Abstract
Dietary supplementation is commonly employed by individuals seeking to improve body composition and exercise performance. The purpose of the present study was to examine the safety and effectiveness of a commercially available dietary supplement designed to promote thermogenesis and fat loss. In a randomized double-blind trial, participants were assigned to consume placebo or a multi-ingredient supplement containing caffeine, green tea extract, l-carnitine, evodiamine and other ingredients that purportedly enhance thermogenesis. The study included acute baseline testing, a 6-week progressive resistance training and supplementation intervention, and post-intervention testing. Laboratory assessments included resting energy expenditure responses to acute supplement ingestion, evaluation of body composition and muscular performance, and analysis of blood variables (metabolic panel, testosterone, estrogen and cortisol). Dependent variables were analyzed using ANOVA with repeated measures. No unfavorable effects of supplementation were reported, and the supplement did not adversely affect safety markers. However, the supplement did not reduce fat mass or increase lean mass relative to placebo. In the supplement group, lower body maximal strength was increased relative to placebo (+18%, d=1.1 vs. +10%, d=0.5), and cortisol concentrations were decreased relative to placebo (-16%; d=-0.4 vs. +15%, d=.75). However, no differences were observed for upper body maximal strength or muscular endurance. REE increased in response to both supplement and placebo ingestion (placebo: +5%; supplement: +11.5%), but the difference between conditions was not statistically significant. Overall, some select parameters may have been beneficially modified by supplementation, but this did not result in superior weight or fat loss over 6 weeks of supplementation and resistance training.
Key points.
A multi-ingredient dietary supplement designed to promote thermogenesis and fat loss appears to be safe to consume for at least 6 weeks in healthy young adults.
Supplement ingestion acutely increases resting energy expenditure, but this was not significantly greater than increases seen with placebo.
When combined with a 6-week resistance training program, daily ingestion of the supplement did not produce superior changes in fat mass or lean mass relative to placebo.
Supplementation for 6 weeks reduced cortisol concentrations relative to placebo, but the physiological impact of this change is likely irrelevant.
Key words: resistance training, metabolism, adipose tissue, skeletal muscle
Introduction
Modifying body composition by increasing muscle mass and reducing fat mass is a common goal of exercising individuals. In pursuit of these goals, active individuals often utilize a combination of regimented exercise, nutritional alterations and dietary supplementation. It is estimated that approximately half of Americans regularly consume dietary supplements, and roughly 20% utilize sports supplements (Dickinson et al., 2014).
Multi-ingredient sports supplements contain a mixture of biologically active compounds designed to improve body composition, performance or recovery. A popular subset of these supplements, termed thermogenic or “cutting” agents, purportedly increase thermogenesis, promote fat oxidation and improve exercise performance (Schwarz et al., 2013). While limited research has been conducted on commercially available multi-ingredient thermogenic supplements, some data are available concerning the effects of common ingredients, including caffeine, green tea extract, l-carnitine, and evodiamine.
Caffeine has been reported to increase serum catecholamine concentrations, energy expenditure and thermogenesis (Astrup et al., 1990; Keijzers et al., 2002; Kim et al., 2011; Norager et al., 2006). Furthermore, caffeine may affect both sides of the energy balance equation by not only increasing energy expenditure, but also decreasing energy intake (Harpaz et al., 2017). Caffeine may impact the hormonal fluctuations associated with exercise as moderate to high doses have been reported to increase the testosterone and cortisol responses to resistance exercise (Beaven et al., 2008; Cook et al., 2012). However, other research has reported reduced cortisol concentrations following exercise with caffeine supplementation (Paton et al., 2010).
Green tea extract, another common ingredient in thermogenic supplements, may exert its effects through catechin polyphenols such as epigallocatechin gallate (EGCG) (Kreider et al., 2010). Dulloo et al. (1999) reported that green tea extract combined with caffeine increases 24-hour energy expenditure and fat utilization to a greater extent than caffeine alone. While meta-analysis has indicated that green tea exerts positive effects on weight loss, the magnitude is likely small (Hursel et al., 2009).
L-carnitine and evodiamine also purportedly exert beneficial effects on body composition. Previous research regarding the efficacy of l-carnitine to promote fat loss is mixed (Jeukendrup and Randell, 2011; Malaguarnera et al., 2007; Pistone et al., 2003; Villani et al., 2000). A recent meta-analysis reported that carnitine is effective at producing small decreases in body weight, although this was primarily in obese and/or diabetic populations (Pooyandjoo et al., 2016). Evodiamine has previously been reported to possess anti-obesity effects in rodent models (Kobayashi et al., 2001; Wang et al., 2008). However, it did not increase energy expenditure or fat oxidation in adult males at rest or during exercise (Schwarz et al., 2013).
Some data indicate that the individual ingredients contained in thermogenic dietary supplements could play a role in enhancing energy expenditure, fat loss and exercise adaptations. Additionally, several recent investigations have provided preliminary information regarding the acute effects of multi-ingredient thermogenic supplements (Bergstrom et al., 2014; Bergstrom et al., 2013; Campbell et al., 2016a; Campbell et al., 2016b; Ryan et al., 2009; Wilborn et al., 2009; Ziegenfuss et al., 2017). However, little is known about the chronic effects of supplementation in the context of a structured exercise program. Therefore, the purpose of the present study was to examine the safety and effectiveness of a commercially available dietary supplement designed to promote thermogenesis and fat loss in the context of a structured resistance training program.
Methods
Overview
The present study was a randomized, double-blind, placebo-controlled trial. Each participant was randomly assigned to consume a commercially-available dietary supplement (SUP) or placebo (PL) daily for 6 weeks. Procedures included acute baseline testing, a 6-week resistance training and supplementation intervention, and post-intervention testing. Laboratory assessments included resting energy expenditure (REE) responses to acute supplement ingestion, evaluation of body composition and muscular performance, and analysis of blood variables. At the baseline testing session, REE was assessed before and after SUP or PL ingestion.
Participants
Prospective participants were recruited via email, recruiting databases, flyers and word-of-mouth. All individuals were screened in order to determine if they met the following eligibility criteria: male, age 18 to 30, resistance trained (defined as training 3 to 5 days per week for at least 1 year), not consuming ergogenic dietary supplements for the previous 6 months, apparently healthy (i.e. no major metabolic or cardiovascular disease), weight stable (no changes in weight greater than 10 pounds in the previous 6 months), and no known allergies to supplement ingredients. Additionally, all participants were considered moderate caffeine users (i.e. they consumed >50 mg/d, but < 200 mg/d). This research was conducted in accordance with the Declaration of Helsinki, and all participants reviewed and signed a university-approved informed consent document prior to participation.
Laboratory assessments
Prior to baseline testing, participants completed a health history questionnaire and had their height and weight assessed using standard procedures. Each participant also completed a 4-day nutrition log in the 2 weeks prior to baseline assessments. Laboratory assessments were performed at baseline and after 6 weeks of resistance training and dietary supplementation. Prior to laboratory assessments, participants were asked to abstain from smoking, caffeine, tobacco, and alcohol consumption. Additionally, participants were instructed not to exercise for 72 hours prior to the visit, to report after a 12-hour overnight fast, and to complete a pre-testing nutrition and lifestyle record. Both of the laboratory visits consisted of identical testing of anthropometric, body composition and hemodynamic variables, a blood draw, and muscular performance testing. At the baseline session, the acute metabolic responses to SUP or PL ingestion were examined. Both testing sessions occurred at the same time of day.
At each visit, height, weight and circumferences of the biceps, chest, waist and thighs were obtained. Body composition was assessed by dual-energy x-ray absorptiometry (DXA; Hologic Wi). Heart rate and blood pressure were assessed via automated blood pressure monitor (OMRON Digital BP Monitor, HEM-907XL). Blood draws were performed via sterile venipuncture of the antecubital vein using standard phlebotomy procedures. Participants were fasted overnight for 12 hours prior to testing and were seated in a phlebotomy chair or laying down in a supine position for all blood draws. Vials of blood were labeled and were prepared based upon the recommended procedures for each assay. Blood samples were analyzed for testosterone, estrogen, cortisol and standard metabolic markers by a commercial testing service (Quest Diagnostics, Madison, NJ). REE was assessed via indirect calorimetry (ParvoMedics TrueOne 2400). For each REE assessment, participants rested in a supine position for 25 minutes while expired air was collected and analyzed. Testing took place in a cool, dark room and participants were not allowed to engage in any activities that could cause stimulation during testing.
At baseline, participants were matched based on lean mass and randomly assigned to PL (maltodextrin capsules) or SUP (MusclePharm Iron Cuts®). After completing the first REE assessment at baseline, participants were instructed to ingest their assigned supplement for evaluation of acute responses to ingestion. Participants consumed one serving (3 capsules) of either PL or SUP with 8 fluid ounces of room-temperature water and remained supine over the next 60 minutes. REE was assessed for the last 25 minutes of each 30-minute segment, providing 30- and 60-minute post-ingestion REE values.
Maximal strength was assessed via 1 repetition maximum (1RM) on the bench press and leg press exercises using the recommendations of the National Strength & Conditioning Association (NSCA, 2000), with 2 minutes of rest separating each attempt. Five minutes after the 1RM was established, muscular endurance was assessed via repetitions to failure using a load corresponding to 85% of the 1RM.
Resistance training
The resistance training program was performed on 4 days per week for 6 weeks. Participants alternated between upper body and lower body sessions. The upper body sessions consisted of 6 upper body exercises and 2 abdominal/core exercises, and each session utilized combinations of barbell bench press, dumbbell bench press, dumbbell incline bench press, pec deck machine, skullcrushers, triceps pushdown, lat pulldown, low row, wide grip rows, shrugs, preacher curls, and dumbbell bicep curls. The lower body sessions consisted of 5 lower body exercises and 2 abdominal/core exercises, and each session utilized combinations of leg press, deadlifts, dumbbell step ups, Romanian deadlift, hamstring curls, walking lunges with dumbbells and calf raises. Four sets of each exercise were performed, and exercises progressed from sets of 12 repetitions at the beginning of the training period to sets of 8 repetitions at the end of the training period, with the exception of abdominal/core exercises. In order to track compliance, participants were required to pick up a workout log from the research laboratory each day prior to performing the resistance training session. The completed log was then returned and evaluated by study personnel following each session. Each individual was required to maintain >90% compliance to the resistance training program to continue participation in the study, and overall compliance was 96.4%.
Dietary supplementation
Following baseline testing, participants were provided with capsules for their assigned condition: PL or SUP. The contents of SUP (MusclePharm Iron Cuts®) are displayed in Table 1. The maltodextrin PL capsules were designed to be identical to SUP in appearance and size. Participants received sufficient capsules for 7 days of consumption and were asked to consume 3 capsules each morning with breakfast. To promote compliance, participants were asked to come to the lab every week to return unused pills and receive additional pills. Based on this weekly distribution method and the return of pill bottles, all individuals exhibited 100% compliance with the supplementation program.
Table 1.
Supplement facts.
| Serving Size: 1 Capsule | Amount per serving |
|---|---|
| Vitamin D (as Cholecalciferol) | 400IU |
| Chromium (as Chromium Picolinate) | 50mcg |
| Thermogenic & Fat Metabolizer: L-Carnitine Tartrate, Green Tea (Camellia Sinensis) Leaf Extract, Caffeine Anhydrous, Panax Ginseng Root Powder, N-Acetyl-L-tyrosine, Thermodiamine™ (98% Evodiamine), Vinpocetine, Inositol. | 930mg |
| Muscle Building Maximizer: Maca 4:1 (Lepidium meyenii) Root Extract, AminoShield® Eriobotrya japonica Leaf Extract Proprietary Blend of 20% Pentacyclic Triterpenoids, Alpha Lipoic Acid, Boron Citrate, Fenugreek (Trigonella Foenum Graecum) Seed Extract 50% Saponins, Pumpkin Seed (Cucurbita Moschata Poiret) Extract. | 900mg |
| Estrogen & Cortisol Metabolizer: Gymnema Sylvestre Leaf Extract, Grape (Vitis vinifera) Seed Extract, Diindolylmethane DIM), Cinnamon (Cinnamomum cassia) Bark powder, Banaba (Lagerstroemia speciosa) Leaf Extract 1% Corosolic Acid, Chromium Picolinate | 383mg |
Statistical analysis
Group characteristics were compared at baseline using independent samples t-tests. Two-way ANOVA (2 groups x 2 time points) with repeated measures was used to compare anthropometric, body composition and performance changes. REE and RQ were analyzed using 2-way ANOVA (2 groups x 3 time points) with repeated measures. Tukey post-hoc was utilized when a significant interaction was observed. An a priori alpha level for detecting significance was set at p ≤ 0.05. Cohen’s d effect sizes were calculated as the mean of the 6-week measurement minus the mean of the baseline measurement, divided by the pooled standard deviation. Traditionally, Cohen’s d effect sizes are categorized as “small” (~0.2), “medium” (~0.5) or “large” (~0.8) (Cohen, 1988). More recently, new categories of “very large” (~1.2) and “huge” (~2.0) have been advocated (Sawilowsky, 2009). However, Rhea proposed new scales specifically for strength training research, stratified by training status (Rhea, 2004). For the recreationally trained individuals used in the present study, this scale includes the categories of trivial (<0.35), small (0.35 – 0.8), moderate (0.8 – 1.5) and large (>1.5).
Results
Twenty participants completed the study (age: 21.10 ± 2.5 y, height: 1.77 ± 0.05 m, weight: 87.2 ± 15.4 kg, body fat: 14.8 ± 5.4%). At baseline, there were no differences between groups for anthropometric, body composition or muscular performance variables (p > 0.25 for all variables). No adverse events occurred as a result of supplementation. Most hemodynamic and safety variables were unaltered with supplementation (Table 2). Group x time interactions were present for total protein and albumin, indicating that these variables decreased in SUP relative to PL. However, the concentrations of each of these markers remained within a normal range. No changes in estrogen, total testosterone or free testosterone were seen in either group (Table 2). However, a group x time interaction was present for cortisol, indicating that reductions were seen in SUP (-16%; d = -0.4) relative to PL (+15%; d = +0.75).
Table 2.
Blood variables. Values displayed as mean (±SD).
| Variable | Group | Pre | Post | p-value (group x time) |
p-value (time) |
Normal Range |
|---|---|---|---|---|---|---|
| Glucose (mg/dL) | SUP | 84.4 (14.0) | 86.4 (8.9) | 0.68 | 0.72 | 65 – 99 |
| PL | 86.3 (8.8) | 86.1 (7.1) | ||||
| Urea Nitrogen (mg/dL) | SUP | 16.4 (3.7) | 15.8 (4.0) | 0.78 | 0.63 | 7 – 25 |
| PL | 16.3 (1.3) | 16.1 (3.4) | ||||
| Creatinine (mg/dL) | SUP | 1.2 (0.1) | 1.2 (0.2) | 0.99 | 0.16 | 0.5 – 1.1 |
| PL | 1.2 (0.1) | 1.2 (0.1) | ||||
| Estimated glomerular filtration rate (mL/min/1.73 m2) | SUP | 91.5 (11.8) | 88.4 (14.6) | 0.88 | 0.19 | >60 |
| PL | 87.6 (11.8) | 83.6 (8.9) | ||||
| Sodium (mEq/L) | SUP | 140.5 (2.3) | 139.9 (2.1) | 0.41 | 0.75 | 135 – 146 |
| PL | 139.6 (1.7) | 139.9 (0.9) | ||||
| Potassium (mEq/L) | SUP | 4.6 (0.5) | 4.6 (0.5) | 0.56 | 0.60 | 3.5 – 5.3 |
| PL | 4.5 (0.3) | 4.7 (0.5) | ||||
| Chloride (mEq/L) | SUP | 104.2 (1.7) | 104.1 (1.6) | 0.23 | 0.24 | 98 – 110 |
| PL | 104.0 (1.4) | 104.0 (1.7) | ||||
| Carbon Dioxide (mEq/L) | SUP | 24.2 (3.9) | 24.7 (1.6) | 0.22 | 0.57 | 19 – 30 |
| PL | 26.1 (1.5) | 24.7 (2.8) | ||||
| Calcium (mg/dL) | SUP | 9.8 (0.4) | 9.8 (0.2) | 0.27 | 0.31 | 8.6 – 10.2 |
| PL | 9.8 (0.2) | 10.0 (0.4) | ||||
| Total Protein (g/dL) | SUP | 7.3 (0.4) | 7.1 (0.4) | 0.04 | 0.70 | 6.1 – 8.1 |
| PL | 7.1 (0.5) | 7.3 (0.4) | ||||
| Albumin (g/dL) | SUP | 4.7 (0.3) | 4.5 (0.2) | 0.05 | 0.99 | 3.6 – 5.1 |
| PL | 4.6 (0.1) | 4.7 (0.2) | ||||
| Globulin (g/dL) | SUP | 2.6 (0.3) | 2.5 (0.4) | 0.15 | 0.49 | 1.9 – 3.7 |
| PL | 2.6 (0.4) | 2.6 (0.3) | ||||
| Albumin:Globulin | SUP | 1.8 (0.2) | 1.8 (0.3) | 0.45 | 0.45 | 1.0 – 2.5 |
| PL | 1.8 (0.3) | 1.8 (0.2) | ||||
| Bilirubin (mg/dL) | SUP | 0.7 (0.3) | 0.6 (0.3) | 0.66 | 0.23 | 0.2 – 1.2 |
| PL | 0.8 (0.4) | 0.7 (0.2) | ||||
| Alkaline Phospatase (IU/L) | SUP | 64.8 (18.4) | 60.3 (13.8) | 0.41 | 0.20 | 33 – 115 |
| PL | 63.4 (20.0) | 62.4 (21.5) | ||||
| Alanine Aminotransferase (IU/L) | SUP | 25.7 (7.9) | 25.0 (8.1) | 0.89 | 0.40 | 6 – 29 |
| PL | 19.4 (5.8) | 18.4 (1.3) | ||||
| Aspartate Aminotransferase (IU/L) | SUP | 22.4 (12.5) | 24.2 (9.5) | 0.38 | 0.90 | 10 – 30 |
| PL | 18.6 (5.7) | 16.3 (3.7) | ||||
| Estrogen (pg/mL) (pg/mL) | SUP | 127.2 (78.9) | 130.2 (50.9) | 0.26 | 0.20 | 60-190 |
| PL | 95.7 (53.3) | 140.0 (49.0) | ||||
| Cortisol (mcg/dL) | SUP | 19.7 (5.3) | 16.7 (7.8) | 0.03 | 0.77 | 6-23 |
| PL | 16.2 (3.8) | 18.6 (2.5) | ||||
| Testosterone (ng/dL) | SUP | 649.5 (166.0) | 686.7 (225.3) | 0.77 | 0.56 | 300-1000 |
| PL | 583.3 (92.4) | 596.0 (34.4) | ||||
| Free Testosterone (pg/mL) | SUP | 156.5 (34.6) | 163.1 (61.0) | 0.53 | 0.99 | 35-155 |
| PL | 138.1 (18.5) | 131.9 (21.9) |
No group x time interactions were present for weight (p = 0.19), fat mass (p = 0.52), lean mass (p = 0.56), or body fat percentage (p = 0.92) (Figure 1). Additionally, no time main effects were seen for weight (p = 0.16), fat mass (p = 0.81), body fat percentage (p = 0.70) or lean mass (p = 0.09).
Figure 1.
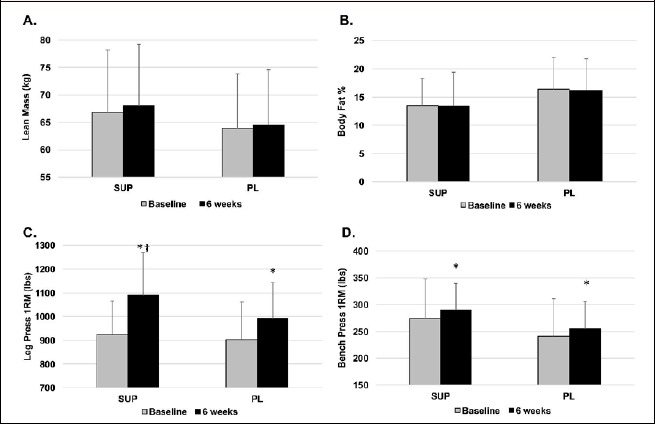
Muscular performance and body composition results. After 6 weeks of resistance training and supplementation, no significant effects were seen for lean mass (A) or body fat percentage (B). Leg press strength increased in both groups, but greater increases were seen with the dietary supplement, as compared to placebo (C). Bench press strength increased similarly in both groups (D).
A significant group x time interaction was present for leg press 1RM (p = 0.05), indicating that lower body strength increased to a greater extent in SUP (d = 1.1; 420.5 ± 63.6 kg to 495.0 ± 72.3 kg) than PL (d=.53; 410.5 ± 82.3 kg to 450.9 ± 69.1 kg) (Figure 1). No group x time interactions were present for bench press 1RM (p = 0.65), bench press repetitions to failure (p = 0.87) or leg press repetitions to failure (p = 0.21). A significant time main effect was present for bench press 1RM (p = 0.005), indicating an increase in upper body strength in both groups. Additionally, effect sizes for bench press 1RM were similar between groups (SUP: d = 0.23; PL: d = 0.20).
No time main effect was present for repetitions to failure for bench press (p = 0.28) or leg press (p = 0.08).
No group x time interactions were present for hemodynamic or metabolic variables in response to acute supplement ingestion: REE (0.17), RQ (p = 0.21), systolic blood pressure (p = 0.32), diastolic blood pressure (p = 0.16), or heart rate (p = 0.37). Significant time main effects were present for REE (p = 0.00006) and RQ (p = 0.018), indicating that both increased in response to SUP or PL ingestion (Figure 2). The effect sizes for the change in REE between 0 and 60 minutes were d = 0.65 in SUP and d = 0.34 in PL. No time main effects were present for systolic blood pressure (p = 0.23), diastolic blood pressure (p = 0.17), or heart rate (p = 0.16).
Figure 2.
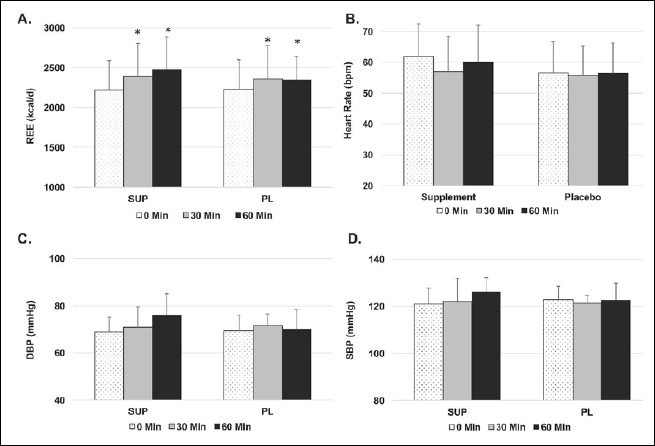
Acute metabolic responses to supplement ingestion. In response supplement ingestion at baseline, a time main effect was present for REE, indicating an increase in both groups at 30 and 60 minutes post-ingestion (A). No effects were seen for heart rate (B), diastolic blood pressure (C) or systolic blood pressure (D).
Discussion
The primary finding of the present study is that a multi-ingredient supplement designed to promote thermogenesis and fat loss appears to be safe to consume over a 6-week period, but does not confer benefits for body composition enhancement nor unequivocally improve adaptations to a resistance training program. The two possible benefits of supplementation observed in the present study were a greater increase in lower body strength and a reduction in cortisol concentrations relative to placebo.
Based on evaluation of the metabolic panel, supplement ingestion did not adversely affect safety markers. Although minor decreases in total protein and albumin were seen in SUP, these remained within normal ranges. Cortisol decreased by 16% with supplementation, but increased by 15% with placebo. However, the physiological impact of these changes is unclear. Although often viewed simply as “catabolic” and therefore detrimental to muscular improvements induced by resistance training, the physiological role of cortisol in training adaptations has not been fully elucidated (Morton et al., 2016; West and Phillips, 2012).
In the present study, supplementation did not improve any indices of body composition relative to placebo during the 6-week resistance training program. Specifically, fat mass, lean mass and body fat percentage did not change in either group over the course of the study, and the effect sizes for these variables were deemed trivial (i.e. d ≤ ±0.1). Maximal leg press strength increased to a greater extent with supplementation, with a moderate effect size (d = 1.1) for SUP and a small effect size (d = 0.5) for PL based on the effect size scale developed for strength training (Rhea, 2004). Maximal bench press strength increased similarly in both groups, but the effect sizes were considered trivial for this population (d = 0.2 for both groups). As it is not expected that the biologically active ingredients contained in the supplement affect upper and lower body musculature differentially, the larger absolute improvements in lower body strength for both groups may have allowed differences between SUP and PL to become more apparent. Additionally, it is possible that the prescribed resistance training program produced greater lower body strength gains due to the training habits of the participants prior to the study. Participants may have previously emphasized upper body exercises rather than lower body exercises in their training, thereby exhibiting a potentially different training status between the upper and lower body at study commencement. If this was the case, the effects of the supplement may have only been demonstrated for lower body strength due to the relatively less trained nature of the lower body musculature. However, this is speculative as the details of the training programs followed by participants prior to study commencement were not specifically examined.
In addition to differential effects on lower body strength and cortisol concentrations, acute energy expenditure responses appeared to differ, despite the lack of statistical interaction. In the first 30 minutes after supplement ingestion, REE increased similarly (PL: +5.8%; SUP: +7.6%). By 60 minutes post-ingestion, REE was 5% higher than baseline in PL (d = 0.34; small to medium effect) and 11.5% higher than baseline in SUP (d = 0.65; medium to large effect). Although the precise reason for the increase in REE after placebo ingestion is unclear, similar increases in REE in response to placebo ingestion have previously been reported (Vukovich et al., 2005). The practical importance of the small potential differences in REE responses between conditions is highly questionable, particularly based on the lack of differences in body weight or fat mass after the 6-week supplementation period. However, the present investigation was relatively small and of a short duration. A larger and longer duration investigation may be necessary to fully elucidate if small apparent differences in energy expenditure in response to supplement ingestion lead to any differences in body weight or body fat. Individual variability in energy expenditure responses is also important to note. In the present investigation, the REE responses to supplement ingestion ranged from -1.1 to +14.7% at 30 minutes post-ingestion and from -1.9 to +21.9% at 60 minutes post-ingestion. This seems to indicate that the combination of ingredients contained in the dietary supplement may impact the energy expenditure response differently among young adult men.
Several recent investigations reported the acute effects of ingesting a thermogenic dietary supplement. These data indicate that multi-ingredient thermogenic supplements may be effective at acutely increasing REE relative to placebo, thereby demonstrating the potential to promote fat loss when consumed chronically (Bergstrom et al., 2013; Campbell et al., 2016a; 2016b; Ryan et al., 2009; Wilborn et al., 2009; Ziegenfuss et al., 2017). However, there is a paucity of information regarding the chronic effects of these supplements. The present study provides longitudinal data that could impact the usage of thermogenic dietary supplements for several reasons. First, the studied supplement did not adversely affect blood safety markers over the course of 6 weeks in healthy young adult men. Therefore, the ingredients used in this supplement, in the proportions studied, may be safe for consumption by this population. Second, despite some potentially desirable responses to supplement ingestion (i.e. decreased cortisol concentrations and an acute increase in REE), body composition benefits of chronic supplementation were not observed. Finally, despite the lack body composition improvements, supplementation produced greater increases in lower body strength as compared to placebo. Therefore, depending on the specific goals of an individual (i.e. body composition improvement vs. strength gain), supplementation may or may not be beneficial. However, some caution should be employed when applying the results of this study to influence the use of other thermogenic dietary supplements due to differences in specific formulations and proportions of ingredients in each product. Additionally, discretion is needed when comparing the results of research studies utilizing different multi-ingredient dietary supplements for the same reasons.
Conclusions
Based on the results of the present investigation, the studied multi-ingredient supplement is apparently safe to consume for 6 weeks in healthy adult males, but does not provide unequivocal advantages relative to placebo. No benefits were observed for body composition, testosterone concentrations or upper body strength. Possible benefits include increased lower body strength after a progressive resistance training program and reductions in cortisol concentrations, although the importance of this hormonal change is questionable. Due to the multi-ingredient nature of the supplement, it is not currently possible to determine which ingredients or combinations of ingredients exerted these effects. Future research should continue to evaluate whether thermogenic supplements possess utility to improve energy expenditure, body composition and performance in active individuals.
Acknowledgements
The study was designed by LT, JM, and JO; data were collected and analyzed by SU, JM, JO, SH, and MS; data interpretation and manuscript preparation were undertaken by GMT, CF, CW, and LT. All authors approved the final version of the paper. This study was supported by a research grant awarded by the International Society of Sports Nutrition to the investigators in the Human Performance Lab at the University of Mary Hardin-Baylor. This research grant was sponsored by MusclePharm Corporation. The sponsoring organization was not involved in the study design, data collection or data analysis. No authors declare conflicts of interest. The reported experiments comply with the current laws of the country in which they were performed.
Biographies

Grant M. TINSLEY
Employment
Assistant Professor at Texas Tech University. Lubbock, TX, USA.
Degree
PhD
Research interests
Body composition, nutrition, resistance training, dietary supplements.
E-mail: grant.tinsley@ttu.edu

Stacie URBINA
Employment
Assistant Director, Human Performance Laboratory at the University of Mary Hardin-Baylor. Belton, TX, USA.
Degree
M.S.Ed.
Research interests
Sports supplementation on body composition, metabolism, and performance.
E-mail: surbina@umhb.edu

Jacy MULLINS
Employment
Exercise Physiologist at Fitness Beyond Training, Georgetown, TX. USA.
Degree
M.S.Ed.
Research interests
Effects of exercise and nutrition on body composition and health.
E-mail: jacy@fitnessbeyondtraining.com

Jordan OUTLAW
Employment
Owner at Outlaw Fitness and Nutrition. Hutto, TX.
Degree
M.S.Ed.
Research interests
Exercise and sports supplementation on body composition and performance.
E-mail: jjoutlaw@mail.umhb.edu

Sara HAYWARD
Employment
Doctor of Physical Therapy Graduate Assistant at the University of Mary Hardin-Baylor. Belton, TX, USA.
Degree
M.S.Ed.
Research interests
Effects of exercise and sports supplementation on body composition, metabolism, and performance.
E-mail: shayward@umhb.edu

Matt STONE
Employment
Graduate Research/Teaching Assistant at the University of Arkansas & Exercise Science Research Center, Fayetteville, AR.
Degree
M.S.Ed.
Research interests
Exercise and sports supplementation on body composition and performance.
E-mail: mss06@email.uark.edu

Cliffa FOSTER
Employment
Professor and Associate Dean of the Exercise & Sport Science Department at the University of Mary Hardin-Baylor. Belton, TX, USA.
Degree
Ed.D.
Research interests
Endurance exercise and resistance exercise training adaptations, and nutritional intervention.
E-mail: cfoster@umhb.edu

Colin WILBORN
Employment
Dean of Graduate School, Professor of Physical Therapy and the Director of the Human Performance Lab at the University of Mary Hardin-Baylor. Belton, TX, USA.
Degree
Ph.D.
Research interests
Effects of exercise and sports supplementation on body composition, metabolism, and performance.
E-mail: cwilborn@umhb.edu

Lem TAYLOR
Employment
Associate Professor of Exercise Physiology and the Director of Research, Human Performance Lab at the University of Mary Hardin-Baylor. Belton, TX, USA.
Degree
PhD
Research interests
Skeletal muscle physiology, protein supplementation, and ergogenic aids.
E-mail: ltaylor@umhb.edu
References
- Astrup A., Toubro S., Cannon S., Hein P., Breum L., Madsen J. (1990) Caffeine: a double-blind, placebo-controlled study of its thermogenic, metabolic, and cardiovascular effects in healthy volunteers. The American Journal Of Clinical Nutrition 51, 759-767. [DOI] [PubMed] [Google Scholar]
- Beaven C.M., Hopkins W.G., Hansen K.T., Wood M.R., Cronin J.B., Lowe T.E. (2008) Dose effect of caffeine on testosterone and cortisol responses to resistance exercise. International Journal of Sport Nutrition & Exercise Metabolism 18, 131-141. [DOI] [PubMed] [Google Scholar]
- Bergstrom H.C., Housh T.J., Traylor D.A., Lewis R.W., Jr., Cochrane K.C., Jenkins N.D., Schmidt R.J., Johnson G.O., Housh D.J., Cramer J.T. (2014) Metabolic, cardiovascular, and perceptual responses to a thermogenic nutritional supplement at rest, during exercise, and recovery in men. Journal of Strength and Conditioning Research 28, 2154-2163. [DOI] [PubMed] [Google Scholar]
- Bergstrom H.C., Housh T.J., Traylor D.A., Lewis R.W., Jr., Jenkins N.D., Cochrane K.C., Schmidt R.J., Johnson G.O., Housh D.J. (2013) Physiologic responses to a thermogenic nutritional supplement at rest, during low-intensity exercise, and during recovery from exercise in college-aged women. Applied Physiology, Nutrition, and Metabolism 38, 988-995. [DOI] [PubMed] [Google Scholar]
- Campbell B.I., Colquhoun R.J., Zito G., Martinez N., Kendall K., Buchanan L., Lehn M., Johnson M., St Louis C., Smith Y., Cloer B. (2016a) The effects of a fat loss supplement on resting metabolic rate and hemodynamic variables in resistance trained males: a randomized, double-blind, placebo-controlled, cross-over trial. Journal of the International Society of Sports Nutrition 13, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B.I., Zito G., Colquhoun R., Martinez N., Kendall K., Buchanan L., Lehn M., Johnson M., St Louis C., Smith Y., Cloer B., Pingel A. (2016b) The effects of a single-dose thermogenic supplement on resting metabolic rate and hemodynamic variables in healthy females--a randomized, double-blind, placebo-controlled, cross-over trial. Journal of the International Society of Sports Nutrition 13, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical power analysis for the behavioral sciences. Second edition Hillsdale, N.J. : L. Erlbaum Associates. [Google Scholar]
- Cook C., Beaven C.M., Kilduff L.P., Drawer S. (2012) Acute caffeine ingestion’s increase of voluntarily chosen resistance-training load after limited sleep. International Journal of Sport Nutrition & Exercise Metabolism 22, 157-164. [DOI] [PubMed] [Google Scholar]
- Dickinson A., Blatman J., El-Dash N., Franco J.C. (2014) Consumer usage and reasons for using dietary supplements: report of a series of surveys. Journal of the American College of Nutrition 33, 176-182. [DOI] [PubMed] [Google Scholar]
- Dulloo A., Duret C., Rohrer D., Girardier L., Mensi N., Fathi M., Chantre P., Vandermander J. (1999) Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. American Journal of Clinical Nutrition 70(6), 1040-1045. [DOI] [PubMed] [Google Scholar]
- Harpaz E., Tamir S., Weinstein A., Weinstein Y. (2017) The effect of caffeine on energy balance. Journal of Basic and Clinical Physiology and Pharmacology 28, 1-10. [DOI] [PubMed] [Google Scholar]
- Hursel R., Viechtbauer W., Westerterp-Plantenga M.S. (2009) The effects of green tea on weight loss and weight maintenance: a meta-analysis. International Journal of Obesity 33, 956-961. [DOI] [PubMed] [Google Scholar]
- Jeukendrup A.E., Randell R. (2011) Fat burners: nutrition supplements that increase fat metabolism. Obesity Reviews 12, 841-851. [DOI] [PubMed] [Google Scholar]
- Keijzers G.B., Galan B.E.D., Tack C.J., Smits P. (2002) Caffeine Can Decrease Insulin Sensitivity in Humans. Diabetes Care 25, 364-369. [DOI] [PubMed] [Google Scholar]
- Kim T.W., Shin Y.O., Lee J.B., Min Y.K., Yang H.M. (2011) Caffeine increases sweating sensitivity via changes in sudomotor activity during physical loading. Journal of Medicinal Food 14, 1448-1455. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Nakano Y., Kizaki M., Hoshikuma K., Yokoo Y., Kamiya T. (2001) Capsaicin-like anti-obese activities of evodiamine from fruits of Evodia rutaecarpa, a vanilloid receptor agonist. Planta Medica 67, 628-633. [DOI] [PubMed] [Google Scholar]
- Kreider R.B., Wilborn C.D., Taylor L., Campbell B., Almada A.L., Collins R., Cooke M., Earnest C.P., Greenwood M., Kalman D.S., Kerksick C.M., Kleiner S.M., Leutholtz B., Lopez H., Lowery L.M., Mendel R., Smith A., Spano M., Wildman R., Willoughby D.S., Ziegenfuss T.N., Antonio J. (2010) ISSN exercise & sport nutrition review: research & recommendations. Journal of the International Society of Sports Nutrition 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaguarnera M., Cammalleri L., Gargante M.P., Vacante M., Colonna V., Motta M. (2007) L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. American Journal of Clinical Nutrition 86, 1738-1744. [DOI] [PubMed] [Google Scholar]
- Morton R.W., Oikawa S.Y., Wavell C.G., Mazara N., McGlory C., Quadrilatero J., Baechler B.L., Baker S.K., Phillips S.M. (2016) Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. Journal of Applied Physiology (1985) 121, 129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norager C.B., Jensen M.B., Weimann A., Madsen M.R. (2006) Metabolic effects of caffeine ingestion and physical work in 75-year old citizens. A randomized, double-blind, placebo-controlled, cross-over study. Clinical Endocrinology 65, 223-228. [DOI] [PubMed] [Google Scholar]
- NSCA. (2000) National Strength & Conditioning Association. Essentials of strength training and conditioning. 2nd ed edition Champaign, Ill: Human Kinetics. [Google Scholar]
- Paton C.D., Lowe T., Irvine A. (2010) Caffeinated chewing gum increases repeated sprint performance and augments increases in testosterone in competitive cyclists. European Journal of Applied Physiology 110, 1243-1250. [DOI] [PubMed] [Google Scholar]
- Pistone G., Marino A., Leotta C., Dell’Arte S., Finocchiaro G., Malaguarnera M. (2003) Levocarnitine administration in elderly subjects with rapid muscle fatigue: effect on body composition, lipid profile and fatigue. Drugs Aging 20, 761-777. [DOI] [PubMed] [Google Scholar]
- Pooyandjoo M., Nouhi M., Shab-Bidar S., Djafarian K., Olyaeemanesh A. (2016) The effect of (L-)carnitine on weight loss in adults: a systematic review and meta-analysis of randomized controlled trials. Obesity Reviews 17, 970-976. [DOI] [PubMed] [Google Scholar]
- Rhea M.R. (2004) Determining the magnitude of treatment effects in strength training research through the use of the effect size. Journal of Strength and Conditioning Research 18, 918-920. [DOI] [PubMed] [Google Scholar]
- Ryan E.D., Beck T.W., Herda T.J., Smith A.E., Walter A.A., Stout J.R., Cramer J.T. (2009) Acute effects of a thermogenic nutritional supplement on energy expenditure and cardiovascular function at rest, during low-intensity exercise, and recovery from exercise. Journal of Strength and Conditioning Research 23, 807-817. [DOI] [PubMed] [Google Scholar]
- Sawilowsky S.S. (2009) New effect size rules of thumb. Journal of Modern Applied Statistical Methods 8, 597-599. [Google Scholar]
- Schwarz N.A., Spillane M., La Bounty P., Grandjean P.W., Leutholtz B., Willoughby D.S. (2013) Capsaicin and evodiamine ingestion does not augment energy expenditure and fat oxidation at rest or after moderately-intense exercise. Nutrition Research 33, 1034-1042. [DOI] [PubMed] [Google Scholar]
- Villani R.G., Gannon J., Self M., Rich P.A. (2000) L-Carnitine supplementation combined with aerobic training does not promote weight loss in moderately obese women. International Journal of Sport Nutrition & Exercise Metabolism 10, 199-207. [DOI] [PubMed] [Google Scholar]
- Vukovich M.D., Schoorman R., Heilman C., Jacob P., Benowitz N.L. (2005) Caffeine–herbal ephedra combination increases resting energy expenditure, heart rate and blood pressure. Clinical and Experimental Pharmacology and Physiology 32, 47-53. [DOI] [PubMed] [Google Scholar]
- Wang T., Wang Y., Kontani Y., Kobayashi Y., Sato Y., Mori N., Yamashita H. (2008) Evodiamine improves diet-induced obesity in a uncoupling protein-1-independent manner: involvement of antiadipogenic mechanism and extracellularly regulated kinase/mitogen-activated protein kinase signaling. Endocrinology 149, 358-366. [DOI] [PubMed] [Google Scholar]
- West D.W., Phillips S.M. (2012) Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. European Journal of Applied Physiology 112, 2693-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilborn C., Taylor L., Poole C., Bushey B., Williams L., Foster C., Campbell B. (2009) Effects of ingesting a commercial thermogenic product on hemodynamic function and energy expenditure at rest in males and females. Applied Physiology, Nutrition, and Metabolism 34, 1073-1078. [DOI] [PubMed] [Google Scholar]
- Ziegenfuss T.N., Lopez H.L., Sandrock J.E., Kedia A.W., Habowski S., Kerksick C. (2017) Effect of a Multi-Nutrient Over-the-Counter Supplement on Changes in Metabolic Rate and Markers of Lipolysis. Journal of Dietary Supplements 14, 288-302. [DOI] [PubMed] [Google Scholar]


