Abstract
Recombinant adeno-associated viral (rAAV) vectors have been widely used in human gene therapy. One major impediment to its broad application is the inability to produce high-quality vectors in mass quantity. Here, an efficient and scalable suspension cell culture system for the production of rAAV vectors is described. In this system, the AAV trans factors, Rep78, Rep52, VP1, VP2, and VP3, were stably integrated into a single vaccinia virus carrier by maximizing the use of alternative codons between genes with identical amino acids, and the cis rAAV genome was carried by an E1/E3 gene-deleted adenovirus. Infection of improved, E1 integrated, suspension-cultured cells with these two viral vectors resulted in the robust production of rAAV vectors. The newly enhanced system can consistently produce ∼1 × 1015 genome containing rAAV vectors per liter of suspension cells. Moreover, the capsid composition of rAAV vectors produced by this system is markedly different from those produced using the traditional system in that the VP1 protein is more abundant than the VP2 protein (19:1 versus 1:1). The unique VP1 superabundant rAAV vectors produced in this new system exhibited improved transduction in vivo after intravitreal injection.
Keywords: AAV, vaccinia virus, VP1, gene therapy, vector production
Introduction
Recombination adeno-associated viral (rAAV) vectors have been widely used as gene-delivery vehicles for both basic scientific research and human gene therapy.1, 2, 3 They have been successfully used in clinical trials and are approved as a drug for human use.4, 5, 6 Although a variety of systems have been proposed to produce rAAV vectors,7, 8, 9, 10, 11, 12, 13 large-scale manufacturing of high-quality rAAV vectors that meet the needs of basic research in large animal models and clinical uses remains a major challenge.14
The common method used for producing rAAV requires co-transfection of adherent HEK293 cells with two or three plasmids carrying the necessary cis and trans components.8, 14, 15, 16 The two inverted terminal repeats (ITRs) are the only cis elements required for rAAV replication and packaging. Although four non-structural proteins are encoded in the AAV genome, Rep78, Rep68, Rep52, and Rep40, only Rep78 and Rep52 are required as trans elements for rAAV production.17 The capsid proteins VP1, VP2, and VP3, are essential for AAV capsid assembly and are therefore required trans elements. Besides coding VP1, VP2, and VP3, the cap gene also encodes an assembly-activating protein (AAP), which may further support AAV assembly.18 The AAV helper viruses, such as adenovirus (Ad), and herpes simplex virus (HSV), provide the additional trans-acting factors required for rAAV production.7 The rAAV vectors produced by the traditional transient transfection have been validated in clinical trial for their safety, convenience, and effectiveness. However, the method itself has limited scalability potential.9
As an alternative to the transfection method, both baculovirus and HSV-based carriers have been developed to produce rAAV vectors using a suspension culture system.10, 11, 12, 13 These viral-based systems have been reported to generate 0.5 ∼1 × 105 vector genomes (vg)/cell of rAAV vector, and when scaled-up to a 100∼200 L volume, can produce 1 × 1016 vg of rAAV vector.14, 19 However, there remains concern regarding the potency of the vectors produced, the possibility of generating replication competent AAV (rcAAV) particles, and the difficulties associated with engineering the carrier viruses, considering their limited use. Recently, a novel rAAV production method using a vaccinia carrier from our group has been described, which can eliminate the generation of rcAAV particles while retaining similar scalability.20 However, this method requires the use of two vaccinia viral (VV) vectors, an Ad-AAV hybrid vector (Ad/AAV), and a wild-type Ad (wtAd), making this system rather complicated.
Traditionally, the AAV capsid is comprised of 60 subunits of VP1, VP2, and VP3 capsid proteins in a ratio of ∼1:1:8. To control the protein ratio, wild-type AAV relies on the use of overlapping, and alternative mRNA splicing combined with the use of a non-canonical start codon (ACG) for VP2 translation. This control mechanism is preserved in the transfection method for rAAV production.8, 15 However, rAAV vectors with an untraditional ratio of capsid proteins have been reported.10 In fact, the presence of VP1 or VP2 is not required for the assembly of intact capsids.21 For example, rAAV5 produced in Sf9 cells only has one copy of VP1 per capsid,13 and rAAV capsids lacking VP2 can be generated by mutating the VP2 start codon.21, 22 However, the absence of VP1 has a high impact on rAAV transduction, while deletion of VP2 does not change the rAAV transduction profile. The VP1-specific protein sequence motifs in the N-terminal region are essential for virus trafficking and cell-specific transduction.23, 24 It is well documented that insufficient VP1 levels will result in reduced rAAV transduction rates,10, 13, 23, 24, 25, 26 and recovery of VP1 expression boosts rAAV5 per-particle infectivity rates.13, 26
In the present study, a novel VV-Ad system was developed that efficiently produces VP1-superabandunt rAAV vectors. This system uses a single VV vector to provide the necessary Rep and Cap proteins and a single Ad/AAV carrying the rAAV genome. Results demonstrated that approximately 2 × 105 vg/cell or 1 × 1015 vg/L of rAAV vector can be produced by simply infecting the E1a/E1b-expressing QW158-7 suspension cells with these two viral vectors. The resulting rAAV vectors carried more VP1 and less VP2 compared with traditional rAAV vectors and displayed higher transduction efficiency after intravitreal injection.
Results
Generation of a Single VV Carrier That Stably Expresses All AAV Packaging Genes
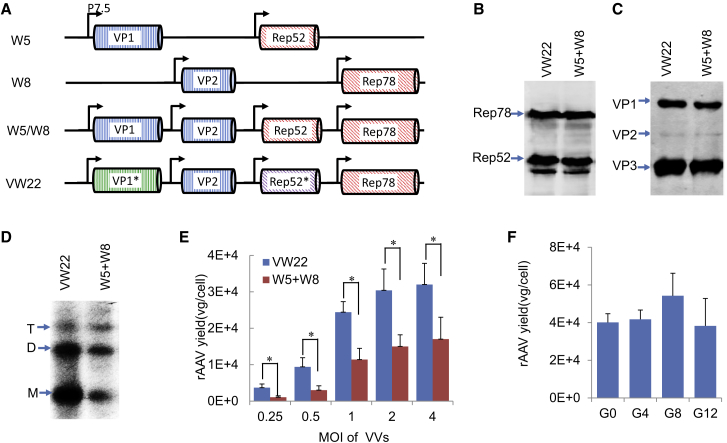
In the previously described VV-Ad system for rAAV production,20 Rep78 and Rep52, and VP1 and VP2, were allocated into two different constructs (Figure 1A; W5 and W8), because the identical nucleotides at their 3′ ends destabilized the VV carrier. Therefore, one VV carrier expressing all four genes was not generated (Figure 1A; W5/W8). Here, a novel strategy was employed that allowed the formation of a single, stable VV carrier, capable of expressing all four genes simultaneously. Through maximization of alternative coding, novel Rep52 (Rep52*) and VP1 (VP1*) genes were designed to express the Rep52 and VP1 proteins (Figures S1 and S2). Although Rep52 and Rep52* express identical polypeptides, the homology of Rep52 and Rep52* at the nucleotide level is only 61.2%. Similarly, VP1 and VP1* share a homology of 59.2% at the nucleotide level, despite coding for identical amino acids. Rep52* and VP1* exhibited similar expression profiles compared with unmodified Rep52 and VP1, controlled either by cytomegalovirus (CMV) promoter or vaccinia P7.5 promoter (Figure S3). As expected, these four genes could be successfully built into a single VV construct (VW22) in which each gene was driven by a P7.5 promoter (Figure 1A).
Figure 1.
Constructing a Stable Single Vaccinia Carrier for rAAV Production
(A) Schematic illustration of vaccinia carriers. W5 and W8 carry AAV trans factors. The hypothetical W5/W8 carrier shows the long direct repeat sequences of Rep52-Rep78 and VP2-VP1 sequences that would have destabilized the vaccinia carrier. VW22 contains Rep52*, the codon-optimized variant of Rep52, and VP1*, the codon-optimized variant of VP1. The arrow stands for p7.5 promoter. (B and C) Immunoblot analysis of Rep (B) and Cap (C) proteins from lysates that were harvested at 24 hr post-VV infection. Mouse anti-Rep and mouse anti-VP1, VP2, and VP3 were used as detecting antibodies. (D) Southern blot analysis of rAAV vector genome replication in vaccinia-Ad-mediated rAAV production system. The Hirt DNA was extracted at 24 hr post-VW22-infection, and the rAAV vector genomes were detected with EGFP-specific probe. The bands corresponding to the linear monomer (M), dimer (D), and tetramer (T) of rAAV genomes are indicated. (E) rAAV vector yield comparison between VW22 and W5 + W8. rAAV vectors were harvested 36 hr post vaccinia carrier infection. The rAAV vector yields were measured by qPCR. (F) Test of the stability of VW22. VW22 were consecutively passed 12 times in BSC-1 cells and tested for the ability to complement rAAV packaging. The x axis indicated the passages of VW22 used for rAAV package. The y axis showed the vector yield. For the experiments in (B)–(F), HeLa-S3 cells were infected with wtAd (MOI = 1) and Ad/AAV-CMV-EGFP (MOI = 50), and VVs were introduced 16 hr later. *p < 0.01. Bars represent the means of three independent experiments.
Infection of HeLa-S3 cells with VW22 resulted in the production of Rep and Cap proteins to similar levels as HeLa-S3 cells co-infected with W5 and W8 (Figures 1B and 1C). Since VP2 is initiated by an ACG start codon, the expression of VP2 is less robust compared to VP3 in this system. When VW22 (MOI = 1) was introduced to cells 16 hr after co-infection with wtAd (MOI = 1) and Ad/AAV (MOI = 50), efficient replication of rAAV genomes from the Ad/AAV hybrid was observed (Figure 1D). The rAAV vector yield is proportional to the MOI of VW22 (Figure 1E). About 3.2 × 104 vg/cell of rAAV vector was generated when MOI was 4, approximately two-fold higher than that of W5 and W8 co-infection. VW22 is stable for at least 12 continuous replication and expansion cycles without losing its ability to complement rAAV production (Figure 1F). Taken together, all the AAV packaging genes are stably and functionally expressed from a single VV carrier.
Eliminating wtAd from the New rAAV Production System
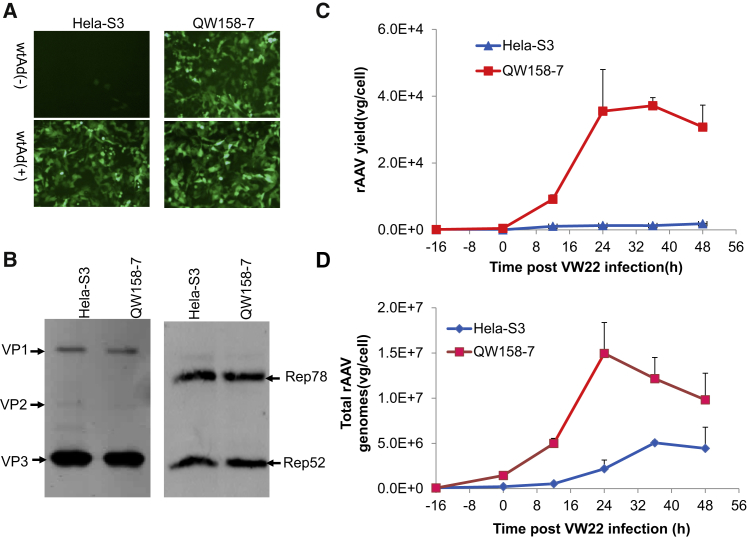
wtAd has been demonstrated to increase rAAV packaging in HeLa-S3 cells when using the previously described VV-Ad system for rAAV production.20 We compared the helper functions of wtAd and an E1 deleted Ad, Ad375, in this system (Figure S4A). Ad375 failed to increase rAAV genome replication and was unable to improve rAAV production, indicating that the E1a/E1b genes, but not the E3 genes of wtAd, are primarily responsible for enhanced replication of rAAV genomes. Therefore, the E1a/E1b genes were introduced into HeLa-S3 cells to eliminate the using of wtAd. The resulting E1a- and E1b-expressing cell line was named QW158-7, which can be easily propagated in suspension and have similar cell morphology as the original HeLa-S3 cells. Not surprisingly, they complemented rAAV vector production quite efficiently in adherent culture conditions (Figure 2A). Results demonstrated that even in the absence of wtAd infection, over 3 × 104 vg/cell of rAAV vector can be produced using suspension-cultured QW158-7 cells when the VW22 helper virus was added at an MOI of 4 (Figure 2C). The expression profile of Cap and Rep proteins from VW22 was similar between QW158-7 and HeLa-S3 cells (Figure 2B). QW158-7 cells provide earlier and more abundant replicated rAAV genomes for AAV packaging (Figure 2D), which are generally positively correlated to rAAV yield.27
Figure 2.
Establishing an Optimized Suspension Cell Line for rAAV Vector Production
HeLa-S3 cells were co-transfected with pCMV-E1a/E1b and pCI-neo (100:1), and the best cell line supporting rAAV production (QW158-7) was selected, identified, and used for rAAV packaging. (A) Adherent cultured QW158-7 cells complemented VW22 for rAAV vector production in the absence of wtAd. Both HeLa-S3 and QW158-7 cells were tested for rAAV vector packaging with or without wtAd infection. The rAAV vectors with EGFP as reporter gene were collected and assayed at 36 hr post-VW22-infection. After three rounds of freezing/thaw and inactivation treatment by heating at 56°C for 1 hr, an aliquot of final vectors were used for comparison of rAAV yield using GM16095 cells. The EGFP+ cells were observed and photographed at 48 hr post-infection. (B) Western blot analysis of Rep and Cap proteins expressed in QW158-7 and HeLa-S3 cells. Cell lysates were harvested and analyzed 24 hr post-VW22-infection. (C) A time course of rAAV vector packaging in 100 mL suspension cultured QW158-7 cells. rAAV vectors were harvested and assayed at various time points post-VW22-infection. HeLa-S3 cells were used as control in the absence of wtAd infection. (D) Comparison of Ad/AAV replication property in QW158-7 cells and HeLa-S3 in the absence of wtAd. In all experiments, Ad/AAV-CMV-EGFP hybrid vectors (MOI = 50) were infected into cells 16 hr before adding VW22 (MOI = 4). The titer of rAAV vector genomes and total rAAV genomes were measured by qPCR as mentioned in the Materials and Methods. Bars represent the means of three independent experiments.
Increasing MOI of the Ad/AAV hybrid from 1 to 50 significantly promoted total rAAV genome accumulation and improved rAAV yield (Figures S5E and S5F). However, a further increase of the Ad/AAV vector failed to substantially enhance rAAV production, presumably due to the increased toxicity to the host cells and interactions between the two types of viruses (Figure S5H). We have systematically evaluated whether individual Ad genes, E1a-13s, E1a-12s, E1b-55k, E1b-19k, E2a, VA I RNA, and E4orf6, would increase rAAV packaging or not. Each gene was expressed by a VV vector that was controlled by a P7.5 promoter. As demonstrated in Figure S6A, adding E1a-13s, E2a, E4orf6 (facilitating AAV DNA replication); E2a and VA I RNA (enhancing the viral mRNA stability and efficiency of translation); E4orf6 and E1b-55k (facilitating the timely transportation of viral mRNAs); and E1b-19K (providing additional anti-apoptotic activity) had no effect, or only a slight increase, on rAAV vector yield in the new system. These results suggested that the Ad/AAV hybrid at MOI of 50 provided sufficient replicated rAAV genomes in QW158-7 cells for vector production.
Modulating VP2/VP3 Expression Can Improve rAAV Production Yield
An increase in MOI of VW22 significantly improved rAAV vector production (Figure S5A). However, a high VW22 MOI was toxic to the host cells. It inhibited the replication of the Ad/AAV hybrid and reduced rAAV genome accumulation (Figures S5B and S5C). In an effort to further improve this system without increasing the MOI of VW22, the effect of individual AAV genes (Rep78, Rep68, Rep52, Rep40, VP1, VP2, VP3, AAP, and X-gene)28, 29 on rAAV packaging was evaluated. The expression levels of VP2 and VP3 were found to be proportional to rAAV vector yield (Figures S6B and S6C). For example, enhancing VP2 expression improved vector yield by 5-fold, and the optimized expression of VP3 lead to a 3-fold increase in vector yield. In contrast, increasing VP1 expression decreased rAAV yield in the VV-Ad system. This result suggested that insufficient VP2 and VP3 might have been the primary cause of inefficient rAAV packaging in the new system.
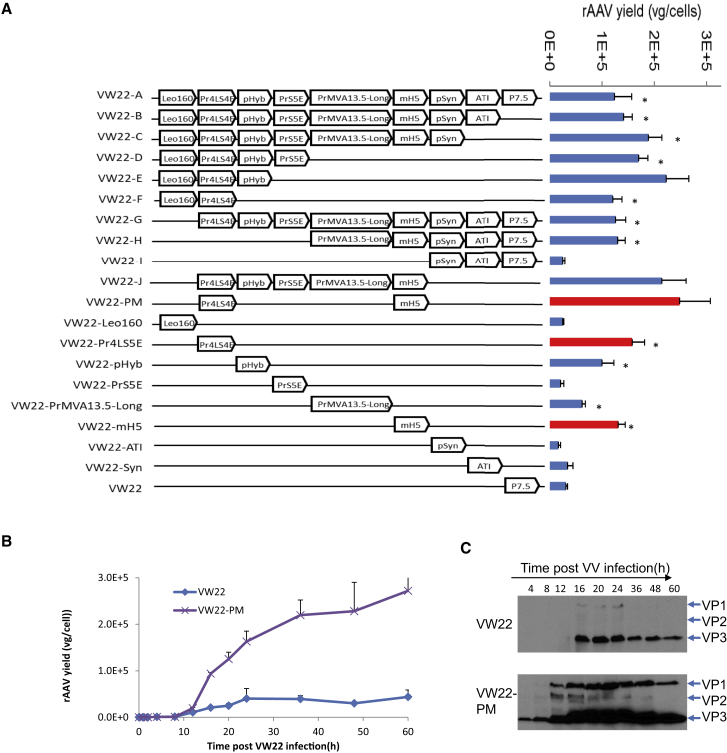
To address the issue of inefficient VP2/VP3 expression, it was hypothesized that the universal adoption of the P7.5 promoter might lead to the unbalanced expression of AAV genes. Since there are a variety of VV promoters available with different expression profiles and potency, eight VV promoters (Leo160,30 Pr4LS5E,31 pHyb,32 PrS5E,31 PrMVA13.5-long,31 mH5,33 pSyn,34 and ATI;35 Table S1) were systematically assessed for their effect on rAAV production. To accomplish this, either individual promoter or various combinations of these promoters were used to drive the VP2 gene (Figure 3A). As presented in Figure 3A, the VW22 carriers containing a Pr4LS5E or an mH5 promoter consistently resulted in higher rAAV yield. In fact, using these promoters resulted in the production of 3∼6-fold more rAAV vectors compared with that of original VW22 carrier in adherent cultured QW158-7 cells. Moreover, the VW22 carrier with a Pr4LS5E-mH5 dual promoter (VW22-PM) led to the highest rAAV yield, 3.5 × 105vg/cells, which is approximately an 8-fold increase over the VW22 (Figure 3A). The rAAV yield with the VW22-PM carrier in suspension QW158-7 cells produced approximately 3 × 105 vg/cell, which is about seven times higher than that of VW22 (Figures 3B and S7A). Earlier expression and accumulation of VP2/VP3 was considered as the likely reason for more efficient replication and packaging of rAAV vectors (Figures 3C and S7B). In contrast, the replication of Ad/AAV and VV in the system was not affected when the Pr4LS5E-mH5 dual promoter was used to control VP2/VP3 expression (Figures S7C and S7D). Based on this data, it was concluded that the use of a strong and early VV promoter improved expression of VP2/VP3 and enhanced rAAV packaging in the new vector production system.
Figure 3.
Regulating VP2/VP3 Expression of VV by Strong and Early Promoters Enhance rAAV Package of VV-Ad System
(A) Schematic representation of the VVs with single or combinatorial promoters (left) and their ability to produce rAAV vectors (right). The used promoters include Leo160, Pr4LS5E, pHyb, PrMVA13.5-long, PrS5E, mH5, pSyn, and ATI (Table S1). The combinatorial promoters made from different combinations of those promoters were also used to drive the expression of VP2 in VW22. The figure on the right showed the relative yield of rAAV vectors 48 hr after the specified vaccinia carrier infection (MOI = 2). (B) Time courses of rAAV yield of VV-Ad system using VW22-PM as Rep and Cap provider (with Pr4LS5E-mH5 driven VP2) in suspension QW158-7 cells. (C) Western blot analysis of Cap protein expression in VV-Ad system during VW22-PM-mediated rAAV production. Mouse anti-VP1, VP2, and VP3 were used as detecting antibodies. In all experiments, Ad/AAV-CMV-EGFP hybrid vectors (MOI = 50) were infected 16 hr before adding VW22s. The titer of rAAV vectors was measured by qPCR. n = 4, *p < 0.01.
An Optimized System for Robust rAAV Production
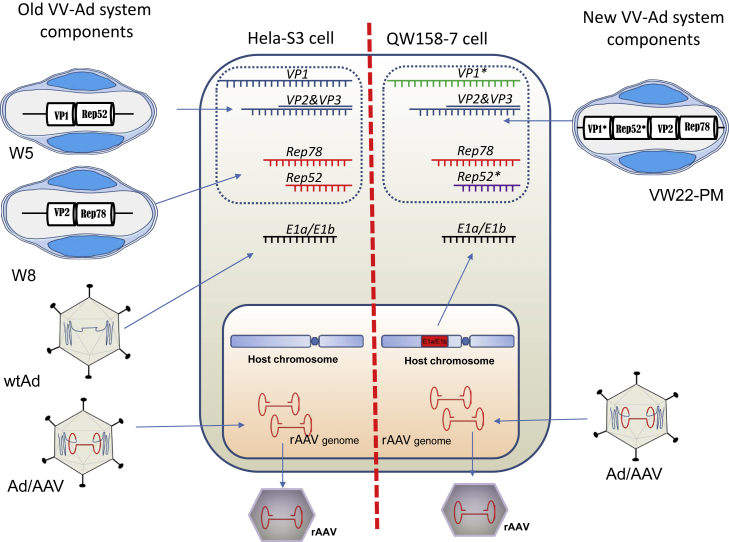
The combination of all the discussed enhancements and optimization resulted in a new VV-Ad system for rAAV vector production using suspension cells without transfection procedures (Figure 4). This new system includes three major components: a single, cytoplasmic VV carrier (VW22-PM) providing AAV helper functions; one Ad/AAV hybrid carrier containing rAAV genomes and providing Ad helper functions; and suspension host QW158-7 cells containing the E1a/E1b functions (Figure 4, right). This is in contrast to the original VV-Ad system that required five components: two VVs (W5 and W8), two Ads (Ad/AAV hybrid and wtAd), and used HeLa-S3 as host cells (Figure 4, left).20 The new system eliminates excessive procedures and cost for additional VV carrier preparation, provides a more direct method, and importantly, removes wtAd from the production system.
Figure 4.
Schematic Comparison of the Original (Left) and New (Right) VV-Ad Systems for rAAV Vector Production
The previous system used two vaccinia vectors (W5 and W8) to provide the required Rep and Cap functions, one Ad/AAV hybrid vector to provide the rAAV genome, and one wtAd to provide E1a/E1b function in HeLa-S3 cells. In the new system, one vaccinia vector (VW22-PM) provides all Rep and Cap functions. It contains two rep genes (Rep78 and Rep52*) and two cap genes (VP1* and VP2). Rep52* and VP1* genes are codon optimized from original Rep52 and VP1 (Figures S1 and S2). The E1a/E1b gene was integrated into the genome of QW158-7 cells.
To further confirm that this new production system is competent for general rAAV vector production, it was used to produce the following rAAV vector-based AAV serotype 2: ssAAV-CMV-LacZ, ssAAV-CB-AAT, dsAAV-CB-EGFP, and dsAAV-CB-Gaussia luciferase (Gluc). Ad/AAV hybrids carrying the corresponding transgene cassettes and AAV ITRs were generated. The AAV genome size for these vectors ranged from 2.6 to 4.7 kb. As presented in Table 1, both single-strand rAAV vectors (ssAAV-CMV-EGFP, ssAAV-CMV-LacZ, and ssAAV-CB-AAT) and self-complementary rAAV vectors (scAAV-CB-EGFP and scAAV-CB-Gluc) were produced successfully. The yield ranged from 0.59 × 105 to 2.76 × 105 vg/cell (Table 1). Moreover, increasing the cell density as high as 5 × 106 cells/mL still allowed for a high yield of rAAV vector per cell. Under this condition, over 1 × 1015 vg rAAV vectors could be produced from 1 L of cell culture.
Table 1.
rAAV Yield of the Optimized VV-Ad System
| Vector Name | Vector Size (kb) | Corresponding Ad/AAV Name | Vector Yield |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Adherent Culture (vg/Cell) | Suspension Culture |
||||||||
| Low Cell Density (l × 106 Cells/mL) |
Modest Cell Density (2.5 × 106 Cells/mL) |
High Cell Density (5 × 106 Cells/mL) |
|||||||
| vg/Cell | vg/L | vg/Cell | vg/L | vg/Cell | vg/L | ||||
| ssAAV2-CMV-EGFP | 4.0 | Ad/AAV-CMV-EGFP | 2.37 ± 0.7 × 105 | 2.01 ± 0.42 × l05 | 2.01 ± 0.42 × l014 | 1.79 ± 0.43 × l05 | 4.48 ± 1.08 × l014 | 1.95 ± 0.22 × 105 | 9.75 ± 1.10 × l014 |
| ssAAV2-CMV-LacZ | 4.7 | Ad/AAV-CMV-lacZ | 0.96 ± 0.14 × 105 | 0.59 ± 0.04 × l05 | 0.59 ± 0.04 × l014 | – | – | – | – |
| dsAAV2-CB-E GFP | 2.1 | Ad/dsAAV-CB-EGFP | 1.78 ± 0.04 × l05 | 1.60 ± 0.01 × l05 | 1.60 ± 0.01 × l014 | – | – | – | – |
| dsAAV2-CB-G luc | 1.8 | Ad/dsAAV-CB-Glue | 1.54 ± 0.27 × l05 | 1.17 ± 0.04 × l05 | 1.17 ± 0.04 × l014 | 1.09 ± 0.13 × 105 | 2.72 ± 0.32 × l014 | – | – |
| ssAAV2-CB-h AAT | 2.6 | Ad/AAV-CB-A AT | 4.04 ± 0.90 × l05 | 2.76 ± 0.11 × l05 | 2.76 ± 0.11 × l014 | – | – | 2.03 ± 0.43 × l05 | 10.2 ± 2.15 × l014 |
Suspension QW158-7 cells were infected with the Ad/AAV hybrid (MOI = 50) and VW22-PM (MOI = 2). The titer of rAAV vectors was measured by qPCR at 48 hr post-VW22-PM infection.
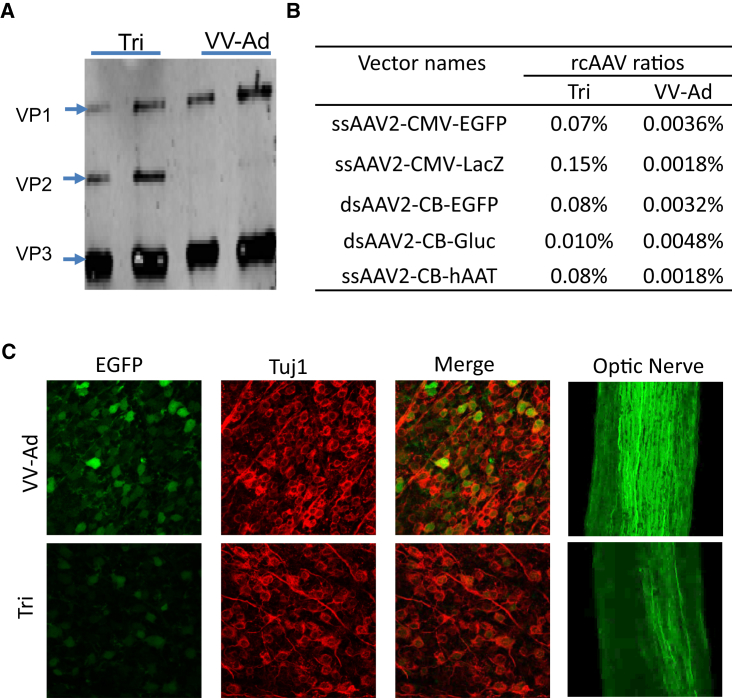
Since the new system uses a VV carrier and an Ad/AAV hybrid to produce rAAV vectors, the chance of residual carrier viruses in the final rAAV preparations is a safety concern. However, no residual VV or wtAd contamination could be detected using a plaque assay (the sensitive of our assay was 1 VV or wtAd out of 1010 AAV particles). The Ad/AAV hybrid has a peak density of 1.344 g/cm3, which is close to the density of empty AAV particles and is easy to inactivate by heating treatment at 56°C for 1 hr (Figures S8F and S8G). Moreover, there were less rcAAV genomes detected in rAAV vectors produced by the VV-Ad system compared with that of the triple-plasmid transfection system (Figures 5B and S8E), which confirms previous results.20
Figure 5.
Comparison of rAAV Vectors Produced by the VV-Ad System and Triple Plasmid Transfection Methods
(A) Capsid composition of rAAV vectors produced by VV-Ad and triple plasmid transfection (Tri) methods. rAAV vectors (∼1 × 1010 particles) purified after two rounds of CsCl ultracentrifuge were electrophoresed and visualized by silver staining. (B) rcAAV contamination in rAAV vectors was determined by measuring the percentage of Rep78 containing genomes in total rAAV genomes. (C) Transduction activity of dsAAV2-CB-EGFP vectors in vivo after intravitreal injection. Confocal images of flat-mounted retinas showing EGFP+ RGCs and Tuj1 immunostaining, and EGFP+ axons in optic nerve (n = 5, *p < 0.05).
VP1 Superabundant rAAV Vectors Efficiently Express Transgene In Vivo
It is notable that rAAV vectors produced by the new system displayed a high abundance of VP1 (VP1 superabundant AAV). For these rAAV vectors, the ratio of VP1:VP2:VP3 is close to 1.9:0.1:8, which differs from the traditional AAV capsid with a ratio of 1:1:8 (Figure 5A). Those VP1 superabundant rAAV vectors demonstrated similar genome status, morphology, thermal stability, and CsCl gradient buoyant density as the canonical rAAV vectors (Figures S8A–S8D). As the abundance of VP1 is critical for infectivity of rAAV,23, 24 the transduction efficacy of VP1 superabundant rAAV vectors were compared to canonical vectors through injection into the vitreous chamber of mice to target the retina cells. At 2 weeks post-injection, it was observed that the VP1-enhanced vectors produce higher expression in retina ganglion cells and optic nerve cells than canonical rAAV vectors (Figure 5C).
Discussion
In the current study, a robust and scalable manufacturing system was described that contained significant improvements over the previous VV-Ad system for rAAV production (Figure 4). In fact, all components of the system have been substantially improved and optimized. A single VV carrier, VW22-PM, was engineered to consolidate all the necessary AAV trans factors. The wtAd was completely eliminated, and its essential functions for rAAV production have been incorporated into a HeLa-S3 derivative cell line, QW158-7 cells, which can be grown in suspension. This new rAAV vector production system performed significantly better than other rAAV production systems that have been previously described.9, 10, 11, 12, 17, 36, 37
The main advantages of the new VV-Ad system include the following: (1) rAAV vectors can be generated in a yield greater than 2 × 105 vg /cell or greater than 1 × 1015 vg/L; (2) the scalable system can be readily implemented, since production cells can be grown in suspension and the helper carrier virus can be propagated easily; (3) it completely avoids rcAAV generation by sequestering rep and cap genes and the rAAV genomes into two separate compartments of the host cell; (4) the presence of different VV promoters (early, intermediate, late) and sequential infection of two helper viral vectors enables temporal regulation of Rep and Cap expression and genome replication, which can closely mimic wtAAV production and increase rAAV production; (5) it avoids the use of wtAd; (6) it generated a VP1 superabundant rAAV vector, which has high in vivo transduction; (7) it is especially flexible for producing chimeric AAV vectors that contain a VP1 from one serotype and VP2 or VP3 from other sources.
One main obstacle of using a single VV carrier to express Rep78 and Rep52, VP1, VP2, and VP3 simultaneously is that these proteins have identical nucleotide sequences in the AAV virus. It was initially determined that viruses harboring large homologous repeats were unstable and limited their expansion for large-scale rAAV production.25, 38 This problem was solved by maximizing the use of alternative codons for Rep52 and VP1 that allowed VP1, Rep52, VP2, and Rep78 proteins to be expressed in a single VV without changing either the amino acid sequences or the expression profile of these proteins (Figure 1). The elimination of the extra VV carrier not only reduces the labor and cost for VV carrier preparation, but also improves the rAAV yield (Figure 1E), presumably by reducing cell cytotoxicity.20 The VV carrier was able to support rAAV production without losing its effectiveness even after 12 consecutive passages. This is significantly better than systems using baculoviruses, which are prone for accumulating mutations and forming revertants.25, 38 The stability of the new VV can be readily adapted for large-scale rAAV production.
For AAV Cap expression using a VV carrier, two expression cassettes are required, as the VP1 failed to produce enough VP2/VP3 (Figure S3). The weak ACG start codon was used to initiate VP2/VP3 translation, as the use of strong AUG start codon significantly decreased VP3 expression and reduced rAAV yield (data not shown). This strategy resulted in the production of much more VP1, but less VP2, compared to that of canonical rAAV vectors (Figure 5A). The varied ratio of VP1:VP2:VP3 was also reported with the use of baculoviruses to express Cap proteins.13, 25, 39 Results indicated that the high expression level of VP1 may inhibit rAAV packaging, since overexpression of VP1 significantly decreased rAAV yield (Figure S6). The VP1 capsid may have a pH-sensitive protease that can catalyze autolytic cleavage of the capsid40 and lower the production yield. The smaller amount of VP2 may not be a critical limitation for rAAV production in this system, as additional supplementation of VP3 only also dramatically improved rAAV package efficiency (Figure S6). Consistently, a complete lack of VP2 did not affect packaging efficiency.21, 22 As the ratio of VP1:VP2:VP3 affects rAAV production, it is important to balance the expression of three capsid proteins. In the current study, it was demonstrated that VP2/VP3 expression could be improved by using a vaccinia promoter with either stronger overall activity or with higher early activity (Figure 3).31, 33 Further mimic wtAAV generation using different VV promoters (early, intermediate, and late; strong and weak) may continuously improve rAAV yields in this system.
The AAP protein was reported to function in assembly of the AAV capsid for some AAV serotypes.41, 42, 43, 44, 45 AAP uses a non-conventional CTG start codon, located between the ACG start codon of VP2 and ATG start codon of VP3. In the current study, the start codon CTG of AAP at 2729 was changed to CCG in the codon-optimized VP1. Thus, there should be no AAP expressed from codon-optimized VP1. Although the open reading frame (ORF) of AAP is still present in VP2 gene, there should be no AAP transcripts when VP2 is expressed in the cytoplasm since there is no cellular promoter for AAP. However, the additional expression of AAP using a vaccinia vector did not lead to a significant increase in rAAV production yield (Figure S6). Nevertheless, the role of AAP in our VV-Ad system remains to be investigated further.
In the current system, the new suspension base cell line, QW158-7, expressed the essential E1a/E1b genes and eliminated the use of wtAd. The complete removal of wtAd from the system greatly simplifies the downstream processing and purification procedures. Unlike the HeLa-S3 cells, QW158-7 cells greatly supported Ad/AAV hybrid replication and provided a sufficient amount of replicated rAAV genomes. VVs were replicated efficiently in QW158-7 cells and expressed abundant Rep and Cap proteins. Moreover, the additional Reps did not significantly increase rAAV yield, which indicated that the Rep78 and Rep52 expressed by VW22 is enough for rAAV genome rescue and replication in QW158-7 cells. The robust accumulation of replicated rAAV genomes and Cap proteins in QW158-7 cells resulted in efficient rAAV packaging (Table 1). Experiments utilizing a modestly higher cell density (5 × 106 cells/mL) conducted in a 6-L stir-tank bioreactor demonstrated a linear increase in vector production. Optimization of rAAV production for high cell density (>1 × 107 cells/mL) should be able to further increase rAAV yield. Moreover, QW158-7 is stable up to 50 passages. All those properties should make QW158-7 an attractive cell line for large-scale rAAV production.
Taken together, a robust system for packaging high-quality rAAV vectors was presented. This novel system is transfection independent, efficient, stable, safe, and easy to scale up. The ability to produce VP1 superabundant and rcAAV-free rAAV vector makes this system optimal for producing the AAV needed for large animal studies or human clinical trials.
Materials and Methods
Cell Lines
All cells (HEK293, HeLa-S3, GM1609, BSC-1, and QW158-7) were grown in DMEM medium supplemented with 10% fetal bovine serum (FBS) (Sigma), and 100 U/mL of penicillin/streptomycin (Invitrogen). All cells were maintained in a humidified, 37°C incubator with 5% CO2. The suspension HeLa-S3 and QW158-7 cells were grown in DMEM media with 10% FBS and 0.1% pluronic F-68 in a 1-l Celstir* Spinner Flask (Wheaton Science Products, Millville, NJ).
Generation of Stable Cell Lines
To generate stable cell lines carrying the Ad E1a/E1b genes (QW158-7), HeLa-S3 cells were co-transfected with pCI-CMV-E1a/E1b and pCI-neo (100:1) by Lipofectamine 2000 and selected using 1,000 μg/mL G418. Single-cell-derived cell lines were then evaluated by their abilities to produce rAAV vectors by infecting with Ad/AAV-CMV-EGFP and VW22 in 24-well plates.
Construction and Generation of Recombinant VV Vectors
The plasmid pRB21 (with P7.5 promoter) was digested with SmaI (NEB) and used as the backbone to construct all plasmids having individual AAV and Ad genes. Rep68, Rep40, X-gene, and AAP were generated using PCR amplified from pH22. E1a-13S, E1b-55K, E1b-19k, E2a, E4orf6, and VA I RNA were amplified using the pFΔ6 plasmid as a template. The PCR products were digested by EcoRV and blunt-end ligated into pRB21. pRB21-E1a-12S plasmid was generated by replacing BsgI/BsmI fragment in E1a-13s with the corresponding sequence from the E1a-12s gene.
Rep52* and VP1* (Figures S1 and S2) genes, together with p7.5 promoter and transcription stop signal, were synthesized by integrated DNA technologies (IDT) and blunt-end ligated into pRB21. To generate pRB21-W8* that contains both VP1* and Rep52* genes, the synthesized fragments were assembled into the pRB21. The pRB21-VW22 construct was obtained by cloning an XhoI-digested fragment from pRB21-W8* into the pRBW1-W5 plasmid, which was used to generate W5 vectors.20 To generate pRB21-VW22 with different promoters, a DNA sequence containing all promoters was synthesized in the following order: Leo160/Pr4LS5E/pHyb/PrMVA13.5-long/PrS5E/mH5/pS/ATI (Table S1). The sequence was used to replace the p7.5 promoter, which is used to control VP2 expression, and obtained VW22-A (Figure 3A). The VW22 constructs with individual promoters or promoter combinations were generated by deleting additional promoter sequences.
To generate VVs, BSC-1 cells were infected by vRB12 at an MOI of 0.05 for 1 hr followed by Polyjet (SignaGen) transfection of pRB21-derived plasmids. Each kind of VV was subject to three rounds of plaque purification and then propagated in BSC-1 cells. The titer of each VV was determined by counting plaques formed in BSC-1 cells after serial dilution. W5, W8, and VVs carrying Rep78, Rep52, VP1, VP2, and VP3 have been previously.20
Ads
Adenoviral vectors used in the current study are based on Ad5. Ad375 was purchased from OD260 Inc. Ad375 is an E1-deleted Ad, with the E3 region intact. All the Ad/AAV hybrids are E1/E3 gene-deleted Ads that contain transgene cassettes flanked on their 5′ and 3′ ends by AAV ITR vector sequences. Ad/dsAAV-CB-EGFP, Ad/dsAAV-CB-Gluc, and Ad/AAV-CB-AAT were obtained from a contract company. The Ad/AAV-CMV-EGFP hybrid vector was purchased from Penn Vector Core (Philadelphia, PA). The Ad/AAV-CMV-LacZ was previously described.20 All Ad vectors used were grown in HEK293 cells and purified by CsCl ultracentrifuge.
Production of rAAV Vectors Using VV-Ad System
To produce rAAV vectors using the VV-Ad system in adherent cells, host cells were infected with Ad/AAV hybrid vectors 16 hr before VV infection. At 36 hr after VV infection, the cells were collected. To produce rAAV vectors in suspension cells, QW158-7 or HeLa-S3 cells were grown in a 1L-Flask (Wheaton Science Products, Millville, NJ). When the cell density reached 1 × 106 cells/mL, Ad/AAV hybrids were added (MOI = 50) and incubated for 16 hr. The cells were then collected and re-suspended in 100 mL sera-free DMEM media. Next, VW22 (MOI = 2) was added and incubated at 37°C for 2 hr. After that, the DMEM media was supplemented with 10% FBS and 0.1% Pluronic F-68 at a total volume of 400 mL. rAAV vectors generated by the new Ad-VV system, as well as by the triple plasmid transfection method,46 were purified by two rounds of CsCl gradient ultracentrifugation. After extensive buffer exchange against PBS with 5% D-sorbitol, the purified vectors were incubated at 56°C for 1 hr and stored at −80°C.
Titration of rAAV Yield, rAAV, Ad/AAV, and VV Genomes Using qPCR
To titer the rAAV genomes in the culture system, 2 mL of cell culture infected with VV and Ad/AAV were removed and sonicated to lyse the cells efficiently. After sonication, 10 μL of each sample were added into 90 μL DNase I solution (500 μg/mL, 5 mM CaCl2, 5 mM MgCl2, 50 mM Tris-HCl [pH 8.0]) and incubated at 37°C for 1 hr. DNase I was inactivated by adding 7 μL 0.5 M EDTA (pH 8.0) followed by incubation at 70°C for 20 min. Next, 93 μL of proteinase K solution (40 μg/mL proteinase K, 1 M NaCl, 1% Sarkosyl) were added and incubated at 55°C for at least 2 hr. Samples were then boiled for 20 min to inactivate the proteinase K. After inactivation, samples were serially diluted, and 10 μL were taken to analyze using qPCR. To measure the total rAAV genomes, Ad/AAV genomes, and VV genomes, samples were treated using the same protocol but without DNase I treatment. For titration of purified rAAV, Ad, or VV vectors, 10 μL of each vector were treated with DNase I and proteinase K. Then, the DNA samples were diluted to different ratios and used for analysis via qPCR. The qPCR was conducted using Sybr green reagents in an Eppendorf MasterCycler RealPlex.46 Samples were denatured at 95°C for 5 min, followed by 40 cycles of amplification (95°C for 10 s, 60°C for 30 s) and terminated after generating a melting curve. A plasmid containing corresponding targeted sequence was used to make the standard curve.
Analysis of rAAV Vector Genomes Using Southern Blot
For detection of replicated rAAV genomes using a Southern blot, Hirt-extracted DNA was digested by DpnI overnight. For detecting rAAV genomes in the vectors, rAAV vectors (∼1 × 1010) were treated with DNase I and proteinase K, and the DNA was extracted using a GeneJET gel purification kit. All samples were electrophoresed on a 0.8% agarose gel, and a Southern blot was performed using a 32P-labeled EGFP-specific probe.
Silver Staining Analysis of rAAV Capsid Components
The capsid composition of the rAAV vectors was determined by silver staining analysis. Briefly, rAAV vectors (∼1 × 1010 particles) were electrophoresed using a 10% SDS-PAGE gel, and the standard silver staining protocol was followed according to the manufacturer’s procedures (Pierce Silver Stain Kit, Thermo Scientific, Rockford, IL).
Western Blot
Total proteins were extracted with lysis buffer, which consisted of 50 mM Tris (pH 8.0), 150 mM NaCl, 1% Triton X-100, 10 mM DTT, and 1× protein inhibitor (Roche, Indianapolis, IN). Cell lysates were resolved on 10% SDS-PAGE gel and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, California). After blocking the membrane with 5% non-fat dry milk in TBST buffer, which contains 25 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.1% Tween 20, the membrane was incubated with the primary antibody, anti-AAV capsid (B1, American Research Products, Belmont, MA), or anti-AAV Rep (303.9, American Research Products) at a dilution of 1:500 at 4°C overnight. The membrane was washed and incubated with a horseradish peroxidase (HRP)-conjugated sheep, anti-mouse immunoglobulin G (IgG) antibody (Sigma, St Louis, MI) at a dilution of 1:10,000. The membrane was developed using an enhanced chemiluminescent substrate (Amersham-Pharmacia Biotech, Piscataway, NJ).
TEM of rAAV Particles
For TEM analysis, formvar was coated on a microscope slide and floated onto a water bath. Cleaned 200 mesh grids were placed on this film and collected using parafilm. Five microliters of purified AAV (∼1 × 1013 vg/mL) was placed on the grid and allowed to dry, then 8 μL of 1% phospho-tungstic acid (Electron Microscopy Science, Hatfield, PA) was applied and drawn off. After drying, the grids were observed using a Philips Transmission Electron Microscope CM 12 (Philips, the Netherlands) with an accelerating voltage of 100 KV and imaged with a DVC detector controlled by AMT software (Danvers, MA, USA).
rAAV Transduction In Vitro
For each experiment, 50,000 viable GM16095 cells were seeded into a 24-well plate 24 hr before transduction. rAAV particles carrying EGFP or Gluc genes were added directly to each well. Transgene expression was examined 48 hr post-infection. EGFP expression was observed 24 hr post-infection using a fluorescent microscope. Gaussia luciferase (Gluc) expression in the medium was determined by adding 5 μL of coelenterazine substrate (CLZ, 200 μg/mL) in 0.1M Tris buffer (pH 7.5) containing 0.5 M NaCl using a POLARstar omega bioluminescence plate reader to measure bioluminescence (BMG Labtech, Germany). All experiments were performed in triplicate, and the results are presented as averages of the experimental data.
rAAV Transduction In Vivo
In vivo transduction experiments were carried out in C57BL/6 mice. All mice were housed in a specific pathogen-free environment, with a normal diet, and treated in accordance with NIH guidelines as approved by IACUC at Temple University. Mice were anesthetized by xylazine and ketamine based on their body weight (0.01 mg xylazine/g + 0.08 mg ketamine/g). For each AAV intravitreal injection, a micropipette was inserted into the peripheral retina of 3-week-old mice just behind the ora serrata and advanced into the vitreous chamber so as to avoid damage to the lens. Approximately 2 μL of the vitreous fluid was removed before injection of 2 μL dsAAV2-CB-EGFP (6.5 × 1012 vg/mL) into the vitreous chamber. Eye ointment containing neomycin (Akorn, Somerset, New Jersey) was applied to protect the cornea after surgery.
Immunohistochemistry with Whole-Mount Retina
Retinas were dissected out from 4% paraformaldehyde (PFA)-fixed mouse eyeballs and washed extensively in PBS before blocking in staining buffer (10% normal goat serum [NGS] and 2% Triton X-100 in PBS) for half an hour. All antibodies were diluted in the same staining buffer. Antibodies used were as follows: mouse neuronal class β-III tubulin (clone TUJ1, 1:200 dilution; Covance). Floating retinas were incubated with primary antibodies overnight at 4°C and washed three times for 30 min each with PBS. Secondary antibodies (Cy3-conjugated) were then applied (1:200; Jackson ImmunoResearch) and incubated for 1 hr at room temperature. Retinas were again washed three times for 30 min each with PBS before a coverslip was attached with Fluoromount-G (Southernbiotech).
Statistical Analyses
Two-tailed Student’s t tests and one-way ANOVA with Bonferroni multiple comparisons post-test were performed. The differences were considered significant when p was < 0.05. The analysis was performed using the SPSS 11.0.
Author Contributions
Q.W. helped with study design, performed experiments, analyzed the data, prepared figures, and wrote the manuscript. Z.W., J.Z., H.W., and Z.Z. performed experiments. L.M. and Y.H. performed animal studies. J.F. and L.L. performed TEM experiments. J.F. and Y.D. helped with manuscript writing and editing. W.X. directed the study and wrote the manuscript.
Acknowledgments
This work is supported by NIH grants (R01HL080789, R01HL114152, and HL130871), the Fujian Class A technology project (JA12012), and the Natural National Science foundation of China (81271691, 81371669, 81371672). We would like to acknowledge Joseph Uknalis for taking the TEM pictures.
Footnotes
Supplemental Information includes eight figures and one table and can be found with this article online at https://doi.org/10.1016/j.omtm.2017.11.002.
Supplemental Information
References
- 1.Hastie E., Samulski R.J. Adeno-associated virus at 50: a golden anniversary of discovery, research, and gene therapy success—a personal perspective. Hum. Gene Ther. 2015;26:257–265. doi: 10.1089/hum.2015.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacLaren R.E., Groppe M., Barnard A.R., Cottriall C.L., Tolmachova T., Seymour L., Clark K.R., During M.J., Cremers F.P., Black G.C. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y., Wang L., Bell P., McMenamin D., He Z., White J., Yu H., Xu C., Morizono H., Musunuru K. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat. Biotechnol. 2016;34:334–338. doi: 10.1038/nbt.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salmon F., Grosios K., Petry H. Safety profile of recombinant adeno-associated viral vectors: focus on alipogene tiparvovec (Glybera®) Expert Rev. Clin. Pharmacol. 2014;7:53–65. doi: 10.1586/17512433.2014.852065. [DOI] [PubMed] [Google Scholar]
- 5.Nathwani A.C., Reiss U.M., Tuddenham E.G., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bainbridge J.W., Mehat M.S., Sundaram V., Robbie S.J., Barker S.E., Ripamonti C., Georgiadis A., Mowat F.M., Beattie S.G., Gardner P.J. Long-term effect of gene therapy on Leber’s congenital amaurosis. N. Engl. J. Med. 2015;372:1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore A.R., Dong B., Chen L., Xiao W. Vaccinia virus as a subhelper for AAV replication and packaging. Mol. Ther. Methods Clin. Dev. 2015;2:15044. doi: 10.1038/mtm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao X., Li J., Samulski R.J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grieger J.C., Soltys S.M., Samulski R.J. Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Mol. Ther. 2016;24:287–297. doi: 10.1038/mt.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mietzsch M., Grasse S., Zurawski C., Weger S., Bennett A., Agbandje-McKenna M., Muzyczka N., Zolotukhin S., Heilbronn R. OneBac: platform for scalable and high-titer production of adeno-associated virus serotype 1-12 vectors for gene therapy. Hum. Gene Ther. 2014;25:212–222. doi: 10.1089/hum.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas D.L., Wang L., Niamke J., Liu J., Kang W., Scotti M.M., Ye G.J., Veres G., Knop D.R. Scalable recombinant adeno-associated virus production using recombinant herpes simplex virus type 1 coinfection of suspension-adapted mammalian cells. Hum. Gene Ther. 2009;20:861–870. doi: 10.1089/hum.2009.004. [DOI] [PubMed] [Google Scholar]
- 12.Kang W., Wang L., Harrell H., Liu J., Thomas D.L., Mayfield T.L., Scotti M.M., Ye G.J., Veres G., Knop D.R. An efficient rHSV-based complementation system for the production of multiple rAAV vector serotypes. Gene Ther. 2009;16:229–239. doi: 10.1038/gt.2008.158. [DOI] [PubMed] [Google Scholar]
- 13.Mietzsch M., Casteleyn V., Weger S., Zolotukhin S., Heilbronn R. OneBac 2.0: Sf9 cell lines for production of AAV5 vectors with enhanced infectivity and minimal encapsidation of foreign DNA. Hum. Gene Ther. 2015;26:688–697. doi: 10.1089/hum.2015.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clément N., Grieger J.C. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol. Ther. Methods Clin. Dev. 2016;3:16002. doi: 10.1038/mtm.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimm D., Kay M.A., Kleinschmidt J.A. Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol. Ther. 2003;7:839–850. doi: 10.1016/s1525-0016(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 16.Kotin R.M. Large-scale recombinant adeno-associated virus production. Hum. Mol. Genet. 2011;20(R1):R2–R6. doi: 10.1093/hmg/ddr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith R.H., Levy J.R., Kotin R.M. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 2009;17:1888–1896. doi: 10.1038/mt.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonntag F., Schmidt K., Kleinschmidt J.A. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc. Natl. Acad. Sci. USA. 2010;107:10220–10225. doi: 10.1073/pnas.1001673107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cecchini S., Virag T., Kotin R.M. Reproducible high yields of recombinant adeno-associated virus produced using invertebrate cells in 0.02- to 200-liter cultures. Hum. Gene Ther. 2011;22:1021–1030. doi: 10.1089/hum.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong B., Moore A.R., Dai J., Roberts S., Chu K., Kapranov P., Moss B., Xiao W. A concept of eliminating nonhomologous recombination for scalable and safe AAV vector generation for human gene therapy. Nucleic Acids Res. 2013;41:6609–6617. doi: 10.1093/nar/gkt404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warrington K.H., Jr., Gorbatyuk O.S., Harrison J.K., Opie S.R., Zolotukhin S., Muzyczka N. Adeno-associated virus type 2 VP2 capsid protein is nonessential and can tolerate large peptide insertions at its N terminus. J. Virol. 2004;78:6595–6609. doi: 10.1128/JVI.78.12.6595-6609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grieger J.C., Samulski R.J. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J. Virol. 2005;79:9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson J.S., Li C., DiPrimio N., Weinberg M.S., McCown T.J., Samulski R.J. Mutagenesis of adeno-associated virus type 2 capsid protein VP1 uncovers new roles for basic amino acids in trafficking and cell-specific transduction. J. Virol. 2010;84:8888–8902. doi: 10.1128/JVI.00687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popa-Wagner R., Porwal M., Kann M., Reuss M., Weimer M., Florin L., Kleinschmidt J.A. Impact of VP1-specific protein sequence motifs on adeno-associated virus type 2 intracellular trafficking and nuclear entry. J. Virol. 2012;86:9163–9174. doi: 10.1128/JVI.00282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohlbrenner E., Aslanidi G., Nash K., Shklyaev S., Campbell-Thompson M., Byrne B.J., Snyder R.O., Muzyczka N., Warrington K.H., Jr., Zolotukhin S. Successful production of pseudotyped rAAV vectors using a modified baculovirus expression system. Mol. Ther. 2005;12:1217–1225. doi: 10.1016/j.ymthe.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urabe M., Nakakura T., Xin K.Q., Obara Y., Mizukami H., Kume A., Kotin R.M., Ozawa K. Scalable generation of high-titer recombinant adeno-associated virus type 5 in insect cells. J. Virol. 2006;80:1874–1885. doi: 10.1128/JVI.80.4.1874-1885.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward P., Clément N., Linden R.M. cis effects in adeno-associated virus type 2 replication. J. Virol. 2007;81:9976–9989. doi: 10.1128/JVI.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao M., Chiriva-Internati M., Hermonat P.L. AAV2 X increases AAV6 rep/cap-driven rAAV production. Virology. 2015;482:84–88. doi: 10.1016/j.virol.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao M., You H., Hermonat P.L. The X gene of adeno-associated virus 2 (AAV2) is involved in viral DNA replication. PLoS ONE. 2014;9:e104596. doi: 10.1371/journal.pone.0104596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Pilato M., Sánchez-Sampedro L., Mejías-Pérez E., Sorzano C.O., Esteban M. Modification of promoter spacer length in vaccinia virus as a strategy to control the antigen expression. J. Gen. Virol. 2015;96:2360–2371. doi: 10.1099/vir.0.000183. [DOI] [PubMed] [Google Scholar]
- 31.Wennier S.T., Brinkmann K., Steinhäußer C., Mayländer N., Mnich C., Wielert U., Dirmeier U., Hausmann J., Chaplin P., Steigerwald R. A novel naturally occurring tandem promoter in modified vaccinia virus ankara drives very early gene expression and potent immune responses. PLoS ONE. 2013;8:e73511. doi: 10.1371/journal.pone.0073511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baur K., Brinkmann K., Schweneker M., Pätzold J., Meisinger-Henschel C., Hermann J., Steigerwald R., Chaplin P., Suter M., Hausmann J. Immediate-early expression of a recombinant antigen by modified vaccinia virus ankara breaks the immunodominance of strong vector-specific B8R antigen in acute and memory CD8 T-cell responses. J. Virol. 2010;84:8743–8752. doi: 10.1128/JVI.00604-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyatt L.S., Shors S.T., Murphy B.R., Moss B. Development of a replication-deficient recombinant vaccinia virus vaccine effective against parainfluenza virus 3 infection in an animal model. Vaccine. 1996;14:1451–1458. doi: 10.1016/s0264-410x(96)00072-2. [DOI] [PubMed] [Google Scholar]
- 34.Chakrabarti S., Sisler J.R., Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 35.Funahashi S., Itamura S., Iinuma H., Nerome K., Sugimoto M., Shida H. Increased expression in vivo and in vitro of foreign genes directed by A-type inclusion body hybrid promoters in recombinant vaccinia viruses. J. Virol. 1991;65:5584–5588. doi: 10.1128/jvi.65.10.5584-5588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H. Intron splicing-mediated expression of AAV Rep and Cap genes and production of AAV vectors in insect cells. Mol. Ther. 2008;16:924–930. doi: 10.1038/mt.2008.35. [DOI] [PubMed] [Google Scholar]
- 37.Chahal P.S., Schulze E., Tran R., Montes J., Kamen A.A. Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. J. Virol. Methods. 2014;196:163–173. doi: 10.1016/j.jviromet.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Negrete A., Yang L.C., Mendez A.F., Levy J.R., Kotin R.M. Economized large-scale production of high yield of rAAV for gene therapy applications exploiting baculovirus expression system. J. Gene Med. 2007;9:938–948. doi: 10.1002/jgm.1092. [DOI] [PubMed] [Google Scholar]
- 39.Urabe M., Ding C., Kotin R.M. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- 40.Salganik M., Venkatakrishnan B., Bennett A., Lins B., Yarbrough J., Muzyczka N., Agbandje-McKenna M., McKenna R. Evidence for pH-dependent protease activity in the adeno-associated virus capsid. J. Virol. 2012;86:11877–11885. doi: 10.1128/JVI.01717-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Earley L.F., Powers J.M., Adachi K., Baumgart J.T., Meyer N.L., Xie Q., Chapman M.S., Nakai H. Adeno-associated virus (AAV) assembly-activating protein is not an essential requirement for capsid assembly of AAV serotypes 4, 5, and 11. J. Virol. 2017;91 doi: 10.1128/JVI.01980-16. e01980-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonntag F., Köther K., Schmidt K., Weghofer M., Raupp C., Nieto K., Kuck A., Gerlach B., Böttcher B., Müller O.J. The assembly-activating protein promotes capsid assembly of different adeno-associated virus serotypes. J. Virol. 2011;85:12686–12697. doi: 10.1128/JVI.05359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Earley L.F., Kawano Y., Adachi K., Sun X.X., Dai M.S., Nakai H. Identification and characterization of nuclear and nucleolar localization signals in the adeno-associated virus serotype 2 assembly-activating protein. J. Virol. 2015;89:3038–3048. doi: 10.1128/JVI.03125-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grosse S., Penaud-Budloo M., Herrmann A.K., Börner K., Fakhiri J., Laketa V., Krämer C., Wiedtke E., Gunkel M., Ménard L. Relevance of assembly-activating protein for adeno-associated virus vector production and capsid protein stability in mammalian and insect cells. J. Virol. 2017;91 doi: 10.1128/JVI.01198-17. e01198-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naumer M., Sonntag F., Schmidt K., Nieto K., Panke C., Davey N.E., Popa-Wagner R., Kleinschmidt J.A. Properties of the adeno-associated virus assembly-activating protein. J. Virol. 2012;86:13038–13048. doi: 10.1128/JVI.01675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Q., Dong B., Firrman J., Roberts S., Moore A.R., Cao W., Diao Y., Kapranov P., Xu R., Xiao W. Efficient production of dual recombinant adeno-associated viral vectors for factor VIII delivery. Hum. Gene Ther. Methods. 2014;25:261–268. doi: 10.1089/hgtb.2014.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.