Abstract
A 22-year-old woman was diagnosed with intermediate risk stage II Hodgkin lymphoma and treated with three cycles of adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by involved-field radiation therapy. A complete metabolic remission was achieved after two cycles of ABVD, which was maintained until three years after completion of treatment. Follow-up FDG-PET/CT four years after completion of treatment, however, showed a new FDG-avid (Deauville score of 4) lesion in the right scapula, suggesting relapsed disease. Computer tomography (CT)-guided biopsy of this lesion was performed and subsequent histological examination revealed a radiation-induced giant cell granuloma.
Keywords: Biopsy, FDG-PET/CT, Giant cell granuloma, Hodgkin lymphoma
Fig. 1.
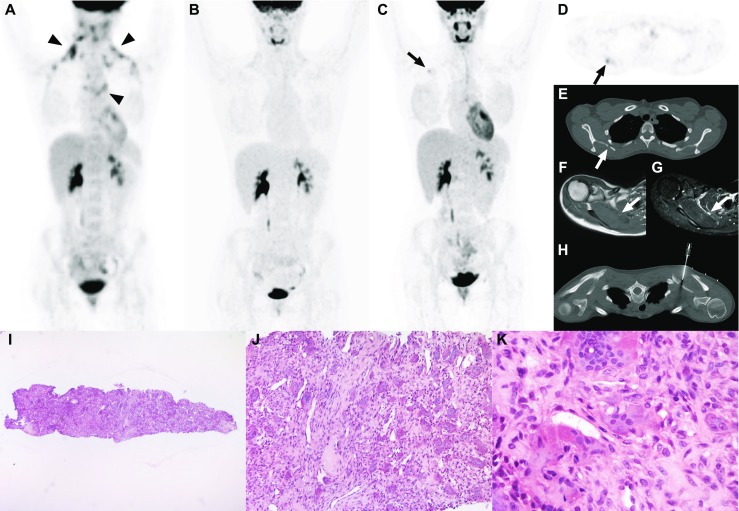
A 22-year-old woman presented with fatigue and enlarged cervical lymph nodes, without B-symptoms. 18F-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography computed tomography (PET/CT) showed bilateral cervical and mediastinal enlarged and FDG-avid lymph nodes (Figure part A, arrowheads). Subsequent histological examination of an excised right cervical lymph node revealed classical nodular sclerosing Hodgkin lymphoma. The patient was allocated intermediate risk stage II disease and treated with three cycles of adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by involved-field radiation therapy. A complete metabolic remission was achieved after two cycles of ABVD, which was maintained until three years after completion of treatment (Figure part B). Follow-up FDG-PET/CT (which was performed as part of a study) four years after completion of treatment, however, showed a new FDG-avid (Deauville score of 4, maximum standardized uptake value of 2.9) lesion in the right scapula (Figure parts C and D, arrows), which appeared lytic at computed tomography (CT) (Figure part E, arrow), hypointense at T1-weighted magnetic resonance imaging (MRI) (Figure part F, arrow), and hyperintense at fat-suppressed T2-weighted MRI (Figure part G, arrow). CT-guided biopsy of this lesion was performed (Figure part H). Biopsy of the right scapula (hematoxylin-eosin [H&E] stain, original magnification × 25), length 4 mm, showed lesional tissue, but no bone (Figure part I). There were numerous, fairly evenly spread multinucleated giant cells of the osteoclast type, set in a dense fibrous background with spindled cells and blood vessels (H&E stain, original magnification × 100) (Figure part J). Detail (H&E stain, original magnification × 400) shows the multinucleated giant cells and the spindled cells, without any identifiable Reed-Sternberg/Hodgkin cells (Figure part K). In addition, CD30 and CD15 were negative (not shown). Therefore, relapsed Hodgkin lymphoma was excluded. The spindled cells and the lack of mutation H3F3A are unusual for a giant cell tumor of bone. The lack of atypia and mitoses as well as its size at imaging make an osteosarcoma also less plausible. A giant cell process as seen in a brown tumor in hyperparathyroidism was excluded biochemically (parathyroid hormone 1,9 pmol/L [normal range: 0,5-4,2 pmol/L]). Considering the exposure of the right scapula to the radiation therapy, a reactive process, such as a giant cell granuloma would be the most likely diagnosis [1], although the giant cells are not typically clustered and rather large. In conclusion, this case demonstrates the non-specificity of FDG-PET/CT in the follow-up setting of Hodgkin lymphoma, the need for biopsy of suspicious FDG-avid lesions before a change in treatment planning can be justified, and that a rare reactive process such as a (probably) radiation-induced giant cell granuloma can mimic relapsed Hodgkin lymphoma
Acknowledgement
We thank the Netherlands Committee on Bone Tumors for assistance in interpreting the histopathological slides.
Compliance with Ethical Standards
Conflict of Interest
Hugo J.A. Adams: no conflict of interest.
John M.H. de Klerk: no conflict of interest.
Josien C. Regelink: no conflict of interest.
Ben. G.F. Heggelman: no conflict of interest.
Stefan V. Dubois: no conflict of interest.
Thomas C. Kwee; no conflict of interest.
Ethical Statement
The study was approved by an institutional review board or equivalent and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects in the study gave written informed consent or the institutional review board waived the need to obtain informed consent.
Funding
This work was financially supported by an Alpe d’HuZes/Dutch Cancer Society Bas Mulder Award for T.C.K. (grant number 5409). Data collection, data analysis, and interpretation of data, writing of the paper, and decision to submit were left to the authors’ discretion and were not influenced by Alpe d’HuZes/Dutch Cancer Society.
Footnotes
This manuscript has not been published before or is not under consideration for publication anywhere else and has been approved by all co-authors.
Reference
- 1.Forte V, Shimotakahara S, Crysdale WS, Thorner P. Recurring giant-cell granuloma at the site of previous radiation therapy. J Otolaryngol. 1990;19:285–287. [PubMed] [Google Scholar]


