Abstract
Purpose
This study examined the lifestyle and clinical risk factors for lymphedema in a cohort of patients who underwent bilateral breast cancer surgery.
Patients and Methods
Between 2013 and 2016, 327 patients who underwent bilateral breast cancer surgery were prospectively screened for arm lymphedema as quantified by the weight-adjusted volume change (WAC) formula. Arm perometry and subjective data were collected preoperatively and at regular intervals postoperatively. At the time of each measurement, patients completed a risk assessment survey that reported the number of blood draws, injections, blood pressure readings, trauma to the at-risk arm, and number of flights since the previous measurement. Generalized estimating equations were applied to ascertain the association among arm volume changes, clinical factors, and risk exposures.
Results
The cohort comprised 327 patients and 654 at-risk arms, with a median postoperative follow-up that ranged from 6.1 to 68.2 months. Of the 654 arms, 83 developed lymphedema, defined as a WAC ≥ 10% relative to baseline. On multivariable analysis, none of the lifestyle risk factors examined through the risk assessment survey were significantly associated with increased WAC. Multivariable analysis demonstrated that having a body mass index ≥ 25 kg/m2 at the time of breast cancer diagnosis (P = .0404), having undergone axillary lymph node dissection (P = .0464), and receipt of adjuvant chemotherapy (P = .0161) were significantly associated with increased arm volume.
Conclusion
Blood pressure readings, blood draws, injections, and number or duration of flights were not significantly associated with increases in arm volume in this cohort. These findings may help to guide patient education about lymphedema risk reduction strategies for those who undergo bilateral breast cancer surgery.
INTRODUCTION
Although surgical and targeted treatments for breast cancer have improved survival, treatment complications remain a significant concern for patients. Breast cancer–related lymphedema (BCRL) is one complication caused by damage to lymph nodes through surgical intervention and/or radiation, which may interrupt the circulation of lymph fluid and precipitate edema of the arm, breast, or trunk.1-3 Associated symptoms, such as decreased arm functionality, pain, heaviness, changes in skin quality, and high rates of infection (eg, cellulitis), may compromise overall quality of life (QOL) and are significant sources of distress in at-risk patients.4,5 Survivors of breast cancer have a lifelong risk of developing lymphedema.6-8
The etiologic factors that contribute to the development of BCRL and potential lifestyle strategies aimed at minimizing lymphedema risk after breast cancer surgery are frequent points of discussion and controversy in the literature.9 Well-defined risk factors include axillary lymph node dissection (ALND), regional lymph node radiation (RLNR), and high body mass index (BMI), with mastectomy, chemotherapy, and older age at diagnosis demonstrating an association on occasion.7,8,10-13 Why some patients with the same demographic, surgical, and treatment-related characteristics develop lymphedema and others do not remains unclear. This lack of knowledge has prompted speculation about whether lifestyle-related risk exposures play a causative role.14 The position statement of the National Lymphedema Network15 outlines precautionary recommendations intended to reduce the risk of developing lymphedema in at-risk breast cancer survivors, which include the avoidance of trauma, temperature extremes, skin infections, venipuncture (eg, blood draws, intravenous infusions), and limb constriction (eg, blood pressure readings) on the ipsilateral arm. These guidelines play a significant role in patient education with regard to post-treatment lifestyle but have not evolved in the face of ongoing surgical and treatment advancements.9
With limited high-level evidence to support or refute the risk of blood pressure measurements and needle punctures in lymphedema development, patients may experience distress because of their risk for BCRL and their need to avoid such medical procedures throughout their life.9,16 These restrictions may prove especially problematic in patients who receive bilateral treatment of breast cancer and who are at bilateral risk for BCRL because optimal health care necessitates procedures in the at-risk arm, which make the evaluation of whether there is a sound scientific basis for precautionary behaviors imperative. Currently, > 20% of all patients with breast cancer undergo bilateral surgery,17-19 and a pressing need exists to provide high-level data and reassurance to these patients about the safety and efficacy of using their arms for medical procedures. Thus, we examined the association between lymphedema and the commonly discussed lifestyle risk exposures for BCRL, including blood pressure readings, blood draws, injections, infusions, and air travel, in a prospective cohort of patients who underwent bilateral breast cancer surgery.
PATIENTS AND METHODS
Study Design
Lymphedema screening program.
With the approval of the Partners Healthcare Institutional Review Board, we used perometry to prospectively screen women with newly diagnosed breast cancer for lymphedema at our institution from 2010 to 2016.20 The perometer is a reliable optoelectronic system that uses infrared lamp light-receiver pairs contained within a frame.21 All patients underwent a preoperative baseline measurement22 and were measured postoperatively and approximately every 3 to 8 months at regular follow-up oncology visits or more frequently at the request of the patient. Patients were also provided at each screening visit the Lymphedema Evaluation Following Treatment of Breast Cancer questionnaire,23 which assesses QOL issues, changes in upper-extremity functionality and use, and fear perception and avoidance behaviors. Lymphedema diagnosis was contingent upon both clinical examination and perometry.24-26 The protocol for prospective lymphedema screening has been well established at our institution.20
Risk assessment survey.
On the basis of a component of the lymphedema screening program, patients were asked to complete a risk assessment survey at the time of every follow-up arm measurement. They reported risk exposures, including the number of blood pressure readings, blood draws, injections, trauma to the at-risk arm (eg, fractures), and number and length of flights since their previous visit. Patients who underwent bilateral breast cancer surgery began completing the risk assessment survey after 2013. The analysis includes patients who underwent bilateral surgery and completed the risk assessment survey during at least one follow-up appointment. Data was incorporated in which both a perometer measurement was obtained and a survey completed.
Quantifying arm volume changes.
To quantitatively determine the percentage arm volume change compared with preoperative baseline, the previously validated weight-adjusted volume change (WAC) formula was used [WAC = (A2W1) / (W2A1) – 1] for patients who undergo bilateral breast surgery and thereby lack a contralateral control arm. A1 is the preoperative and A2 the postoperative at-risk arm volume, and W1 and W2 are the patient’s weight that corresponds to these time points. The WAC formula allows for the calculation of independent left and right arm volumes and takes into account changes in the patient’s weight, which can contribute to increases or decreases in arm volume unrelated to lymphedema.27 Lymphedema was defined as a WAC of ≥ 10% that occurred > 3 months after surgery.28,29
Patient Population
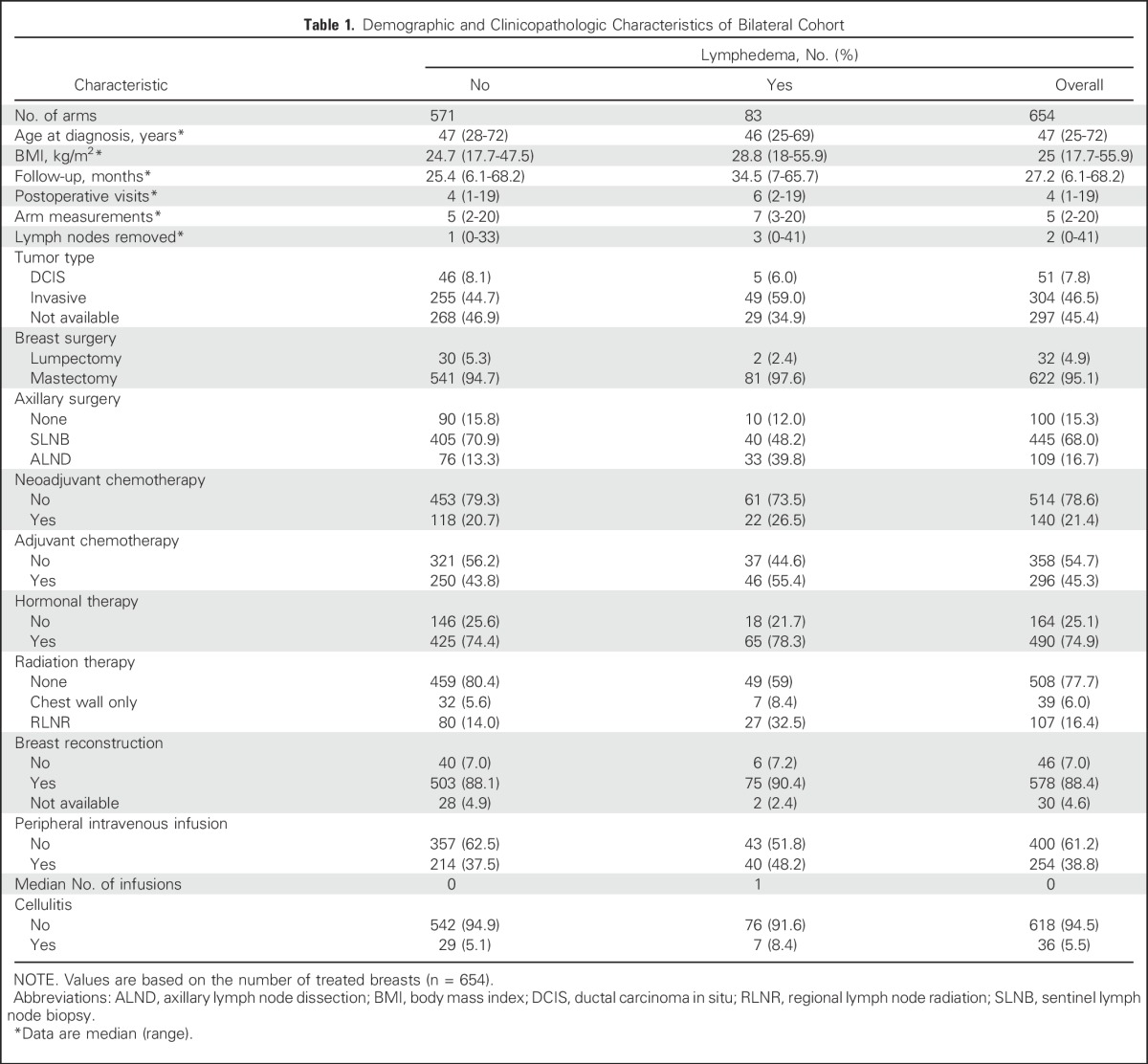
This study included 327 patients who underwent bilateral surgery for a diagnosis of primary breast cancer between 2013 and 2016. A total of 654 at-risk arms were evaluated; 622 of these represented mastectomies (95.1%) and 32 lumpectomies (4.9%), of which 54% (355 of 654) were performed for the treatment of breast cancer and 46% (297 of 654) prophylactically. The number of at-risk arms was 654 because each breast was considered individually. Patients who undergo prophylactic contralateral mastectomy at our hospital typically have a sentinel lymph node biopsy (SLNB) performed on their contralateral breast to stage an occult breast cancer, which thus puts both arms at risk for lymphedema.
All patients had a preoperative perometer measurement and at least 6 months of postoperative follow-up. Only measurements that occurred ≥ 3 months after surgery were used to determine the incidence of BCRL because arm volume increases recorded within 3 months of surgery may be attributed to transient postsurgical swelling.2,14 Demographic, clinicopathologic, and treatment-related characteristics were obtained through medical record review. To avoid potential confounding factors, measurements obtained after local recurrence or distant metastases were excluded.
Statistical Analysis
Generalized estimating equations were used to assess the association among WAC measured as a continuous variable, clinical risk factors, and nonprecautionary behaviors. These models account for correlation within the same patient and for patients who underwent bilateral breast surgery, on the same side of the body. Longitudinal WAC measurement was used as the response variable. At each measurement, the number of blood draws, injections, blood pressure readings, and traumas were analyzed as binary variables categorized according to whether patients reported having had one or more events versus none. Number of flights and hours spent on flights in total since the last follow-up were analyzed as dichotomous and trichotomous variables, respectively. Univariable model results were used to estimate and plot the mean WAC within each subgroup for categorical clinical and risk factors along with the 95% CI for the mean and the P value associated with the comparison of means. The multivariable model was chosen in a backward selection fashion by starting with a full model that included all variables that had a P < .10 in the univariable analysis and variables considered to be known confounders and by removing one variable at a time until only variables with P < .05 remained.
The Kaplan-Meier method was used to estimate the 2-year cumulative incidence of lymphedema. Statistical analyses were conducted with SAS 9.4 software (SAS Institute, Cary, NC) and R programming language (www.R-project.org).
RESULTS
Patient Population
Median postoperative follow-up time in the cohort was 27 months (range, 6 to 68 months), whereas median age at diagnosis was 47 years (range, 25 to 72 years). Median BMI was 25 kg/m2 (range, 18 to 56 kg/m2). Medical record review indicated that of 654 treated breasts, 36 episodes of cellulitis in the breast and/or the arm required treatment with oral or intravenous antibiotics (Table 1). The 2-year cumulative incidence of BCRL, defined as WAC ≥ 10%,28,29 was determined to be 11.8% (95% CI, 9.4% to 14.8%).
Table 1.
Demographic and Clinicopathologic Characteristics of Bilateral Cohort
Precautionary Behaviors
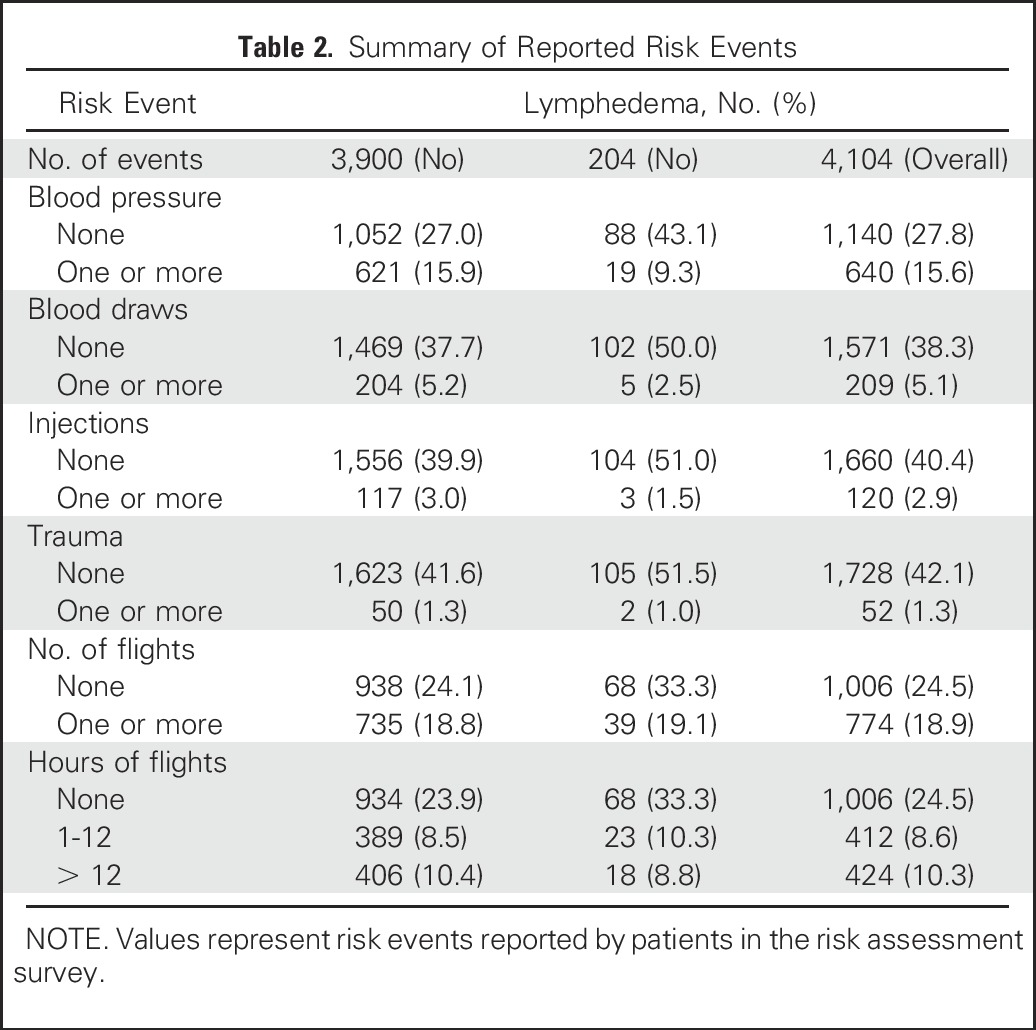
Through the risk assessment survey, 1,795 events were reported over 4,104 responses. Of the total of 4,102 responses, 209 patients (5.1%) reported having one or more blood draws in their affected arms since their last measurement, 640 (15.6%) reported blood pressure readings, 120 (2.9%) reported injections, and 52 (1.3%) reported trauma events (eg, bruising, fractures). Nineteen percent of responses (n = 774) indicated one or more flights since the patients’ last measurement (Table 2).
Table 2.
Summary of Reported Risk Events
Univariable and Multivariable Analysis Results
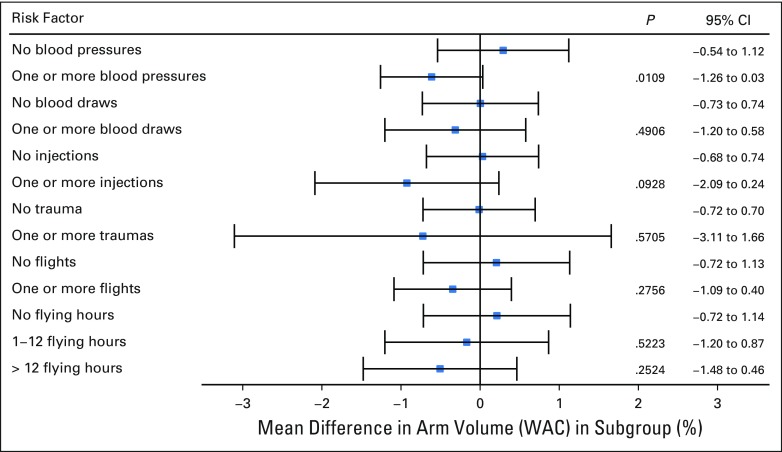
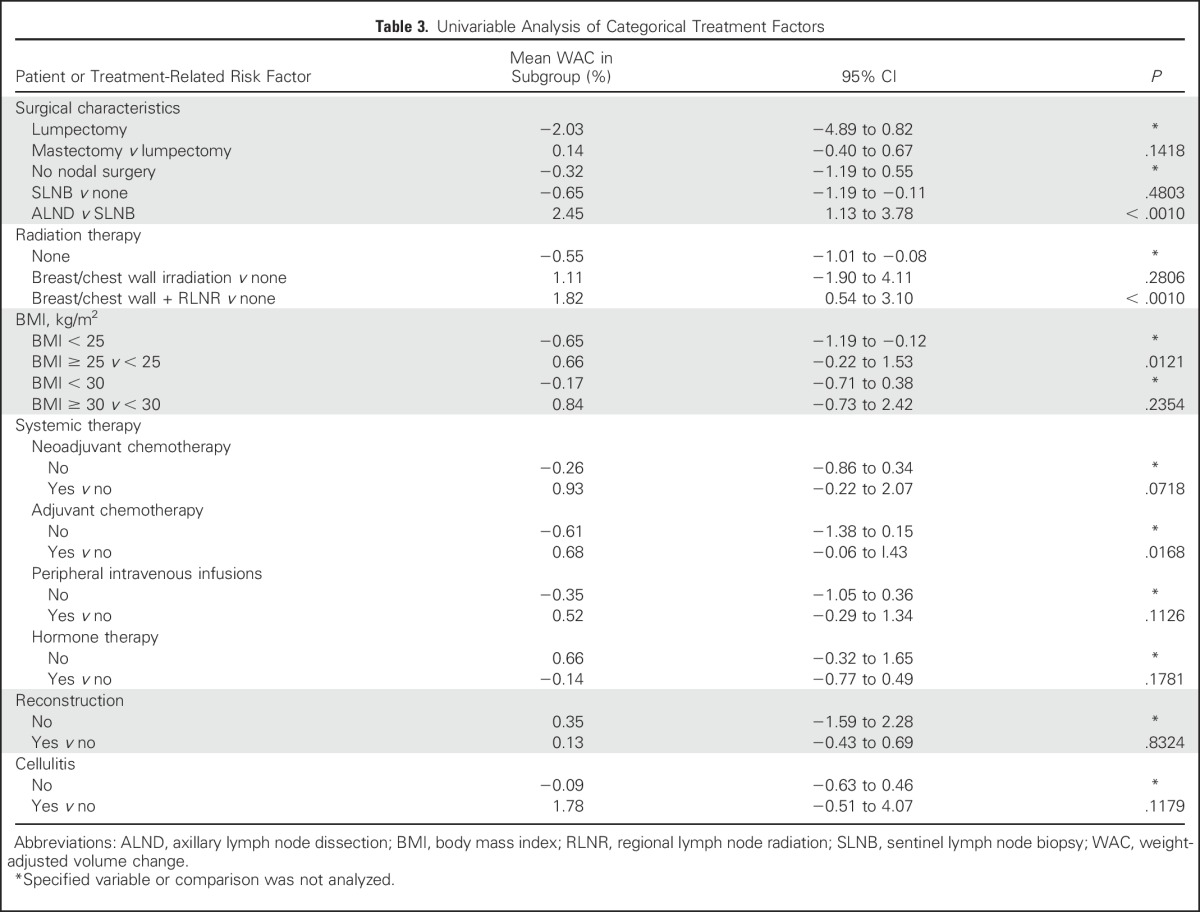
By univariable analysis, no significant association was found between an increased WAC and having undergone one or more blood draws versus no blood draws (P = .4906), one or more injections versus no injections (P = .0928), one or more incidents of trauma to the at-risk arms versus no trauma (P = .5705), and number (P = .2756) or duration (1 to 12 hours [P = .5223] and ≥ 12 hours [P = .2524]) of flights versus none (Fig 1). In addition, cellulitis infections were not found to be significantly associated with increased WAC (P = .1179; Table 3). Factors significantly associated with increased WAC were ALND compared with SLNB (P < .001), breast/chest wall radiation and RLNR compared with no radiation (P < .001), BMI ≥ 25 kg/m2 compared with < 25 kg/m2 (P = .0121), and adjuvant chemotherapy versus no adjuvant chemotherapy (P = .0168; Table 3). Univariable analysis showed that having one or more blood pressure measurements versus none was significantly associated with decreased WAC (P = .0109; 95% CI, −1.26 to 0.03; Fig 1); this was no longer significant upon multivariable analysis.
Fig 1.
Univariable analysis. WAC, weight-adjusted volume change.
Table 3.
Univariable Analysis of Categorical Treatment Factors
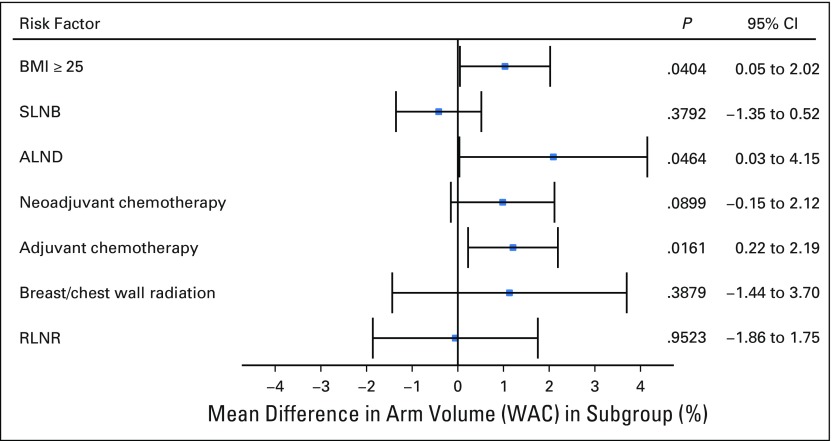
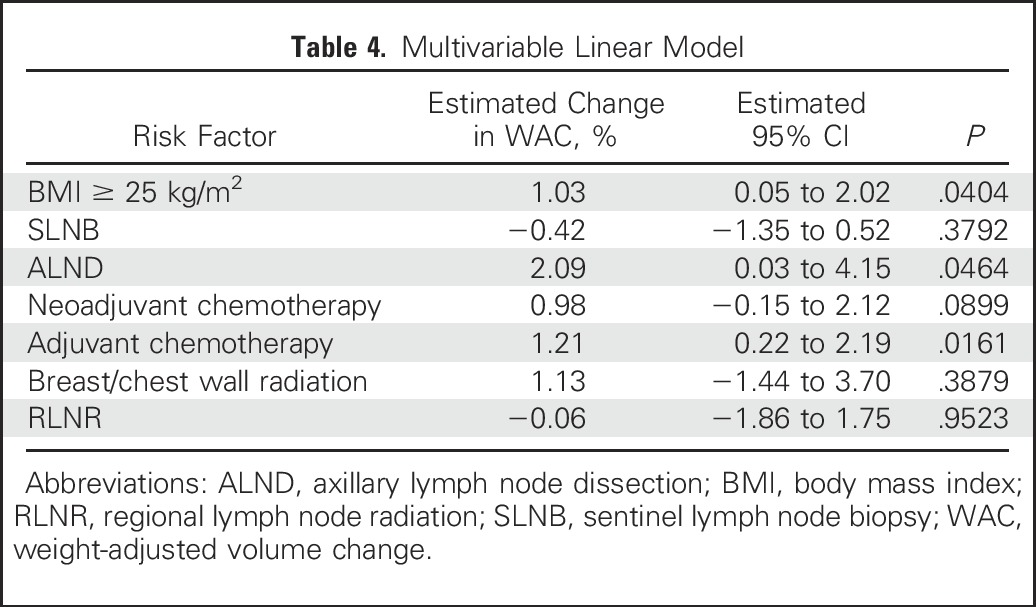
The only variables that retained significance upon multivariable analysis were BMI ≥ 25 kg/m2 (P = .0404), ALND (P = .0464), and adjuvant chemotherapy (P = .0161). None of the risk exposures analyzed were significantly associated with WAC in the multivariable analysis (Fig 2; Table 4).
Fig 2.
Multivariable analysis. ALND, axillary lymph node dissection; BMI, body mass index; RLNR, regional lymph node radiation; SLNB, sentinel lymph node biopsy; WAC, weight-adjusted volume change.
Table 4.
Multivariable Linear Model
DISCUSSION
This study examined the association between the risk of lymphedema in patients who underwent bilateral breast cancer surgery and lifestyle/clinical risk exposures for lymphedema. Precautionary strategies aimed at minimizing BCRL risk after breast cancer surgery are frequent discussion points in the literature. Although risk reduction guidelines as described in the National Lymphedema Network position statement15 are based on clinical reasoning, limited high-level research supports or refutes their effectiveness. Studies that sought answers about risk reduction guideline utility are restricted in scope and are predominantly small, retrospective, and single-site reports with cohorts that underwent mostly ALND.9,30-32 Historically, studies demonstrated that women who undergo bilateral ALND do not have an increased risk of BCRL compared with those with unilateral lymph node dissection.33,34
To examine the current evidence, our team carried out a comprehensive review of 31 studies that examined the association between these exposures and BCRL. Only eight were prospective cohort studies,10,14,35-40 among which four demonstrated a statistically significant correlation between BCRL and commonly reported lifestyle-based risk factors, namely infections,10,14 sauna use,39 and skin puncture.40 In a small number of patients who developed lymphedema after skin puncture, Clark et al40 found a correlation between skin puncture and lymphedema in patients who underwent ALND (n = 188). The authors did not look into the temporal relationship between skin puncture and lymphedema development, so the association could not be used to identify whether the punctures themselves were a risk factor for swelling. Clark et al noted in their discussion that replication of their work with a larger sample that includes patients with SLNB is needed. All but one14 of the studies in our comprehensive review had cohorts that underwent predominantly ALND, which made them higher-risk populations because ALND contributes to an approximately fourfold increased incidence of lymphedema compared with SLNB.8 Of note, other studies have demonstrated that ipsilateral skin puncture does not represent a risk factor for lymphedema; furthermore, surgical procedures on the at-risk arm have been shown to not contribute to permanent arm swelling.14,39,41,42
With regard to limb constriction through the use of blood pressure cuffs, no high-level scientific evidence suggests a relationship between ipsilateral blood pressure measurements and arm swelling or long-term adverse effects of tourniquet use on limb volume.43 Surveyed breast oncology surgeons and clinicians have regularly demonstrated a willingness to use tourniquets on the ipsilateral arm during elective hand surgeries.44,45 This lack of evidential support extends to air travel. Among eight studies that analyzed flight risk to date, only one questionnaire-based study demonstrated that air travel increases the risk for lymphedema.46 Several other objective studies have not identified a causative link between flights and lymphedema and have not demonstrated whether prophylactic compression confers a benefit to at-risk women.11,14,36,40,46,47
In a recent prospective analysis, Ferguson et al14 assessed whether lifestyle and behavioral risk exposures, including air travel, blood draws, injections, blood pressure measurements, and cellulitis infections, on the ipsilateral arm confer a risk for the development of lymphedema. The analysis included 632 patients who had undergone unilateral or bilateral surgery, each with a preoperative baseline measurement and an overall median follow-up of 24 months. By multivariable analysis, none of the lifestyle risk factors examined were associated with increased arm volume. The only factors found to be significantly associated with arm volume increase were a BMI ≥ 25 kg/m2, ALND, RLNR, and cellulitis. Limitations of the study were a median follow-up time of 24 months, low incidence of risk events, potential for recall bias, and lack of information about patients’ receipt of physical therapy.
In the current cohort of patients who underwent bilateral breast cancer surgery, although the univariable analysis showed that one or more blood pressure measurements had a significantly lower WAC than no blood pressure measurements, neither group independently demonstrated a significant arm volume change from baseline. Furthermore, the significant difference was no longer observed upon multivariable analysis. The factors found to be significantly associated with arm swelling by multivariable analysis were BMI ≥ 25 kg/m2 at diagnosis, ALND, and adjuvant chemotherapy. High BMI and ALND are well-substantiated risk factors for BCRL in the literature.5,7,8,10,11,23,48-53 In the current cohort, adjuvant chemotherapy also was found to be a significant risk factor for the development of arm swelling, which may be a reflection of transient fluctuations in arm swelling as a result of taxane-based chemotherapy regimens that have been found to contribute to mild arm swelling.54 Limitations of this study are similar to those of Ferguson et al,14 namely, the potential for recall bias as a result of the provision of the risk assessment survey at inconsistent intervals and low incidence of risk events. Our median postoperative follow-up, however, was longer at 25.4 months for patients who did not develop lymphedema and even longer (34.5 months) for patients who developed lymphedema. Furthermore, our study collected data on breast and/or arm cellulitis, which may play a causative role in the development or worsening of arm swelling.55-60 We found that cellulitis infections are not significantly associated with the development of BCRL. Some of these patients may not have had readings right around the time of their cellulitis (given the 3- to 8-month interval between measurements) and/or may have had their swelling recede as the infection cleared or as they received treatment by a certified lymphedema therapist before their next perometry reading. As Ferguson et al and other studies10,59-61 have found, cellulitis is significantly associated with the development of arm swelling, and the importance of maintaining skin integrity and avoiding infection is critical.
Unlike the study by Ferguson et al14 that only had a small proportion of patients who underwent bilateral breast cancer surgery, the current study represents an analysis of a large cohort of patients at bilateral risk for BCRL to determine whether an association between risk exposures and the development of lymphedema in this higher risk population exists. With consideration of current developments in the surgical treatment of breast cancer, specifically the increasing trend in younger women who undergo contralateral prophylactic mastectomy and that approximately 20% of all patients with breast cancer undergo bilateral mastectomy,17-19 we specifically analyzed this risk in a population where the practice of precautionary behaviors may be difficult to implement. For example, in patients with bilateral axillary surgery, if at-risk arms are to be avoided, blood pressure readings may need to be taken in the leg, which introduces potential inaccuracies.62
Interpretation of Ferguson et al14 has prompted controversy and discussion. Some practitioners misinterpreted the study as evidence to support changes in clinical practice and the complete dismissal of all such precautions.63-68 Because the results of the current study also demonstrate a lack of association between these risk exposures and lymphedema development, we strongly emphasize that we do not consider these findings as sufficient data to do away with current precautions provided to patients, and we do not support changing clinical practice with regard to risk reduction patient education. Our goal is to add to the research base and bring reasonable doubt to current guidelines to ensure that any recommendations we give to patients are well-substantiated and individualized to prevent unnecessary distress in light of historical research and improved treatment modalities. Currently, the proportion of patients who undergo SLNB for the assessment of lymph node status in clinically node-negative breast cancer is increasing, and this low-risk group now comprises approximately 80% of this population.69 Patients at different risk for BCRL should be provided different precautionary guidelines, and we hope to see a move toward a risk-adjusted approach in the application of these guidelines pending high-level research. This topic must be further studied prospectively through large-scale clinical trials. The goal of our work on precautionary measures is to foster a dialogue about these guidelines, but current guidelines should remain the standard of care until definitive evidence is available. The generation of new studies and collaborative discussion will allow for progress in improving patient QOL throughout survivorship.
Footnotes
Supported by National Cancer Institute grants R01CA139118 and P50CA089393 (to A.G.T.) and the Adele McKinnon Research Fund for Breast Cancer-Related Lymphedema.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Clinical trial information: NCT01521741.
AUTHOR CONTRIBUTIONS
Conception and design: Maria S. Asdourian, Meyha N. Swaroop, Hoda E. Sayegh, Cheryl L. Brunelle, Melissa N. Skolny, Alphonse G. Taghian
Collection and assembly of data: Maria S. Asdourian, Meyha N. Swaroop, Hoda E. Sayegh, Melissa N. Skolny, Alphonse G. Taghian
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Association Between Precautionary Behaviors and Breast Cancer–Related Lymphedema in Patients Undergoing Bilateral Surgery
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Maria S. Asdourian
No relationship to disclose
Meyha N. Swaroop
No relationship to disclose
Hoda E. Sayegh
No relationship to disclose
Cheryl L. Brunelle
No relationship to disclose
Amir I. Mina
No relationship to disclose
Hui Zheng
No relationship to disclose
Melissa N. Skolny
No relationship to disclose
Alphonse G. Taghian
Honoraria: UpToDate
Consulting or Advisory Role: VisionRT
REFERENCES
- 1.Erickson VS, Pearson ML, Ganz PA, et al. : Arm edema in breast cancer patients. J Natl Cancer Inst 93:96-111, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Armer JM, Radina ME, Porock D, et al. : Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res 52:370-379, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Shih YC, Xu Y, Cormier JN, et al. : Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: A 2-year follow-up study. J Clin Oncol 27:2007-2014, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Ridner SH: Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer 13:904-911, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Cormier JN, Xing Y, Zaniletti I, et al. : Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology 42:161-175, 2009 [PMC free article] [PubMed] [Google Scholar]
- 6.Armer JM, Stewart BR: Post-breast cancer lymphedema: Incidence increases from 12 to 30 to 60 months. Lymphology 43:118-127, 2010 [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai RJ, Dennis LK, Lynch CF, et al. : The risk of developing arm lymphedema among breast cancer survivors: A meta-analysis of treatment factors. Ann Surg Oncol 16:1959-1972, 2009 [DOI] [PubMed] [Google Scholar]
- 8.DiSipio T, Rye S, Newman B, et al. : Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol 14:500-515, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Asdourian MS, Skolny MN, Brunelle C, et al. : Precautions for breast cancer-related lymphoedema: Risk from air travel, ipsilateral arm blood pressure measurements, skin puncture, extreme temperatures, and cellulitis. Lancet Oncol 17:e392-e405, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Bevilacqua JL, Kattan MW, Changhong Y, et al. : Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol 19:2580-2589, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Swenson KK, Nissen MJ, Leach JW, et al. : Case-control study to evaluate predictors of lymphedema after breast cancer surgery. Oncol Nurs Forum 36:185-193, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Warren LE, Miller CL, Horick N, et al. : The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: A prospective cohort study. Int J Radiat Oncol Biol Phys 88:565-571, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jammallo LS, Miller CL, Singer M, et al. : Impact of body mass index and weight fluctuation on lymphedema risk in patients treated for breast cancer. Breast Cancer Res Treat 142:59-67, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson CM, Swaroop MN, Horick N, et al. : Impact of ipsilateral blood draws, injections, blood pressure measurements, and air travel on the risk of lymphedema for patients treated for breast cancer. J Clin Oncol 34:691-698, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Lymphedema Network: Lymphedema risk reduction practices, 2012. http://www.lymphnet.org/pdfDocs/nlnriskreduction.pdf.
- 16.Nielsen I, Gordon S, Selby A: Breast cancer-related lymphoedema risk reduction advice: A challenge for health professionals. Cancer Treat Rev 34:621-628, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Tuttle TM, Habermann EB, Grund EH, et al. : Increasing use of contralateral prophylactic mastectomy for breast cancer patients: A trend toward more aggressive surgical treatment. J Clin Oncol 25:5203-5209, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Mahmood U, Hanlon AL, Koshy M, et al. : Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann Surg Oncol 20:1436-1443, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Kummerow KL, Du L, Penson DF, et al. : Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 150:9-16, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Brunelle C, Skolny M, Ferguson C, et al. : Establishing and sustaining a prospective screening program for breast cancer-related lymphedema at the Massachusetts General Hospital: Lessons learned. J Pers Med 5:153-164, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tierney S, Aslam M, Rennie K, et al. : Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg 12:412-417, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Sun F, Skolny MN, Swaroop MN, et al. : The need for preoperative baseline arm measurement to accurately quantify breast cancer-related lymphedema. Breast Cancer Res Treat 157:229-240, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Jammallo LS, Miller CL, Horick NK, et al. : Factors associated with fear of lymphedema after treatment for breast cancer. Oncol Nurs Forum 41:473-483, 2014 [DOI] [PubMed] [Google Scholar]
- 24.O’Toole J, Jammallo LS, Skolny MN, et al. : Lymphedema following treatment for breast cancer: A new approach to an old problem. Crit Rev Oncol Hematol 88:437-446, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Toole JA, Ferguson CM, Swaroop MN, et al. : The impact of breast cancer-related lymphedema on the ability to perform upper extremity activities of daily living. Breast Cancer Res Treat 150:381-388, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Miller CL, Specht MC, Skolny MN, et al. : Sentinel lymph node biopsy at the time of mastectomy does not increase the risk of lymphedema: Implications for prophylactic surgery. Breast Cancer Res Treat 135:781-789, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller CL, Specht MC, Horick N, et al. : A novel, validated method to quantify breast cancer-related lymphedema (BCRL) following bilateral breast surgery. Lymphology 46:64-74, 2013 [PubMed] [Google Scholar]
- 28.Armer JM, Stewart BR: A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol 3:208-217, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Armer JM, Stewart BR, Shook RP: 30-month post-breast cancer treatment lymphoedema. J Lymphoedema 4:14-18, 2009 [PMC free article] [PubMed] [Google Scholar]
- 30.Cemal Y, Pusic A, Mehrara BJ: Preventative measures for lymphedema: Separating fact from fiction. J Am Coll Surg 213:543-551, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng CT, Deitch JM, Haines IE, et al. : Do medical procedures in the arm increase the risk of lymphoedema after axillary surgery? A review. ANZ J Surg 84:510-514, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Jakes AD, Twelves C: Breast cancer-related lymphoedema and venepuncture: A review and evidence-based recommendations. Breast Cancer Res Treat 154:455-461, 2015 [DOI] [PubMed] [Google Scholar]
- 33. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2776::aid-cncr25>3.0.co;2-v. Petrek JA, Heelan MC: Incidence of breast carcinoma-related lymphedema. Cancer 83:2776-2781, 1998 (suppl S12B) [DOI] [PubMed] [Google Scholar]
- 34.Loudon L, Petrek J: Lymphedema in women treated for breast cancer. Cancer Pract 8:65-71, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Kilbreath SL, Ward LC, Lane K, et al. : Effect of air travel on lymphedema risk in women with history of breast cancer. Breast Cancer Res Treat 120:649-654, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Chang TS, Han LY, Gan JL, et al. : Microwave: An alternative to electric heating in the treatment of peripheral lymphedema. Lymphology 22:20-24, 1989 [PubMed] [Google Scholar]
- 37.Liu NF, Olszewski W: The influence of local hyperthermia on lymphedema and lymphedematous skin of the human leg. Lymphology 26:28-37, 1993 [PubMed] [Google Scholar]
- 38. doi: 10.1097/00000637-199606000-00003. Gan JL, Li SL, Cai RX, et al: Microwave heating in the management of postmastectomy upper limb lymphedema. Ann Plast Surg 36:576-580, 1996; discussion 580-581. [DOI] [PubMed] [Google Scholar]
- 39.Showalter SL, Brown JC, Cheville AL, et al. : Lifestyle risk factors associated with arm swelling among women with breast cancer. Ann Surg Oncol 20:842-849, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark B, Sitzia J, Harlow W: Incidence and risk of arm oedema following treatment for breast cancer: A three-year follow-up study. QJM 98:343-348, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Dawson WJ, Elenz DR, Winchester DP, et al. : Elective hand surgery in the breast cancer patient with prior ipsilateral axillary dissection. Ann Surg Oncol 2:132-137, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Hershko DD, Stahl S: Safety of elective hand surgery following axillary lymph node dissection for breast cancer. Breast J 13:287-290, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Assmus H, Staub F: Postmastectomy lymphedema and carpal tunnel syndrome. Surgical considerations and advice for patients [in German]. Handchir Mikrochir Plast Chir 36:237-240, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Fulford D, Dalal S, Winstanley J, et al. : Hand surgery after axillary lymph node clearance for breast cancer: Contra-indication to surgery? Ann R Coll Surg Engl 92:573-576, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gharbaoui IS, Netscher DT, Thornby J, et al. : Safety of upper extremity surgery after prior treatment for ipsilateral breast cancer: Results of an American Society for Surgery of the Hand membership survey and literature review. J Am Soc Surg Hand 5:232-238, 2005 [Google Scholar]
- 46.Casley-Smith JR, Casley-Smith JR: Lymphedema initiated by aircraft flights. Aviat Space Environ Med 67:52-56, 1996 [PubMed] [Google Scholar]
- 47.Graham PH: Compression prophylaxis may increase the potential for flight-associated lymphoedema after breast cancer treatment. Breast 11:66-71, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Mak SS, Yeo W, Lee YM, et al: Risk factors for the initiation and aggravation of lymphoedema after axillary lymph node dissection for breast cancer. Hong Kong Med J 15:8-12, 2009 (suppl 4) [PubMed] [Google Scholar]
- 49.Park JH, Lee WH, Chung HS: Incidence and risk factors of breast cancer lymphoedema. J Clin Nurs 17:1450-1459, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Norman SA, Localio AR, Kallan MJ, et al. : Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomarkers Prev 19:2734-2746, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bar Ad V, Cheville A, Solin LJ, et al. : Time course of mild arm lymphedema after breast conservation treatment for early-stage breast cancer. Int J Radiat Oncol Biol Phys 76:85-90, 2010 [DOI] [PubMed] [Google Scholar]
- 52.McLaughlin SA, Wright MJ, Morris KT, et al. : Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J Clin Oncol 26:5213-5219, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrek JA, Senie RT, Peters M, et al. : Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 92:1368-1377, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Swaroop MN, Ferguson CM, Horick NK, et al. : Impact of adjuvant taxane-based chemotherapy on development of breast cancer-related lymphedema: Results from a large prospective cohort. Breast Cancer Res Treat 151:393-403, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mallon E, Powell S, Mortimer P, et al. : Evidence for altered cell-mediated immunity in postmastectomy lymphoedema. Br J Dermatol 137:928-933, 1997 [PubMed] [Google Scholar]
- 56.Woo PC, Lum PN, Wong SS, et al. : Cellulitis complicating lymphoedema. Eur J Clin Microbiol Infect Dis 19:294-297, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Al-Niaimi F, Cox N: Cellulitis and lymphoedema: A vicious cycle. J Lymphoedema 4:38-42, 2009 [Google Scholar]
- 58.Hughes LL, Styblo TM, Thoms WW, et al. : Cellulitis of the breast as a complication of breast-conserving surgery and irradiation. Am J Clin Oncol 20:338-341, 1997 [DOI] [PubMed] [Google Scholar]
- 59.Soran A, D’Angelo G, Begovic M, et al. : Breast cancer-related lymphedema—What are the significant predictors and how they affect the severity of lymphedema? Breast J 12:536-543, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Indelicato DJ, Grobmyer SR, Newlin H, et al. : Delayed breast cellulitis: An evolving complication of breast conservation. Int J Radiat Oncol Biol Phys 66:1339-1346, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Mak SS, Yeo W, Lee YM, et al. : Predictors of lymphedema in patients with breast cancer undergoing axillary lymph node dissection in Hong Kong. Nurs Res 57:416-425, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Malhotra A, Cohen D, Syms C, et al. : Blood pressure changes in the leg on standing. J Clin Hypertens (Greenwich) 4:350-354, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahn S, Port ER: Lymphedema precautions: Time to abandon old practices? J Clin Oncol 34:655-658, 2016 [DOI] [PubMed] [Google Scholar]
- 64.Nudelman J: Do no harm: Lymphedema risk reduction behaviors. J Clin Oncol 34:3109-3110, 2016 [DOI] [PubMed] [Google Scholar]
- 65.Asdourian MS, Skolny MN, Brunelle C, et al. : Reply to J. Nudelman. J Clin Oncol 34:3111-3112, 2016 [DOI] [PubMed] [Google Scholar]
- 66. Stout NL: #Lymphchat4: Risk reduction (recap) [National Lymphedema Network E-Channel Twitter Chat]. https://storify.com/lymphnet/new-story-559d375c04ba787972fd8f8e.
- 67.Nudelman J: Debunking lymphedema risk-reduction behaviors: Risky conclusions. Lymphat Res Biol 14:124-126, 2016 [DOI] [PubMed] [Google Scholar]
- 68. doi: 10.1089/lrb.2017.0016. Brunelle CL, Swaroop MN, Asdourian MS, et al: Precautionary behaviors and breast cancer-related lymphedema. Lymphat Res Biol 10.1089/lrb.2017.0016 [epub ahead of print on September 7, 2017] [DOI] [PubMed] [Google Scholar]
- 69.Donker M, van Tienhoven G, Straver ME, et al. : Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 15:1303-1310, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]