Abstract
We report a case with altered biodistribution of 99mTc-dicarboxypropane diphosphonate (99mTc-DPD) on whole body bone scan after intravenous iron supplement therapy. A 47-year-old male patient who had recently been detected with a hepatic mass suggestive of hepatocellular carcinoma underwent bone scan as staging work-up before surgery. Bone scan images at 3 h after injection of 99mTc-DPD demonstrated unusually increased blood pool activities in the heart, liver, and spleen with usual skeletal uptakes. The patient had been treated for severe anemia from hemorrhoid with two intravenous administration of ferric hydroxide carboxymaltose complex at approximately 22 h and 2 h prior to the 99mTc-DPD injection, which we consider as the most probable cause of altered biodistribution of 99mTc-DPD.
Keywords: Bone scan, 99mTc-DPD, Iron supplement, Biodistribution
Introduction
Bone scan is one of the most common nuclear imaging modalities, with high sensitivity for bone tumors, infection, and trauma, as well as an ability to image the entire skeleton at a reasonable cost [1]. The uptake of radiopharmaceutical occurs via chemisorption of phosphate compound to calcium hydroxyapatite, which is abundant in the osseous matrix of bone. Several radiopharmaceuticals are available for bone scan, including 99mTc-methylenediphosphonate (99mTc-MDP), 99mTc-hydroxymethylene diphosphonate (99mTc-HMDP), and 99mTc-dicarboxypropane diphosphonate (99mTc-DPD). Although these methods show comparable detection strength for metastatic foci, 99mTc-DPD is superior in terms of blood clearance, bone-to-soft tissue ratio, and subjectively assessed image quality [2, 3].
It is generally recommended that the skeletal images be obtained at 3–4 h after the injection of radiopharmaceutical to allow for clearance of soft tissue or blood pool activities. The biodistribution and clearance of bone-seeking radiopharmaceuticals may be affected by several factors such as radiopharmaceutical preparation, formulation, administration techniques and procedures, pathophysiological and biochemical changes, medical procedures, and drug therapy or drug interactions [4, 5]. In terms of drug interactions, various aluminum-containing drugs [6], cortisone [7], nephrotoxic drugs [8], and diphosphonate compounds [9] have been reported to significantly alter the biodistribution of bone-seeking radiopharmaceuticals. Altered biodistribution by these factors may lead to insufficient radioactivity at target organs, unnecessary radiation exposure due to repeated examination, and even misdiagnosis. Therefore, knowing these factors is important for nuclear physicians in order to make appropriate interpretation. Here, we report a case with altered biodistribution of 99mTc-DPD on bone scan after intravenous (IV) iron supplement therapy.
Case Report
A 47-year-old male with hepatitis B virus (HBV)-associated liver cirrhosis was referred to our surgery department for a recently found 2.6 cm mass in the segment III of the liver on ultrasonography. He had history of hematochezia 3 month ago due to internal hemorrhoid. Physical examination was unremarkable at the time of admission. Routine hematology tests revealed anemia with hemoglobin level of 8.3 g/dl (normal range: 13–17) and hematocrit level of 31.3% (normal range: 39–52). All initial laboratory test results were within normal range, except for a slightly elevated level of aspartate aminotransferase of 55 IU/L (normal range: 0–40) and alanine aminotransferase of 58 IU/L (normal range: 0–40). Blood testing revealed positive for hepatitis surface antigen and negative for hepatitis surface antibody. HBV DNA level was significantly higher at 3.6 x105 IU/ml (normal range: <15). The patient showed an alpha fetoprotein level of 7.0 ng/mL (normal range: 0–7.5) and elevated level of protein induced by vitamin K absence/antagonist-II (PIVKA-II) of 481 mAU/mL (normal range: 0–40). Abdominal computed tomography (CT) showed a 2.6 cm mass with arterial enhancement and delayed washout in segment III of the liver, suggestive of hepatocellular carcinoma. Underlying liver showed cirrhotic features including fissural widening and edge blunting. The echocardiography findings were normal.
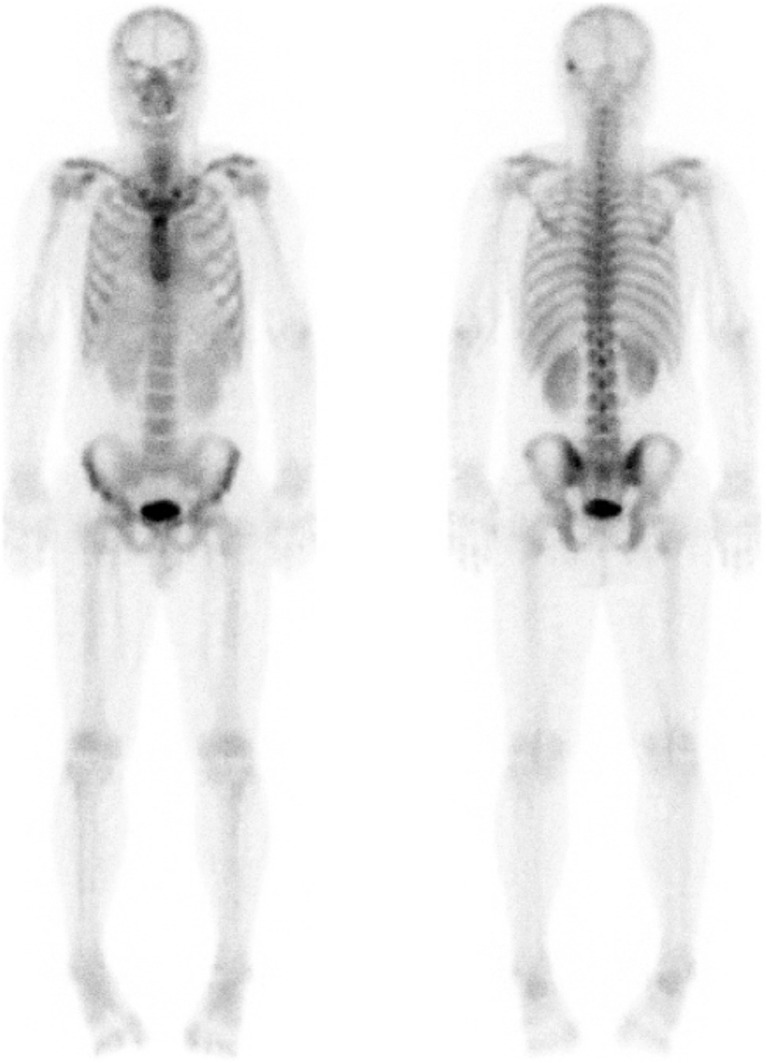
Bone scan was performed as a staging work-up before hepatic mass surgery. The images were obtained at approximately 3 h after the intravenous injection of 780 MBq 99mTc-DPD, and revealed focally increased uptake in left temporal bone, which was thought to be a benign bone lesion rather than skeletal metastasis. The imaging also showed diffusely increased radiotracer activities in the heart, liver, and spleen (Fig. 1).
Fig. 1.
Anterior (left) and posterior (right) view of preoperative 99mTc-DPD bone scan showing unusual, diffusely increased activities in the heart, liver, and spleen. A focal increased uptake in the left temporal bone may be a benign bone lesion rather than skeletal metastasis
Review of the overall preparation of radiopharmaceuticals, compounding, and administration procedures did not reveal remarkable steps. However, we found that 1800 mg (iron 500 mg) of ferric hydroxide carboxymaltose complex (®Ferinject injection, Choongwae Pharma Co., Seoul, Korea) mixed with normal saline was intravenously administered to the patient twice—the first dose at approximately 22 h before and the second dose at 2 h before 99mTc-DPD injection.
On the next day of bone scan, the patient underwent left lateral segmentectomy of the liver for hepatocellular carcinoma, and his postoperative hospital course was unremarkable.
Discussion
In this case report, we describe a patient whose 99mTc-DPD bone scan showed increased blood pool activities after having received intravenous iron supplement. After excluding possible causes of increased extraosseous uptake on bone scan, such as free pertechnetate due to presence of air in container, a long-standing preparation, an inappropriate amount of stannous ion, colloid formation due to aluminum, and high pH in the preparation, we suspected drug interaction. Diphosphonates, etidronate, iron or chemotherapy agents can cause non-osseous uptakes on bone scan [10], and in the present case, we suspect that intravenous iron supplement was associated with increased blood pool activities on bone scan as previously reported on other bone-seeking radiopharmaceuticals.
Several reports have focused on extraskeletal radiotracer activity associated with iron supplement in bone scan using 99mTc-labeled phosphates. Van Antwerp et al. [11] reported intramuscular 99mTc-phosphates activity at the intramuscular injection site of iron-dextran, and suggested that the mechanism of this extraskeletal activity may be a combination of reduced technetium with ferric hydroxide, as it is released from the iron dextran complex. MacDonald et al. [12] reported some cases with diffuse hepatic activity following intravenous injection of iron colloid solutions, and hypothesized that a 99mTc-iron-colloid complex can be formed through transchelation of the 99mTc-MDP, thus yielding a compound with different organ specificity and biological fate.
99mTc-labeled bone seeking agents including 99mTc-hydroxydiphosphonate (99mTc-HDP) [13, 14], 99mTc-hydroxyethylidene diphosphonate (99mTc-HEDP) [15], 99mTc-pyrophosphate (99mTc--PYP) [15], and 99mTc-HMDP [16] have also been reported to be associated with increased blood pool activity after intravenous injection of iron supplement. This finding might be explained by the occupation of unfilled coordination sites in the iron present in iron supplement, resulting in chemisorption of the phosphorus compounds and deposition of technetium, possibly as TcO2•2H2O [17]. It has also been proposed that metal ions such as iron could bring about changes in tissue distribution of 99mTc-labeled phosphates through the exchange of ligands at a given technetium oxidation level [18].
Based on our observation, we carefully suggest that the common phosphate-carbon-phosphate bonds in 99mTc-labeled phosphonates including MDP, HMDP, and DPD [19] might lead to similarly altered biodistribution on bone scan when iron is overloaded. Therefore, iron overload can be considered as a culprit when bone scan shows unusually increased blood pool activities.
Acknowledgements
None.
Compliance with Ethical Standards
Conflict of Interest
Eonwoo Shin, Minyoung Oh, Changhwan Sung, Ki-Hun Kim, and Jin-Sook Ryu declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the respective institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
The institutional review board of our institute approved this retrospective study, and the requirement to obtain informed consent was waived.
References
- 1.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 2.Bergqvist L, Brismar J, Cederquist E, Darte L, Naversten Y, Palmer J. Clinical comparison of bone scintigraphy with 99Tcm-DPD, 99Tcm-HDP and 99Tcm-MDP. Acta Radiol Diagn (Stockh) 1984;25:217–223. doi: 10.1177/028418518402500310. [DOI] [PubMed] [Google Scholar]
- 3.Vorne M, Vahatalo S, Lantto T. A clinical comparison of 99mTc-DPD and two 99mTc-MDP agents. Eur J Nucl Med. 1983;8:395–397. doi: 10.1007/BF00253214. [DOI] [PubMed] [Google Scholar]
- 4.Hesslewood S, Leung E. Drug interactions with radiopharmaceuticals. Eur J Nucl Med. 1994;21:348–356. doi: 10.1007/BF00947972. [DOI] [PubMed] [Google Scholar]
- 5.Vallabhajosula S, Killeen RP, Osborne JR. Altered biodistribution of radiopharmaceuticals: role of radiochemical/pharmaceutical purity, physiological, and pharmacologic factors. Semin Nucl Med. 2010;40:220–241. doi: 10.1053/j.semnuclmed.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Zimmer AM, Pavel DG. Experimental investigations of the possible cause of liver appearance during bone scanning. Radiology. 1978;126:813–816. doi: 10.1148/126.3.813. [DOI] [PubMed] [Google Scholar]
- 7.Alazraki N, Scott S, Manaster B, Wooten W, Murphy K. Effect of glucocorticoids on sensitivity of Tc-99m phosphonate bone imaging for detecting bone trauma. J Nucl Med. 1987;28:606. [Google Scholar]
- 8.Lutrin CL, McDougall IR, Goris ML. Intense concentration of technetium-99m pyrophosphate in the kidneys of children treated with chemotherapeutic drugs for malignant disease. Radiology. 1978;128:165–167. doi: 10.1148/128.1.165. [DOI] [PubMed] [Google Scholar]
- 9.Sandler ED, Parisi MT, Hattner RS. Duration of etidronate effect demonstrated by serial bone scintigraphy. J Nucl Med. 1991;32:1782–1784. [PubMed] [Google Scholar]
- 10.Loutfi I, Collier BD, Mohammed AM. Nonosseous abnormalities on bone scans. J Nucl Med Technol. 2003;31:149–153. [PubMed] [Google Scholar]
- 11.VanAntwerp J, Hall J, OMara R, Hilts S. Bone scan abnormality produced by interaction of Tc-99m diphosphonate with iron dextran (Imferon) [abstract] J Nucl Med. 1975;16:577. [Google Scholar]
- 12.MacDonald J. Idiopathic hepatic uptake of (99m)Tc methylene diphosphonate: a case report. J Nucl Med Technol. 2001;29:32–36. [PubMed] [Google Scholar]
- 13.Byun HH, Rodman SG, Chung KE. Soft-tissue concentration of 99mTc-phosphates associated with injections of iron dextran complex. J Nucl Med. 1976;17:374–375. [PubMed] [Google Scholar]
- 14.Parker JA, Jones AG, Davis MA, Mcilmoyle G, Tow DE. Reduced uptake of bone-seeking radiopharmaceuticals related to iron excess. Clin Nucl Med. 1976;1:267–268. doi: 10.1097/00003072-197612000-00006. [DOI] [Google Scholar]
- 15.Choy D, Murray IP, Hoschl R. The effect of iron on the biodistribution of bone scanning agents in humans. Radiology. 1981;140:197–202. doi: 10.1148/radiology.140.1.6264545. [DOI] [PubMed] [Google Scholar]
- 16.Forauer AR, Grossman SJ, Joyce JM. Altered biodistribution of Tc-99m HMDP on bone scintigraphy from recent intravenous iron therapy. Clin Nucl Med. 1994;19:817–818. doi: 10.1097/00003072-199409000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Jones AG, Francis MD, Davis MA. Bone scanning: radionuclidic reaction mechanisms. Semin Nucl Med. 1976;6:3–18. doi: 10.1016/S0001-2998(76)80032-3. [DOI] [PubMed] [Google Scholar]
- 18.McRae J, Hambright P, Valk P, Bearden AJ. Chemistry of 99mTc tracers. II. In vitro conversion of tagged HEDP and pyrophosphate (bone-seekers) into gluconate (renal agent). Effects of ca and Fe (ii) on in vivo distribution. J Nucl Med. 1976;17:208–211. [PubMed] [Google Scholar]
- 19.Pauwels EK, Blom J, Camps JA, Hermans J, Rijke AM. A comparison between the diagnostic efficacy of 99mTc-MDP, 99mTc-DPD and 99mTc-HDP for the detection of bone metastases. Eur J Nucl Med. 1983;8:118–122. doi: 10.1007/BF00256735. [DOI] [PubMed] [Google Scholar]