healing effects were observed when the empty TyroSphere-gel was applied to an autograft every other day starting on POD 0 (B) or on POD 2 (C).
In experiment 2, STSGs treated with 2.0 mL of CSA-TyroSpheres-gel dressings demonstrated impaired healing and maceration by POD 4, progressing to sloughing and graft loss by POD 8. At PODs 2 and 4, the STSG had taken 100% to the new recipient site. The degree of bruising observed was within normal limits for STSGs and consistent with untreated control STSGs (data not shown) and indicates that the graft microvasculature, which is subject to trauma during the harvest procedure, had established connection with the vasculature of the recipient wound bed. However, by POD 6, significant thinning and paling of the STSGs was observed in addition to maceration of the STSG (Figure 4) and progressed to complete loss by POD 8. This phenomenon was not observed in groups treated with TyroSpheres-gel (vehicle only, experiment 1) dressings. CSA levels in the blood were negligible throughout the duration of the study. All values were below the sensitivity limit of the commercial assay used (Table 3).
Figure 4.
No deleterious effect was observed clinically in autografts treated with 0.4% CSA-TyroSpheres-gel every other day. The grafts were observed on POD 0 (A), POD 2 (B), POD 4 (C), and POD 6 (D).
Table 3.
No systemic absorption of CSA was observed at any time point postoperatively when autografts were treated with 0.4% CSA-TyroSpheres-gel every other day
| POD | Systemic CSA Level (ng/ml) |
|---|---|
| 0 | 0.0 |
| 2 | 0.0–0.4 |
| 4 | 0.0–1.7 |
| 6 | 0.0–3.5 |
CSA, cyclosporine-A; POD, postoperative day.
In experiment 3, the 1.0 mL CSA-TyroSpheres-gel dressing–treated STSG showed signs of maceration by POD 6 (Figure 5) and became ulcerated by POD 8. Thereafter, 50% of the STSG was lost, while the remainder gradually healed. No deleterious effects were observed in STSGs treated with 0.2 mL and 0.5 mL CSA-TyroSpheres-gel dressings, and no significant difference in appearance was noted between these and the untreated control STSG. Consistent with the result observed in experiment 2, CSA could not be detected in peripheral blood at any time point. Results of this experiment suggested that a dose of 0.2 mL or 0.5 mL would avoid negative effects on STSG healing and would be suitable for use in experiment 4 to lightly and sufficiently coat the STSG with minimal excess.
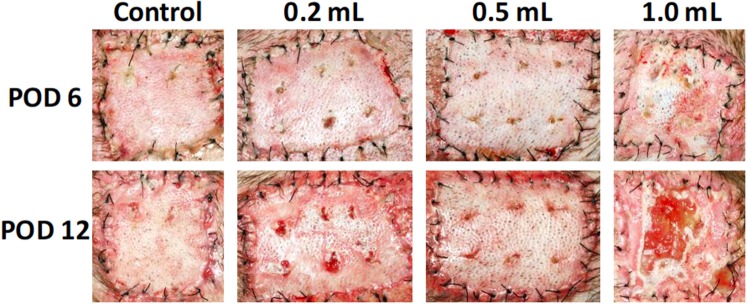
Figure 5.
A dose titration of autografts treated with 0.4% CSA-TyroSpheres-gel every other day demonstrated no adverse effect on graft healing when using 0.2 ml or 0.5 ml of the TyroSpheres-gel per graft. However, when 1.0 ml of the TyroSpheres-gel was used, the autografts became macerated and sloughed by POD 12. An untreated control was performed for comparison.
Clinical Efficacy Study
Following rejection of xenogeneic STSGs, all wound beds were debrided and covered with allogeneic STSGs. 0.5 ml CSA- and FK506-TyroSpheres-gel dressings were utilized.
In groups 1A and 2A, xenogeneic STSGs from GalT-KO swine survived a median of 12.5 and 12 days when treated topically with CSA- and FK506-TyroSpheres-gel dressings, respectively, compared with a median of 14 days when treated with standard STSG dressing, that is, BacitracinTM ointment (P = 0.27; P = 0.16). Allogeneic STSGs survived a median of 14 days when treated with CSA- and FK506-TyroSpheres-gel dressings compared with a median of 13 days when treated with standard BacitracinTM dressing (P = 0.80; P = 0.80; Figure 6). Biopsies of CSA-TyroSpheres-gel dressings–treated STSGs also revealed ischemia with clinical appearances similar to control BacitracinTM–treated STSGs (Figure 6A, B).
Figure 6.
A. Kaplan-Meier survival analysis for xenogeneic and allogeneic skin grafts treated with standard BacitracinTM or CSA/FK506-TyroSpheres-gel. Representative histology at 400× magnification showing hematoxylin and eosin–stained slide from a biopsy taken on POD 6 of an allograft treated with CSA-TyroSpheres-gel (B). Diffuse dermal rejection (*) with ischemic changes in the epidermis is seen in this graft. (C) Shows a 400× magnification hematoxylin and eosin–stained slide from a biopsy taken on POD 4 of an allograft treated with the standard wound care agent, BacitracinTM. This graft demonstrated early epidermal changes (†) consistent with ischemia and early rejection.
In groups 1B and 2B, xenogeneic STSGs had significantly shorter survival with a median of 10 days each when treated topically with CSA- and FK506-TyroSpheres-gel dressings, which was significantly shorter compared with BacitracinTM–treated control STSGs (median = 14 days; P = 0.0002; P = 0.0002), despite pretreatment with CSA- and FK506-TyroSpheres-gel dressings into the wound bed. Allogeneic STSGs survived a median of 11 and 12 days when treated with CSA- and FK506-TyroSpheres-gel dressings, compared with a median of 14 and 13 days, respectively, when treated with standard BacitracinTM dressing (P = 0.0009; P = 0.0011). The STSGs appeared to take clinically but rapidly became ischemic and sloughed off by POD 8 (Figure 7). Compared with control-treated grafts however, FK506-TyroSphere-gel dressings led to profoundly reduced inflammation in both the graft and wound bed (Figure 7A, B).
Figure 7.
A. Kaplan-Meier survival analysis for xenogeneic and allogeneic skin grafts treated with standard BacitracinTM or CSA/FK506-TyroSpheres-gel after initial treatment of the underlying wound bed. Representative histology at 400× magnification showing hematoxylin and eosin–stained slide from biopsies taken on POD 4 of xenografts treated with CSA- “underlay” TyroSphere-gel and topical CSA-TyroSpheres-gel (B) or BacitracinTM (C). The CSA-TyroSphere-gel–treated grafts demonstrated some ischemic changes in the epidermis (†), but there is evidence of epithelial reconstitution. Also, there is some mild dermal inflammation (*). The Bacitracin-treated control graft demonstrates diffuse dermal inflammation (*).
DISCUSSION
Burns are extremely common and can have lasting physical and psychological effects on patients. According to the latest annual report of the American Burn Association, more than 450,000 patients received medical treatment for burn injuries, with more than 40,000 of these patients hospitalized.34 While burn care and treatment have dramatically improved over the past 20 years, more than 3000 deaths in 2015 were associated with burn injuries.34 In addition, from 2003 to 2007, the burn unit at Fort Sam Houston (USAISR) had 1497 hospitalizations, including 656 military patients, of which 540 were related to the conflict in Iraq.35
Allograft skin is the current accepted standard for the treatment of burn wounds when autologous donor sites are insufficient or not available while xenograft skin is an alternative option. Either way, rapid wound closure is necessary to avoid the sequelae of temperature dysregulation, fluid and electrolyte imbalance, and the risks of infection resulting from the loss of the skin barrier. Both allogeneic and xenogeneic skin grafts are typically rejected in a matter of days to weeks however, and further immunosuppression to halt the rejection process is deemed an unacceptable risk in burn patients who are already at increased risk of infection. Moreover, systemic immunosuppression is associated with other side effects including hypertension, nephrotoxicity, metabolic syndrome, and cancer.36 Herein, we investigated the safety and efficacy of the topical delivery of immunosuppression (CSA, FK506) using biodegradable nanospheres (TyroSpheres) to prolong allogeneic and xenogeneic STSG survival while avoiding systemic absorption and exposure to immunosuppressants.
The first 3 experiments demonstrated that CSA- and FK506-TyroSpheres-gel could be applied in the form of a topical dressing (0.2–0.5 ml) to STSGs from both allogeneic and xenogeneic sources without the risks of systemic exposure and consequent complications. However, no significant prolongation of STSG survival was observed in vivo with topical or topical and wound bed application of CSA- or FK506-TyroSpheres-gel dressing. Of note, histology revealed that both xenogeneic and allogeneic STSGs placed on wound beds pretreated with FK506-TyroSpheres-gel had markedly reduced numbers of inflammatory cells present, whereas CSA-treated wound beds were minimally affected. It is unclear whether the dose of the current immunosuppressants was not optimal for prolonging skin graft survival or if the drugs themselves were unable to prolong skin graft survival.
Previous, related studies have investigated the topical application of both CSA and FK506 after allo-STSG in murine37–39 models. Untreated control grafts were typically lost within 7 days but prolonged survival (up to > 120 days) could be achieved when treated with daily topical CSA or FK506. Of note, increased allo-STSG survival with topical CSA was associated with systemic absorption of CSA (250–500 ng/ml)39 but not with topical FK506 (< 0.5 ng/ml).38 Interestingly, the reduction in cellular infiltration of STSGs was also observed in the study with topical CSA (with systemic absorption).39 The resistance of alloreactive leucocytes to topical CSA may account for this phenomenon, although it may subsequently be overcome by increased dosaging (and presumably, systemic absorption).40 In contrast, systemic absorption of CSA and FK506 was not observed in our study even though decreased cellular infiltration was observed with FK506-TyroSpheres. Perhaps the difference in skin structure between animal models and immunosuppressant carrier for topical delivery in these studies may account for these discrepancies.
We therefore postulate that the dramatic reduction in inflammatory cells observed in our study impaired angiogenesis and vascularization of the STSGs, ultimately leading to non-take due to ischemia, suggesting that early graft loss was not mediated by adaptive immunity. To conclude, topical delivery of slow-release immunosuppressants through TyroSpheres alone does not significantly prolong either allograft or xenograft survival on full-thickness wound beds; however, preapplication to the wound bed was effective at reducing subsequent inflammation both in the wound bed itself and the STSG.
Future work will focus on the delivery of different immunosuppressive agent(s) with different mechanism(s) of action in vivo. Potential drugs include those that inhibit T cell targets, which are considered important in the rejection of skin.41 We will also study the effect of these topical, slow release immunosuppressant-loaded products on the immunological reaction to and survival of allogenically transplanted primarily vascularized skin flaps.
ACKNOWLEDGEMENTS
This work was supported by the Armed Forces Institute of Regenerative Medicine, Department of Defense, under Award No. W81XWH-08-2-0034. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702–5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. We also acknowledge funding from the National Institute of Health Grant No.1C 06 RR 20135-01 for production of the Miniature Swine breading facility.
This work was supported by the Armed Forces Institute of Regenerative Medicine, Department of Defense, under Award No. W81XWH-08-2-0034.
REFERENCES
- 1. Grunwald TB, Garner WL. Acute burns. Plast Reconstr Surg 2008;121:311e–9e. [DOI] [PubMed] [Google Scholar]
- 2. Sheridan RL. Burn care: results of technical and organizational progress. JAMA 2003;290:719–22. [DOI] [PubMed] [Google Scholar]
- 3. Garner WL, Magee W. Acute burn injury. Clin Plast Surg 2005;32:187–93. [DOI] [PubMed] [Google Scholar]
- 4. Sheridan RL. Comprehensive treatment of burns. Curr Probl Surg 2001;38:657–756. [PubMed] [Google Scholar]
- 5. Greenhalgh DG, Saffle JR, Holmes JH 4th, et al. . American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res 2007;28(6):776–790. [DOI] [PubMed] [Google Scholar]
- 6. Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet 2004;363:1895–902. [DOI] [PubMed] [Google Scholar]
- 7. Jewell L, Guerrero R, Quesada AR, Chan LS, Garner WL. Rate of healing in skin-grafted burn wounds. Plast Reconstr Surg 2007;120:451–6. [DOI] [PubMed] [Google Scholar]
- 8. Chester DL, Balderson DS, Papini RP. A review of keratinocyte delivery to the wound bed. J Burn Care Rehabil 2004;25:266–75. [DOI] [PubMed] [Google Scholar]
- 9. Saffle JR. Closure of the excised burn wound: temporary skin substitutes. Clin Plast Surg 2009;36:627–41. [DOI] [PubMed] [Google Scholar]
- 10. Chiu T, Burd A. “Xenograft” dressing in the treatment of burns. Clin Dermatol 2005;23:419–23. [DOI] [PubMed] [Google Scholar]
- 11. Lineen E, Namias N. Biologic dressing in burns. J Craniofac Surg 2008;19:923–8. [DOI] [PubMed] [Google Scholar]
- 12. Weiner J, Yamada K, Ishikawa Y, et al. . Prolonged survival of GalT-KO swine skin on baboons. Xenotransplantation 2010;17:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vodicka P, Smetana K Jr, Dvoránková B, et al. . The miniature pig as an animal model in biomedical research. Ann N Y Acad Sci 2005;1049:161–71. [DOI] [PubMed] [Google Scholar]
- 14. Elliott RA Jr, Hoehn JG. Use of commercial porcine skin for wound dressings. Plast Reconstr Surg 1973;52:401–5. [PubMed] [Google Scholar]
- 15. Song IC, Bromberg BE, Mohn MP, Koehnlein E. Heterografts as biological dressings for large skin wounds. Surgery 1966;59:576–83. [PubMed] [Google Scholar]
- 16. Galili U, Wang L, LaTemple DC, Radic MZ. The natural anti-Gal antibody. Subcell Biochem 1999;32:79–106. [DOI] [PubMed] [Google Scholar]
- 17. Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem 1988;263:17755–62. [PubMed] [Google Scholar]
- 18. Dor FJ, Tseng YL, Cheng J, et al. . alpha1,3-Galactosyltransferase gene-knockout miniature swine produce natural cytotoxic anti-Gal antibodies. Transplantation 2004;78:15–20. [DOI] [PubMed] [Google Scholar]
- 19. Tseng YL, Kuwaki K, Dor FJ, et al. . alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation 2005;80:1493–500. [DOI] [PubMed] [Google Scholar]
- 20. Kuwaki K, Tseng YL, Dor FJ, et al. . Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med 2005;11:29–31. [DOI] [PubMed] [Google Scholar]
- 21. Leto Barone AA, Mastroianni M, Farkash EA, et al. . Genetically modified porcine split-thickness skin grafts as an alternative to allograft for provision of temporary wound coverage: preliminary characterization. Burns 2015;41:565–74. [DOI] [PubMed] [Google Scholar]
- 22. Ninnemann JL, Fisher JC, Frank HA. Prolonged survival of human skin allografts following thermal injury. Transplantation 1978;25:69–72. [DOI] [PubMed] [Google Scholar]
- 23. Heuschkel S, Goebel A, Neubert RH. Microemulsions—modern colloidal carrier for dermal and transdermal drug delivery. J Pharm Sci 2008;97:603–31. [DOI] [PubMed] [Google Scholar]
- 24. Luengo J, Weiss B, Schneider M, et al. . Influence of nanoencapsulation on human skin transport of flufenamic acid. Skin Pharmacol Physiol 2006;19:190–7. [DOI] [PubMed] [Google Scholar]
- 25. Liu H, Li S, Wang Y, Yao H, Zhang Y. Effect of vehicles and enhancers on the topical delivery of cyclosporin A. Int J Pharm 2006;311:182–6. [DOI] [PubMed] [Google Scholar]
- 26. Batheja P, Sheihet L, Kohn J, Singer AJ, Michniak-Kohn B. Topical drug delivery by a polymeric nanosphere gel: formulation optimization and in vitro and in vivo skin distribution studies. J Control Release 2011;149:159–67. [DOI] [PubMed] [Google Scholar]
- 27. Goyal R, Macri L, Kohn J. Formulation strategy for the delivery of cyclosporine A: comparison of two polymeric nanospheres. Sci Rep 2015;5:13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sheihet L, Garbuzenko OB, Bushman J, Gounder MK, Minko T, Kohn J. Paclitaxel in tyrosine-derived nanospheres as a potential anti-cancer agent: in vivo evaluation of toxicity and efficacy in comparison with paclitaxel in Cremophor. Eur J Pharm Sci 2012;45:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sheihet L, Piotrowska K, Dubin RA, Kohn J, Devore D. Effect of tyrosine-derived triblock copolymer compositions on nanosphere self-assembly and drug delivery. Biomacromolecules 2007;8:998–1003. [DOI] [PubMed] [Google Scholar]
- 30. Sheihet L, Chandra P, Batheja P, Devore D, Kohn J, Michniak B. Tyrosine-derived nanospheres for enhanced topical skin penetration. Int J Pharm 2008;350:312–9. [DOI] [PubMed] [Google Scholar]
- 31. Committee for the Update of the Guide for the Care and Use of Laboratory Animals; Institute for Laboratory Animal Research; Division on Earth and Life Studies; National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academies Press (US); 2011. [Google Scholar]
- 32. Albritton A, Leonard DA, Leto Barone A, et al. . Lack of cross-sensitization between α-1,3-galactosyltransferase knockout porcine and allogeneic skin grafts permits serial grafting. Transplantation 2014;97:1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leight GS, Kirkman R, Rasmusen BA, et al. . Transplantation in miniature swine. III: effects of MSLA and A-O blood group matching on skin allograft survival. Tissue Antigens 1978;12:65–74. [DOI] [PubMed] [Google Scholar]
- 34. American Burn Association. Burn incidence and treatment in the United States; 2016. Available at http://www.ameriburn.org/resources_factsheet.php. Accessed March 26, 2016.
- 35. Renz EM, Cancio LC, Barillo DJ, et al. . Long range transport of war-related burn casualties. J Trauma 2008;64(2 Suppl):S136–44; discussion S144–5. [DOI] [PubMed] [Google Scholar]
- 36. Ciarcia R, Damiano S, Fiorito F, et al. . Hydrocortisone attenuates cyclosporin A-induced nephrotoxicity in rats. J Cell Biochem 2012;113:997–1004. [DOI] [PubMed] [Google Scholar]
- 37. Yuzawa K, Taniguchi H, Seino K, Otsuka M, Fukao K. Topical immunosuppression in skin grafting with FK 506 ointment. Transplant Proc 1996;28:1387–9. [PubMed] [Google Scholar]
- 38. Fujita T, Takahashi S, Yagihashi A, Jimbow K, Sato N. Prolonged survival of rat skin allograft by treatment with FK506 ointment. Transplantation 1997;64:922–5. [DOI] [PubMed] [Google Scholar]
- 39. Lai CS, Wesseler TA, Alexander JW, Babcock GF. Long-term survival of skin allografts in rats treated with topical cyclosporine. Transplantation 1987;44:83–7. [DOI] [PubMed] [Google Scholar]
- 40. Patam MA, Tran HS, Llull R, Chrzanowski FA Jr, Black KS, Hewitt CW. Site-specific immunosuppression: mechanisms of cellular immunosuppression that are operative at local and systemic levels. J Burn Care Rehabil 2000;21(1 Pt 1):10–9. [PubMed] [Google Scholar]
- 41. Shao K, Lu Y, Wang J, et al. . Different effects of tacrolimus on innate and adaptive immune cells in the allograft transplantation. Scand J Immunol 2016;83:119–27. [DOI] [PubMed] [Google Scholar]