Abstract
Follicular dendritic cell sarcoma (FDCS) is an extremely rare tumor with only 67 cases of head and neck FDCS reported in the literature. A 65-year-old female had a 6-cm follicular dendritic cell sarcoma resected from the left parotid gland with close margins. It recurred 1 year later as a 5-cm mass that was intensely [18F] fluoro-2-deoxy-D-glucose (18F-FDG) avid on positron emission tomography/computed tomography (PET/CT) and was re-excised. A follow-up PET/CT did not show any metastatic disease. The use of 18F-FDG PET/CT in the management of FDCS warrants further research. We present the 18F-FDG PET/CT imaging findings of this rare tumor.
Keywords: Follicular dendritic cell sarcoma, FDCS, Parotid gland, 18F-FDG, Fluorodeoxyglucose, PET/CT
Introduction
Soft tissue sarcomas are uncommon tumors that arise from the embryonic mesoderm and account for approximately 0.7% of all adult malignancies. They have a high mortality rate and account for 3–4% of annual cancer-related deaths [1]. Diagnostic imaging plays a major role in the evaluation of patients with sarcoma. Anatomic imaging, which is used in the evaluation of sarcomas, includes computed tomography (CT), magnetic resonance imaging (MRI) and bone scintigraphy. During the initial workup, MRI provides a better description and definition of the extent of the tumor than CT or PET/CT [2]. 18F-FDG PET/CT imaging has been investigated in soft tissue sarcomas for biopsy guidance [3], response assessment [4], grading [5], follow-up [6] and prognostication [7]. Several recent studies have found a correlation between the maximum standardized uptake value (SUVmax) of sarcoma tumors and histological characteristics such as histologic grade, mitotic counts, and presence of tumor necrosis [8]. 18F-FDG PET/CT can also be used as a prognostic indicator for progression-free survival and overall survival [9].
FDCS is a very rare type of sarcoma of low to intermediate malignant potential, originating from follicular dendritic cells, which are non-lymphoid and non-phagocytic accessory cells of the lymphoid system [10, 11]. Most cases arise within lymph nodes, but extranodal sites including the mediastinum, gastrointestinal tract, liver and spleen have been described. Only 67 cases of head and neck FDCS have been reported in the literature, with none in the parotid gland [12–16]. The use of 18F-FDG PET/CT imaging in the evaluation of head and neck FDCS has not been previously described.
Case Report
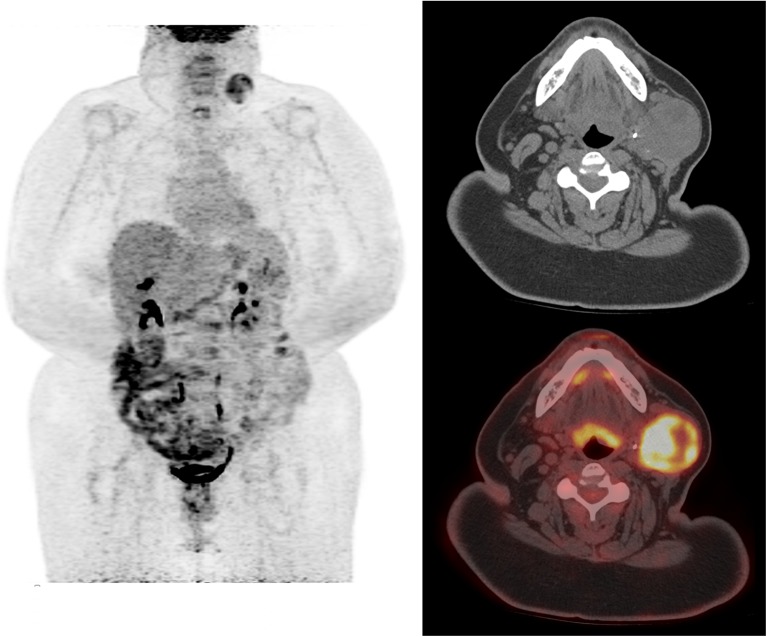
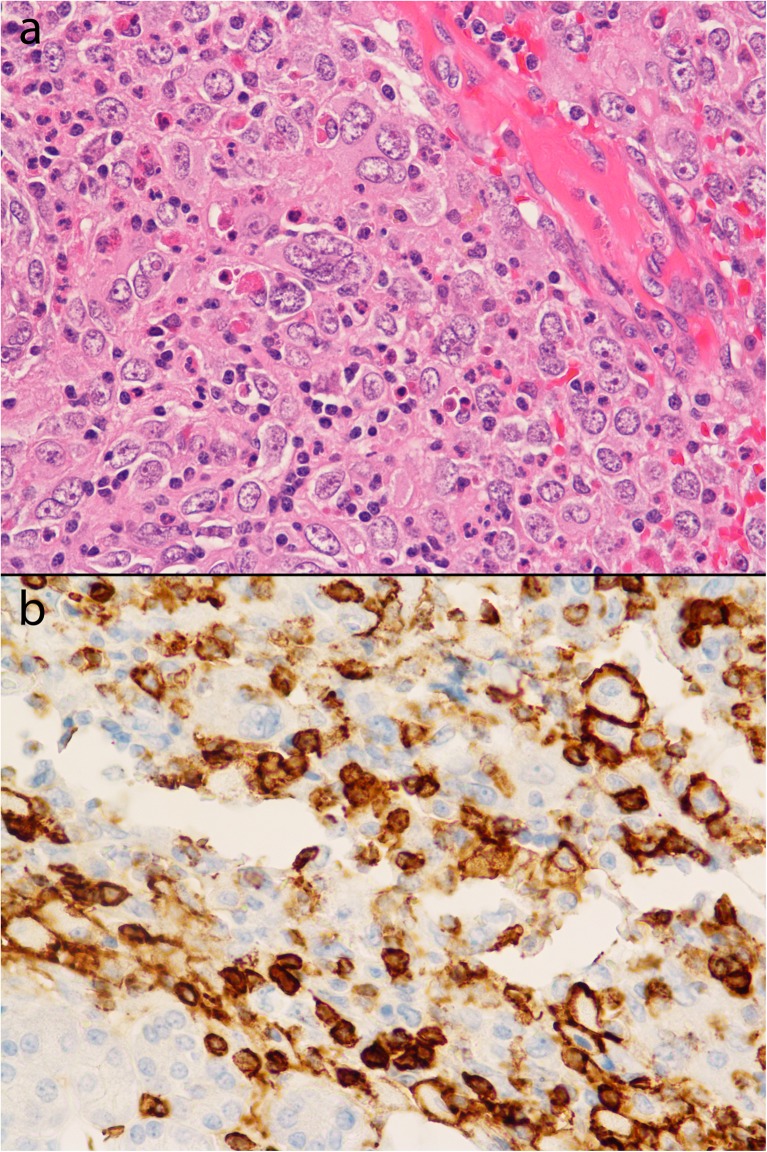
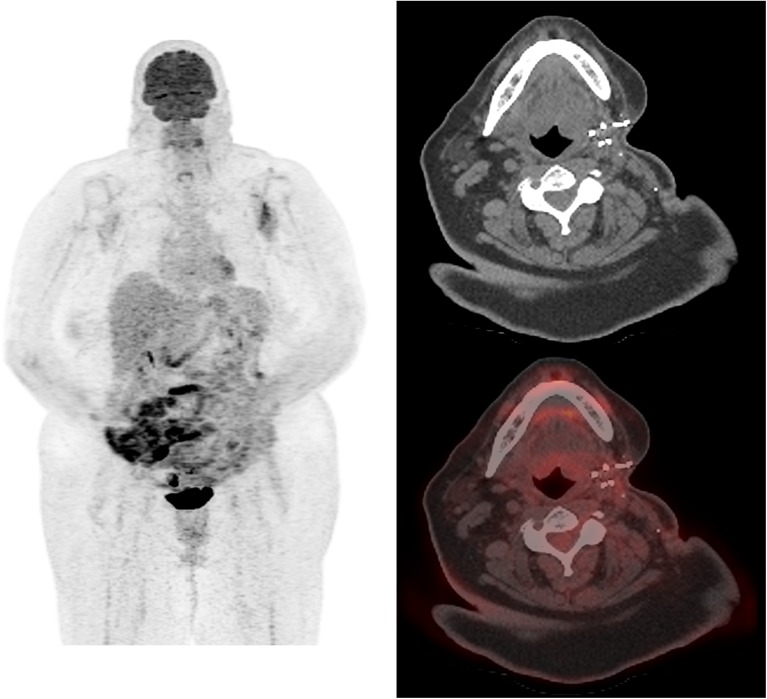
A 65-year-old female had a 6-cm FDCS tumor resected from the left parotid gland. The tumor was arising from the gland, and the margins were negative but close (less than 0.5 mm from both the deep and superficial margins of the resection). A follow-up 18F-FDG PET/CT (dose: 604 MBq) done 18 months later showed a new left parotid gland mass measuring 5.0 × 4.2 cm, which was intensely 18F-FDG avid with a SUVmax of 9.1, consistent with sarcoma recurrence. Maximum intensity projection (MIP) and transaxial images showed the 18F-FDG-avid left parotid sarcoma recurrence with no evidence of nodal involvement or metastatic disease (Fig. 1). Excision of the mass showed a well-circumscribed, lobulated 5-cm tumor with multiple hemorrhagic areas consistent with recurrent FDCS. The proliferative index by MIB-1 was 10%. Histological evaluation showed tumor cells with histiocytoid appearance, pleomorphic nuclei, and small-to-intermediate-sized nucleoli (on H&E stain under 400× magnification) (Fig.2a). Immunohistochemical staining showed CD15-positive malignant cells (Fig.2b). The immunomorphologic features were consistent with FDCS. A follow-up PET/CT performed 9 months later did not show any evidence of recurrent disease (Fig. 3).
Fig. 1.
PET/CT MIP, transaxial CT, and PET/CT fusion images showing an 18F-FDG-avid left parotid mass, consistent with recurrent FDCS
Fig. 2.
a Histopathological evaluation showed tumor cells with histiocytoid appearance, pleomorphic nuclei, and small-to-intermediate-sized nucleoli. H&E stain, magnification 400×. b Immunohistochemical staining showing CD15-positive malignant cells, cytoplasmic staining. The immunomorphologic features were consistent with FDCS
Fig. 3.
Follow-up PET/CT performed 9 months later; MIP, transaxial CT and PET/CT fusion images do not show any evidence of recurrent disease in the left parotid surgical bed
Discussion
FDCS is a very rare neoplasm with 129 cases reported in the literature, including 67 cases in the head and neck [12–16]. It affects males and females equally and at all ages with a mean age at presentation of 46. Approximately two thirds arise in a lymph node and one third arise in extranodal sites, including the oral cavity, GI tract, liver and spleen. Most extranodal sites occur in the head and neck; however, FDCS arising in the parotid gland has not been previously reported in the literature [17–19]. FDCS was traditionally understood to behave more like a low-grade sarcoma than lymphoma [20], with a local-regional recurrence rate of 40% and distant metastases of 25% (lymph nodes, lung, liver); however, recent studies suggest that FDCS tumors have a more aggressive clinical course than previously recognized [11, 16, 21, 22]. Radical surgery is curative in up to two thirds of cases [13], and radiotherapy and chemotherapy do not seem to be effective in improving patient’s disease-free survival after radical tumor excision [11, 17]. The use of 18F-FDG PET/CT in the staging of FDCS has only been described in two cases of mediastinal FDCS [23, 24], and its use in the staging or surveillance of head and neck FDCS has not been previously described in the literature and warrants further research.
Acknowledgements
None
Compliance with ethical standards
Conflict of Interest
William Makis, EW Hudson and Brian Chiu declare that they have no conflict of interest.
Ethical statement
The study was approved by an institutional review board or equivalent and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects in the study gave written informed consent or the institutional review board waived the need to obtain informed consent.
References
- 1.Charest M, Hickeson M, Lisbona R, Novales-Diaz JA, Derbekyan V, Turcotte RE. FDG PET/CT imaging in primary osseous and soft tissue sarcomas: a retrospective review of 212 cases. Eur J Nucl Med Mol Imaging. 2009;36:1944–51. doi: 10.1007/s00259-009-1203-0. [DOI] [PubMed] [Google Scholar]
- 2.Brisse H, Ollivier L, Edeline V, Pacquement H, Michon J, Glorion C, et al. Imaging of malignant tumors of the long bones in children: monitoring response to neoadjuvant chemotherapy and preoperative assessment. Pediatr Radiol. 2004;34:595–605. doi: 10.1007/s00247-004-1192-x. [DOI] [PubMed] [Google Scholar]
- 3.Hicks RJ, Toner GC, Choong PF. Clinical applications of molecular imaging in sarcoma evaluation. Cancer Imaging. 2005;5:66–72. doi: 10.1102/1470-7330.2005.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benz MR, Czernin J, Allen-Auerbach MS, Tap WD, Dry SM, Elashoff D, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2009;15:2856–63. doi: 10.1158/1078-0432.CCR-08-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folpe AL, Lyles RH, Sprouse JT, Conrad EU, III, Eary JF. (F-18) fluorodeoxyglucose positron emission tomography as a predictor of pathologic grade and other prognostic variables in bone and soft tissue sarcoma. Clin Cancer Res. 2000;6:1279–87. [PubMed] [Google Scholar]
- 6.Bastiaannet E, Groen H, Jager PL, Cobben DC, van der Graaf WT, Vaalburg W, et al. The value of FDG-PET in the detection, grading and response to therapy of soft tissue and bone sarcomas; a systematic review and metaanalysis. Cancer Treat Rev. 2004;30:83–101. doi: 10.1016/j.ctrv.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Eary JF, O’Sullivan F, Powitan Y, Chandhury KR, Vernon C, Bruckner JD, et al. Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med Mol Imaging. 2002;29:1149–54. doi: 10.1007/s00259-002-0859-5. [DOI] [PubMed] [Google Scholar]
- 8.Rakheja R, Makis W, Skamene S, Nahal A, Brimo F, Azoulay L, et al. Correlating metabolic activity on 18F-FDG PET/CT with histopathologic characteristics of osseous and soft tissue sarcomas: a retrospective review of 136 patients. AJR. 2012;198:1409–16. doi: 10.2214/AJR.11.7560. [DOI] [PubMed] [Google Scholar]
- 9.Costelloe CM, Macapinlac HA, Madewell JE, Fitzgerald NE, Mawlawi OR, Rohren EM, et al. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med. 2009;50:340–7. doi: 10.2967/jnumed.108.058461. [DOI] [PubMed] [Google Scholar]
- 10.Chan JK, Fletcher CD, Nayler SJ, Cooper K. Follicular dendritic cell sarcoma. Cancer. 1997;79:294–313. doi: 10.1002/(SICI)1097-0142(19970115)79:2<294::AID-CNCR13>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 11.Pileri SA, Grogan TM, Harris NL, Banks P, Campo E, Chan JK, et al. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology. 2002;41:1–29. doi: 10.1046/j.1365-2559.2002.01418.x. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Ordonez B, Erlandson RA, Rosai J. Follicular dendritic cell tumor: report of 13 additional cases of a distinctive entity. Am J Surg Pathol. 1996;20:944–55. doi: 10.1097/00000478-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 13.De Pas T, Spitaleri G, Pruneri G, Curigliano G, Noberasco C, Luini A, et al. Dendritic cell sarcoma: an analytic overview of the literature and presentation of original five cases. Crit Rev Oncol Hematol. 2008;65:1–7. doi: 10.1016/j.critrevonc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Chera BS, Orlando C, Villaret DB, Mendenhall WM. Follicular dentritic cell sarcoma of the head and neck: case report and literature review. Laryngoscope. 2008;118:1607–12. doi: 10.1097/MLG.0b013e31817aec58. [DOI] [PubMed] [Google Scholar]
- 15.Leipsic JA, McAdams HP, Sporn TA. Follicular dendritic cell sarcoma of the mediastinum. AJR. 2007;188:W554–6. doi: 10.2214/AJR.04.1530. [DOI] [PubMed] [Google Scholar]
- 16.Vargas H, Mouzakes J, Purdy SS, Cohn AS, Parnes SM. Follicular dendritic cell tumor: an aggressive head and neck tumor. Am J Otolaryngol. 2002;23:93–8. doi: 10.1053/ajot.2002.30781. [DOI] [PubMed] [Google Scholar]
- 17.Satoh K, Hibi G, Yamamoto Y, Urano M, Kuroda M, Nakamura S. Follicular dendritic cell tumor in the oro-pharyngeal region: report of a case and a review of the literature. Oral Oncol. 2003;39:415–9. doi: 10.1016/S1368-8375(02)00138-0. [DOI] [PubMed] [Google Scholar]
- 18.Biddle DA, Ro JY, Yoon GS, Yong YW, Ayala AG, Ordonez NG, Ro J. Extranodal follicular dendritic cell sarcoma of the head and neck region: three new cases, with a review of the literature. Mod Pathol. 2002;15:50–8. doi: 10.1038/modpathol.3880489. [DOI] [PubMed] [Google Scholar]
- 19.Desai S, Deshpande RB, Jambhekar N. Follicular dendritic cell tumor of the parapharyngeal region. Head Neck. 1999;21:164–7. doi: 10.1002/(SICI)1097-0347(199903)21:2<164::AID-HED11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca R, Tefferi A, Strickler JG. Follicular dendritic cell sarcoma mimicking diffuse large cell lymphoma: a case report. Am J Hematol. 1997;55:148–55. doi: 10.1002/(SICI)1096-8652(199707)55:3<148::AID-AJH6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Soriano AO, Thompson MA, Admirand JH, Fayad LE, Rodriguez AM, Romaguera JE, et al. Follicular dendritic cell sarcoma: a report of 14 cases and a review of the literature. Am J Hematol. 2007;82:725–8. doi: 10.1002/ajh.20852. [DOI] [PubMed] [Google Scholar]
- 22.Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001;25:721–31. doi: 10.1097/00000478-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Krober SF, Marx A, Aebert H, Dohmen BM, Kaiserling E. Sarcoma of follicular dendritic cells in the dorsal mediastinum. Hum Pathol. 2004;35:259–63. doi: 10.1016/j.humpath.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Subesinghe M, Smith JT, Chowdhury FU. F-18 FDG PET/CT imaging of follicular dendritic cell sarcoma of the mediastinum. Clin Nucl Med. 2012;37:204–5. doi: 10.1097/RLU.0b013e31823ea1a5. [DOI] [PubMed] [Google Scholar]