Abstract
To describe patterns of depressive symptoms across 10-years by HIV status and to determine the associations between depressive symptom patterns, HIV status, and clinical profiles of persons living with HIV from the Multicenter AIDS Cohort Study (N = 980) and Women’s Interagency HIV Study (N = 1744). Group-based trajectory models were used to identify depressive symptoms patterns between 2004 and 2013. Multinomial logistic regressions were conducted to determine associations of depression risk patterns. A 3-group model emerged among HIV-negative women (low: 58%; moderate: 31%; severe: 11%); 5-groups emerged among HIV-positive women (low: 28%; moderate: 31%; high: 25%; decreased: 7%; severe: 9%). A 4-group model emerged among HIV-negative (low: 52%; moderate: 15%; high: 23%; severe: 10%) and HIV-positive men (low: 34%; moderate: 34%; high: 22%; severe: 10%). HIV+ women had higher odds for moderate (adjusted odds ratio [AOR] 2.10, 95% CI 1.63–2.70) and severe (AOR 1.96, 95% CI 1.33–2.91) depression risk groups, compared to low depression risk. HIV+ men had higher odds for moderate depression risk (AOR 3.23, 95% CI 2.22–4.69), compared to low risk. The Framingham Risk Score, ART use, and unsuppressed viral load were associated with depressive symptom patterns. Clinicians should consider the impact that depressive symptoms may have on HIV prognosis and clinical indicators of comorbid illnesses.
Keywords: HIV, Depression, Longitudinal, Comorbidities
Introduction
Depression is the most common psychological illness among persons living with HIV (PLWH), affecting between 18 and 81% in a systematic review [1]. A recent review found major depressive disorder to be 2–4 times more likely in PLWH than in HIV-negative persons [2]. As HIV transitions from an acute infection to a highly manageable long-term illness, long-term depressive symptoms and associated clinical indicators are becoming salient concerns for patients and clinicians. While depression has been suggested as a risk factor for health behaviors associated with HIV transmission [3–5] and may be associated with adjustment to the acquisition of new HIV-infection [6], depression may be evident during all stages of HIV-infection, making the long-term monitoring of symptoms paramount to treatment [2].
The majority of research related to depression has assessed relationships with health behavior, such as increased alcohol and substance use [7–10] and decreased ART adherence [11–13]. Research focusing on the relationship between depression and clinical profiles is sparse. As this population ages, the burden of age-related long-term illness may increase depression among PLWH and vice versa [14]. For instance, Schwartz et al. [15] found that women with significant depressive symptoms (75% or more of their follow-up visits) across nearly 10 years had higher Framingham Risk Scores, compared to women without long-term depressive symptoms. Among participants of the Veterans Aging Cohort, HIV+ male veterans with depression at baseline were more likely to experience heart failure nearly 6 years later [16]. Conversely, Parruti et al. [17] found no significant cross-sectional association between depressive symptoms and cardiovascular risk (carotid artery thickness and presence of arterial plaques). While these studies provide a framework for how depression may affect future clinical profiles, it is currently uncertain how comorbidities may affect depressive symptoms longitudinally.
Most studies that focused on risk factors for depression among PLWH are limited in the ability to assess individual change in symptoms overtime, as they used cross-sectional designs or cumulative depression scores and not patterns of depression. For example, Pyra et al. [18] aimed to assess long-term depressive symptom differences by sexual identity among women living with and without HIV, using generalized estimating equations [19], which models marginal (or population level) effects. Other studies have used cross-sectional methods [20] or have calculated long-term depression by creating a cumulative depression variable [15]. While these studies and others provide a platform for which to conceptually understand risk factors for depression, they do not provide insight on how symptoms change over time.
Although women constitute nearly 25% of new HIV-infections in the United States [21], most studies of depression among PLWH have focused on majority male samples [1]. The preponderance of evidence from research among the general population suggests that there are significant sex differences in the prevalence and long-term severity of depressive symptoms [22]. Currently, it is unclear how depressive symptoms change over time among PLWH, if depressive symptom patterns differ by HIV status, and whether depressive symptoms vary by clinical comorbidities among PLWH.
Given the relatively little longitudinal research on depressive symptoms and comorbidities among PLWH, the goals of this analysis are to (1) describe patterns of depressive symptoms over a 10-year period by sex and HIV status through group-based trajectory modeling (GBTM), (2) determine whether differences in depressive symptom trajectory exist by HIV status, and (3) assess the association between depressive symptom trajectories and the clinical profiles of PLWH.
There are several advantages of using group-based trajectory modeling over traditional longitudinal methods, such as generalized estimating equations (GEE) and generalized linear mixed models (GLMM). The most notable benefit is the ability to identify latent strata that reflect group differences in patterns [23]. While both GEE and GLMM control for individual differences, these methods either focus specifically on a marginal pattern of change (i.e., GEE) or rely on differences in change by specific risk factors (GLMM). Therefore, GBTM allows us to take a more person-based approach to analyze change, and to identify distinct patterns of change that are sub-stantively meaningful, and that do not assume a general mean change pattern of an entire sample.
We hypothesized that distinct patterns of depressive symptoms would emerge, and that PLWH would have significantly greater long-term depressive symptoms compared to HIV-negative persons. We further hypothesized that poor clinical profiles (e.g., diabetes, increased BMI status and Framingham Risk Score, lower CD4 count and unsuppressed HIV viral load) would be associated with higher depressive symptom patterns. Clinical associations of long-term depressive symptoms would provide clinicians with the means to identify those with the greatest need for early intervention and sustained treatment.
Methods
Participants
The Multicenter AIDS Cohort Study (MACS) [24–26] and Women’s Interagency HIV Study (WIHS) [27, 28] are well-established, national multicenter cohorts of men who have sex with men (MSM) and of women, respectively, living with or at risk for HIV-infection. Participants from MACS were recruited from the following metropolitan areas: Baltimore, MD; Washington, DC; Chicago, IL; Pittsburgh, PA; and Los Angeles, CA. Participants from WIHS were recruited from the following metropolitan areas: Brooklyn and Bronx, NY; Washington, DC; Chicago, IL; and Los Angeles and San Francisco, CA. The MACS recruited MSM across 3 waves in 1984–1985 (n = 4954), 1987–1991 (n = 668), and 2001–2003 (n = 1350). Women were recruited in WIHS across 2 waves in 1994–1995 (n = 2625) and 2001–2002 (n = 1141). The data from these studies were collected from structured interviews and standardized physical, psychological, and laboratory assessments. Written informed consent was obtained prior to each semiannual assessment for both cohorts. IRB approval for the current analyses was obtained from the University of Florida. The questionnaires are available online for MACS at www.statepi.jhsph.edu/macs/forms.html and for WIHS at https://statepiaps.jhsph.edu/wihs/index-forms.htm.
The current study utilized data from participants of the cardiovascular sub-studies (enrollment in 2004) of the MACS and WIHS, to understand the associations between clinical profiles including cardiovascular disease risk factors (i.e., Framingham risk score, BMI, diabetes) and depressive symptoms prior to cardiovascular disease or related events. Therefore, this study focuses on depressive symptom patterns from 2004 to 2013. The cardiovascular substudy of WIHS included 1321 HIV+ and 606 HIV− women aged 25–60 years. The cardiovascular substudy of MACS included 828 HIV+ and 392 HIV− MSM older than 40 years of age and less than 300 lbs. Because one of the aims is to assess the effect of HIV status on depressive symptom patterns, we excluded those who seroconverted during the study follow-up. Further, those with fewer than 4 depression assessments were excluded. Death during follow-up occurred in 6.7% (n = 127) of WIHS and 5.1% (n = 52) of MACS participants. Those who died were more likely to have less than 4 depression measurements in WIHS (31% vs.≥4 measurements 5%, P < .001) and MACS (25% vs. ≥4 measurements 5%, P < .001). The mean person-years in the study was 8.2 years (interquartile range [IQR], 7.0–10.0 years) for WIHS and 8.1 years (IQR, 7.5–10.0 years) for MACS.
Main Outcome Measure
Depressive symptoms were assessed at each semiannual visit with the Center for Epidemiologic Studies Depression Scale (CES-D) [29]. The scores for each item were summed to create an overall score, ranging from 0 to 60. Higher scores indicated increased depressive symptoms. The validity of the CES-D has been reported for the general population and clinical subgroups, with a Cronbach a ranging from .84 to .93 [29–31]. Further, the CES-D performs well among PLWH, with a Cronbach α > .80 [32].
Independent Variables
Clinical Factors
HIV status was assessed by enzyme-linked immunosorbent assay, with Western blot for confirmation at baseline for HIV+ participants and semiannually for HIV− participants. Seroconversion was confirmed by testing HIV− participants at each semiannual visit using the aforementioned tests. For HIV+ participants, cumulative use of ART was calculated in years of use, multiplied by ART adherence, by the baseline of the 10-year follow-up period. Plasma HIV RNA viral load and CD4+ T cell count were measured using standard laboratory techniques. HIV RNA viral load was subsequently categorized as suppressed (<200 copies/mL) or unsuppressed (≥200 copies/mL) [33]. In order to capture varying levels of immune function, CD4+ T-cell count was categorized as high (≥500 cells/mm3), medium (300–500 cells/mm3), or low (<300 cells/mm3). Diabetes was dichotomized as having ever been diagnosed by baseline vs no history of diabetes. The Framingham Risk Score [34] was calculated, using the sex-based algorithms, including the following variables: age, total cholesterol level, high-density lipoprotein cholesterol level, systolic blood pressure, use of blood pressure lowering agents, and smoking status at baseline. Therefore, we did not adjust for these variables outside of this risk score. The Framingham Risk Score values range from negative to positive, with negative values indicating low risk and positive indicating high risk. Body mass index (BMI) was based on weight and height and was measured as a continuous variable.
Confounding variables
Race was self-reported and categorized as white, black, and other minority race (Asian/ Pacific Islander or Native American/Alaskan). Annual income was self-reported and categorized, based on natural cut-offs in the data, as <$10,000, $10,000–$30,000, or ≥$30,000 a year. Receipt of mental health treatment was assessed through self-reported use of an antidepressant medication or receipt of psychological treatment at baseline. Alcohol use was measured by asking about the average frequency (number of days per week) and quantity (number of drinks per drinking day) of alcohol use. The average number of drinks per week reported were calculated by multiplying the frequency by the quantity of drinks consumed, and consumption was categorized as having less than 1 drink per week, 1–7 for women or 1–14 drinks per week for men, and >7 for women or >14 drinks per week for men [35]. Self-reported illicit drug use was dichotomous and measured by asking whether participants used any of the following: crack or any form of cocaine; uppers (including crystal, methamphetamines, speed, or ice); or heroin or other opiates.
Data Analyses
Univariate and bivariate analyses were conducted to assess frequencies and proportions, as well as differences in characteristics by HIV status. The χ2 (categorical variables) and t (continuous by categorical variables) tests were used to examine statistical significance.
Group Based Trajectory Modeling
We conducted GBTM by cohort and HIV status. In the first modeling step, we assessed linear patterns of 1 to 6 groups, based on the number or groups found in extent literature [36–38]. All GBTMs were modeled as censored normal, which allows us to model trajectories of a variable with a truncated distribution. Models with a group(s) with less than 5% of the sample were rejected. Once the best fitting number of groups was identified, we assessed the best fitting change structure for each group (linear, quadratic, cubic). Goodness-of-fit was assessed at each step using the Akaike Information Criteria (AIC; the smaller the value, the better the model) and Bayesian Information Criteria (BIC; the smaller the value, the better the model), group posterior probabilities (PP ≥ 0.7 is indicative of sufficient internal reliability), and mean model entropy (≥0.7 is optimal; summed PP/number of groups). The PP estimate is the probability a group-based trajectory adequately captures the individual patterns. Models with PP and/or model entropy values less than 0.7 were rejected [39]. The 95% CIs of the resulting patterns were used to qualitatively assess the stability of the trajectories. Models with small 95% CIs of trajectories were favored over wide 95% CIs. Model fit information between tested models are available as supplemental data.
Multinomial Logistic Regression
Multinomial logistic regressions were conducted to assess differences of the depressive symptom patterns by HIV status, stratified by cohort. A second model including only HIV+ participants was conducted, also stratified by cohort. Clinical factors were considered significantly associated with depressive symptom patterns at the P < .05 cutoff.
Missing Data
Missing data were assessed and variables associated with missing data were identified. In MACS, 67% had less than 10% missing data. Missing greater than 10% was significantly associated with greater smoking (38% vs. 26%), illicit drug use (26% vs. 18%), higher CD4+ T-cell count (44% ≥ 500 cells/mm3 vs. 23%), and fewer cumulative years on ART (5.3 vs. 8.2), compared to those with less than 10% missing. In WIHS, 64% had less than 10% missing data. Missing greater than 10% was significantly associated with race (White 27% vs. 18%, Black 57% vs. 65%, Other minority 16% vs. 17%), lower BMI status (28.5 vs. 30.9), diabetes (21% vs. 28%), CD4+ T-cell count (31% ≥ 500 cells/mm3 vs. 42%), and fewer years on ART (4.2 vs. 6.8). Missingness was not associated with depressive symptoms (continuously or categorically) in MACS or WIHS. In order to address the potential for bias we conducted multiple imputation, averaged across 10 imputations.
All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc).
Results
Baseline characteristics by cohort and HIV status are presented in Table 1.
Table 1.
Baseline characteristics by cohort and HIV status
| Baseline characteristics | Column No. (%) Mean (SD) |
P Value | ||||
|---|---|---|---|---|---|---|
| WIHS (N = 1744) |
MACS (N = 980) |
|||||
| HIV− (n = 552) |
HIV+ (n = 1192) |
P Value | HIV− (n = 386) |
HIV+ (n = 594) |
||
| Race | .005 | <.001 | ||||
| White | 93 (17) | 277 (23) | 257 (67) | 307 (52) | ||
| African American/black | 370 (67) | 713 (60) | 105 (27) | 226 (38) | ||
| Other | 89 (16) | 202 (17) | 24 (6) | 61 (10) | ||
| Annual income, $ | .001 | <.001 | ||||
| <$10,000 | 232 (42) | 612 (51) | 65 (17) | 157 (26) | ||
| $10,000–$30,000 | 206 (37) | 385 (32) | 85 (22) | 211 (36) | ||
| ≥$30,000 | 114 (21) | 195 (16) | 236 (61) | 226 (38) | ||
| Receipt of mental health services | <.001 | <.001 | ||||
| No | 495 (90) | 939 (79) | 341 (88) | 465 (78) | ||
| Yes | 57 (10) | 253 (21) | 45 (12) | 129 (22) | ||
| Alcohol consumptiona | <.001 | .04 | ||||
| <1 drink/week | 219 (40) | 630 (53) | 43 (11) | 92 (16) | ||
| 1–7 [14] drinks/week | 275 (50) | 483 (40) | 312 (81) | 471 (79) | ||
| >7 [14] drinks/week | 58 (11) | 79 (7) | 31 (8) | 31 (5) | ||
| Illicit drug useb | <.001 | .01 | ||||
| No | 321 (69) | 955 (80) | 316 (84) | 441 (77) | ||
| Yes | 141 (30) | 237 (20) | 62 (16) | 131 (23) | ||
| Framingham risk score, mean (SD) | 8.1 (6.8) | 8.7 (5.9) | .08 | 13.4 (1.6) | 13.5 (1.6) | .23 |
| Ever diagnosed as having diabetes | .54 | .02 | ||||
| No | 416 (75) | 882 (74) | 227 (72) | 384 (65) | ||
| Yes | 136 (25) | 310 (26) | 109 (38) | 210 (35) | ||
| Body mass index, mean (SD) | 30.5 (4.8) | 29.4 (10.5) | .002 | 26.6 (4.1) | 25.5 (3.7) | <.001 |
| HIV RNA suppressionc | NA | NA | ||||
| Suppressed (<200 copies/mL) | 539 (45) | 417 (70) | ||||
| Unsuppressed (≥200 copies/mL) | 653 (55) | 177 (30) | ||||
| CD4+ T-cell countc | NA | NA | ||||
| ≥500 cells/mm3 | 452 (38) | 260 (44) | ||||
| 300–500 cells/mm3 | 342 (29) | 156 (26) | ||||
| <300 cells/mm3 | 398 (33) | 178 (30) | ||||
| Cumulative ART exposure, mean years (SD)c | NA | 5.8 (2.6) | NA | 7.2 (2.8) | ||
| Depressive risk pattern | <.001 | <.001 | ||||
| Low | 319 (58) | 335 (45) | 202 (52) | 201 (34) | ||
| Moderate | 174 (31) | 366 (31) | 58 (15) | 201 (34) | ||
| High | N/A | 304 (25) | 89 (23) | 130 (22) | ||
| Decreased | N/A | 79 (7) | N/A | N/A | ||
| Severe | 59 (11) | 108 (9) | 37 (10) | 62 (10) | ||
WIHS women’s interagency HIV study, MACS multicenter AIDS cohort study, ART antiretroviral therapy
1–7 drinks/week for women, 1–14 drinks/week for men; >7 drinks/week for women, >14 drinks/week for men
Includes crack cocaine, other forms of cocaine, methamphetamines (speed, meth, or ice), and ecstasy
Among HIV-seropositive participants only
Depressive Symptom Patterns
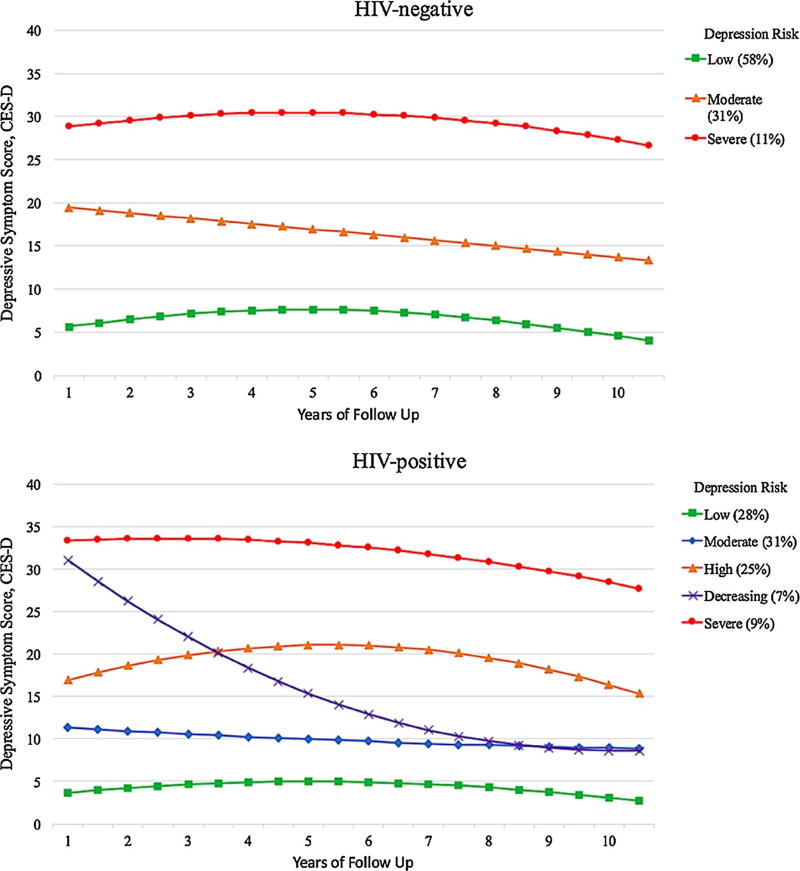
A 3-group trajectory model emerged as the best-fitting model for HIV-negative women (Fig. 1; model entropy, 0.96). Depression risk patterns were labeled as “low” (58%; PP 0.98, IQR .99–1.0; CES-D < 10 during most of 10-years), “moderate” (31%; PP 0.95, IQR .99–1.0; CES-D > 10 and <16 during most of 10-years), and “severe” (11%; PP 0.95, IQR .96–1.0; CES-D > 23 during most of 10-years). A 5-group trajectory model was the best-fitting model among HIV-positive women (Fig. 1; model entropy, 0.92): low (28%; PP 0.93, IQR .92–1.0), moderate (31%; PP 0.85, IQR .79–.99), high (25%; PP 0.92, IQR .83–.99; CES-D > 16 and <23 during most of 10-years), decreased (7%; PP 0.92, IQR .90–1.0; severe depressive symptoms that decrease over time), and severe (9%; PP .97, IQR .99–1.0).
Fig. 1.
Depressive symptom trajectories in the women’s interagency HIV study (WIHS) stratified by human immunodeficiency virus (HIV) status
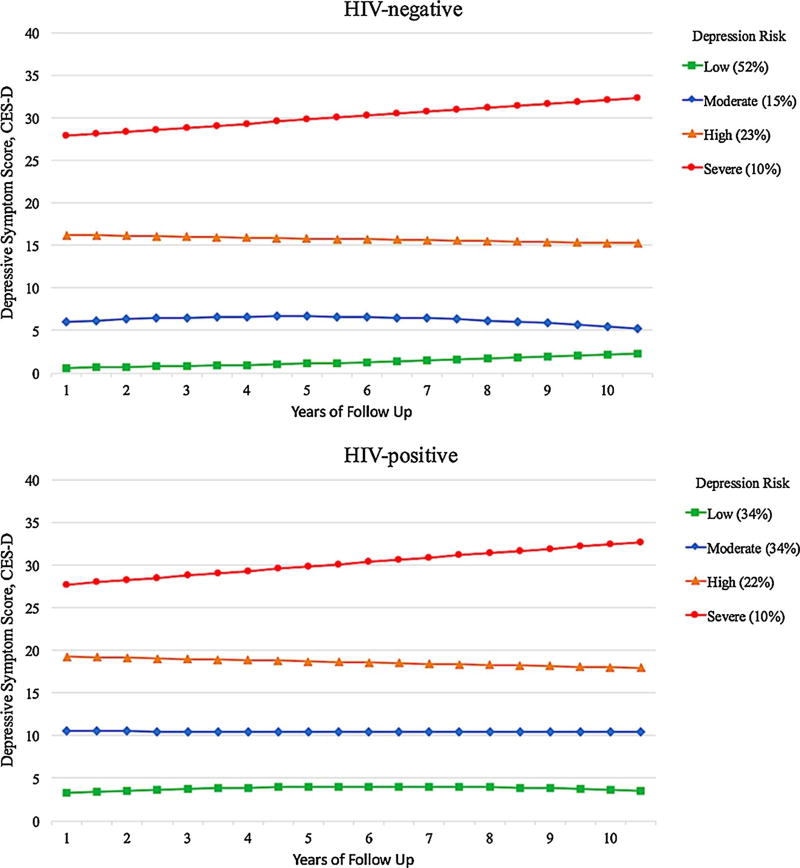
A 4-group trajectory model emerged as the best-fitting model for HIV-negative men (Fig. 2; model entropy, 0.96): low (52%; PP 0.96, IQR .99–1.0), moderate (15%; PP 0.96, IQR .98–1.0), high (23%, PP .97, IQR .99–1.0) and severe (10%; PP 0.97, IQR .99–1.0). A 4-group trajectory model was the best-fitting model among HIV-positive men (Fig. 2; model entropy, 0.95): low (34%; PP 0.97, IQR .99–1.0), moderate (34%; PP 0.92, IQR .88–.1.0), high (22%; PP 0.94, IQR .94–1.0), and severe (10%; PP .97, IQR .99–1.0).
Fig. 2.
Depressive symptom trajectories in the multicenter AIDS cohort study (MACS) stratified by human immunodeficiency virus (HIV) status
Multivariable Analysis of Depressive Symptom Patterns and HIV Status
Among women living with and without HIV (Table 2), HIV+ status was associated with 2.10 times higher odds for membership in the moderate depression risk group, compared to the low depression risk group (95% CI 1.63–2.70; P < .001). Likewise, HIV+ status was associated with 1.96 times higher odds for being in the severe depression risk group compared with the low risk group (95% CI 1.33–2.91; P < .001). Among men living with and without HIV, HIV+ status was associated with 3.23 times higher odds for membership in the moderate depression risk group (95% CI 2.22–4.69; P < .001), compared to the low risk group. In men, HIV status was not statistically significantly associated with membership in the high risk (adjusted odds ratio [AOR] 0.98, 95% CI 0.67–1.43) or severe risk group (AOR 0.97, 95% 0.58–1.64), compared to the low risk group.
Table 2.
The effect of HIV-status on depressive mood trajectory group membership among women and men living with and without HIV infection
| Reference group | Women
|
Men
|
|||
|---|---|---|---|---|---|
| Low risk (n = 654)
|
Low risk (n = 403)
|
||||
| Moderate risk (n = 540) |
Severe risk (n = 167) |
Moderate risk (n = 259) |
High risk (n = 219) |
Severe risk (n = 99) |
|
|
|
|
||||
| AOR (95% CI) | AOR (95% CI) | ||||
| HIV + status (reference = HIV-negative) | 2.10 (1.63–2.70)*** | 1.96 (1.33–2.91)*** | 3.23 (2.22–4.69)*** | 0.98 (0.67–1.43) | 0.97 (0.58–1.64) |
AOR adjusted odds ratio, 95% CI confidence interval
P < 0.10,
P < 0.05,
P < 0.01,
P < 0.001
Controlling for race, income, receipt of mental health services, alcohol consumption, illicit drug use, BMI, diabetes, and Framingham risk score
Multivariable Analysis of Depressive Symptom Patterns and Clinical Profiles Among HIV1 Women
Results of full models are shown in Table 3. Controlling for race, annual income, receipt of mental health services, alcohol consumption, and illicit drug use, each unit increase in the Framingham Risk Score was associated with a 3% increased odds for membership in the moderate (95% CI 1.00–1.06, P < .05) and high risk groups (95% 1.00–1.06, P < .05) and and 5% increased odds for membership in the severe (95% CI 1.00–1.09, P < .05) depression risk group, compared to the low risk group. Body mass index and diabetes were not statistically significantly associated with depressive symptoms patterns. Each increased year on ART was associated with 7% times lower odds for membership in the high risk group (95% 0.87–0.99, P < .05) and 14% lower odds for membership in the severe risk group (95% 0.79–0.94, P < .001), compared to the low risk group. Unsuppressed viral load was associated with 1.58 times higher risk in the high depression risk group (95% 1.10–2.28, P < .05), compared to the low risk group. Further, unsuppressed viral load was associated with increased odds for membership in the decreased risk group, compared to the high depression risk group (AOR 2.29, 95% 1.11–4.72, P < .05). Those with diabetes had 0.49 times lower odds for membership in the decreased risk group compared to the high risk group; however, statistical significance was not met (95% CI 0.22–1.08, P < .10).
Table 3.
Multivariable analysis of baseline predictors of depressive mood trajectory group membership among women living with HIV infection (N = 1192)
| Reference group | Low (n = 335)
|
High (n = 108) | ||
|---|---|---|---|---|
| Moderate (n = 366) | High (n = 304) | Severe (n = 108) | Decreased (n = 79) | |
|
|
||||
| AOR (95% CI) | AOR (95% CI) | |||
| Race (reference = white) | ||||
| African American/black | 0.92 (0.64–1.33) | 0.99 (0.66–1.50) | 0.74 (0.41–1.34) | 1.42 (0.58–3.47) |
| Other | 0.91 (0.55–1.53) | 1.45 (0.85–2.50) | 1.89 (0.93–3.82)† | 1.56 (0.56–4.39) |
| Annual income (reference ≥ $30,000) | ||||
| <$10,000 | 1.80 (1.19–2.72)** | 5.60 (3.29–9.53)*** | 9.89 (3.73–26.2)*** | 0.37 (0.10–1.43) |
| $10,000–$30,000 | 1.74 (1.15–2.63)** | 3.17 (1.83–5.52)*** | 4.95 (1.81–13.6)** | 0.54 (0.13–2.20) |
| Receipt of mental health services (reference = no) | 1.23 (0.81–1.86) | 2.27 (1.49–3.46)*** | 1.92 (1.09–3.38)* | 0.76 (0.35–1.64) |
| Alcohol consumption (reference < 1 drink/week) | ||||
| 1–14 drinks/week | 0.95 (0.69–1.31) | 0.86 (0.61–1.22) | 1.63 (1.00–2.67)* | 0.46 (0.23–0.93)* |
| ≥14 drinks/week | 2.09 (0.85–5.16) | 2.45 (0.99–6.09)* | 4.81 (1.68–13.7)** | 0.84 (0.30–2.40) |
| Illicit drug use (reference = no)a | 3.36 (1.34–8.41)** | 3.46 (1.38–8.69)** | 4.70 (1.73–12.7)** | 0.66 (0.26–1.65) |
| Body mass index, continuous | 1.00 (0.99–1.02) | 1.00 (0.98–1.02) | 0.98 (0.95–1.02) | 1.00 (0.95–1.06) |
| Diabetes (reference = no) | 1.08 (0.75–1.57) | 1.22 (0.82–1.80) | 1.39 (0.81–2.37) | 0.49 (0.22–1.08)† |
| Framingham risk score (continuous) | 1.03 (1.00–1.06)* | 1.03 (1.00–1.06)* | 1.05 (1.00–1.09)* | 1.01 (0.94–1.07) |
| Cumulative ART exposure, years | 0.99 (0.93–1.06) | 0.93 (0.87–0.99)* | 0.86 (0.79–0.94)*** | 1.10 (0.97–1.24) |
| Unsuppressed viral load (reference = no) | 1.27 (0.90–1.79) | 1.58 (1.10–2.28)* | 1.17 (0.71–1.93) | 2.29 (1.11–4.72)* |
| CD4+ T-cell count (reference ≥ 500) | ||||
| < 300 | 0.93 (0.63–1.37) | 1.03 (0.68–1.55) | 1.45 (0.83–2.53) | 0.67 (0.30–1.50) |
| 300–500 | 1.33 (0.92–1.94) | 1.01 (0.66–1.53) | 0.95 (0.51–1.77) | 0.69 (0.28–1.70) |
AOR adjusted odds ratio, 95% CI confidence interval
P < 0.10,
P < 0.05,
P < 0.01,
P < 0.001
Includes crack cocaine, other forms of cocaine, methamphetamines (speed, meth, or ice), and ecstasy
Multivariable Analysis of Depressive Symptom Patterns and Clinical Profiles Among HIV+ Men
Results of full models are shown in Table 4. Controlling for race, annual income, receipt of mental health services, alcohol consumption, and illicit drug use, each unit increase in the Framingham Risk Score was associated with 20% lower odds for membership in the severe depression risk group (95% CI 0.65–0.99, P < .05), compared to the low risk group. Diabetes was associated with 1.61 times higher odds for membership in the high depression risk group compared to the low risk group, however this did not reach statistical significant (95% CI 0.94–2.77, P < .10). Body mass index was not statistically significantly associated with depressive symptoms patterns. Each increased year of ART use was associated with 14% higher odds for membership in the moderate risk group (95% 1.05–1.25, P < .01), compared to the low risk group. Unsuppressed viral load was associated with 1.81 times higher risk in the high depression risk group (95% 1.03–3.18, P < .05), compared to the low risk group. A CD4+ T-cell count <300 (compared to >500) was associated with 1.82 times higher odds for membership in the high depression risk group (95% CI 0.97–3.43, P < .10) compared to the low risk group, however this association did not reach statistical significant.
Table 4.
Multivariable analysis of baseline predictors of depressive mood trajectory group membership among men living with HIV infection (N = 594)
| Reference groups | Low risk (n = 201)
|
||
|---|---|---|---|
| Moderate risk (n = 201) | High risk (n = 130) | Severe depression (n = 62) | |
|
|
|||
| AOR (95% CI) | |||
| Race/ethnicity (reference = white) | |||
| African American/black | 1.62 (0.95–2.78)† | 1.32 (0.70–2.49) | 0.48 (0.20–1.13)† |
| Other | 0.72 (0.33–1.55) | 0.65 (0.25–1.65) | 0.91 (0.35–2.36) |
| Annual income (reference ≥ $30,000) | |||
| <$10,000 | 2.00 (1.07–3.70)* | 2.30 (1.14–4.62)* | 8.59 (3.38–21.9)*** |
| $10,000–$30,000 | 2.09 (1.23–3.55)*** | 1.63 (0.85–3.13) | 4.10 (1.65–10.2)** |
| Receipt of mental health services (reference = no) | 1.84 (1.01–3.34)* | 4.75 (2.57–8.79)*** | 5.54 (2.62–11.7)*** |
| Alcohol consumption (reference < 1 drink/week) | |||
| 1–14 drinks/week | 1.67 (0.90–3.07)† | 0.81 (0.41–1.59) | 0.92 (0.38–2.23) |
| ≥14 drinks/week | 2.45 (0.85–7.06)† | 1.45 (0.43–4.89) | 1.09 (0.18–6.55) |
| Illicit drug use (reference = no)a | 1.67 (0.94–2.97)† | 2.89 (1.54–5.41)*** | 1.86 (0.83–1.18) |
| Body mass index, continuous | 1.04 (0.98–1.11) | 1.00 (0.94–1.08) | 0.99 (0.90–1.08) |
| Diabetes (reference = no) | 1.41 (0.89–2.25) | 1.61 (0.94–2.77)† | 1.25 (0.61–2.54) |
| Framingham risk score (continuous) | 1.03 (0.90–1.18) | 0.96 (0.82–1.13) | 0.80 (0.65–0.99)* |
| Cumulative HAART exposure, years | 1.14 (1.05–1.25)** | 1.01 (0.92–1.11) | 1.02 (0.91–1.15) |
| Unsuppressed viral load (reference = no) | 1.10 (0.66–1.85) | 1.81 (1.03–3.18)* | 1.54 (0.74–3.23) |
| CD4+ T-cell Count (reference >500) | |||
| <300 | 1.54 (0.91–2.63) | 1.82 (0.97–3.43)† | 1.71 (0.77–3.79) |
| 300–500 | 0.95 (0.57–1.59) | 1.38 (0.76–3.79) | 1.34 (0.62–2.90) |
AOR adjusted odds ratio, 95% CI confidence interval
P < 0.10;
P < 0.05;
P < 0.01;
P < 0.001
Includes crack cocaine, other forms of cocaine, methamphetamines (speed, meth, or ice), and ecstasy
Discussion
We aimed to describe depressive symptom patterns over time and the associations of HIV status and baseline clinical profiles. Our study found long term patterns that are consistent with high risk for depression (CESD >16) [29, 32] or indicative of severe risk or probable depression (CESD > 23) [40] in PLWH. Patterns considered long-term high risk or greater were found in 34% of HIV+ women and 32% of HIV+ men. While many studies have found significant differences of depression by gender [41–44], we found that HIV+ women and men had similar proportions of stable high/severe symptomology. The long-term stability of depressive symptom patterns found in this study indicate that depressive symptoms may remain increased years after diagnosis. This finding is consistent with a study with a shorter period of follow-up, showing that among newly diagnosed persons, depressive symptoms did not decrease over the course of nearly 5 years, even after starting ART [6]. Our study also showed that some HIV+ women had a decreasing pattern of depressive symptoms over time. As hypothesized, HIV+ status was a significant independent predictor of long-term high and severe symptoms, particularly among women, and was a distinct predictor of moderate symptoms among men.
Of the clinical indicators, the Framingham Risk Score was consistently associated with increased risk for membership in all trajectories compared with long-term low symptoms among HIV+ women. Diabetes and low CD4-count trended toward increased odds for high depression risk in men only. Among women, increased years taking ART seemed to be protective against high and severe long-term depression risk. In men ART use was associated with increased odds for moderate depression risk. In women and men, unsuppressed viral load was positively associated with high depression risk. Related research has found that depression is high among those lost to HIV care, with subsequently high viral load and low CD4 count [45].
The readers should consider some limitations of the current study. First, depressive symptoms were assessed using a self-report measure not intended for clinical or diagnostic purposes. However, the CES-D is a standardized tool that has been shown to have good reliability and validity in detecting significant depressive symptoms in a variety of populations, including persons living with and without HIV. Second, use of the Framingham Risk Score among PLWH has been questioned [46], as other risk scores have been developed. The D:A:D score [47] is one such measure that is tailored for use in HIV+ samples and includes specific types of ART that confer increased risk for cardiovascular disease. We chose to use the Framingham Risk Score to control for cardiovascular risk as a clinical indicator among HIV+ and HIV− participants. Third, because the outcome of depressive symptom patterns in some cases exceed 10%, odds ratios are an overestimate of the risk ratio. However, we do not attempt to interpret any of the odds ratios as risk ratios. Lastly, because data used for this analysis were during the post-ART era, we could not assess differences in depression by ART type as the majority of those on ART were on highly active ART.
A significant strength of the current analysis was the inclusion of HIV− participants who were recruited from similar populations as HIV+ participants, in accordance with the HIV epidemic by sex, allowing us to directly compare depressive symptoms by HIV status. The current study adds to existing literature on the proportion of HIV+ persons living with depressive symptoms. This study also provids novel information regarding changes in depressive symptoms over 10 years and the clinical factors that are associated with those at risk for depressive symptoms over time.
Conclusions
Clinicians should consider the impact that depressive symptoms may have on HIV prognosis and clinical indicators of comorbid illnesses and vis-à-vis. The current literature should be extended to include social support variables and time-varying effects of factors that change over time to further identify factors associated with depressive symptoms. Future research could also focus on specific time periods of increased depressive symptom risk, such as menopause and post-menopause in HIV-infected women.
Supplementary Material
Acknowledgments
This work was supported by Grant F31 AA024064 from the National Institute of Alcoholism and Alcohol Abuse of the National Institutes of Health (Natalie Kelso-Chichetto), Grant U01-AI-103397 from the Miami Women’s Interagency HIV Study (WIHS) (support for Robert Cook), and Grant F31 DA039810 from the National Institute on Drug Abuse (Chukwuemeka Okafor).
Additional Information WIHS (Principal Investigators): Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I-WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA). MACS (Principal Investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The MACS website is located at http://aidscohortstudy.org/.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10461-017-1822-6) contains supplementary material, which is available to authorized users.
Disclaimer Data in this manuscript were collected by the WIHS and Multicenter AIDS Cohort Study (MACS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH); Johns Hopkins Institute for Clinical and Translational Research, or National Center for Advancing Translational Sciences.
Compliance with Ethical Standards
Ethical Approval All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The secondary use of the MACS and WIHS data was approved by the Institutional Review Board of the University of Florida, Gainesville FL.
Conflict of interest The authors declare that they have no conflict of interest.
Informed Consent All participants of MACS and WIHS completed informed consent.
References
- 1.Arseniou S, Arvaniti A, Samakouri M. HIV infection and depression. Psychiatry Clin Neurosci. 2014;68(2):96–109. doi: 10.1111/pcn.12097. [DOI] [PubMed] [Google Scholar]
- 2.Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV infected patients: a review. Curr Psychiatry Rep. 2014;17(1):1–11. doi: 10.1007/s11920-014-0530-4. [DOI] [PubMed] [Google Scholar]
- 3.Beidas RS, Birkett M, Newcomb ME, Mustanski B. Do psychiatric disorders moderate the relationship between psychological distress and sexual risk-taking behaviors in young men who have sex with men? A longitudinal perspective. AIDS Patient Care STDS. 2012;26(6):366–74. doi: 10.1089/apc.2011.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seth P, Raiji PT, DiClemente RJ, Wingood GM, Rose E. Psychological distress as a correlate of a biologically confirmed STI, risky sexual practices, self-efficacy and communication with male sex partners in African-American female adolescents. Psychol Health Med. 2009;14:291–300. doi: 10.1080/13548500902730119. [DOI] [PubMed] [Google Scholar]
- 5.Williams CT, Latkin CA. The role of depressive symptoms in predicting sex with multiple and high-risk partners. JAIDS. 2005;38:69–73. doi: 10.1097/00126334-200501010-00013. [DOI] [PubMed] [Google Scholar]
- 6.Gold JA, Grill M, Peterson J, et al. Longitudinal characterization of depression and mood states beginning in primary HIV infection. AIDS Behav. 2014;18(6):1124–32. doi: 10.1007/s10461-013-0688-5. [DOI] [PubMed] [Google Scholar]
- 7.Braithwaite RS, Fang Y, Tate J, et al. Do alcohol misuse, smoking, and depression vary concordantly or sequentially? A longitudinal study of HIV-infected and matched uninfected veterans in care. AIDS Behav. 2016;20(3):566–72. doi: 10.1007/s10461-015-1117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palfai TP, Cheng DM, Coleman SM, Bridden C, Krupitsky E, Samet JH. The influence of depressive symptoms on alcohol use among HIV-infected Russian drinkers. Drug Alcohol Depend. 2014;134:85–91. doi: 10.1016/j.drugalcdep.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chander G, Himelhoch S, Moore R. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66(6):769–89. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 10.Lightfoot M, Rogers T, Goldstein R, et al. Predictors of substance use frequency and reductions in seriousness of use among persons living with HIV. Drug Alcohol Depend. 2005;77(2):129–38. doi: 10.1016/j.drugalcdep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. JAIDS. 2013;58(2):181–7. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Springer SA, Dushaj A, Azar MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS Behav. 2012;16(8):2119–43. doi: 10.1007/s10461-012-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horberg MA, Silverberg MJ, Hurley LB, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. JAIDS. 2008;47(3):384–90. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- 14.Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, Catz S. Chronic illness burden and quality of life in an aging HIV population. AIDS Care. 2013;25(4):451–8. doi: 10.1080/09540121.2012.712669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz RM, Mansoor A, Wilson TE, et al. Chronic depressive symptoms and Framingham coronary risk in HIV-infected and HIV-uninfected women. AIDS Care. 2012;24(3):394–403. doi: 10.1080/09540121.2011.608791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White JR, Chang CC, So-Armah KA, et al. Depression and human immunodeficiency virus infection are risk factors for incident heart failure among veterans: veterans Aging Cohort Study. Circulation. 2015;132(17):1630–8. doi: 10.1161/CIRCULATIONAHA.114.014443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parruti G, Vadini F, Sozio F, et al. Psychological factors, including alexithymia, in the prediction of cardiovascular risk in HIV infected patients: results of a cohort study. PLoS ONE. 2013;8(1):e54555. doi: 10.1371/journal.pone.0054555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pyra M, Weber KM, Wilson TE, et al. Sexual minority women and depressive symptoms throughout adulthood. Am J Public Health. 2014;104(12):e83–90. doi: 10.2105/AJPH.2014.302259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 20.Richardson J, Barkan S, Cohen M, et al. Experience and covariates of depressive symptoms among a cohort of HIV infected women. Soc Work Health Care. 2001;32(4):93–111. doi: 10.1300/J010v32n04_05. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. [Accessed January 25, 2016];HIV among women. Division of HIV/AIDS Prevention, National Center for HIV/ AIDS, Viral Hepatitis, Sexual Transmitted Diseases and Tuberculosis Prevention, Centers for Disease Control and Prevention. 2015 http://www.cdc.gov/hiv/group/gender/women/index.html#refa.
- 22.Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35(3):320–30. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jennings WG, Meade C. Group-based trajectory modeling. Oxford Handbooks Online; 2016. [Access December 17, 2016]. at http://www.oxfordhandbooks.com/view/10.1093/oxfordhb/9780199935383.001.0001/oxfordhb-9780199935383-e-96?print=pdf. [Google Scholar]
- 24.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR. The multicenter AIDS cohort study: rationale, organization, and selected characteristics of the participants. Am J Epidemiology. 1987;126(2):310–8. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 25.Detels R, Phair JP, Saah AJ, Rinaldo CR, Murioz A, Kaslow RA, et al. Recent scientific contributions to understanding HIV/AIDS from the multicenter AIDS cohort study. J Epidemiol. 1992;2(2 supp):11–9. [Google Scholar]
- 26.Dudley J, Jin S, Hoover D, Metz S, Thackeray R, Chmiel J. The multicenter AIDS cohort study: retention after 9 1/2 years. Am J Epidemiol. 1995;142(3):323–30. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 27.Barkan SE, Melnick SL, Preston-Martin S, et al. The women’s interagency HIV study: wIHS collaborative study group. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 28.Bacon MC, von Wyl V, Alden C, et al. The women’s interagency HIV study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–9. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radloff LS. The CES-D Scale A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 30.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46(5):437–43. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 31.Verdier-Taillefer MH, Gourlet V, Fuhrer R, Alpérovitch A. Psychometric properties of the Center for Epidemiologic Studies-Depression scale in multiple sclerosis. Neuroepidemiology. 2001;20(4):262–7. doi: 10.1159/000054800. [DOI] [PubMed] [Google Scholar]
- 32.Gay CL, Kottorp A, Lerdal A, Lee KA. Psychometric limitations of the Center for Epidemiologic Studies-Depression Scale for assessing depressive symptoms among adults with HIV/AIDS: a Rasch analysis. Depress Res Treat. 2016;2016:2824595. doi: 10.1155/2016/2824595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.AIDSinfo. Virologic failure: adult and adolescent ARV guidelines. 2016 Retrieved from https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/15/virologic-failure.
- 34.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 35.Center for Disease Prevention and Control. Alcohol & Public Health. 2016 Retrieved at https://www.cdc.gov/alcohol/faqs.htm.
- 36.Hsu H-C. Group-based trajectories of depressive symptoms and the predictors in the older population. Int J Geriatr Psychiatry. 2012;27(8):854–62. doi: 10.1002/gps.2796. [DOI] [PubMed] [Google Scholar]
- 37.Liang J, Xu X, Quiñones AR, Bennett JM, Ye W. Multiple trajectories of depressive symptoms in middle and late life: racial/ ethnic variations. Psychol Aging. 2011;26(4):761–77. doi: 10.1037/a0023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montagnier D, Dartigues J-F, Rouillon F, Pérès K, Falissard B, Onen F. Ageing and trajectories of depressive symptoms in community-dwelling men and women. Int J Geriatr Psychiatry. 2014;29(7):720–9. doi: 10.1002/gps.4054. [DOI] [PubMed] [Google Scholar]
- 39.Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent class growth modeling: a tutorial. Tutor Quant Methods Psychol. 2009;5:11–24. [Google Scholar]
- 40.Choi SKY, Boyle E, Burchell AN, Gardner S, Collins E, Grootendorst P, et al. Validation of six short and ultra-short screening instruments for depression for people living with HIV in Ontario: results from the Ontario HIV treatment network cohort study. PLoS ONE. 2015;10(11):e0142706. doi: 10.1371/journal.pone.0142706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization collaborative study on psychological problems in general health care. Arch Gen Psychiatry. 1998;55(5):405–13. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- 42.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the united states: results from the national comorbidity survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 43.Weissman MM, Bland RC, Canino GJ, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276(4):293–9. [PubMed] [Google Scholar]
- 44.Reis RK, Haas VJ, dos Santos CB, Teles SA, Galvão MTG, Gir E. Symptoms of depression and quality of life of people living with HIV/AIDS. Rev Lat Am Enfermagem. 2011;19(4):874–81. doi: 10.1590/s0104-11692011000400004. [DOI] [PubMed] [Google Scholar]
- 45.Pecoraro A, Mimiaga M, O’Cleirigh C, et al. Depression, substance use, viral load, and CD4+ count among patients who continued or left antiretroviral therapy for HIV in St Petersburg, Russian Federation. AIDS Care. 2015;27(1):86–92. doi: 10.1080/09540121.2014.959464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mateen FJ, Post WS, Sacktor N, et al. Multicenter AIDS cohort study (MACS) investigators. Long-term predictive value of the Framingham risk score for stroke in HIV-positive vs HIV-negative men. Neurology. 2013;81(24):2094–102. doi: 10.1212/01.wnl.0000437296.97946.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friis-Møller N, Thiébaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17(5):491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.