Abstract
Purpose
Dopamine transporter imaging is suggested to be a useful imaging biomarker for Parkinson’s disease (PD) progression and monitoring drug effects. We investigated the longitudinal decline characteristics of striatal [18F]FP-CIT uptake in PD.
Methods
We retrospectively reviewed 35 PD patients and 9 non-PD patients. All patients underwent [18F]FP-CIT PET at the initial diagnosis and follow-up. PET images were spatially normalized and analyzed with eight striatal and one occipital VOI templates. We measured the specific to non-specific binding ratio (SNBR) of the striatal subregions and calculated the absolute annual reduction (AAR) and relative annual reduction (%RAR) of the SNBRs.
Results
Total striatal SNBRs in PD patients were significantly lower than those in non-PD patients, with the most significant difference in the posterior putamen. Both AAR (0.26 ± 0.14 vs. 0.09 ± 0.19, p < 0.05) and %RAR (6.9 ± 3.5 vs. 1.2 ± 2.7, p < 0.001) of total striatal SNBRs were significantly greater in PD than non-PD patients. There were no significant differences in the AAR and %RAR of total striatal SNBRs between elderly and young onset PD. The AARs of the posterior putamen were higher in early PD than in advanced PD. Conversely, the %RARs were not significantly different between early and more advanced PD. The disease duration was significantly negatively correlated with the AAR but not with the %RAR of the posterior putamen.
Conclusions
The longitudinal decline of striatal [18F]FP-CIT uptake in PD was nonlinear and significantly faster than that in non-PD, with a different rate of decline among the striatal subregions.
Keywords: [18F]FP-CIT pet, Parkinson’s disease, Longitudinal decline, Dopamine transporter imaging
Introduction
Parkinson’s disease (PD) is a slow and progressive disease characterized by dopaminergic neuronal degeneration in the substantia nigra. Conservative management strategies replacing de novo dopamine (e.g., levodopa or dopamine receptor agonists) are currently used but not proven to be effective in the alteration of the disease course. Even the results of the ELLDOPA trial suggest that L-dopa downregulates the binding of the striatal dopamine transporter [1]. There are also several ongoing clinical trials regarding the potential of neuroprotective therapy to decrease dopaminergic neuronal degeneration. To evaluate the effectiveness of disease-modulating therapy, a surrogate marker that is sensitive to clinical progression, reproducible, and resilient to confounding factors is required. However, clinical parameters (e.g., the Hoehn-Yahr stage or UPDRS score) are not appropriate for the objective evaluation of disease progression and the effect of neuroprotective drugs, since symptoms fluctuate over time and the majority of patient assessments are subjective [2].
Numerous reports have demonstrated that dopamine transporter imaging (e.g., [123I]β-CIT SPECT or [123I]FP-CIT SPECT) is a useful imaging biomarker for the evaluation of disease progression by a quantitative analysis of dopamine neuronal degeneration [3–5]. Marek et al. reported that there was an annual decline rate of approximately 11% for the [123I]β-CIT uptake in the striatum of patients with PD, and Winogrodzka et al. found that the annual decline rate of [123I]FP-CIT uptake was approximately 8% [5, 6]. Moreover, these values are similar to those of neuronal cell degeneration in the substantia nigra observed in a pathologic study [7].
[18F]FDOPA PET is also an imaging modality that represents dopaminergic neuronal degeneration in PD. [18F]FDOPA uptake in the striatum reflects the dopa-carboxylase activity in the dopaminergic nerve terminals and exhibits a similar decreasing pattern, which has been correlated with disease severity in patients with PD [8]. Two longitudinal studies found that in PD, the uptake of [18F]FDOPA decreases faster than that occurring during the normal aging process [9, 10]. Moreover, Morrish et al. suggested that the decreasing rate of [18F]FDOPA uptake in recent onset PD is significantly greater than that in advanced PD patients, indicating that the degenerative process slows over the disease course [11]. However, [18F]FDOPA PET is an indirect method of measuring the degeneration of presynaptic dopaminergic neurons by aromatic L-amino acid decarboxylase (AADC) activity. In fact, there have been some reports regarding the upregulation of AADC activity during the early phase of PD caused by a dopamine deficiency [12].
[18F]fluorinated-N-3-fluoropropyl-2-b-carboxymethoxy-3-b-(4-iodophenyl)nortropane ([18F]FP-CIT) is a PET tracer that binds to the dopamine transporter (DAT) and directly reflects presynaptic dopaminergic neuronal degeneration. With a newly developed radiochemistry method (i.e., protic solvent system), [18F]FP-CIT is widely used in the diagnosis of PD [13]. Since it is a PET tracer, a more accurate subregional and quantitative analysis is possible and its metabolites are not radioactive, facilitating superior quantification than DAT SPECT [14]. With these advantages, [18F]FP-CIT PET is expected to be useful for evaluating striatal dopaminergic neural loss and the therapeutic response of neuroprotective drugs. For the successful performance of clinical trials for developing disease-modifying new drugs in patients with PD using [18F]FP-CIT PET, information regarding the natural progression pattern of striatal [18F]FP-CIT uptake in PD is required to determine the number of subjects to be enrolled and duration of follow-up and to evaluate drug efficacy. As we know, there has not been a study regarding the longitudinal decline pattern of striatal [18F]FP-CIT uptake in PD. Therefore, we investigated the characteristics associated with the longitudinal decline of striatal [18F]FP-CIT uptake in PD.
Materials and Methods
Subjects
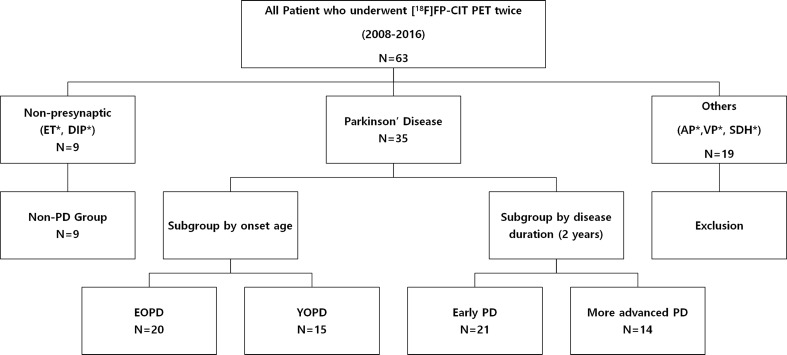
Figure 1 is a flow diagram presenting the inclusion and exclusion criteria with the number of subjects in each group. A total of 63 patients have undergone [18F]FP-CIT PET/CT twice since 2008 at our center. Of these, 35 patients were diagnosed with PD and 9 with drug-induced parkinsonism (DIP) or essential tremor (ET), designated as non-PD in this study. The other 18 patients were diagnosed with atypical parkinsonism (e.g., PSP, MSA, and CBD or vascular parkinsonism) and were excluded from the analysis. One patient was found to have a subdural hematoma on the second [18F]FP-CIT PET/CT and was also excluded from our analysis. Moreover, in the PD group, we performed two subgroup analyses according to the age at onset and disease duration. First, in accordance with the National Parkinson Foundation recommendations [15], we defined patients whose symptom onset was below 50 years of age as young onset PD (YOPD) and the others as elderly onset PD (EOPD). Second, we designated patients with a disease duration of less than two years as early PD and those with disease duration of longer than two years as advanced PD. This study was approved by the institutional review board, and written informed consent was obtained from each subject.
Fig. 1.
Flow diagram showing the inclusion and exclusion criteria of the Parkinson’s disease (PD) and non-PD groups with each number of subjects. The subgroups of PD patients were divided by the age at onset and disease duration. ET, essential tremor; DIP, drug-induced parkinsonism; AP, atypical parkinsonism; VP, vascular parkinsonism; SDH, subdural hematoma
[18F]FP-CIT PET/CT
[18F]FP-CIT was synthesized as previously described [13]. Brain [18F]FP-CIT PET images were obtained using a PET/CT scanner (Biograph TruePoint 40, Siemens Medical System) 180 min after an injection of 185 MBq [18F]FP-CIT. The PET images were acquired for 10 min in a three-dimensional mode immediately after a brain computed tomography (CT) scan for attenuation correction and image fusion. CT scanning was performed at 120 kVp and 228 mAs with a slice thickness of 1.5 mm. The [18F]FP-CIT PET images were reconstructed with a TrueX algorithm and an all-pass filter using a 336 × 336 matrix.
Quantitative Analyses
Image processing was performed using SPM2 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London) within MATLAB R2013a for Windows (The MathWorks, Inc.) and MRIcro version 1.40 (Chris Rorden, Columbia, SC; http://www.mricro.com). All reconstructed PET images were spatially normalized to a Talairach space using a standard [18F]FP-CIT PET template, which was developed in-house as described previously [2]. The images were reoriented so that the striatum contralateral to the symptomatic side was on the left. If there was no laterality, the anatomic left became the left side of the standard space. Quantitative analyses were based on eight volume-of-interest (VOI) templates of bilateral striatal subregions [ventral striatum (VS), caudate (CA), anterior putamen (AP), and posterior putamen (PP)] and one of the occipital subregions. The anterior commissure coronal plane divided the putamen into the AP and PP [2]. The automatically normalized VOI template was adjusted manually by one of the authors under the supervision of a nuclear medicine physician with 20 years of experience using our in-house VOI editing software [16].
The level of concentrated activity in each VOI was calculated. The specific to non-specific binding ratio (SNBR) was defined as follows: [mean standardized uptake value (SUV) of the striatal subregional VOI – mean SUV of the occipital VOI]/mean SUV of the occipital VOI], considering the occipital uptake to be non-specific binding. For each subregion, the absolute annual reduction (AAR) was calculated as follows: (follow-up SNBR – initial SNBR)/test interval (year). Moreover, the relative annual reduction was calculated by dividing the AAR by the initial SNBR (%RAR = 100 × AAR/initial SNBR).
Subgroup Analyses
We performed additional subgroup analyses to understand the decreasing pattern of striatal [18F]FP-CIT uptake in detail using the age at onset and disease duration. First, we examined whether there were differences in the quantitative parameters, including the AAR and %RAR between the YOPD and EOPD groups. Second, we searched for differences in the quantitative parameters between the early PD and the more advanced PD groups in the same manner. In addition to the differences in the quantitative parameters, correlations between the disease duration and quantitative parameters were investigated.
Statistical Analyses
We used a Mann-Whitney U test for the comparison of the quantitative parameters between the two groups. Moreover, the Spearman correlation coefficients between the variables were calculated. The Statistical Package for the Social Sciences (SPSS) for Windows (version 21.0; SPSS Inc.) was used for the statistical analyses, and a P value of less than 0.05 was considered statistically significant. Data for the study variables were expressed as the mean ± standard deviation (SD).
Results
Patient Characteristics
The clinical data of the patients are presented in Table 1. PD patients were significantly younger than non-PD patients and had a longer test interval (P < 0.001); however, there was no significant difference in the disease duration between the PD and non-PD groups. Tables 2 and 3 list the clinical characteristics of the PD patient subgroups categorized by the age at onset or disease duration as mentioned above. Apart from age, there were no significant differences in the clinical characteristics between the EOPD and YOPD groups. Moreover, except for the disease duration and the initial Hoehn-Yahr stage, there were no significant differences in the clinical characteristics between the early PD and more advanced PD groups.
Table 1.
Clinical characteristics of Parkinson’s disease (PD) and non-PD patients
| Characteristic | PD (n = 35) | Non-PD (n = 9) | P value |
|---|---|---|---|
| Age at 1st PET (years) | 55 ± 12 | 70 ± 8 | < 0.001 |
| Age at 2nd PET (years) | 60 ± 11 | 74 ± 8 | < 0.05 |
| Sex (M/F), n | 21/14 | 2/7 | NA |
| Disease duration (months) | 30 ± 34 | 32 ± 37 | NS |
| Test interval (months) | 56 ± 19 | 36 ± 14 | < 0.001 |
| Hoehn-Yahr stage at 1st PET | 1.6 ± 0.6 | NA | NA |
| Hoehn-Yahr stage at 2nd PET | 2.6 ± 0.8 | NA | NA |
NA not applicable; NS not significant
Table 2.
Clinical characteristics of elderly onset Parkinson’s disease (EOPD) and young onset Parkinson’s disease (YOPD) patients
| Characteristic | EOPD (n = 20) | YOPD (n = 15) | P value |
|---|---|---|---|
| Age at 1st PET (years) | 64 ± 7 | 44 ± 7 | < 0.001 |
| Age at 2nd PET (years) | 68 ± 7 | 49 ± 6 | < 0.001 |
| Sex (M/F), n | 12/8 | 9/6 | NA |
| Disease duration (months) | 30 ± 36 | 30 ± 33 | NS |
| Test interval (months) | 51 ± 21 | 62 ± 15 | 0.115 |
| Hoehn-Yahr stage at 1st PET | 1.8 ± 0.6 | 1.4 ± 0.6 | 0.09 |
| Hoehn-Yahr stage at 2nd PET | 2.7 ± 0.7 | 2.5 ± 0.8 | NS |
NA not applicable; NS not significant
Table 3.
Clinical characteristics of early stage of Parkinson’s disease (PD) and more advanced stage of PD patients
| Characteristic | Early PD (n = 21) | More advanced PD (n = 14) | P value |
|---|---|---|---|
| Age at 1st PET (years) | 53 ± 13 | 59 ± 10 | NS |
| Age at 2nd PET (years) | 58 ± 12 | 63 ± 10 | NS |
| Sex (M/F), n | 13/8 | 8/6 | NA |
| Disease duration (months) | 11 ± 8 | 57 ± 40 | < 0.001 |
| Test interval (months) | 60 ± 21 | 50 ± 14 | NS |
| Hoehn-Yahr stage at 1st PET | 1.4 ± 0.5 | 1.9 ± 0.6 | 0.007 |
| Hoehn-Yahr stage at 2nd PET | 2.4 ± 0.7 | 2.9 ± 0.8 | NS |
NA not applicable; NS not significant
Quantitative Parameters
The initial SNBRs of the total striatum in the PD group were significantly lower than those in the non-PD group (4.0 ± 1.2 vs. 6.4 ± 0.8; p < 0.001). In the PD group, the initial SNBRs of PP exhibited the lowest value of 2.6 ± 1.1 followed by AP (4.0 ± 1.4), VS (4.9 ± 1.1), and CA (5.0 ± 1.5). Among these, the VS, AP, and PP values exhibited significant differences compared to those of the non-PD group. However, there was no significant difference in CA (Table 4). The AAR (0.26 ± 0.14 vs. 0.09 ± 0.19; total striatum p < 0.05) and %RAR (6.9 ± 3.5 vs. 1.2 ± 2.7; total striatum p < 0.001) of the PD group had significantly faster values than those of the non-PD group. The %RAR value of PP in the PD group was approximately eight times greater at 9.6 ± 3.6 than 1.2 ± 2.7 of the non-PD group (Table 4).
Table 4.
Initial specific to non-specific binding ratios (SNBRs), absolute annual reduction (AAR), and relative annual reduction (RAR) of the striatal subregions in the Parkinson’s disease (PD) and non-PD groups
| Initial SNBR | AAR (/year) | RAR (%/year) | |||||
|---|---|---|---|---|---|---|---|
| Region | Side | PD | Non-PD | PD | Non-PD | PD | Non-PD |
| Total striatum | Contralateral | 3.8 ± 1.2** | 6.3 ± 0.8 | 0.25 ± 0.14* | 0.10 ± 0.17 | 7.1 ± 3.6** | 1.3 ± 2.4 |
| Ipsilateral | 4.2 ± 1.2** | 6.4 ± 0.8 | 0.27 ± 0.14* | 0.08 ± 0.22 | 6.7 ± 3.5** | 1.0 ± 3.2 | |
| Mean | 4.0 ± 1.2** | 6.4 ± 0.8 | 0.26 ± 0.14* | 0.09 ± 0.19 | 6.9 ± 3.5** | 1.2 ± 2.7 | |
| Subregion | |||||||
| CA | Contralateral | 4.8 ± 1.6 | 5.6 ± 0.8 | 0.29 ± 0.20* | 0.12 ± 0.16 | 6.6 ± 4.4* | 2.0 ± 2.6 |
| Ipsilateral | 5.1 ± 1.5 | 5.7 ± 0.9 | 0.26 ± 0.21 | 0.09 ± 0.22 | 5.4 ± 4.3* | 1.4 ± 3.9 | |
| Mean | 5.0 ± 1.5 | 5.6 ± 0.8 | 0.28 ± 0.20* | 0.11 ± 0.18 | 6.0 ± 4.2* | 1.7 ± 3.0 | |
| VS | Contralateral | 4.8 ± 1.2* | 6.1 ± 0.9 | 0.22 ± 0.19 | 0.10 ± 0.16 | 4.9 ± 3.9* | 1.5 ± 2.4 |
| Ipsilateral | 5.0 ± 1.1* | 6.2 ± 0.9 | 0.21 ± 0.21* | −0.01 ± 0.20 | 4.4 ± 4.2* | −0.2 ± 3.0 | |
| Mean | 4.9 ± 1.1* | 6.2 ± 0.9 | 0.22 ± 0.19* | 0.04 ± 0.16 | 4.6 ± 3.9* | 0.7 ± 2.5 | |
| AP | Contralateral | 3.7 ± 1.3** | 7.2 ± 1.1 | 0.28 ± 0.14* | 0.07 ± 0.24 | 8.4 ± 4.3** | 0.8 ± 3.1 |
| Ipsilateral | 4.3 ± 1.5** | 7.2 ± 1.1 | 0.33 ± 0.20* | 0.10 ± 0.27 | 8.2 ± 4.8** | 1.2 ± 3.5 | |
| Mean | 4.0 ± 1.4** | 7.2 ± 1.1 | 0.31 ± 0.16* | 0.09 ± 0.24 | 8.3 ± 4.3** | 1.0 ± 3.0 | |
| PP | Contralateral | 2.3 ± 1.0** | 6.7 ± 0.9 | 0.21 ± 0.13* | 0.09 ± 0.18 | 9.4 ± 4.2** | 1.1 ± 2.3 |
| Ipsilateral | 2.9 ± 1.2** | 6.8 ± 0.9 | 0.27 ± 0.12* | 0.09 ± 0.23 | 9.7 ± 3.6** | 1.2 ± 3.1 | |
| Mean | 2.6 ± 1.1** | 6.7 ± 0.9 | 0.24 ± 0.11* | 0.09 ± 0.20 | 9.6 ± 3.6** | 1.2 ± 2.7 | |
*< 0.05, **< 0.001; CA caudate nucleus; VS ventral striatum; AP anterior putamen; PP posterior putamen
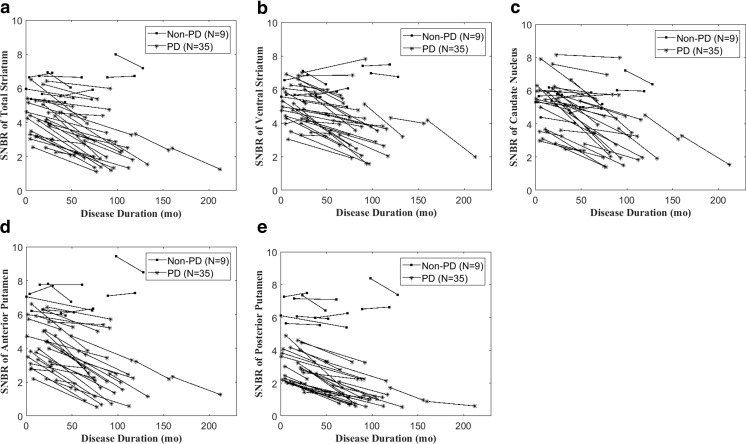
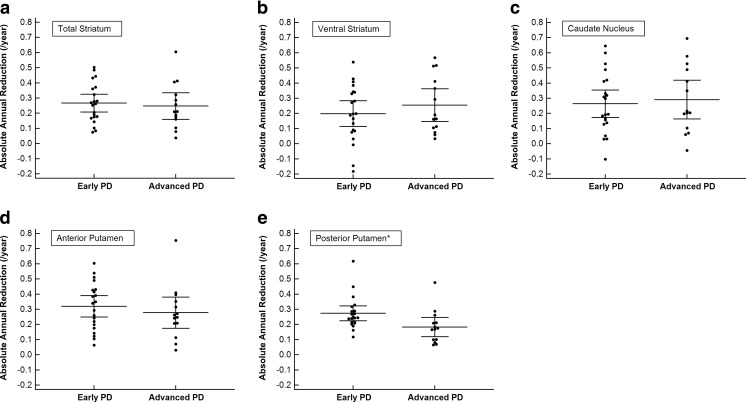
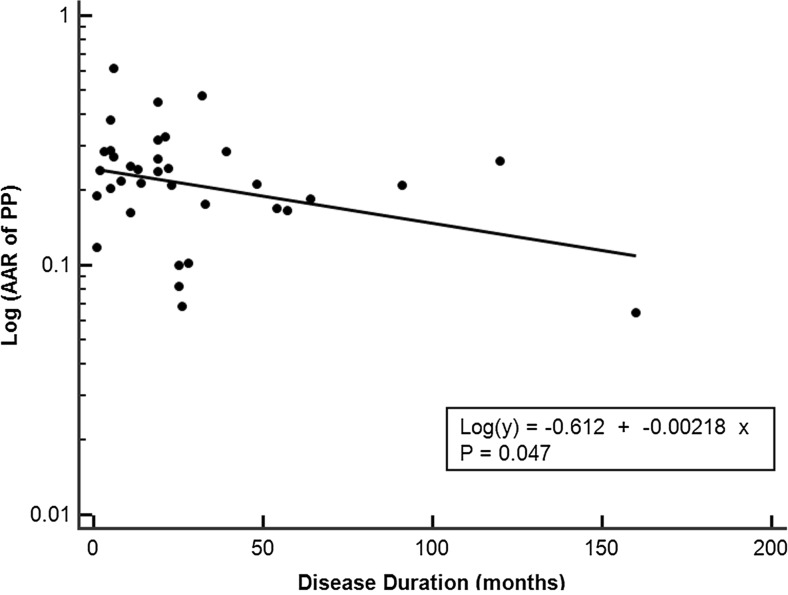
Figure 2 presents the overall trend of the SNBR changes with disease progression in the PD and non-PD groups. All PD patients exhibited a decreasing pattern with disease progression. All of the non-PD patients exhibited higher SNBRs of PP at both the initial and follow-up [18F]FP-CIT PET. All PD patients demonstrated a downward SNBR slope of PP with disease progression, and the slopes flattened further in the patients with a longer disease duration. The AAR of PP was the only parameter among all subregional quantitative parameters that showed a significant difference between the early stage of PD and more advanced stage of PD groups (P < 0.05) (Fig. 3). A scatter plot of AAR of PP with log-scale showed a significant negative correlation with the disease duration (Fig. 4). However, the %RARs of PP were not significantly different between the early stage of PD and more advanced stage of PD groups (Table 6).
Fig. 2.
SNBR changes of striatal subregions and total striatum with disease progression in the Parkinson’s disease (PD) and non-PD groups. a Total striatum, b ventral striatum, c caudate nucleus, d anterior putamen, and e posterior putamen (PP). All non-PD patients exhibited higher SNBRs of PP at both the initial and follow-up [18F]FP-CIT PET. All PD patients exhibited a downward slope of the SNBR for the PP with disease progression, and the slopes were further flattened in patients with a longer disease duration
Fig. 3.
Comparison of absolute annual reduction (AAR) between early stage of Parkinson’s disease (PD) and more advanced stage of PD groups in the striatal subregions and total striatum. a Total striatum, b ventral striatum, c caudate nucleus, d anterior putamen, and e posterior putamen. Only the AAR of the PP exhibited a significant difference between early stage of PD and a more advanced stage of PD groups (*P < 0.05)
Fig. 4.
Scatter plot of absolute annual reduction (AAR) with log-scale in the posterior putamen (PP) according to the disease duration. There was a significant negative correlation suggesting a decreasing pattern of the exponential curve
Table 6.
Initial specific to non-specific binding ratios (SNBRs), absolute annual reduction (AAR), and relative annual reduction (RAR) of striatal subregions in the early stage of Parkinson’s disease (PD) and more advanced stage of PD groups
| Initial SNBR | AAR (/year) | RAR (%/year) | |||||
|---|---|---|---|---|---|---|---|
| Region | Side | Early | Advanced | Early | Advanced | Early | Advanced |
| Total striatum | Contralateral | 4.2 ± 1.2* | 3.2 ± 0.9 | 0.26 ± 0.13 | 0.24 ± 0.15 | 6.6 ± 3.3 | 7.8 ± 4.1 |
| Ipsilateral | 4.7 ± 1.4* | 3.6 ± 0.8 | 0.28 ± 0.14 | 0.25 ± 0.15 | 6.5 ± 3.2 | 7.0 ± 4.0 | |
| Mean | 4.4 ± 1.2* | 3.4 ± 0.8 | 0.27 ± 0.13 | 0.25 ± 0.15 | 6.6 ± 3.1 | 7.3 ± 4.0 | |
| Subregion | |||||||
| CA | Contralateral | 5.2 ± 1.6 | 4.2 ± 1.4 | 0.28 ± 0.20 | 0.31 ± 0.21 | 5.7 ± 3.8 | 7.9 ± 4.9 |
| Ipsilateral | 5.5 ± 1.6 | 4.6 ± 1.2 | 0.26 ± 0.20 | 0.27 ± 0.24 | 5.2 ± 3.8 | 5.8 ± 5.1 | |
| Mean | 5.4 ± 1.6 | 4.4 ± 1.3 | 0.27 ± 0.20 | 0.29 ± 0.22 | 5.4 ± 3.7 | 6.8 ± 4.9 | |
| VS | Contralateral | 5.0 ± 1.2 | 4.5 ± 1.0 | 0.20 ± 0.18 | 0.25 ± 0.21 | 4.2 ± 3.7 | 5.5 ± 4.1 |
| Ipsilateral | 5.3 ± 1.3 | 4.7 ± 0.7 | 0.19 ± 0.22 | 0.26 ± 0.18 | 3.8 ± 4.4 | 5.4 ± 3.7 | |
| Mean | 5.2 ± 1.3 | 4.6 ± 0.9 | 0.19 ± 0.19 | 0.26 ± 0.19 | 4.0 ± 4.0 | 5.4 ± 3.7 | |
| AP | Contralateral | 4.0 ± 1.3* | 3.1 ± 1.2 | 0.29 ± 0.14 | 0.26 ± 0.15 | 7.9 ± 4.2 | 9.1 ± 4.5 |
| Ipsilateral | 4.7 ± 1.6* | 3.7 ± 1.1 | 0.35 ± 0.19 | 0.30 ± 0.23 | 8.3 ± 4.8 | 8.2 ± 5.0 | |
| Mean | 4.4 ± 1.4* | 3.4 ± 1.1 | 0.32 ± 0.15 | 0.28 ± 0.18 | 8.1 ± 4.2 | 8.6 ± 4.6 | |
| PP | Contralateral | 2.7 ± 0.9* | 1.7 ± 0.7 | 0.25 ± 0.12* | 0.16 ± 0.12 | 9.4 ± 3.4 | 9.3 ± 5.2 |
| Ipsilateral | 3.4 ± 1.2* | 2.1 ± 0.7 | 0.30 ± 0.11* | 0.21 ± 0.11 | 9.5 ± 3.2 | 9.9 ± 4.2 | |
| Mean | 3.1 ± 1.0** | 1.9 ± 0.7 | 0.28 ± 0.10* | 0.18 ± 0.11 | 9.5 ± 3.0 | 9.7 ± 4.5 | |
*< 0.05, **< 0.001; CA caudate nucleus; VS ventral striatum; AP anterior putamen; PP posterior putamen
In the comparison between the YOPD and EOPD groups, the initial SNBRs of all subregions except for PP were lower in the EOPD group than in the YOPD group. There were no differences in any of the other quantitative parameters, including the AARs and %RARs of the striatal subregions (Table 5).
Table 5.
Initial specific to non-specific binding ratios (SNBRs), absolute annual reduction (AAR), and relative annual reduction (RAR) of striatal subregions in elderly onset Parkinson’s disease (EOPD) and young onset Parkinson’s disease (YOPD) patients
| Initial SNBR | AAR (/year) | RAR (%/year) | |||||
|---|---|---|---|---|---|---|---|
| Region | Side | EOPD | YOPD | EOPD | YOPD | EOPD | YOPD |
| Total striatum | Contralateral | 3.3 ± 1.0* | 4.4 ± 1.1 | 0.23 ± 0.13 | 0.28 ± 0.15 | 7.1 ± 3.3 | 7.0 ± 4.1 |
| Ipsilateral | 3.7 ± 1.0** | 4.9 ± 1.2 | 0.24 ± 0.13 | 0.30 ± 0.16 | 6.8 ± 3.2 | 6.6 ± 4.0 | |
| Mean | 3.5 ± 1.0** | 4.7 ± 1.1 | 0.24 ± 0.12 | 0.29 ± 0.15 | 7.0 ± 3.2 | 6.8 ± 4.0 | |
| Subregion | |||||||
| CA | Contralateral | 4.1 ± 1.4* | 5.9 ± 1.3 | 0.25 ± 0.19 | 0.35 ± 0.21 | 6.6 ± 4.4 | 6.5 ± 4.5 |
| Ipsilateral | 4.3 ± 1.2* | 6.1 ± 1.2 | 0.23 ± 0.19 | 0.30 ± 0.24 | 5.5 ± 4.3 | 5.3 ± 4.4 | |
| Mean | 4.2 ± 1.3* | 6.0 ± 1.2 | 0.24 ± 0.19 | 0.32 ± 0.22 | 6.0 ± 4.2 | 5.9 ± 4.4 | |
| VS | Contralateral | 4.4 ± 1.1* | 5.4 ± 1.1 | 0.21 ± 0.18 | 0.23 ± 0.21 | 4.9 ± 3.8 | 4.4 ± 4.1 |
| Ipsilateral | 4.6 ± 1.1* | 5.6 ± 1.0 | 0.21 ± 0.20 | 0.22 ± 0.22 | 4.5 ± 4.3 | 5.3 ± 4.4 | |
| Mean | 4.5 ± 1.1* | 5.5 ± 1.0 | 0.21 ± 0.18 | 0.22 ± 021 | 4.7 ± 3.9 | 4.4 ± 4.0 | |
| AP | Contralateral | 3.2 ± 1.1* | 4.3 ± 1.3 | 0.25 ± 0.15 | 0.32 ± 0.13 | 8.3 ± 4.2 | 9.4 ± 4.9 |
| Ipsilateral | 3.8 ± 1.3* | 5.1 ± 1.4 | 0.30 ± 0.21 | 0.36 ± 0.19 | 8.3 ± 4.4 | 8.1 ± 5.0 | |
| Mean | 3.5 ± 1.2* | 4.7 ± 1.4 | 0.28 ± 0.16 | 0.34 ± 0.16 | 8.4 ± 4.2 | 8.3 ± 4.6 | |
| PP | Contralateral | 2.1 ± 0.8 | 2.6 ± 1.2 | 0.20 ± 0.11 | 0.23 ± 0.15 | 9.4 ± 3.7 | 9.4 ± 4.9 |
| Ipsilateral | 2.5 ± 1.0 | 3.4 ± 1.3 | 0.24 ± 0.10 | 0.30 ± 0.13 | 9.7 ± 3.4 | 9.7 ± 4.0 | |
| Mean | 2.3 ± 0.9 | 3.0 ± 1.2 | 0.22 ± 0.10 | 0.27 ± 0.13 | 9.6 ± 3.2 | 9.6 ± 4.2 | |
*< 0.05, **< 0.001; CA caudate nucleus; VS ventral striatum; AP anterior putamen; PP posterior putamen
In the subgroup analysis by disease duration, the initial SNBRs of the striatal subregions in the early PD group were greater than those in the more advanced PD group, with significant differences in only the AP, PP, and total striatum. The subregional AARs of the AP and PP in the early PD group were greater than those in the more advanced PD group, whereas the CA and VS values of the early PD group were lower than those in the more advanced PD group. Only the AAR of PP demonstrated a significant difference between these values (0.28 ± 0.10/year vs. 0.18 ± 0.11/year; P = 0.013). Both the contralateral and ipsilateral PP sides were significantly different (0.25 ± 0.12/year vs. 0.16 ± 0.12/year; contralateral side P = 0.039; 0.30 ± 0.11/year vs. 0.21 ± 0.11/year; ipsilateral side P = 0.01). In the %RAR comparison, the more advanced PD group had a faster value than the early PD group (except for the ipsilateral AP and contralateral PP); however, none of the subregions exhibited significant differences (7.3 ± 4.0%/year vs. 6.6 ± 3.1%/year; total striatum P = 0.526) (Table 6).
Discussion
This is the first longitudinal study to investigate the decreasing pattern of striatal [18F]FP-CIT uptake. The initial [18F]FP-CIT uptake of the PD group was significantly lower than that of the non-PD group, except for CA. The initial SNBR of PP for the PD group was only 39% of the non-PD group, exhibiting the lowest initial uptake among the subregions, followed by AP (56% of the non-PD group) and CA (89% of the non-PD group) (Table 4). This finding is compatible with reports that PP is the most severely affected subregion in the striatum, whereas CA is a relatively spared subregion [17–19]. Moreover, the ventrolateral aspect of the substantia nigra, which is the projecting dopaminergic neuron to the posterior side of the putamen, is the most severely affected region in the mid-brain [7, 20–22]. Nurmi et al. reported that the initial [18F]CFT uptake of striatal subregions is approximately 27% (posterior putamen), 45% (anterior putamen), and 71% (caudate nucleus) of healthy controls, respectively [23]. The initial [18F]FP-CIT uptake of the striatal subregions was greater than those reported in a [18F]CFT PET study; however, the mean of disease duration of the PD group (30 ± 34 months) was longer in this study compared to 1.7 ± 1.1 years in the [18F]CFT PET study [23]. In this study, the mean age of the non-PD group (70 ± 8 years) was significantly older than that of the PD group (55 ± 12 years). In addition, the non-PD group was not composed of healthy controls but rather patients with DIP or ET. The use of a different type of radiotracer or other methodological differences may also be reasons for this discrepancy.
The annual rate of decline for the [18F]FP-CIT uptake of the total striatum was approximately 6.9%/year in this study. This result is a slower rate compared to those of other studies. In a previous study of dopamine transporter imaging with [18F]CFT PET, the rates of annual decline of the baseline mean in the putamen were 13.1% and 12.5% in the caudate nucleus [24]. Moreover, it was reported that the rate of decline was 8%/year for [123I]FP-CIT SPECT and 11%/year for [123I]β-CIT SPECT [5, 6]. There are some suggested explanations for this discrepancy. First, the average disease duration (30 ± 34 months) and test interval (56 ± 19 months) were longer in this study compared to those in previous studies (2.5 years and 12 months in the [123I]FP-CIT SPECT study and 2.1 years and 2 years in the [18F]CFT PET study). Therefore, patients in this retrospective study were in a more advanced stage of disease. Second, the proportion of de novo patients was small in this study. All of the patients in the [123I]FP-CIT SPECT and [18F]CFT PET studies were de novo at the initial scan compared to only 10 of 35 total patients in this study. There is some controversy regarding the effect of levodopa on the degeneration of dopaminergic neurons. Nurmi et al. reported that PD patients treated with levodopa for 3 months exhibited no significant effect regarding [18F]CFT uptake in any striatal subregion between the two PET scans [25]. However, the Parkinson Study Group suggests that levodopa accelerates the loss of nigrostriatal dopamine nerve terminals or that its pharmacologic effects modify the dopamine transporter [26]. Considering the acceleration effect of levodopa, de novo patients may exhibit a faster rate of decline because they usually initiate antiparkinson drugs (including levodopa) after the initial diagnostic imaging. Different sensitivities or resolution of SPECT or PET, variability in the types of radiotracer, and other methodological diversity can be the reasons for the discrepancy.
There were significant differences in the progression rate among the various striatal subregions in this study. PP exhibited the fastest rate of 9.6%/year, and VS was associated with the slowest rate of 4.6%/year; however, this result may be due to the lower initial SNBR of PP. Indeed, the AAR of AP (0.31 ± 0.16/year; P = 0.016) was greater than that of PP (0.24 ± 0.11/year), and the AARs of CA (0.28 ± 0.20/year; P = 0.539) and VS (0.22 ± 0.19/year; P = 0.600) were not significantly different from that of PP. The CALM-PD study with [123I]β-CIT SPECT previously reported that the decrease in the β-CIT binding of the putamen over 46 months (22.5%) was greater than that of the caudate (19.6%); however, this result may also be due to the lower baseline β-CIT binding of the putamen. The absolute change in β-CIT binding over 46 months was greater in the caudate than in the putamen [3].
We performed several subgroup analyses in this study according to the age at onset and disease duration. First, the initial SNBRs of all subregions apart from the PP of the EOPD group were lower than those of the YOPD group (Table 5). The clinical characteristics of the two groups were not significantly different, except for age (Table 2). Therefore, the differences in the initial SNBRs of CA, VS, and AP are thought to be caused by the aging effect rather than a more severe pathologic involvement. Moreover, this result is in line with a previously reported imbalanced aging effect of [18F]FP-CIT binding in the striatum of PD [27]. None of the %RARs and AARs of the subregions were significantly different between the EOPD and YOPD groups. Thus, the striatal [18F]FP-CIT uptake and decreasing pattern of the two groups appear to be similar, apart from the aging effect. There are several reports regarding the different clinicopathologic characteristics of early onset PD, even though the definition of early onset differs somewhat between the studies. Selikhova et al. suggested that the earlier disease onset group had the longest duration to death and the greatest delay to the onset of falls and cognitive decline [28]. In addition, Gibb et al. reported that young onset cases more often presented with muscular stiffness and exhibited a 24% greater nigral cell loss, but no differences in the basic Lewy body pathology [29]. The pathophysiologic difference between EOPD and YOPD requires further investigation.
The other subgroup analysis was performed based on disease duration. The early PD and more advanced PD groups did not significantly differ in terms of clinical characteristics (e.g., age and test interval), with the exception of disease duration (Table 3). The initial SNBRs of the total striatum (3.4 ± 0.8 vs. 4.4 ± 1.2; P < 0.05), AP (3.4 ± 1.1 vs. 4.4 ± 1.4; P < 0.05), and PP (1.9 ± 0.7 vs. 3.1 ± 1.0; P < 0.001) of the more advanced PD group were significantly lower than those of the early PD group. However, CA and VS were not different between the two subgroups (Table 6). This finding also confirms that the putamen is the most severely affected and the representative subregion in PD and the quantitative value correlates well with the clinical course of the disease.
In the analyses of the decreasing patterns, the AAR of PP in the more advanced PD group was significantly lower than that of the early PD group (0.18 ± 0.11/year vs. 0.28 ± 0.10/year; P < 0.05); however, the %RAR, calculated by dividing the AAR by the initial SNBR, demonstrated a similar rate of decline of about 10%/year. The AAR and %RAR of CA, VS, and AP exhibited no significant differences between the two subgroups. In the comparison of AP and PP, the AAR of PP was significantly lower than that of AP (0.24 ± 0.11/year vs. 0.31 ± 0.16/year; P = 0.004). Furthermore, the AARs of AP (0.33 ± 0.20/year vs. 0.28 ± 0.14/year; P = 0.036) and PP (0.27 ± 0.12/year vs. 0.21 ± 0.13/year; P = 0.006) on the ipsilateral side were significantly greater than those on the contralateral side. From these results, we concluded that the absolute annual degeneration could be greater in patients and/or subregions with more remaining dopaminergic neurons. Therefore, the decreasing pattern of dopaminergic neuronal degeneration appears to be an exponential curve rather than a simple linear graph. There have also been some reports describing the exponential decreasing pattern of the dopaminergic neurons identified on [123I]β-CIT SPECT [30] and [18F]FDOPA PET [9, 30]. A pathologic study suggested that the pigmented neurons in the pars compacta of the substantia nigra also revealed an exponential loss associated with disease progression [7]. The finding that there was a significant negative correlation between the disease duration with the AAR of PP but not with the %RAR also supports the decreasing pattern of the exponential curve.
In the non-PD group, the rate of decline for the striatal [18F]FP-CIT uptake was 1.2%/year, and the figure was much greater than the previously reported aging effect [27]. In this study, the non-PD group patients with an essential tremor or drug-induced parkinsonism were associated with difficulty in the differential diagnosis with PD. In fact, six out of the nine patients with an essential tremor or drug-induced parkinsonism discontinued levodopa after the second [18F]FP-CIT PET confirmed the diagnosis. In addition, the rates of decline of the total striatum and PP of these six patients were significantly greater than those of the other three patients without a history of levodopa medication. As mentioned above, the acceleration effect of levodopa [26] might be one of the causes of a faster decline rate than the healthy controls or the combined preclinical PD. Furthermore, the small number of patients in the non-PD group made accurate comparison difficult.
This study has some limitations associated with the fact that it is a retrospective study. The patients who underwent [18F]FP-CIT PET twice cannot be generalized to all PD patients. In addition, the PD patients in our study were younger than those in the general population, and this study had a higher proportion of YOPD patients. Among the total 35 PD patients, 15 underwent a second [18F]FP-CIT PET for a preoperative test before deep brain stimulation. Other patients underwent a second [18F]FP-CIT PET to confirm the diagnosis or for changing management for patients with a low response.
Since there were a limited number of patients who underwent [18F]FP-CIT PET twice without nigrostriatal involvement, we designated both ET and DIP patients as the non-PD group. However, these two disease entities may differ in the striatal [18F]FP-CIT uptake. Four DIP patients were diagnosed with DIP caused by valproic acid, levosulpiride, and unidentified gastrointestinal motility modulating drugs. Moreover, the overall striatal [18F]FP-CIT uptakes of the four DIP patients were somewhat lower than those of the five ET patients without subregional selectivity. The effects of these drugs on the striatal [18F]FP-CIT uptake are not yet understood; thus, further investigation is required. Furthermore, research regarding the test-retest reproducibility of [18F]FP-CIT PET has not been conducted and is another area of future investigation. Since this study was performed retrospectively, the UPDRS score was not measured at the time of [18F]FP-CIT PET, and the lack of a correlation between the changes in clinical symptoms and [18F]FP-CIT uptake is one of the limitations of this study.
Although this retrospective study has several limitations, including selection bias and a small number of subjects, our findings may be useful for determining test intervals and sample size in future clinical trials investigating the efficacy of neuroprotective drugs.
Conclusion
The longitudinal decline of striatal [18F]FP-CIT uptake in PD was significantly faster than that in non-PD, with a different annual rate of decline among the striatal subregions. The posterior putamen exhibited the lowest initial [18F]FP-CIT uptake but also the fastest annual rate of decline. The absolute annual reduction of [18F]FP-CIT uptake of the posterior putamen decreases with disease progression, and it reflects the nonlinear decline pattern of striatal [18F]FP-CIT uptake in PD.
Acknowledgements
This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C2768).
Compliance with Ethical Standards
Conflict of Interest
Changhwan Sung, Jai Hyuen Lee, Jungsu S Oh, Minyoung Oh, Sang Ju Lee, Seung Jun Oh, Sun Ju Chung, Chong Sik Lee, and Jae Seung Kim declare that they have no conflict of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by our Institutional Review Board (IRB no. S2016–2189-0001).
Informed Consent
The institutional review board of our institute approved this retrospective study, and the requirement to obtain informed consent was waived.
References
- 1.Fahn S. Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J Neurol. 2005;252(Suppl 4):iv37–iv42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- 2.Oh M, Kim JS, Kim JY, Shin KH, Park SH, Kim HO, et al. Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med. 2012;53:399–406. doi: 10.2967/jnumed.111.095224. [DOI] [PubMed] [Google Scholar]
- 3.Parkinson Study Group Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. 2002;287:1653–1661. doi: 10.1001/jama.287.13.1653. [DOI] [PubMed] [Google Scholar]
- 4.Seibyl JP, Marek K, Sheff K, Zoghbi S, Baldwin RM, Charney DS, et al. Iodine-123-beta-CIT and iodine-123-FPCIT SPECT measurement of dopamine transporters in healthy subjects and Parkinson’s patients. J Nucl Med. 1998;39:1500–1508. [PubMed] [Google Scholar]
- 5.Winogrodzka A, Bergmans P, Booij J, van Royen EA, Janssen AG, Wolters EC. [123I]FP-CIT SPECT is a useful method to monitor the rate of dopaminergic degeneration in early-stage Parkinson’s disease. J Neural Transm (Vienna). 2001;108:1011–1019. doi: 10.1007/s007020170019. [DOI] [PubMed] [Google Scholar]
- 6.Marek K, Innis R, van Dyck C, Fussell B, Early M, Eberly S, et al. [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology. 2001;57:2089–2094. doi: 10.1212/WNL.57.11.2089. [DOI] [PubMed] [Google Scholar]
- 7.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 8.Brooks DJ, Salmon EP, Mathias CJ, Quinn N, Leenders KL, Bannister R, et al. The relationship between locomotor disability, autonomic dysfunction, and the integrity of the striatal dopaminergic system in patients with multiple system atrophy, pure autonomic failure, and Parkinson’s disease, studied with PET. Brain. 1990;113(Pt 5):1539–1552. doi: 10.1093/brain/113.5.1539. [DOI] [PubMed] [Google Scholar]
- 9.Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson’s disease. Brain. 1996;119(Pt 2):585–591. doi: 10.1093/brain/119.2.585. [DOI] [PubMed] [Google Scholar]
- 10.Vingerhoets FJ, Snow BJ, Lee CS, Schulzer M, Mak E, Calne DB. Longitudinal fluorodopa positron emission tomographic studies of the evolution of idiopathic parkinsonism. Ann Neurol. 1994;36:759–764. doi: 10.1002/ana.410360512. [DOI] [PubMed] [Google Scholar]
- 11.Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson’s disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry. 1998;64:314–319. doi: 10.1136/jnnp.64.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol. 2000;47:493–503. doi: 10.1002/1531-8249(200004)47:4<493::AID-ANA13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Oh SJ, Chi DY, Kang SH, Kil HS, Kim JS, et al. One-step high-radiochemical-yield synthesis of [18F]FP-CIT using a protic solvent system. Nucl Med Biol. 2007;34:345–351. doi: 10.1016/j.nucmedbio.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Park E, Hwang YM, Lee CN, Kim S, Oh SY, Kim YC, et al. Differential diagnosis of patients with inconclusive Parkinsonian features using [(18)F]FP-CIT PET/CT. Nucl Med Mol Imaging. 2014;48:106–113. doi: 10.1007/s13139-013-0253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foundation NP. Available from: http://www.parkinson.org/understanding-parkinsons/what-is-parkinsons/young-onset-parkinsons
- 16.Kim HW, Kim JS, Oh M, Oh JS, Lee SJ, Oh SJ, et al. Different loss of dopamine transporter according to subtype of multiple system atrophy. Eur J Nucl Med Mol Imaging. 2016;43:517–525. doi: 10.1007/s00259-015-3191-6. [DOI] [PubMed] [Google Scholar]
- 17.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 18.Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, et al. Differing patterns of striatal 18F-dopa uptake in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol. 1990;28:547–555. doi: 10.1002/ana.410280412. [DOI] [PubMed] [Google Scholar]
- 19.Brooks DJ. Functional imaging in relation to parkinsonian syndromes. J Neurol Sci. 1993;115:1–17. doi: 10.1016/0022-510X(93)90061-3. [DOI] [PubMed] [Google Scholar]
- 20.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510X(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 21.German DC, Manaye K, Smith WK, Woodward DJ, Saper CB. Midbrain dopaminergic cell loss in Parkinson’s disease: computer visualization. Ann Neurol. 1989;26:507–514. doi: 10.1002/ana.410260403. [DOI] [PubMed] [Google Scholar]
- 22.Rinne JO, Rummukainen J, Paljarvi L, Rinne UK. Dementia in Parkinson’s disease is related to neuronal loss in the medial substantia nigra. Ann Neurol. 1989;26:47–50. doi: 10.1002/ana.410260107. [DOI] [PubMed] [Google Scholar]
- 23.Nurmi E, Bergman J, Eskola O, Solin O, Vahlberg T, Sonninen P, et al. Progression of dopaminergic hypofunction in striatal subregions in Parkinson’s disease using [18F]CFT PET. Synapse. 2003;48:109–115. doi: 10.1002/syn.10192. [DOI] [PubMed] [Google Scholar]
- 24.Nurmi E, Ruottinen HM, Kaasinen V, Bergman J, Haaparanta M, Solin O, et al. Progression in Parkinson’s disease: a positron emission tomography study with a dopamine transporter ligand [18F]CFT. Ann Neurol. 2000;47:804–808. doi: 10.1002/1531-8249(200006)47:6<804::AID-ANA14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 25.Nurmi E, Bergman J, Eskola O, Solin O, Hinkka SM, Sonninen P, et al. Reproducibility and effect of levodopa on dopamine transporter function measurements: a [18F]CFT PET study. J Cereb Blood Flow Metab. 2000;20:1604–1609. doi: 10.1097/00004647-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 27.Lee CS, Kim S-J, Oh SJ, Kim HO, Yun S-C, Doudet D, et al. Uneven age effects of [18F] FP-CIT binding in the striatum of Parkinson’s disease. Ann Nucl Med. 2014;28:874–879. doi: 10.1007/s12149-014-0882-1. [DOI] [PubMed] [Google Scholar]
- 28.Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132(Pt 11):2947–2957. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 29.Gibb WR, Lees AJ. A comparison of clinical and pathological features of young- and old-onset Parkinson’s disease. Neurology. 1988;38:1402–1406. doi: 10.1212/WNL.38.9.1402. [DOI] [PubMed] [Google Scholar]
- 30.Staffen W, Mair A, Unterrainer J, Trinka E, Ladurner G. Measuring the progression of idiopathic Parkinson’s disease with [123I] beta-CIT SPECT. J Neural Transm (Vienna) 2000;107:543–552. doi: 10.1007/s007020070077. [DOI] [PubMed] [Google Scholar]