Abstract
Purpose
Skeletal-related events (SREs) such as pathologic fracture, spinal cord compression, or the necessity for radiation or surgery to bone metastasis cause considerable morbidity, decrements in quality of life, and costs to the health care system. The results of a recent large randomized trial (Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology [CALGB/Alliance 70604]) showed that zoledronic acid (ZA) every 3 months was noninferior to monthly ZA in reducing the risks of SREs. We sought to determine the cost-effectiveness (CE) of monthly ZA, ZA every 3 months, and monthly denosumab in women with breast cancer and skeletal metastases.
Methods
Using a Markov model, costs per SRE avoided were calculated for the three treatments. Sensitivity analyses were performed where denosumab SRE probabilities were assumed to be 50%, 75%, and 90% lower than the ZA SRE probabilities. Quality-adjusted life-years were also calculated. The analysis was from the US payer perspective.
Results
The mean costs of the denosumab treatment strategy are nine-fold higher than generic ZA every 3 months. Quality-adjusted life-years were virtually identical in all the three treatment arms; hence, the optimal treatment would be ZA every 3 months because it was the least costly treatment. The sensitivity analyses showed that relative to ZA every 3 months, the incremental costs per mean SRE avoided for denosumab ranged from $162,918 to $347,655.
Conclusion
ZA every 3 months was more CE in reducing the risks of SRE than monthly denosumab. This analysis was one of the first to incorporate the costs of generic ZA and one of the first independent CE analyses not sponsored by either Novartis or Amgen, the makers of ZA and denosumab, respectively. ZA every 3 months is the more CE option and more reasonable alternative to monthly denosumab.
INTRODUCTION
Bone metastases occur in 65% to 75% of women with metastatic breast cancer and cause significant morbidity, including pain, pathologic fracture, spinal cord compression, and hypercalcemia.1 Surgery and radiation therapy may be required to prevent or treat the complications of bone metastases.2 Zoledronic acid (ZA), a third-generation aminobisphosphonate given monthly, reduces the risk of pain and skeletal-related events (SREs) up to 40%.1,3 After 9 to 12 doses of monthly ZA, ZA every 3 months is noninferior to monthly ZA in women with breast cancer with skeletal metastases.4,5 In the Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology (CALGB/Alliance) 70604 trial, 1,822 individuals with skeletal metastases (breast cancer [n = 855], prostate cancer [n = 689], and multiple myeloma [n = 278]) were randomly assigned to receive either monthly ZA or ZA every 3 months, starting with the first dose, for 2 years.6 ZA every 3 months was noninferior to monthly ZA in SREs overall and in the three disease sites in prespecified analyses.
Denosumab, a human monoclonal antibody that inhibits osteoclasts by binding to receptor activator of nuclear factor kappa B ligand,7 delays the onset of first and subsequent SREs to a greater extent than ZA in women with bone metastases from breast cancer.8 Both drugs can cause osteonecrosis of the jaw (ONJ) and hypocalcemia. ZA can also cause renal toxicity depending primarily on dose, duration of infusion, and other factors.9
There are ongoing noninferiority trials that compare monthly denosumab with denosumab every 3 months. The open-label phase III trial Swiss Group for Clinical Cancer Research (SAKK) 96/12: REDUSE (ClinicalTrials.gov identifier: NCT02051218) randomly assigns patients with metastatic breast or prostate cancer to receive denosumab every 4 weeks or every 12 weeks. Likewise, the Rethinking Clinical Trials (REaCT) bone-targeting agents (BTAs; ClinicalTrials.gov identifier: NCT02721433) is another open-label, phase III trial that randomly assigns patients with metastatic breast or prostate cancer to either 4-week or 12-week denosumab, pamidronate, or ZA. Both trials are actively recruiting. Until the publication of the results, it is unknown whether denosumab every 3 months is noninferior to monthly dosing.
In addition to their effects on quality of life, the costs to the health care system of preventing and treating SREs are considerable.10 Since March 2013, ZA has been available as a generic drug.11 Denosumab is more expensive than ZA, and despite being superior to ZA in time to development of first and subsequent SREs,8 it may not be cost effective (CE).12 On the basis of the 70604 trial,6 we hypothesized that ZA administered every 3 months is more CE than monthly ZA or monthly denosumab in women with bone metastases from breast cancer.
METHODS
Patients
Each 70604 trial participant signed an institutional review board–approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Model Design
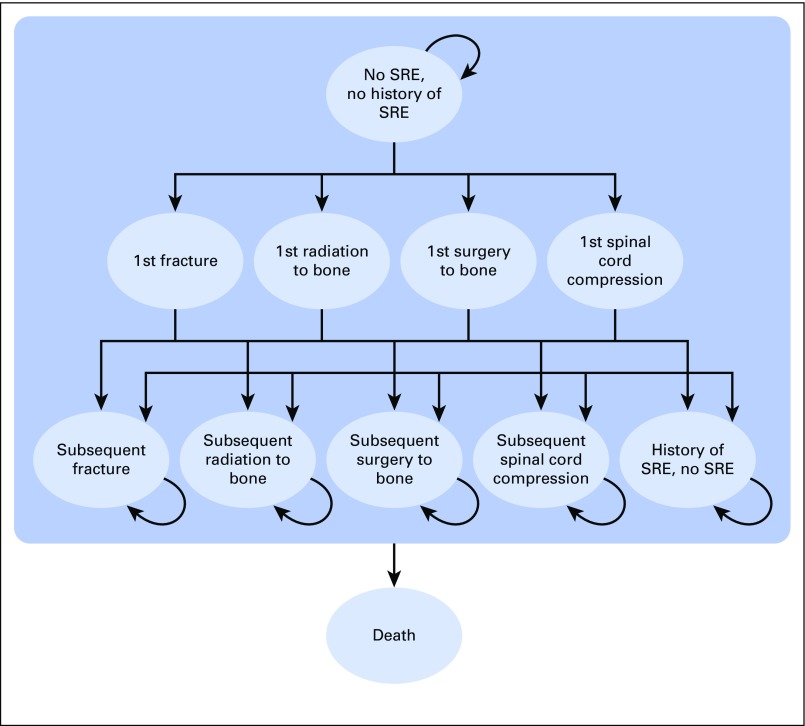
A Markov model created in TreeAge Pro 2014 (TreeAge Software, Williamstown, MA) was used to analyze the CE of three treatment strategies (monthly ZA, ZA every 3 months, and monthly denosumab), each using a hypothetical cohort of 10,000 women with breast cancer and bone metastases for SRE prevention. Figure 1 describes the model design on the basis of the CE analysis by Xie et al.12 The primary reason for replicating the Xie et al12 model was that it looked at pathologic fractures without differentiating vertebral and nonvertebral fractures. The CE analysis was conducted from the US payer’s perspective.
Fig 1.
Markov model. All patients started in the “No SRE, no history of SRE” stage. SRE, skeletal-related event.
The model consisted of 11 distinct health states differentiated by SRE status and SRE type (Fig 1). SRE status included no current SRE, the first onset of SRE, subsequent SRE, and no current SRE but having a history of SRE. The SRE types were a pathologic bone fracture, radiation to bone, surgery to bone, and spinal cord compression. A 2-year time horizon was used to correspond with the length of the trial comparing monthly ZA with ZA every 3 months. Each Markov cycle (the calibration period for the model parameters) was 1 month. Thus, Markov cycles result in 24 simulated months.
Transition Probability Parameters
Transition probabilities (the probability of being in a given state in the next cycle on the basis of the current state) came from multiple sources (Table 1). Annual probabilities of first SREs and subsequent SREs associated with denosumab were from the CE analysis by Xie et al.12 The Xie et al12 model based the SRE probabilities on the randomized trial by Stopeck et al.8 For monthly ZA and ZA every 3 months, annual probability estimates of the first and subsequent SREs were from the 70604 trial.6 The following assumptions were made for monthly ZA, ZA every 3 months, and monthly denosumab. For patients with no current SREs but with a history of an SRE, the type of subsequent SRE was not dependent on the first SRE type, and the overall mortality did not differ between the three treatments.4,8,12
Table 1.
Model Parameter Values
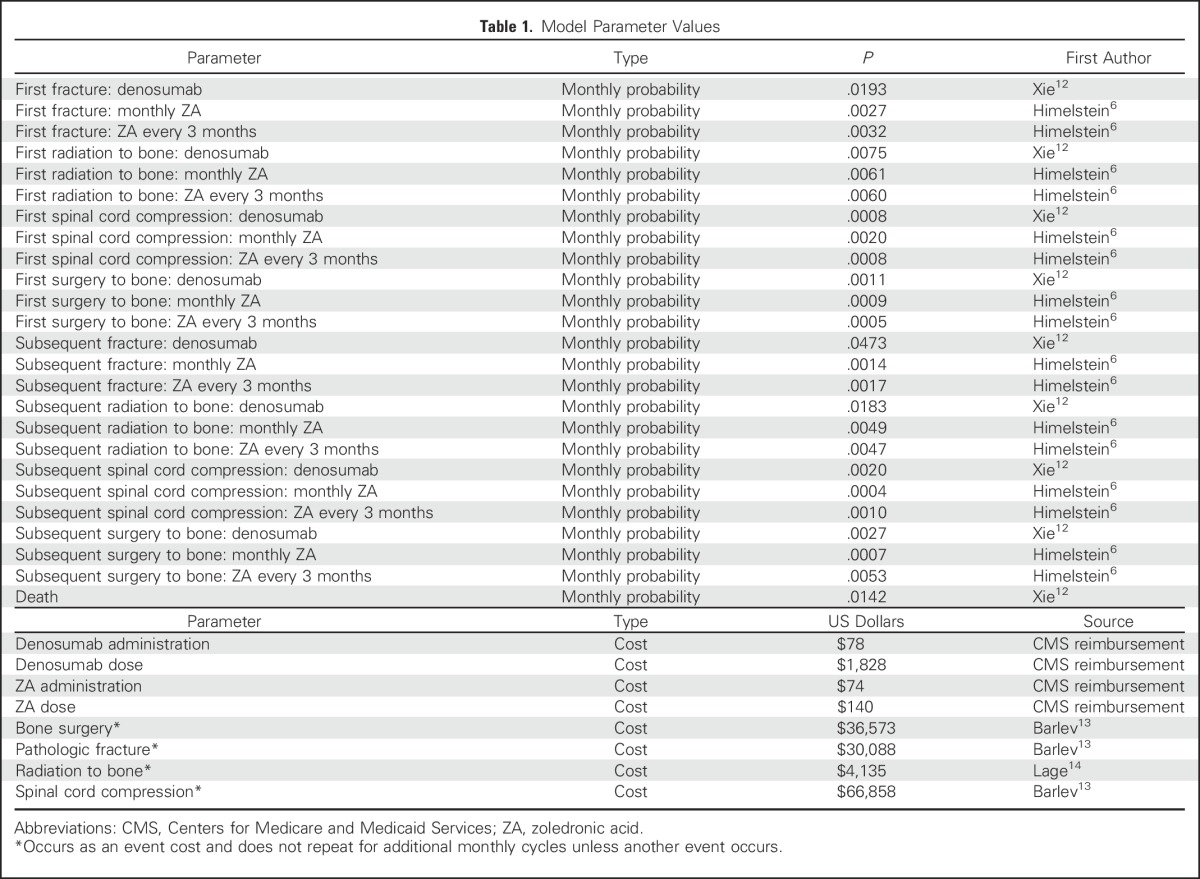
Cost Parameters
The costs for denosumab and ZA included the costs of the drug and its administration. Denosumab costs were for the 120-mg subcutaneous (SQ) dose, which is the dose based on the drug label.11,12 The ZA cost was for the standard 4-mg intravenous (IV) dose. The estimated costs of SREs came from multiple sources. The costs of bone surgery, pathologic fracture, and spinal cord compression were from Barlev et al.13 The costs for radiation to bone were from Lage et al.14 Costs as a result of death were not included. Because mortality was similar among the three treatment groups, excluding costs as a result of death would not affect the incremental costs. The average cost of a treatment strategy (Table 2) was defined as the average of the drug cost, its administration cost, and the cost associated with having an SRE.
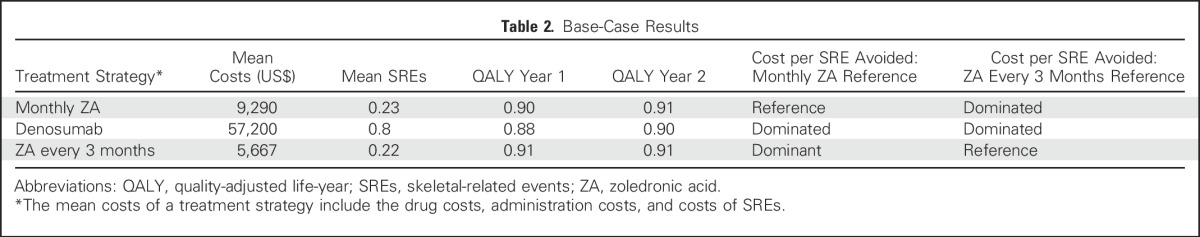
Table 2.
Base-Case Results
Costs from the literature were valued on the basis of differing years of US dollars. As such, all costs before 2015 were inflated to 2015 US dollars using the gross domestic product implicit price deflator.15 Drug costs were based on 2015 Centers for Medicare and Medicaid Services reimbursement rates and did not require inflation adjustment. Given that the model projects 24 months into the future, the future costs are discounted back to a common time frame. Therefore, costs projected by the model are discounted at an annual rate of 3%.
Sensitivity Analyses
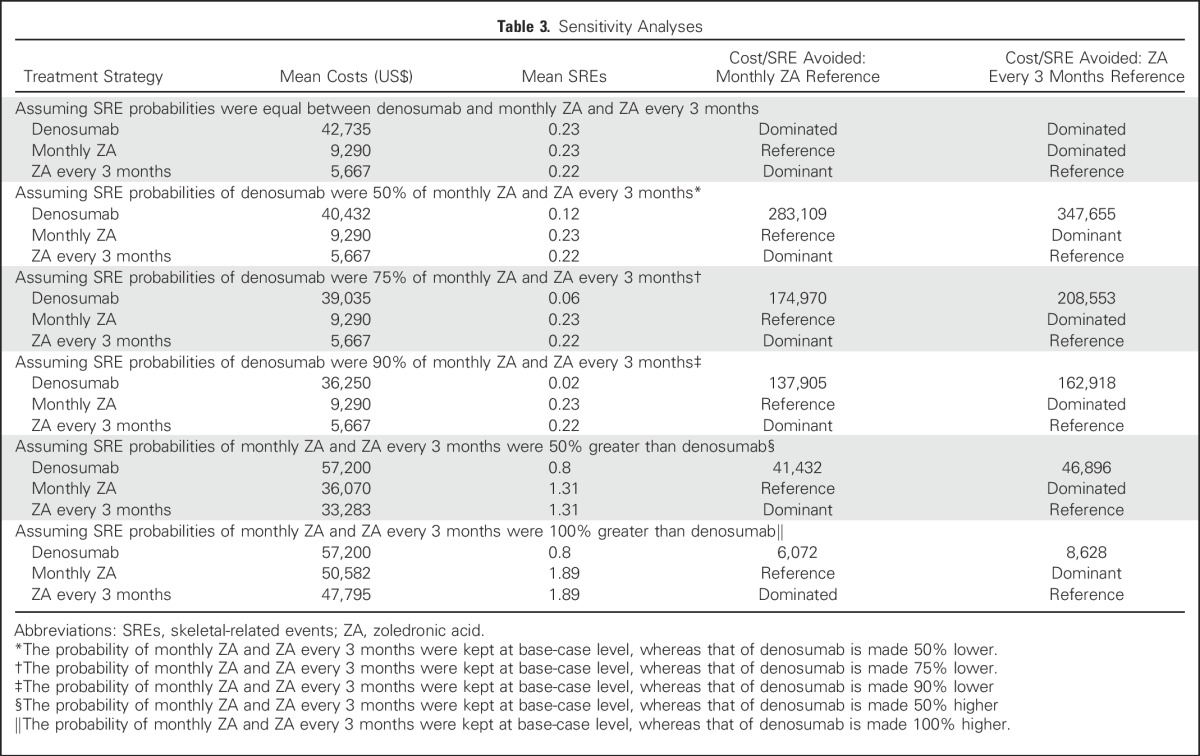
Table 2 lists the differences in the observed SRE probabilities between the randomized trial of monthly denosumab versus ZA8 and trial 70604.6 A series of sensitivity analyses were undertaken on the basis of the relative probabilities of monthly denosumab, monthly ZA, and ZA every 3 months. The sensitivity analyses intended to create scenarios in which the SRE probabilities were based on factors derived from the same underlying data source, whether it was Xie et al12 or the 70604 trial.6 The primary difference between treatments is the significantly higher costs of the denosumab treatment strategy. These sensitivity analyses could better determine the tradeoff between the increased costs of the denosumab treatment strategy and lower costs of treatment every 3 months and the SREs avoided.
Table 3 lists the sensitivity analyses using the following scenarios for first and subsequent SRE probabilities. Denosumab SRE probabilities were assumed to be the same as monthly ZA. Denosumab SRE probabilities were assumed to be 50%, 75%, and 90% lower than monthly ZA or ZA every 3 months; alternatively, monthly ZA and ZA every 3 months SRE probabilities were considered to be higher than the base-case denosumab by 50% or 100%.
Table 3.
Sensitivity Analyses
Finally, to investigate how results may differ when incorporating quality of life, we also conducted sensitivity analyses using quality-adjusted life-years (QALYs) as our outcome measure rather than SREs avoided. QALYs were calculated using transition probabilities of the base-case analysis. Health state utility values came from Snedecor et al.16 This study reported utility values that would be experienced in the first year and could potentially increase to a constant value in the second year. The sensitivity analysis used constant utility values throughout the model time horizon. The analyses were performed separately using the first- and second-year utility values reported by Snedecor et al.16
RESULTS
Table 2 lists the base-case results on the basis of the three treatment strategies. Denosumab had the greatest mean costs and mean number of SREs and is dominated, relative to monthly ZA and ZA every 3 months. ZA every 3 months was less expensive and had slightly fewer SREs than monthly ZA and would be considered the dominant option in base-case analyses. QALYs were virtually identical in all three treatments regardless of whether using year 1 or year 2 utilities.16 Consequently, if equivalent outcomes of QALYs were assumed, the optimal treatment would be the 3-month ZA, as it was the least costly treatment.
Table 3 lists the results of the sensitivity analysis assuming monthly denosumab had transition probabilities equal to monthly ZA and ZA every 3 months in the base-case scenario. Although this assumption results in denosumab having fewer SREs than monthly ZA or ZA every 3 months, and consequently lower costs, the overall findings were unchanged from the base-case analysis. ZA every 3 months still had the lowest costs.
Table 3 also lists sets of sensitivity analyses assuming denosumab SRE probabilities were 50%, 75%, and 90% lower than monthly ZA and ZA every 3 months in the base-case scenario. As expected, these sensitivity analyses all resulted in denosumab having fewer SREs than monthly ZA and ZA every 3 months. However, this did not lead to denosumab being less costly. Compared with monthly ZA, the mean incremental costs per mean SRE avoided for denosumab ranged from $137,905 to $283,109. Likewise, compared with ZA every 3 months, the mean incremental costs per mean SRE avoided for denosumab ranged from $162,918 to $347,655.
The results of the final set of sensitivity analyses assuming monthly ZA and ZA every 3 months SRE probabilities were higher than monthly denosumab by 50% and 100% of the base-case scenario is described in Table 3. The incremental differences in SREs for monthly ZA and ZA every 3 months relative to denosumab were high enough to make the mean costs more comparable. The mean incremental costs per SRE avoided for denosumab ranged from $6,072 to $41,432. Similarly, compared with 3-month ZA mean incremental costs per SRE avoided for denosumab ranged from $8,628 to $46,896.
DISCUSSION
In this CE analysis, from the US payer perspective, the base-case analysis revealed ZA every 3 months to be dominant compared with monthly ZA or monthly denosumab (Table 2). In sensitivity analyses where the SRE probabilities of denosumab were lower than the base-case ZA SRE probabilities, denosumab resulted in fewer SREs but still at substantial costs (Table 3).
Among the strengths of this study is that, to our knowledge, this was the first CE analysis to incorporate the costs of generic ZA, whereas denosumab is patented until 2022 to 2025. There is only one published abstract (in 2014) of a CE analysis comparing monthly generic ZA and monthly denosumab that came to the same conclusion.17 Because the mean cost of the treatment strategy is nine-fold higher for denosumab than generic ZA every 3 months (Table 2), the average costs of SREs with ZA every 3 months would need to approach that nine-fold higher cost difference in drug pricing. This does not begin to be the case until the average number of SREs for ZA is at least 50% to 100% greater than that of denosumab (Table 3). The risks of multiple SREs (first and subsequent) reported by Stopeck et al8 are 23% fewer SREs with monthly denosumab than with monthly ZA. This is similar to the results of sensitivity analysis in which the SRE probabilities of denosumab are assumed to 75% of those of ZA every 3 months (Table 3), in which the cost of denosumab per SRE avoided was over $200,000.
Amgen or Novartis sponsored nearly all the extant CE analyses comparing ZA and denosumab (Table 4). The two analyses supported by Novartis12,16 (the makers of ZA) concluded that, although denosumab was superior to ZA, it was not CE. In contrast, the CE analyses supported Amgen18,19 (the makers of denosumab), concluding that denosumab was CE relative to ZA. Our analysis was independent of any pharmaceutical company and as such it was free of potential pharmaceutical company bias. This bias has been pointed out by others.20
Table 4.
CE Analyses Comparing Monthly ZA, ZA Every 3 Months, and Denosumab
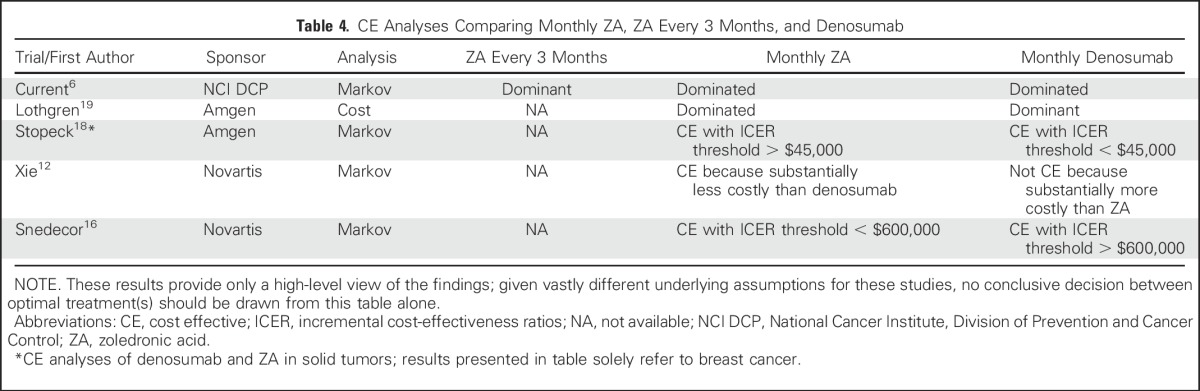
There are substantial differences in underlying model assumptions and study designs of the CE analyses listed in Table 4. For example, the time horizons of the Markov models differed between CE analyses. One CE analysis incorporated the potential to go off treatment, and some CE analyses allowed for improving the quality of life after an SRE, whereas others did not. In short, the underlying differences in these studies make it difficult for direct comparisons, and the CE analyses listed in Table 4 should be interpreted with caution.
It is uncertain what accounts for the higher mean SRE probabilities in the Stopeck et al8 trial (Table 2). One possible explanation is trial design. The Stopeck et al8 trial was a double-blind, placebo-controlled, pharmaceutical company–sponsored trial with international trial sites and a protocol-specified requirement for imaging every 3 months. Thus, detection bias likely led to earlier detection, treatment of SREs, and hence a higher SRE probability.21 In contrast, trial 70604,6 a North American Cooperative Group trial, was without any prespecified imaging requirement, and more than 50% of accruals were from physicians in community practice. The trial design of 70604, although not as scientifically rigorous as the Stopeck et al8 trial, has the advantage of reflecting more real-world scenarios.
Less likely, but still plausible, is the nature of the primary end point, SREs. The development of spinal cord compression and pathologic fractures are hard end points, and when they occur, definitive treatment is rendered. Other SREs rely on physician judgment. For example, whether to radiate skeletal metastases, how to define an impending pathologic fracture, and when to perform surgery or radiation varies among treating physicians. The randomization should take into account physician judgment within each trial, but what if practice patterns and thresholds for treatment differed among physicians between the trials? Given the early detection of SREs with imaging every 3 months, this, too, may have led to higher detection and treatment of SREs.
This CE analysis has several weaknesses. First, we only considered a 2-year time line. The median overall survival of women with metastatic breast cancer is improving,22 especially in women with skeletal metastases only, and in women with human epidermal growth factor receptor 2 overexpressing breast cancers. With longer follow-up, the results might change for denosumab. However, the cost differential is so significant between denosumab and ZA every 3 months that the main conclusions are unlikely to change substantially.
Another limitation is the lack of differentiation between a vertebral and a nonvertebral (hip) fracture. However, none of the major randomized trials evaluating ZA or denosumab3-6, 8 captured data on the particular type of fracture. Because we had no primary source data that differentiated vertebral and nonvertebral fractures, we chose the model by Xie et al12 that used pathologic fracture, regardless of type, as one of the contributors to SREs.
Also not considered were the costs of ONJ and atypical femoral fractures. The overall incidence of ONJ was similar between monthly ZA and denosumab. In the Stopeck et al8 trial, the incidence of ONJ was 2% and 1% in the denosumab and ZA monthly treatment arms, respectively. In the 70604 trial,6 the incidence of ONJ was 2% for the monthly ZA and 1% for ZA every 3 months. The costs of ONJ would not substantially alter the results and conclusions, and we chose to omit these data. There were no atypical femoral fractures observed in either of the trials. Likely, this is related to the 2-year time frame, because atypical fractures increase with longer durations of therapy, and the rarity of this complication.23
Finally, not considered were the costs of the laboratory tests associated with each treatment. The Centers for Medicare and Medicaid Services Current Procedural Terminology codes 96413 and 96401 were the source for the average drug costs and administration costs, respectively. These codes did not include the costs of collecting blood and running the assays for creatinine and calcium. It is likely that if we added the costs of blood drawing and assays for creatinine before administering ZA every 3 months (eight times over 24 months) and calcium tests before monthly denosumab (24 times over 24 months), the costs would be lower with ZA every 3 months. Even if the costs of blood collections and specific tests were included, it would not change the main results and conclusions.
The preference for the routes of administration of these drugs (IV infusion or SQ injection for ZA and denosumab, respectively) was not taken into consideration, because the primary clinical trials did not capture this information. There are other trials that demonstrate SQ injection is overwhelmingly preferred by patients,24 is less costly, and takes less time to administer.25 Preference for SQ injection, however, may be dependent on the individual. For example, for a woman who already has a mediport may prefer the IV route, whereas a woman who does not have a mediport will likely prefer an SQ injection.
In 2011, the ASCO clinical practice guidelines on bone-modifying agents (BMAs) in breast cancer26 stated no preference for one BMA over another. Now that three phase III randomized trials showed that ZA every 3 months is noninferior to monthly ZA,4-6 there is high-level evidence to support ZA every 3 months as an alternative to monthly ZA or monthly denosumab. As such, the 2017 update of BMA in patients with breast cancer with skeletal metastases jointly sponsored by ASCO and Cancer Care Ontario stated that treatment options now include ZA every 3 months, monthly ZA, and monthly denosumab, and there is still insufficient evidence to support the use of one BMA over another.27
This CE analysis shows that despite monthly denosumab being approximately 23% better than monthly ZA in reducing the time to first and subsequent SRE,8 the price differential is nine-fold greater than ZA every 3 months, and cost per SRE avoided for denosumab ranges from $162,918 to $347,655. As we move toward a value-based health care model, ZA every 3 months may be a viable alternative to monthly denosumab when costs are considered.
Footnotes
Supported by the National Cancer Institute of the National Institutes of Health under Grants No. UG1CA189823 (Alliance for Clinical Trials in Oncology Community Oncology Research Program Grant), U10CA180790, U10CA180838, and UG1CA189819. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical trial information: NCT00869206.
Listen to the podcast by Dr Levêque at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Charles L. Shapiro, James P. Moriarty, Bijan J. Borah
Provision of study materials or patients: Charles L. Shapiro, Andrew L. Himelstein, Stephen S. Grubbs
Collection and assembly of data: Charles L. Shapiro, James P. Moriarty, Stacie Dusetzina, Jared C. Foster, Stephen S. Grubbs, Paul J. Novotny
Data analysis and interpretation: Charles L. Shapiro, James P. Moriarty, Stacie Dusetzina, Andrew L. Himelstein, Jared C. Foster, Paul J. Novotny, Bijan J. Borah
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cost-Effectiveness Analysis of Monthly Zoledronic Acid, Zoledronic Acid Every 3 Months, and Monthly Denosumab in Women With Breast Cancer and Skeletal Metastases: CALGB 70604 (Alliance)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Charles L. Shapiro
No relationship to disclose
James P. Moriarty
Research Funding: HalioDx
Stacie Dusetzina
No relationship to disclose
Andrew L. Himelstein
No relationship to disclose
Jared C. Foster
No relationship to disclose
Stephen S. Grubbs
No relationship to disclose
Paul J. Novotny
No relationship to disclose
Bijan J. Borah
No relationship to disclose
REFERENCES
- 1.Wong MH, Stockler MR, Pavlakis N: Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev 2:CD003474, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE: Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12:6243s-6249s, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Kohno N, Aogi K, Minami H, et al. : Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: A randomized, placebo-controlled trial. J Clin Oncol 23:3314-3321, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Amadori D, Aglietta M, Alessi B, et al. : Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): A phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol 14:663-670, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Hortobagyi GN, Van Poznak C, Harker WG, et al. : Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone: The OPTIMIZE-2 randomized clinical trial. JAMA Oncol 3:906-912, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Himelstein AL, Foster JC, Khatcheressian JL, et al. : Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: A randomized clinical trial. JAMA 317:48-58, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gül G, Sendur MA, Aksoy S, et al. : A comprehensive review of denosumab for bone metastasis in patients with solid tumors. Curr Med Res Opin 32:133-145, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Stopeck AT, Lipton A, Body JJ, et al. : Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J Clin Oncol 28:5132-5139, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Bergner R, Diel IJ, Henrich D, et al. : Differences in nephrotoxicity of intravenous bisphosphonates for the treatment of malignancy-related bone disease. Onkologie 29:534-540, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Carter JA, Ji X, Botteman MF: Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res 13:483-496, 2013 [DOI] [PubMed] [Google Scholar]
- 11. U.S. Food and Drug Administration: First-time generic drug approvals March 2013. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/ANDAGenericDrugApprovals/
- 12.Xie J, Diener M, Sorg R, et al. : Cost-effectiveness of denosumab compared with zoledronic acid in patients with breast cancer and bone metastases. Clin Breast Cancer 12:247-258, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Barlev A, Song X, Ivanov B, et al. : Payer costs for inpatient treatment of pathologic fracture, surgery to bone, and spinal cord compression among patients with multiple myeloma or bone metastasis secondary to prostate or breast cancer. J Manag Care Pharm 16:693-702, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lage MJ, Barber BL, Harrison DJ, et al. : The cost of treating skeletal-related events in patients with prostate cancer. Am J Manag Care 14:317-322, 2008 [PubMed] [Google Scholar]
- 15. Quality AfHRa: Using appropriate price indices for analyses of health care expenditures or income across multiple years (MEPS: Medical Expenditure Panel Survey). https://meps.ahrq.gov/
- 16.Snedecor SJ, Carter JA, Kaura S, et al. : Cost-effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin Ther 34:1334-1349, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Bektur C, Nurgozhin T: Cost-effectiveness of denosumab vs. brand or generic zoledronic acid in patients with breast cancer in Kazakhstan. Value Health 17:A773, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Stopeck A, Rader M, Henry D, et al. : Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ 15:712-723, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Lothgren M, Ribnicsek E, Schmidt L, et al. : Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: An analysis for Austria, Sweden and Switzerland. Eur J Hosp Pharm Sci Pract 20:227-231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo K, Lam K, Mittmann N, et al. : Comparing cost-effectiveness analyses of denosumab versus zoledronic acid for the treatment of bone metastases. Support Care Cancer 21:1785-1791, 2013 [DOI] [PubMed] [Google Scholar]
- 21. Cochrane Methods: Assessing risk bias in included studies. http://methods.cochrane.org/bias/assessing-risk-bias-included-studies.
- 22.Sundquist M, Brudin L, Tejler G: Improved survival in metastatic breast cancer 1985-2016. Breast 31:46-50, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Edwards BJ, Bunta AD, Lane J, et al. : Bisphosphonates and nonhealing femoral fractures: Analysis of the FDA Adverse Event Reporting System (FAERS) and international safety efforts: A systematic review from the Research on Adverse Drug Events And Reports (RADAR) project. J Bone Joint Surg Am 95:297-307, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pivot X, Gligorov J, Müller V, et al. : Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: Final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol 25:1979-1987, 2014 [DOI] [PubMed] [Google Scholar]
- 25. doi: 10.1007/s12094-017-1684-4. Lopez-Vivanco G, Salvador J, Diez R, et al: Cost minimization analysis of treatment with intravenous or subcutaneous trastuzumab in patients with HER2-positive breast cancer in Spain. Clin Transl Oncol 10.1007/s12094-017-1684-4 [epub ahead of print on June 2, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Poznak CH, Temin S, Yee GC, et al. : American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol 29:1221-1227, 2011 [DOI] [PubMed] [Google Scholar]
- 27. doi: 10.1200/JOP.2017.027672. Van Poznak CH, Somerfield MR, Barlow WE, et al: The role of bone-modifying agents in metastatic breast cancer: An American Society of Clinical Oncology–Cancer Care Ontario focused guideline update. J Clin Oncol (in press) [DOI] [PubMed] [Google Scholar]