Abstract
Purpose
We propose a quantitative Tc-99m diethylenetriaminepentaacetic acid (DTPA) single-photon emission computed tomography/computed tomography (SPECT/CT) for glomerular filtration rate (GFR) measurement.
Methods
Quantitative SPECT/CT data obtained at 2–3 min post-Tc-99m DTPA injection (370 MBq) were used to determine % injected doses (%IDs) for individual kidneys. The reproducibility of %ID measurement was tested and compared with planar scintigraphy. Cr-51 ethylenediaminetetraacetic acid (EDTA) GFR was used as reference standard. Nine young volunteers, representing normal GFR, and ten older volunteers, reflecting impaired GFR, were enrolled. The established GFR equation derived from these volunteers was applied to 19 renal tumor patients post-partial nephrectomy.
Results
At 2−3 min, %ID was most reproducible with the highest intraclass correlation (ICC) (0.9379) and lowest % coefficient of variation (CV) (6.5259%), which were more reliable than the ICC (0.9368) and %CV (6.7689%) of planar scintigraphy. Cr-51 EDTA GFR (93.16 ± 24.81 ml/min) correlated significantly with %ID (7.66 ± 2.15%, r = 0.7906, p = 0.0001), yielding an equation: Cr-51 EDTA GFR (ml/min) = (%ID × 9.1462) + 23.0653. This equation revealed significant decreases in total and nephrectomized kidney GFR (p = 0.0012 and p < 0.0001, respectively) from preoperative to 3-month postoperative measurements.
Conclusions
Quantitative Tc-99m DTPA SPECT/CT produces reliable and clinically applicable %ID estimates that translate to the GFR of individual kidneys.
Electronic supplementary material
The online version of this article (10.1007/s13139-017-0491-8) contains supplementary material, which is available to authorized users.
Keywords: Computed tomography, Cr-51 EDTA, Glomerular filtration rate, Single-photon emission computed tomography, Tc-99m DTPA
Introduction
Glomerular filtration rate (GFR) is the crucial indicator of kidney function, and impaired GFR is closely associated with increased morbidity and mortality [1, 2]; therefore, GFR measurement is of paramount importance in the management of patients who are at risk of impaired renal function due to underlying disease, such as diabetes, or the administration of chemotherapeutics for malignant diseases [3, 4]. In addition to total bilateral renal function, individual renal function after nephrectomy has emerged as a major long-term prognostic indicator in patients with renal tumors and in kidney donors [5–7].
A number of clinical and laboratory tools for measuring GFR are available. Inulin urinary clearance is considered the gold standard for GFR estimation [8]; however, given the technical difficulty of inulin testing, several alternative methods have been proposed. These include modified inulin tests [9, 10], filtration agent methods [8, 11], serum creatinine-based estimation methods [1, 12], and image-based GFR measurement [13, 14].
Gamma camera-based GFR measurement was introduced in the early 1980s by Gates et al. under the assumption that the percentage injected dose (%ID) of technetium-99m diethylenetriaminepentaacetic acid (Tc-99m DTPA), measured for 1 min at 2–3 min after intravenous injection, would correlate strongly with 24-h urinary creatinine clearance [15, 16]. Gates’ original formula has since been updated or improved by many investigators [17–21], but the original assumption (i.e., %ID of Tc-99m DTPA at 2–3 min post-injection as a surrogate for GFR) persisted until the recent publication of studies using gamma cameras [14, 19–23]. However, the above-mentioned GFR scans were conducted in two dimensions, and radioactivity measured in the kidneys was not appropriately corrected for attenuation, scatter, and the distance variation between patients and detectors. Therefore, only the relative renal function of each kidney, rather than the absolute GFR values of individual or bilateral kidneys, has been used in patient management.
The recent worldwide installation of integrated single-photon emission computed tomography (SPECT) and computed tomography (CT), hereinafter SPECT/CT, has provided a novel opportunity for accurate quantitation of gamma-camera imaging [24, 25]. State-of-the-art SPECT/CT scanners include CT-based attenuation correction, scatter correction, and collimator-detector response correction (resolution recovery) [24]. Consequently, accurate %ID quantitation using SPECT/CT systems is now possible [26–28]. As Tc-99m DTPA is a well-known GFR agent and quantitative SPECT/CT can now provide unprecedented accuracy for radioactivity quantitation, quantitative SPECT/CT for GFR measurement can be developed, but this three-dimensional approach has yet to be reported.
In the current study, we attempted to apply quantitative SPECT/CT to post-Tc-99m DTPA injection %ID measurement for the estimation of GFR. First, to prove the feasibility of quantitative SPECT/CT, the reproducibility of %ID measurement was investigated. Second, the %ID value obtained from quantitative SPECT/CT was compared to the GFR reference standard obtained using the chromium-51 ethylenediaminetetraacetic acid (Cr-51 EDTA) test, and an equation for estimating GFR was developed. Finally, this quantitative SPECT/CT was performed in patients with renal tumors before and after partial nephrectomy to demonstrate its clinical usefulness.
Materials and Methods
Subjects
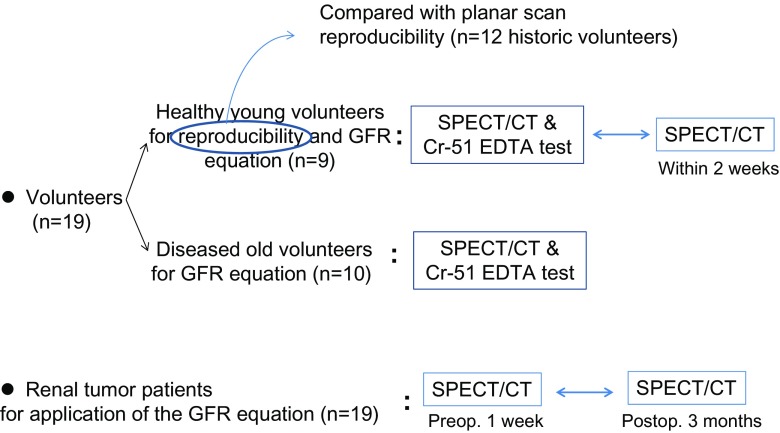
This study included two subject groups: volunteers (n = 19) and renal tumor patients (n = 19) (Table 1). The volunteers comprised typically young (5 males and 4 females; mean age: 25.7 ± 5.9 years) people without any underlying diseases and older individuals (10 males; mean age: 59.9 ± 10.8 years) with a variety of underlying diseases and impaired GFR. The young volunteers underwent quantitative SPECT/CT twice within a 2-week period for reproducibility assessment (Fig. 1). This reproducibility test was performed because it was not clear whether the SPECT acquisition with moving detectors would allow reliable %ID estimates from the dynamic phase data post-Tc-99m DTPA injection. The young volunteers also underwent Cr-51 GFR testing once for GFR equation establishment on the same day of one of the SPECT/CT studies. The older volunteers underwent both quantitative SPECT/CT and Cr-51 EDTA GFR testing on the same day, once, for establishing the GFR equation (Fig. 1).
Table 1.
Characteristics of the volunteers and the renal tumor patients
| Volunteers (n = 19) | Renal tumor patients (n = 19) | P value (statistical test) | ||
|---|---|---|---|---|
| Young volunteers (n = 9) | Old volunteers (n = 10) | Preoperative data | ||
| Age (years) | 25.7 ± 5.9 | 59.9 ± 10.8 | 53.6 ± 13.1 | 0.0001 (Kruskal-Wallis, lowest in young volunteers) |
| Male:female ratio | 5:4 | 10:0 | 11:8 | 0.0433 (chi-square test) |
| Blood chemistry | ||||
| Serum creatinine (mg/dl) | 0.78 ± 0.15 | 1.26 ± 0.33 | 0.85 ± 0.27 | 0.001 (ANOVA, highest in old volunteers) |
| eGFRa (ml/min/1.73 m2) | 108.6 ± 10.2 | 64.3 ± 20.2 | 91.3 ± 20.3 | < 0.001 (ANOVA, lowest in old volunteers) |
| Total protein (g/dl) | 7.12 ± 0.34 | 7.29 ± 0.33 | 7.22 ± 0.52 | NS§ |
| Albumin (g/dl) | 4.51 ± 0.33 | 4.44 ± 0.17 | 4.34 ± 0.29 | NS |
| AST† (IU/l) | 20.4 ± 4.59 | 23.6 ± 6.12 | 24.7 ± 13.0 | NS |
| ALT‡ (IU/l) | 21.6 ± 14.8 | 22.6 ± 10.7 | 30.1 ± 31.3 | NS |
| Urinalysis | ||||
| Albuminuria (yes:no) | 0:9 | 2:8 | 0:19 | NS |
| Glycosuria (yes:no) | 0:9 | 0:10 | 1:18 | NS |
| Underlying diseases | ||||
| Diabetes (yes:no) | 0:9 | 3:7 | 2:17 | NS |
| Hypertension (yes:no) | 0:9 | 4:6 | 5:14 | NS |
AST aspartate aminotransferase, ALT alanine aminotransferase, NS not significant
aEstimated GFR using the abbreviated Modification of Diet in Renal Disease formula: eGFR (ml/min/1.73 m2) = 175 × sCr-1.154 × age-0.203 × (0.742 if female)
Fig. 1.
Study scheme
The other subject group comprised renal tumor patients (n = 19) (Table 1). They had renal cell carcinoma (n = 17), transitional cell carcinoma (n = 1) or simple a renal cyst (n = 1), leading to partial nephrectomy. These patients (11 males and 8 females; mean age: 53.6 ± 13.1 years) were included to apply the established equation in a real-world clinical situation. They underwent quantitative SPECT/CT twice within 1 week (4.0 ± 4.6 days) prior to partial nephrectomy and again approximately 3 months (107.0 ± 24.3 days) after surgery (Fig. 1). The serum creatinine level was higher (p = 0.001) and the eGFR was lower (p < 0.001) in the older volunteers than in the young volunteers and the renal tumor patients (Table 1). The current study was approved by the institutional review board, and all the volunteers provided informed consent.
Quantitative SPECT/CT for %ID Measurements
First, the SPECT/CT scanner equipped with a low-energy high-resolution (LEHR) collimator in the current study (NM/CT670; GE Healthcare, Pittsburgh, PA) was calibrated to the dose calibrator (CRC-15R; CAPINTEC, Ramsey, NJ), and the system sensitivity for Tc-99m was determined using a phantom study. To determine the system sensitivity of the SPECT/CT scanner for converting the measured count rate [counts per second (cps)] to radioactivity [decay per second (dps)], we performed phantom studies in triplicate. Tc-99m pertechnetate was eluted from the Mo-99/Tc-99m generator (Technetium-99 m Generator; Samyoung Unitech). Three doses of Tc-99m pertechnetate (37 MBq, 185 MBq, and 370 MBq) were prepared using the dose calibrator, which was calibrated monthly using Cs-137 and Ba-133 as reference standards. A uniform cylinder phantom (diameter: 20 cm; length: 18 cm) was used in the phantom studies. Each Tc-99m pertechnetate dose was injected into the cylinder phantom, and SPECT/CT images were obtained after the doses had been mixed with water for 5 min. For SPECT/CT image acquisition, the phantom was located at the center of the field of view and 25 cm from the individual detectors. A 360-degree SPECT image was acquired for 1 min using a continuous mode with a 20% (±10%) energy window at the center of the 140-KeV energy peak (range, 126–154 KeV). Next, a spiral CT study was performed using the following parameters: tube potential, 120 kVp; tube current, 30 mA; beam collimation, 20 mm (1.25 × 16); pitch, 0.938:1; coverage speed, 37 mm/s; and tube rotation time, 0.5 s. CT images were reconstructed into a 512 × 512 matrix. The thicknesses of image slices in the transaxial, coronal, and sagittal planes were 2.5 mm, 0.98 mm, and 0.98 mm, respectively. SPECT images were iteratively reconstructed using the ordered subset expectation maximization (OSEM) algorithm with two iterations and ten subsets. During SPECT reconstruction, CT-based attenuation correction, lower energy (115–125 KeV) window-based scatter correction, and depth-dependent collimator-detector response correction were performed (Volumetrix MITM; GE Healthcare). A post-reconstruction Butterworth filter with a cutoff frequency of 0.48 and an order of ten was used. Reconstructed SPECT image slices were 2.95 mm thick with a zoom factor of 1.5 and were displayed in a 128 × 128 matrix. SPECT/CT images were analyzed on a dedicated workstation (Xeleris 3.1; GE Healthcare).
The phantom study results showed that the counts/s corrected for decay, attenuation, scatter, and collimator-detector response correlated strongly with radioactivity in the range of 37–370 MBq, with a R2 of 0.9996, and the system sensitivity was determined to be 275 counts/s per MBq (= 10,176 counts/s per mCi). The phantom study protocol is described elsewhere [27].
Quantitative SPECT/CT for %ID measurement was performed after injecting patients with Tc-99m DTPA. First, a cold vial of DTPA (TechneScan® DTPA; Mallinckrodt Pharmaceuticals, Dublin, Ireland) was reconstituted using Tc-99m pertechnetate eluted from the Mo-99/Tc-99m generator. The labeling efficiency of reconstituted Tc-99m DTPA was always >99%, as assessed using a thin-layer chromatography (TLC) scanner (AR-2000; BIOSCAN, Washington, DC).
The subjects were not placed under dietary restrictions. Thirty minutes prior to the SPECT/CT study, subjects were instructed to drink 500 ml of water. Tc-99m DTPA (dose: 370 MBq = 10 mCi) was injected through the antecubital vein while the subject lay in a head-first supine position on the SPECT/CT scanner table. The energy window was set at a peak of 140 KeV with ±10% (126–154 KeV). For the healthy young volunteers, 1 min after injection, three consecutive 1-min SPECT images were acquired at 1–2 min, 2–3 min, and 3–4 min using a continuous acquisition mode to determine the best time for %ID measurement during SPECT studies. The two detectors were positioned opposite each other (i.e., 180°) at first and subsequently rotated in a half-circle for 1 min to ensure 360° coverage by the two detectors. The 1–2 min and 3–4 min SPECT images were acquired with clockwise rotation, whereas the 2–3 min SPECT image was acquired with counterclockwise rotation. For the older volunteers and the renal tumor patients, the 1-min SPECT was obtained only at 2−3 min post-injection with counterclockwise rotation.
SPECT images were iteratively reconstructed using an ordered subset expectation maximization (OSEM) algorithm, with two iterations and ten subsets. During SPECT reconstruction, CT-based attenuation correction, lower energy (115–125 KeV) window-based scatter correction, and collimator-detector response correction (resolution recovery) were performed (Volumetrix MI™; GE Healthcare). A post-reconstruction Butterworth filter was used with a cutoff frequency of 0.48 and an order of 10. Reconstructed SPECT images had a 3.45-mm slice thickness and were displayed in a 128 × 128 matrix with a zoom factor of 1.28. Spiral CT was performed after the completion of SPECT acquisitions. (See Supplemental Material for CT acquisition parameters.) SPECT/CT images were analyzed on a dedicated workstation (Xeleris 3.1; GE Healthcare).
Kidney Segmentation and %ID Quantitation
Multiple single-slice regions of interest (ROIs) were manually drawn on coronal CT images of individual kidneys. These ROIs were immediately reflected on SPECT and SPECT/CT images. The final volume of interest was generated by integrating multiple single-slice ROIs. Approximately 30 slices were required to cover an entire kidney. The %ID values of individual kidneys were calculated using dosimetry software (Dosimetry Toolkit™; GE Healthcare) from the following data: pre-injection radioactivity (MBq), pre-injection radioactivity measurement time, injection time, residual radioactivity post-injection (MBq), post-injection radioactivity measurement time, image acquisition start time, and system sensitivity (275 counts/s per MBq = 10,176 counts/s per mCi).
Feasibility of Quantitative SPECT/CT
The feasibility of quantitative SPECT/CT was investigated in two aspects. First, the most reproducible time among three consecutive 1-min SPECT images (1–2 min, 2–3 min, and 3–4 min) was determined in the nine young volunteers who underwent SPECT/CT twice within a 2-week period (Table 1 and Fig. 1). The reproducibilities for %ID measurement were determined by calculating intraclass correlation (ICC) and percent coefficient of variation (%CV).
Second, the reproducibility of SPECT/CT acquisition was compared with that of planar GFR scintigraphy, using data from 12 historic volunteers (6 males and 6 females; mean age: 25.0 ± 6.34 years) (Fig. 1 and Supplemental Table 1) who had undergone traditional planar GFR scintigraphy twice within a 1-week period [21]. The planar GFR scintigraphy procedure is described in the Supplemental Material. Although the %ID values measured in the historic 12 volunteers have been reported previously, only the total %IDs of bilateral kidneys were used and compared with serum creatinine levels in that study [21]. In the current study, individual kidney %ID values obtained via planar scintigraphy were compared with those obtained using the current SPECT/CT protocol. The SPECT/CT and planar scintigraphy volunteer groups did not differ significantly in terms of demographic and biochemical characteristics (Table 1 and Supplemental Table 1).
Cr-51 EDTA GFR Test
Please refer to the supplemental material for the description of Cr-51 EDTA GFR test.
Statistical Analysis
Data are presented as means ± standard deviations. Reproducibility was statistically assessed by comparing the ICC values (2-way random model and absolute agreement) using the Konishi-Gupta modified Z-test [29]. Parametric analyses (ANOVA or paired t-test) were used for the group comparisons if the assumption of normal distribution was not rejected; otherwise, non-parametric tests (Mann-Whitney test, Wilcoxon test, or chi-square test) were employed. Pearson’s correlation coefficient was used to compare Cr-51 EDTA GFR and SPECT/CT %ID. MedCalc statistical software (version 12.4.0.0, MedCalc Software, Ostend, Belgium) was used for all analyses. A P value of <0.05 was considered statistically significant.
Results
Reproducibility of Quantitative SPECT/CT
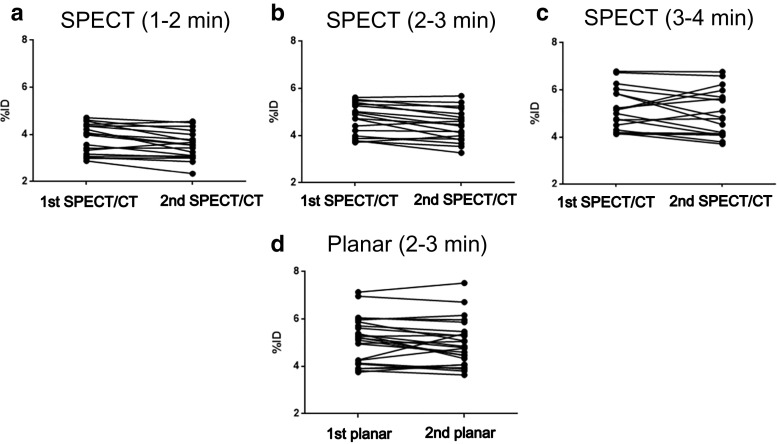
To determine the most reliable time for %ID measurement, SPECT data collected at 1–2 min, 2–3 min, and 3–4 min after Tc-99m DTPA injection were assessed. A total of 18 kidneys from 9 young volunteers (Table 1 and Fig. 1) were analyzed to determine the reproducibility of SPECT/CT %ID measurements, which were obtained within a 2-week period. The %ID values tended to increase gradually from 1–2 min (Fig. 2a) to 2−3 min (Fig. 2b) and 3–4 min (Fig. 2c) post-injection, but no differences were found in %ID values at any time point between the first and the second SPECT acquisitions (3.82% ± 0.63% versus 3.56% ± 0.61% at 1–2 min, 4.71% ± 0.68% versus 4.43% ± 0.68% at 2–3 min, and 5.17% ± 0.89% versus 4.99% ± 0.99% at 3–4 min; all p > 0.05; Fig. 2). The time point of 2–3 min (Fig. 2b) after Tc-99m DTPA injection was found to be most acceptable for SPECT/CT %ID measurements, because the %ID at this time point exhibited the lowest variation (%CV) and greatest ICC between the first and the second measurements [%CV = 6.5259% (lowest), ICC = 0.9379 (highest); 95% CI, 0.8339–0.9768], although there was not a statistically significant difference among the three time points (Konishi-Gupta modified Z-test for ICC, p > 0.05) [29].
Fig. 2.
Comparison of the reproducibility of %ID determined by SPECT/CT (n = 18 individual kidneys from 9 healthy young volunteers) and planar scintigraphy (n = 24 individual kidneys). Upper panels show the SPECT/CT results at 1–2 min (a), 2–3 min (b), and 3–4 min (c) after Tc-99m DTPA injection. The %CVs of the SPECT/CT were 8.0918%, 6.5259%, and 8.8466% for 1−2 min, 2−3 min, and 3−4 min, respectively. The ICCs (95% confidence interval) of the SPECT/CT were 0.9212 (0.7893–0.9705), 0.9379 (0.8339–0.9768), and 0.8750 (0.6657–0.9532) for 1−2 min, 2−3 min, and 3−4 min, respectively. The most acceptable SPECT/CT measurement was obtained at 2–3 min post-injection, at which time point the reproducibility was better than that obtained with planar scintigraphy at 2–3 min (d) post-injection [%CV = 6.7689%, and ICC (95% CI) = 0.9368 (0.8539–0.9727)]
Furthermore, the SPECT/CT %ID values obtained at 2–3 min (Fig. 2b) post-injection had lower %CV and greater ICC values than did planar scintigraphy at 2–3 min (Fig. 2d) post-injection (%CV = 6.7689%, ICC = 0.9368; 95% CI, 0.8539–0.9727; Fig. 2), although this difference was also not statistically significant (Konishi-Gupta modified Z-test for ICC, p > 0.05) [29]. It is likely that a statistically significant difference would have been observed if the sample size had been larger, because the 2−3 min %IDs are thought to be less affected by blood flow activity at an earlier time point (around 1−2 min, Fig. 2a) and by urine activity at a later time point (around 3−4 min, Fig. 2c). Using a similar approach, we had found that 2−3-min post-Tc-99m DTPA injection was the most stable and reliable time period for %ID measurement in planar GFR scintigraphy [21]. In addition, the 2–3 min %ID values calculated using SPECT/CT did not differ significantly from those obtained at 2−3 min using planar scintigraphy (5.12% ± 0.96%, p = 0.1371). Accordingly, 2–3 min after Tc-99m DTPA injection was determined to be the optimal time for %ID calculation in SPECT/CT studies, as well as in planar GFR scintigraphy.
Establishment of an Equation for GFR Estimation
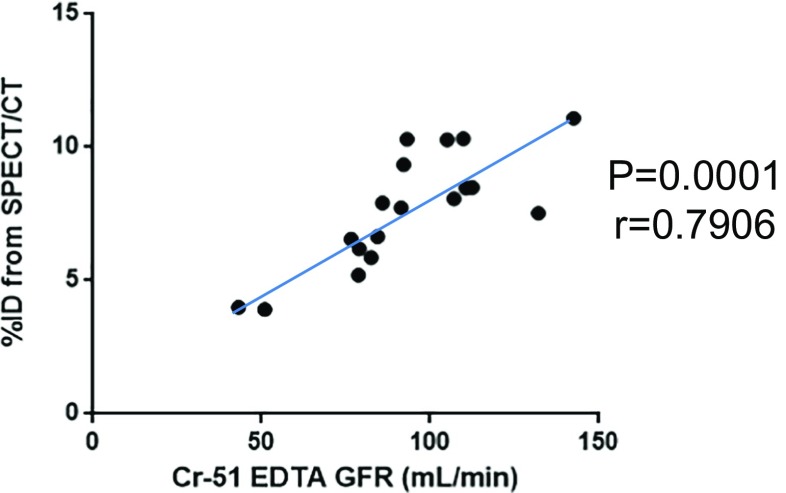
Reference GFR data from Cr-51 EDTA tests were obtained from eight healthy young volunteers (1 of the 9 volunteers was excluded because of technical failure), who comprised the normal GFR group, and ten older volunteers, who comprised the impaired GFR group. The Cr-51 EDTA GFR was 93.16 ± 24.81 ml/min (range: 43.18–142.54 ml/min), and the %ID (sum of bilateral kidney %IDs) was 7.66 ± 2.15% (range: 3.92–11.09%). A significant correlation was observed between Cr-51 EDTA GFR and SPECT/CT %ID (linear correlation coefficient r = 0.7906, p = 0.0001; Fig. 3). Using linear regression analysis, the following new equation for GFR estimation was derived from the SPECT/CT %ID data:
Fig. 3.
Correlations between the GFR measured with Cr-51 EDTA testing and SPECT/CT %ID (n = 18 patients). Eight healthy young volunteers and ten older volunteers were analyzed to determine the correlation between Cr-51 EDTA GFR and SPECT/CT %ID. A significant correlation was observed (r = 0.7906, p = 0.0001). The Cr-51 EDTA GFR could be estimated using the following equation: GFR (ml/min) = (%ID × 9.1462) + 23.0653
Application of Quantitative SPECT/CT to Partial Nephrectomy Patients
Nineteen renal tumor patients (Table 1) underwent preoperative quantitative SPECT/CT within 1 week (mean interval: 4.0 ± 4.6 days) before partial nephrectomy and a follow-up SPECT/CT was performed approximately 3 months (mean interval: 107.0 ± 24.3 days) after surgery (Fig. 1).
Both the total and single kidney GFRs could be assessed using quantitative SPECT/CT. The total GFR was significantly decreased after partial nephrectomy according to SPECT/CT (preoperative: 125.8 ± 19.1 ml/min/1.73 m2 versus postoperative: 116.0 ± 17.9 ml/min/1.73 m2, p = 0.0012; Fig. 4a). The GFR of nephrectomized kidneys was also significantly decreased after surgery (preoperative: 64.2 ± 10.5 ml/min versus postoperative: 52.5 ± 10.1, p < 0.0001; Fig. 4b), whereas the GFR of contralateral kidneys did not differ significantly between the preoperative (61.5 ± 9.8 ml/min) and postoperative time points (63.3 ± 10.7 ml/min, p = 0.3923; Fig. 4c). A typical case is demonstrated in Fig. 5.
Fig. 4.
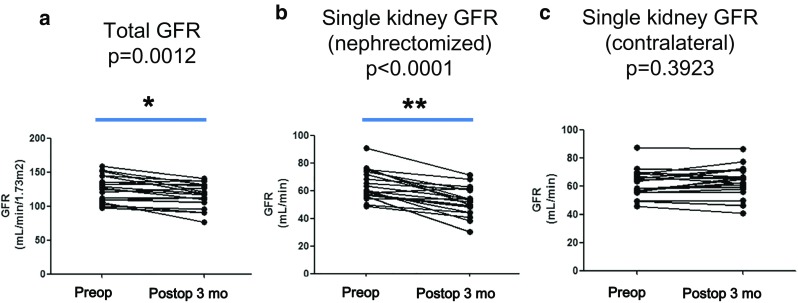
GFR changes measured by SPECT/CT after partial nephrectomy (n = 19). Total GFRs were significantly reduced from the preoperative SPECT/CT to the postoperative 3-month SPECT/CT (p = 0.0012) (a), and the GFRs of nephrectomized kidneys were also significantly decreased after surgery (p < 0.0001) (b). However, the GFRs of the contralateral kidneys did not significantly change after the surgery (p = 0.3923) (c). *p = 0.0012; **p < 0.0001
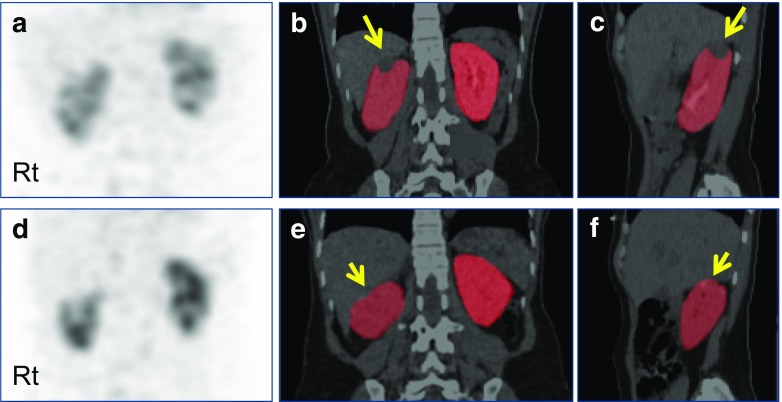
Fig. 5.
Quantitative SPECT/CT for perioperative GFR assessment in a patient with a renal tumor. The 45-year-old female patient had a renal cell carcinoma in the upper pole of the right kidney (long arrows, b and c). The right kidney was subjected to robot-assisted partial nephrectomy, and postoperative quantitative SPECT/CT was acquired 3 months after surgery (resection site is highlighted by short arrows, e and f). Compared with the preoperative quantitative SPECT/CT, the postoperative quantitative SPECT/CT showed a substantial change in GFR in the nephrectomized right kidney from 56.61 ml/min to 48.48 ml/min. On the other hand, the contralateral left kidney exhibited similar single kidney GFR values before (58.34 ml/min) and after surgery (61.08 ml/min). (a) Preoperative maximum-intensity projection (MIP) anterior image; (b) preoperative coronal SPECT/CT image with kidney segmentation; (c) preoperative sagittal SPECT/CT image with kidney segmentation showing right kidney; (d) postoperative MIP anterior image; (e) postoperative coronal SPECT/CT image with kidney segmentation; (f) postoperative sagittal SPECT/CT image with kidney segmentation showing right kidney
Discussion
The current study proved the feasibility of quantitative SPECT/CT as a promising imaging modality for GFR estimation. The measurement of the Tc-99m DTPA %ID during quantitative SPECT/CT was found to be most acceptable at 2–3 min after injection. Therefore, this three-dimensional SPECT/CT study confirms the existing premise that the %ID at 2–3 min post-Tc-99m DTPA injection is a surrogate for GFR in the two-dimensional planar gamma camera imaging [15, 16, 19–21]. Furthermore, the quantitative SPECT/CT %ID measurements were better than those obtained by planar scintigraphy (Fig. 2), which indicates the robustness of SPECT/CT relative to that of planar scintigraphy. If the Gates’ assumption (the %ID of Tc-99m DTPA measured at 2–3 min post-injection correlates strongly with GFR) is biologically relevant, this novel quantitative SPECT/CT imaging technology may provide clinically applicable and reliable GFR measurements.
Despite the proven feasibility of the quantitative SPECT/CT for %ID measurement, a concern remains regarding the accuracy of the obtained equation for GFR estimation. This issue arises from the relatively small number of enrolled patients for GFR equation determination (n = 18) and the lack of patients with severely impaired GFR (i.e., less than 30 ml/min; Fig. 3). If we had enrolled more patients with variable degrees of GFR impairment, the equation from SPECT/CT %ID would have had stronger power for GFR estimation. In this regard, this study’s results warrant further larger scale studies in future.
Another implication of the current study is that quantitative SPECT/CT acquisition is feasible even at a dynamic phase post-Tc-99m DTPA injection. %ID or similar quantitative parameters, such as a standardized uptake value for bone SPECT/CT has been measured at 3−4 h post-injection of Tc-99m phosphonates, when the agents are likely to have accumulated stably [26, 27]. On the other hand, the 2−3 min post-Tc-99m DTPA injection is the time when the injected Tc-99m DTPA moves rapidly through the renal parenchyma from the intravascular space to the urinary system. It was not clear whether a collimated SPECT scanner with limited temporal resolution can produce a reliable %ID estimate in this context [30]. Furthermore, the detectors should not stand still, but should move around the patient to obtain tomographic acquisition, sampling radioactive signals from the rapidly moving Tc-99m DTPA. However, it should be noted that the required temporal resolution in this type of GFR study was only on the scale of minutes rather than seconds. The integrated radioactivity over 1 min (specifically, 2–3 min post-Tc-99m DTPA injection according to the Gates’ suggestion) was necessary for the calculation of %ID. In fact, this 1-min SPECT requirement was the main reason for using continuous mode acquisition rather than the conventional step-and-shoot mode in the current study. The conventional step-and-shoot mode using the same SPECT/CT scanner takes at least 4 min for 360° SPECT acquisition, which is not compatible with the Gates’ assumption for GFR measurement. In the current quantitative SPECT/CT, the counts acquired by the two detectors during a 1-min period were integrated to produce SPECT images, which were then corrected for attenuation, scattering, and variations in the distance between the patient and detector. The resulting SPECT/CT %ID values were quantitatively robust and reliable (Fig. 2). The GFR equation derived from the SPECT/CT %ID, [GFR (ml/min) = (%ID × 9.1462) + 23.0653], was comparable to equations obtained using established protocols of planar scintigraphy (Supplemental Figure 1). Furthermore, perioperative changes in GFR could be relevantly assessed using the quantitative SPECT/CT (Figs. 4 and 5). Despite the inherent limitation of a collimated SPECT scanner, quantitative SPECT/CT has been proven to be a feasible method for estimating GFR from the dynamic studies.
Although SPECT/CT technology was introduced more than 2 decades ago [31–33], this modality did not appear to be a game changer until the recent acknowledgement of its quantitative capability [25–28]. Of course, it is hard to tell that the accurate measurement of %ID has been fully accomplished using only the triple correction methods (attenuation/scatter/detector geometric variation) because further corrections for partial volume effect, dead-time loss, and motion are often required in some other conditions. Therefore, the correction method in this study is a prerequisite of all the corrections needed. Furthermore, the GFR equation derived from the current study may not be considered the final gold standard for GFR estimation from the %ID post Tc-99m DTPA injection. However, the feasibility of quantitative SPECT/CT has been shown clearly. Further studies are required to expand the clinical utility of quantitative SPECT/CT.
Conclusion
Quantitative SPECT/CT generated reliable %ID values at 2–3 min post-Tc-99m DTPA injection. SPECT/CT %ID and Cr-51 EDTA GFR data were compared to generate an equation for GFR estimation. Perioperative GFR changes were successfully assessed using the novel GFR equation in renal tumor patients. Quantitative SPECT/CT holds promise as an imaging tool for GFR estimation.
Electronic supplementary material
(DOCX 27 kb)
(PDF 68 kb)
(GIF 79 kb)
Compliance with Ethical Standards
Conflicts of Interest
Yeon-koo Kang, So Hyun Park, Min Seok Suh, Seok-Soo Byun, Dong-Wan Chae, and Won Woo Lee declare that they have no conflicts of interest. This study was supported in part by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2015R1D1A1A01059146) and by the Seoul National University Bundang Hospital Research Fund (14–2016-012).
Ethical Approval
The study was approved by an institutional review board and was performed in accordance with the ethical standards set in the 1964 Declaration of Helsinki and its later amendments.
Informed Consents
Informed consent was obtained from all volunteers included in the study.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13139-017-0491-8) contains supplementary material, which is available to authorized users.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 3.Humphreys BD, Soiffer RJ, Magee CC. Renal failure associated with cancer and its treatment: an update. J Am Soc Nephrol. 2005;16:151–161. doi: 10.1681/ASN.2004100843. [DOI] [PubMed] [Google Scholar]
- 4.Kramer H, Molitch ME. Screening for kidney disease in adults with diabetes. Diabetes Care. 2005;28:1813–1816. doi: 10.2337/diacare.28.7.1813. [DOI] [PubMed] [Google Scholar]
- 5.Cohney S, Kanellis J, Howell M, CARI The CARI guidelines. Donor renal function. Nephrology (Carlton) 2010;15(Suppl 1):S137–S145. doi: 10.1111/j.1440-1797.2009.01223.x. [DOI] [PubMed] [Google Scholar]
- 6.Lane BR, Gill IS, Fergany AF, Larson BT, Campbell SC. Limited warm ischemia during elective partial nephrectomy has only a marginal impact on renal functional outcomes. J Urol. 2011;185:1598–1603. doi: 10.1016/j.juro.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 7.Simmons MN, Fergany AF, Campbell SC. Effect of parenchymal volume preservation on kidney function after partial nephrectomy. J Urol. 2011;186:405–410. doi: 10.1016/j.juro.2011.03.154. [DOI] [PubMed] [Google Scholar]
- 8.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20:2305–2313. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- 9.Prescott LF, Freestone S, McAuslane JA. Reassessment of the single intravenous injection method with inulin for measurement of the glomerular filtration rate in man. Clin Sci (Lond) 1991;80:167–176. doi: 10.1042/cs0800167. [DOI] [PubMed] [Google Scholar]
- 10.Lee CS, Cha RH, Lim YH, Kim H, Song KH, Gu N, et al. Ethnic coefficients for glomerular filtration rate estimation by the modification of diet in renal disease study equations in the Korean population. J Korean Med Sci. 2010;25:1616–1625. doi: 10.3346/jkms.2010.25.11.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perrone RD, Steinman TI, Beck GJ, Skibinski CI, Royal HD, Lawlor M, et al. Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I-iothalamate, 169Yb-DTPA, 99mTc-DTPA, and inulin. The modification of diet in renal disease study. Am J Kidney Dis. 1990;16:224–235. doi: 10.1016/S0272-6386(12)81022-5. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Funahashi Y, Hattori R, Yamamoto T, Kamihira O, Kato K, Gotoh M. Ischemic renal damage after nephron-sparing surgery in patients with normal contralateral kidney. Eur Urol. 2009;55:209–215. doi: 10.1016/j.eururo.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 14.Choi JD, Park JW, Choi JY, Kim HS, Jeong BC, Jeon SS, et al. Renal damage caused by warm ischaemia during laparoscopic and robot-assisted partial nephrectomy: an assessment using Tc 99m-DTPA glomerular filtration rate. Eur Urol. 2010;58:900–905. doi: 10.1016/j.eururo.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 15.Gates GF. Glomerular filtration rate: estimation from fractional renal accumulation of 99mTc-DTPA (stannous) AJR Am J Roentgenol. 1982;138:565–570. doi: 10.2214/ajr.138.3.565. [DOI] [PubMed] [Google Scholar]
- 16.Gates GF. Computation of glomerular filtration rate with Tc-99m DTPA: an in-house computer program. J Nucl Med. 1984;25:613–618. [PubMed] [Google Scholar]
- 17.Chachati A, Meyers A, Godon JP, Rigo P. Rapid method for the measurement of differential renal function: validation. J Nucl Med. 1987;28:829–836. [PubMed] [Google Scholar]
- 18.Taylor A, Lewis C, Giacometti A, Hall EC, Barefield KP. Improved formulas for the estimation of renal depth in adults. J Nucl Med. 1993;34:1766–1769. [PubMed] [Google Scholar]
- 19.Li Q, Zhang CL, Fu ZL, Wang RF, Ma YC, Zuo L. Development of formulae for accurate measurement of the glomerular filtration rate by renal dynamic imaging. Nucl Med Commun. 2007;28:407–413. doi: 10.1097/MNM.0b013e3280a02f8b. [DOI] [PubMed] [Google Scholar]
- 20.Ma YC, Zuo L, Zhang CL, Wang M, Wang RF, Wang HY. Comparison of 99mTc-DTPA renal dynamic imaging with modified MDRD equation for glomerular filtration rate estimation in Chinese patients in different stages of chronic kidney disease. Nephrol Dial Transplant. 2007;22:417–423. doi: 10.1093/ndt/gfl603. [DOI] [PubMed] [Google Scholar]
- 21.Kim YI, Ha S, So Y, Lee WW, Byun SS, Kim SE. Improved measurement of the glomerular filtration rate from Tc-99m DTPA scintigraphy in patients following nephrectomy. Eur Radiol. 2014;24:413–422. doi: 10.1007/s00330-013-3039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh K. Comparison of methods for determination of glomerular filtration rate: Tc-99m-DTPA renography, predicted creatinine clearance method and plasma sample method. Ann Nucl Med. 2003;17:561–565. doi: 10.1007/BF03006669. [DOI] [PubMed] [Google Scholar]
- 23.Kim HO, Chae SY, Baek S, Moon DH. Factors affecting changes in the glomerular filtration rate after unilateral nephrectomy in living kidney donors and patients with renal disease. Nucl Med Mol Imaging. 2010;44:69–74. doi: 10.1007/s13139-009-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritt P, Vija H, Hornegger J, Kuwert T. Absolute quantification in SPECT. Eur J Nucl Med Mol Imaging. 2011;38(Suppl 1):S69–S77. doi: 10.1007/s00259-011-1770-8. [DOI] [PubMed] [Google Scholar]
- 25.Bailey DL, Willowson KP. An evidence-based review of quantitative SPECT imaging and potential clinical applications. J Nucl Med. 2013;54:83–89. doi: 10.2967/jnumed.112.111476. [DOI] [PubMed] [Google Scholar]
- 26.Cachovan M, Vija AH, Hornegger J, Kuwert T. Quantification of 99mTc-DPD concentration in the lumbar spine with SPECT/CT. EJNMMI Res. 2013;3:45. doi: 10.1186/2191-219X-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh MS, Lee WW, Kim YK, Yun PY, Kim SE. Maximum standardized uptake value of (99m)Tc Hydroxymethylene diphosphonate SPECT/CT for the evaluation of temporomandibular joint disorder. Radiology. 2016;280:890–896. doi: 10.1148/radiol.2016152294. [DOI] [PubMed] [Google Scholar]
- 28.Lee H, Kim JH, Kang YK, Moon JH, So Y, Lee WW. Quantitative single-photon emission computed tomography/computed tomography for technetium pertechnetate thyroid uptake measurement. Medicine (Baltimore) 2016;95:e4170. doi: 10.1097/MD.0000000000004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donner A, Zou G. Interval estimation for a difference between intraclass kappa statistics. Biometrics. 2002;58:209–215. doi: 10.1111/j.0006-341X.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- 30.Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun. 2008;29:193–207. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- 31.Lang TF, Hasegawa BH, Liew SC, Brown JK, Blankespoor SC, Reilly SM, et al. Description of a prototype emission-transmission computed tomography imaging system. J Nucl Med. 1992;33:1881–1887. [PubMed] [Google Scholar]
- 32.Seo HJ, Ryu YH, Lee I, Min HS, Kang KW, Lee DS, et al. Usefulness of (131)I-SPECT/CT and (18)F-FDG PET/CT in evaluating successful (131)I and retinoic acid combined therapy in a patient with metastatic struma ovarii. Nucl Med Mol Imaging. 2015;49:52–56. doi: 10.1007/s13139-014-0295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Jafar H, Al-Shemmeri E, Al-Shemmeri J, Aytglu L, Afzal U, Al-Enizi S. Precision of SPECT/CT allows the diagnosis of a hidden Brodie's abscess of the talus in a patient with sickle cell disease. Nucl Med Mol Imaging. 2015;49:153–156. doi: 10.1007/s13139-014-0311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 27 kb)
(PDF 68 kb)
(GIF 79 kb)