Abstract
Purpose
Early cardiac toxicity is a risk associated with adjuvant chemotherapy plus trastuzumab. However, objective measures of cardiac function and health-related quality of life are lacking in long-term follow-up of patients who remain cancer free after completion of adjuvant treatment.
Patients and Methods
Patients in NSABP Protocol B-31 received anthracycline and taxane chemotherapy with or without trastuzumab for adjuvant treatment of node-positive, human epidermal growth factor receptor 2–positive early-stage breast cancer. A long-term follow-up assessment was undertaken for patients who were alive and disease free, which included measurement of left ventricular ejection fraction by multigated acquisition scan along with patient-reported outcomes using the Duke Activity Status Index (DASI), the Medical Outcomes Study questionnaire, and a review of current medications and comorbid conditions.
Results
At a median follow-up of 8.8 years among eligible participants, five (4.5%) of 110 in the control group and 10 (3.4%) of 297 in the trastuzumab group had a > 10% decline in left ventricular ejection fraction from baseline to a value < 50%. Lower DASI scores correlated with age and use of medications for hypertension, cardiac conditions, diabetes, and hyperlipidemia, but not with whether patients had received trastuzumab.
Conclusion
In patients without underlying cardiac disease at baseline, the addition of trastuzumab to adjuvant anthracycline and taxane-based chemotherapy does not result in long-term worsening of cardiac function, cardiac symptoms, or health-related quality of life. The DASI questionnaire may provide a simple and useful tool for monitoring patient-reported changes that reflect cardiac function.
INTRODUCTION
NSABP (National Surgical Adjuvant Breast and Bowel Project) Protocol B-31 tested whether the addition of the monoclonal antibody trastuzumab to standard paclitaxel after doxorubicin and cyclophosphamide (AC) chemotherapy would improve disease-free or overall survival in patients with human epidermal growth factor receptor 2 (HER2)–positive breast cancer. Planned combined efficacy joint analysis with the similar NCCTG (North Central Cancer Treatment Group) N9831 trial demonstrated a 10-year overall survival of 84% with a hazard ratio of 0.63 with the addition of the antibody to adjuvant chemotherapy.1 Cardiotoxicity was recognized as an important potential toxicity in the design of B-31,2,3 and with 7 years of follow-up, the cumulative incidence of severe cardiac events was significantly increased (4.0% v 1.3%) with the addition of trastuzumab. However, severe cardiotoxicity seemed to be an early event, because only two cases of congestive heart failure (CHF) occurred > 2 years after initiation of trastuzumab.4
Little is known about the cardiac function, health-related quality of life (HR-QoL), and symptoms in long-term survivors of anthracycline-based adjuvant chemotherapy with or without trastuzumab. The B-31 trial provided an opportunity to assess these outcomes in a well-characterized cohort of patients. We report here the results of an amendment to the B-31 protocol, which recruited breast cancer–free survivors for long-term follow-up (LTF) assessments of cardiac function, patient-reported outcomes (PROs) related to HR-QoL, and cardiac-related symptoms and morbidity.
PATIENTS AND METHODS
Patients in B-31 had HER2-positive, node-positive operable breast cancer and received four cycles of AC (60/600 mg/m2 every 3 weeks) followed by paclitaxel (175 mg/m2 every 3 weeks for four cycles or 80 mg/m2 weekly for 12 weeks). Patients randomly assigned to the investigational group also received trastuzumab at 4 mg/kg concurrently with the first dose of paclitaxel followed by 2 mg/kg weekly for a total of 52 weeks. Those with hormone receptor–positive tumors initiated adjuvant endocrine therapy after completion of chemotherapy. Patients with a history or current diagnosis of the following were excluded: angina pectoris, cardiac arrhythmia, severe conduction abnormality, significant valvular disease, cardiomegaly, ventricular hypertrophy, uncontrolled hypertension, myocardial infarction, CHF, or cardiomyopathy. Cardiac function was monitored using left ventricular ejection fraction (LVEF) measured by multigated acquisition (MUGA) scans at 3, 6, 9, and 18 months after random assignment. Rules for holding or stopping trastuzumab were based on the 3-, 6-, and 9-month assessments. Although MUGA scans were not planned beyond 18 months, cardiac history forms were collected every 6 months for the first 5 years and annually thereafter to identify late cardiac morbidity.
The protocol was amended in 2010 to offer survivors without recurrence the opportunity to respond to a single PRO questionnaire and undergo MUGA scans between 5 and 10 years after random assignment. To be eligible for the LTF study, participants had to be disease free from breast cancer or second primary malignancy, have 18-month LVEF data available, have received only the assigned B-31 therapy (ie, patients in the control group not having received trastuzumab subsequent to reporting of the initial B-31/N9831 results), and provide written informed consent for the LTF study.
Recruitment of Sites and Participants
Participating institutions were provided the amended protocol and consent form for submission to their institutional review boards (IRBs) with a list of potential patients for LTF evaluation at their sites. After IRB approval, sites were requested to approach all potentially eligible patients and obtain signed consent forms from those who agreed to participate. Patients who consented were requested to complete the PRO questionnaire and then undergo LVEF assessment by MUGA scan. Patients were also asked to complete an updated cardiac history form within a 2-month window around PRO questionnaire completion.
Questionnaire Survey Content
The survey battery included four instruments. The first was the 36-item Medical Outcomes Study short-form questionnaire (MOS-SF36),5 which has eight subscales with normative data available from the general population.6 MOS-SF36 can also be scored as two component summary scales that measure physical (PCS) and mental (MCS) functioning. These are presented as T scores, with a normal healthy population mean score of 50 and standard deviation (SD) of 10. The Duke Activity Status Index (DASI),7,8 a validated 12-item self-report questionnaire widely used to determine functional capacity among individuals with cardiac disease, was also used. The DASI score correlates with function and oxygen capacity during exercise testing and provides metabolic equivalents for level of activity.7 DASI scores range from 0 to 58.2, with higher scores indicating better functional capacity.9 Chronic comorbid conditions were assessed with a questionnaire developed by Sangha et al.10 Finally, participants were asked separately about common symptoms associated with cardiac disease, with items taken from the Breast Cancer Prevention Trial symptom checklist (chest pain, feeling of suffocation, difficulty breathing, shortness of breath, swelling of hands and feet, and dizziness or faintness), which were rated as follows for severity of bother: not at all, 0; slightly, 1; moderately, 2; quite a bit, 3; and extremely, 4.11
Clinical staff also recorded data on the cardiac history form used throughout the B-31 study. This form asked whether the patient had taken medication for any of the following conditions since the last follow-up: hypertension, angina, myocardial infarction, CHF, cardiomyopathy, atrial fibrillation or atrial arrhythmias, ventricular arrhythmias, diabetes, or elevated fasting lipid profile. Smoking activity was also assessed.
Statistical Methods
The primary aim of the LTF evaluation was to assess the long-term effect of trastuzumab on LVEF 5 to 10 years after study entry by comparing the proportion of patients who received chemotherapy plus trastuzumab and experienced a significant LVEF decline (absolute drop in LVEF from baseline ≥ 10% to a value < 50%) at the LTF evaluation with the proportion in the control group treated with chemotherapy without trastuzumab. Given our obtained sample size, we had 80% power to detect a difference in proportions of 0.073 between treatment groups at a two-sided significance level of .05. A secondary aim was to compare the proportions of patients with LVEF values > 50% at 18 months that declined to < 40% at LTF between the two treatment groups.
Other secondary aims were to determine the long-term effects of the B-31 treatments on HR-QoL, cardiac performance, and comorbid health problems among disease-free survivors. We evaluated the descriptive statistics of the MOS-SF36 MCS and PCS scores and the DASI score overall and by treatment group. Responses to the questions regarding current cardiac medications, cardiac symptoms, and comorbid conditions by treatment group were also compared using the χ2 test. An overall score of the comorbidity questionnaire was calculated according to the method by Sangha et al.10 Briefly, a patient can receive three points for each condition: one point for the presence of the condition, one point for treatment, and one point if the condition limits activities. The possible range of scores is 0 to 60, with higher score indicating a greater impact of comorbidities.
We also explored the distribution of DASI scores after noting the large SD and range of the scores. Although a majority of patients reported few limitations in functioning, there was a substantial group for whom poor functioning was observed. We therefore examined the relationship between the lower tertile scores on DASI and the remainder of the sample to characterize those women reporting relevant cardiac dysfunction at this LTF assessment. Treatment group; age at entry; baseline use of hypertension, diabetes, or hyperlipidemia medications; smoking history; side irradiated; and LVEF values at baseline, 18 months, and LTF were compared between the two DASI groups using the χ2 test. Mean PCS, MCS, and metabolic equivalent scores as well as current use of cardiac medications were also compared between the two DASI groups. We also used multivariable logistic regression to identify predictors of poorer DASI score. The explanatory variables considered for inclusion were treatment group; age at entry; baseline use of hypertension, diabetes, or hyperlipidemia medications; smoking history; side irradiated; and LVEF values at baseline, 18 months, and LTF. Time from random assignment to completion of the PRO questionnaire and assigned treatment group were also included in the final model to control for potential confounding. All P values reported were two sided, with a significance level of .05. Analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC).
RESULTS
Patient Eligibility and Baseline Characteristics
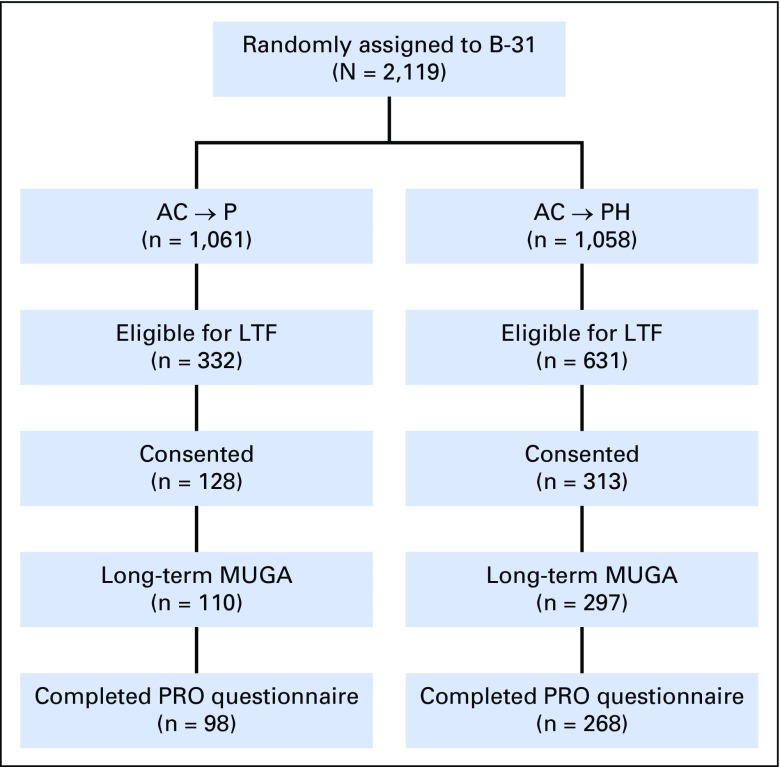
Of the 2,119 patients enrolled in B-31, 963 were eligible to participate in the LTF study (Fig 1). Fewer patients in the control group were eligible as a result of a greater likelihood of breast cancer recurrence, as well as the subsequent receipt of trastuzumab after the results of the B-31/N9831 analysis were made public. Recruitment of eligible long-term survivors was not as successful as hoped, which has been reported in similar efforts.12,13 Of those eligible to participate, 441 (45.8%) consented to participate (control group, 38.6%; trastuzumab group, 49.6%) and are the subject of this report.
Fig 1.
Flow of participants of NSABP (National Surgical Adjuvant Breast and Bowel Project) B-31 long-term cardiac follow-up. AC, doxorubicin and cyclophosphamide; H, trastuzumab; LTF, long-term follow-up; MUGA, multigated acquisition; P, paclitaxel; PRO, patient-reported outcome.
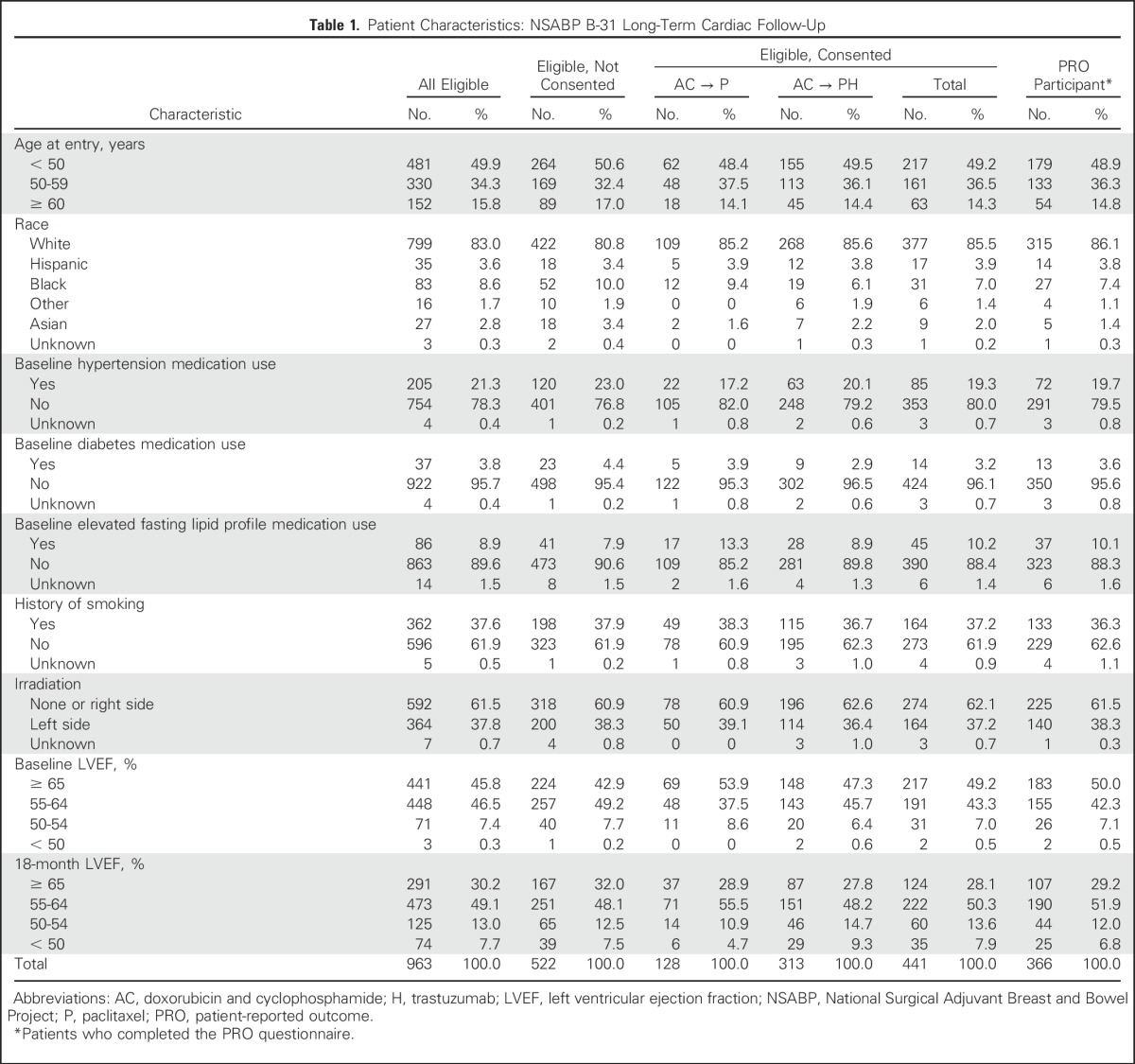
Patient characteristics between consented patients and the entire cohort of eligible LTF patients and between the two treatment groups among those who consented to participate in LTF were similar (Table 1). Also, the baseline characteristics of the LTF cohort were similar to those of the entire B-31 population, as previously reported.14 We received LTF MUGA scan results from 407 of the 441 consented patients (control group, n = 110; trastuzumab group, n = 297). At the time of the LTF assessment, the mean age of the patients was 58.3 years, and median follow-up was 8.8 years (range, 5.6 to 13.8 years).
Table 1.
Patient Characteristics: NSABP B-31 Long-Term Cardiac Follow-Up
LVEF Results and Cardiovascular Medication Use at LTF
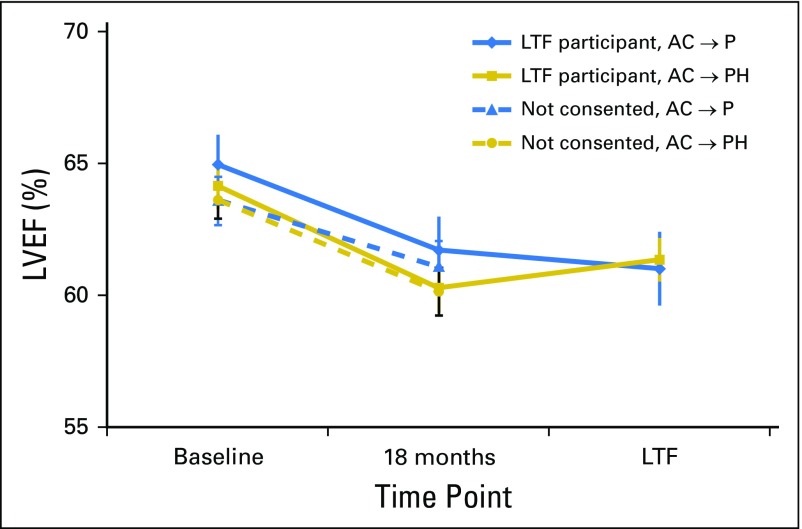
The mean LVEF measurements at baseline, 18 months, and LTF assessments are shown in Figure 2. At 18 months, the mean decline from baseline was 3.2% in the control group and 3.9% in the trastuzumab group. At the LTF assessments, the mean decline from baseline was 3.9% versus 2.8%, respectively.
Fig 2.
Mean left ventricular ejection fraction (LVEF) measurements over time for National Surgical Adjuvant Breast and Bowel Project B-31 long-term cardiac follow-up participants and those who were eligible but did not consent. Vertical bars represent 95% CIs. AC, doxorubicin and cyclophosphamide; H, trastuzumab; LTF, long-term follow-up; P, paclitaxel.
Among the control group LTF participants, three (2.7%) of 110 had a reported decline of ≥ 10% to < 50% at their 18-month assessment compared with 23 (7.7%) of 297 in the trastuzumab group. At the LTF assessment, this decline was reported in five (4.5%) of 110 in the control group versus 10 (3.4%) of 297 in the trastuzumab group. Only one patient in the control group and two in the trastuzumab group with LVEF measurements > 50% at 18 months had measurements < 40% at LTF (Appendix Table A1, online only).
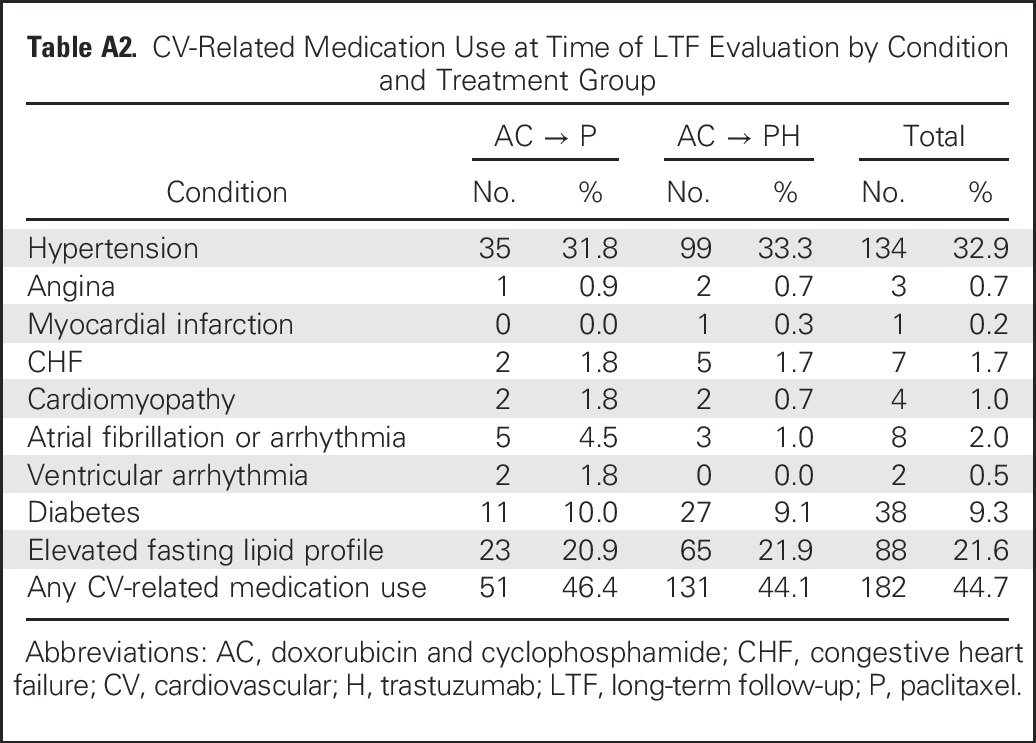
Approximately 19% of the LTF participants were receiving antihypertensive medications at entry in B-31. At the LTF assessment, antihypertensive medications were being used by 31.8% in the control group and 33.3% in the trastuzumab group. Specific reasons for medication use are listed in Appendix Table A2 (online only). Nonsmokers comprised 92.6% of the LTF patients (data not shown).
In our previous report,4 37 patients in the trastuzumab group had experienced a cardiac event, including one cardiac death. Of these, 23 were eligible for the LTF study, and only eight consented to participate. Six of these eight had LTF LVEF measurements > 50%, and five were within 5 percentage points of their baseline values (data not shown). Ten of the 37 patients have died, four subsequent to our previous report. One of these patients was not eligible for the LTF study because of an earlier second primary cancer diagnosis (cause of death unknown), two were eligible but did not consent (one cause of death was unknown, and the other resulted from injury), and one participated in the LTF but later died as a result of lung cancer.
QoL and PROs
Of the 407 patients with LTF LVEF assessments, 366 (89.9%) also completed PRO questionnaires (control group, n = 98; trastuzumab group, n = 268). The median time from random assignment to questionnaire completion was 8.7 years (range, 5.5 to 14 years). We first examined the descriptive statistics for the MCS and PCS scores of MOS-SF36 and the DASI score according to treatment assignment. The scores for both groups were high (favorable), reflecting higher-than-expected physical and mental functioning compared with reference populations of the same age. The mean PCS score for all patients was 49.7 (SD, 9.1), and the mean MCS score was 52.9 (SD, 9.4). The mean DASI score was 45.6 (SD, 13.8; range, 7.2 to 58.2) for the total sample. We also compared common cardiac symptoms and lifetime comorbid conditions by treatment group. We found no statistically significant differences between treatment groups for any individual symptom or condition or for the overall comorbidity score. The mean comorbidity score was 4.77 (SD, 3.61) in the control arm and 4.87 (SD, 4.22) in the trastuzumab arm (data not shown).
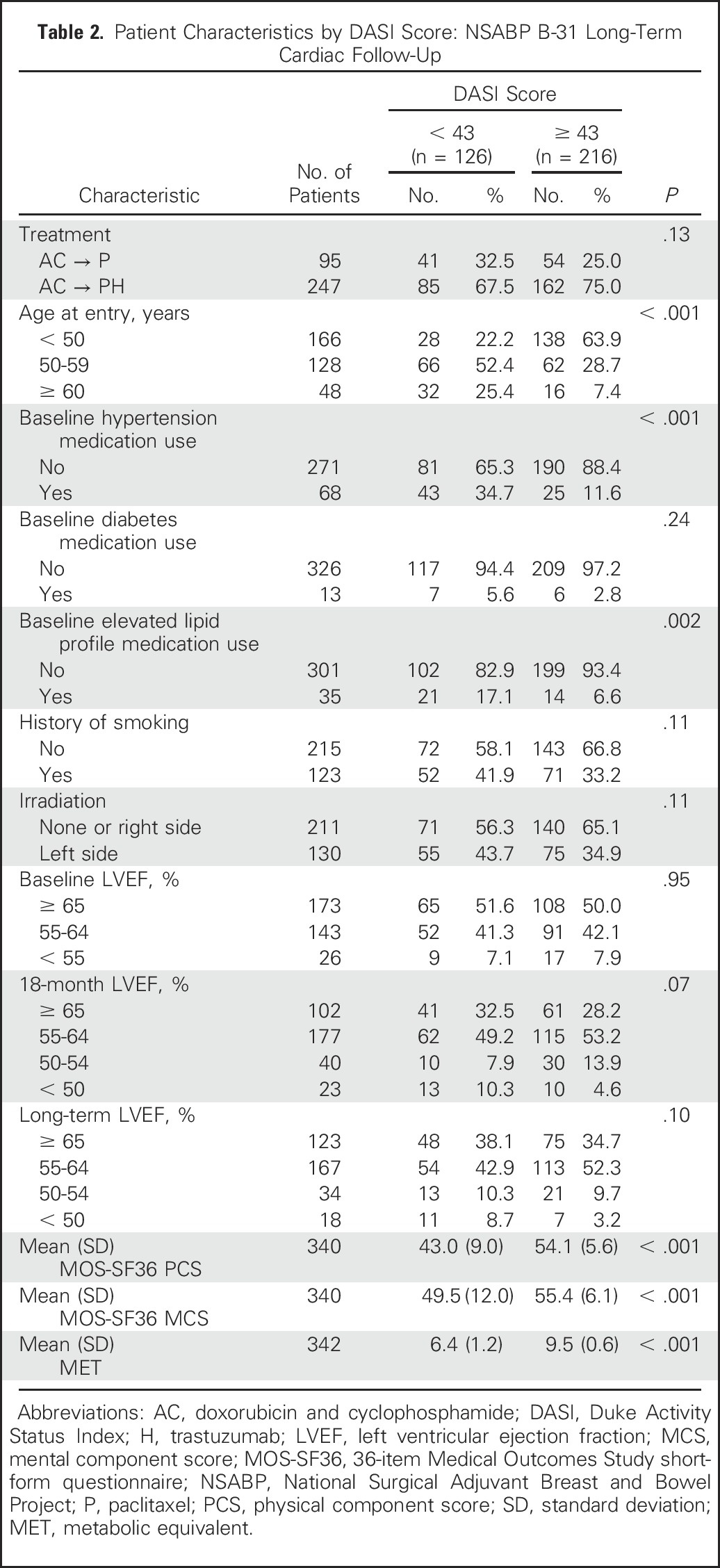
On the basis of the wide distribution of the DASI scores, we categorized patients into two groups representing those with the lowest tertile of DASI scores (< 43) versus the remainder of the sample (≥ 43) to characterize women reporting relevant cardiac dysfunction at the LTF assessment. Table 2 shows the relationship between key patient characteristics according to the categorized DASI score. There were statistically significant relationships between age group (P < .001), baseline hypertensive medication use (P < .001), baseline hyperlipidemia medication use (P = .002), and DASI score, with older patients and patients using medication at baseline reporting more dysfunction. Those with the lowest DASI scores had a lower but nonsignificant 18-month LVEF (P = .07). There were also no significant differences by treatment assignment, baseline diabetes medication use, smoking history, left-sided irradiation, baseline LVEF, or LTF LVEF. The patients in the lowest tertile of DASI scores demonstrated poorer physical and mental MOS-SF36 scores than those with higher DASI scores (P < .001).
Table 2.
Patient Characteristics by DASI Score: NSABP B-31 Long-Term Cardiac Follow-Up
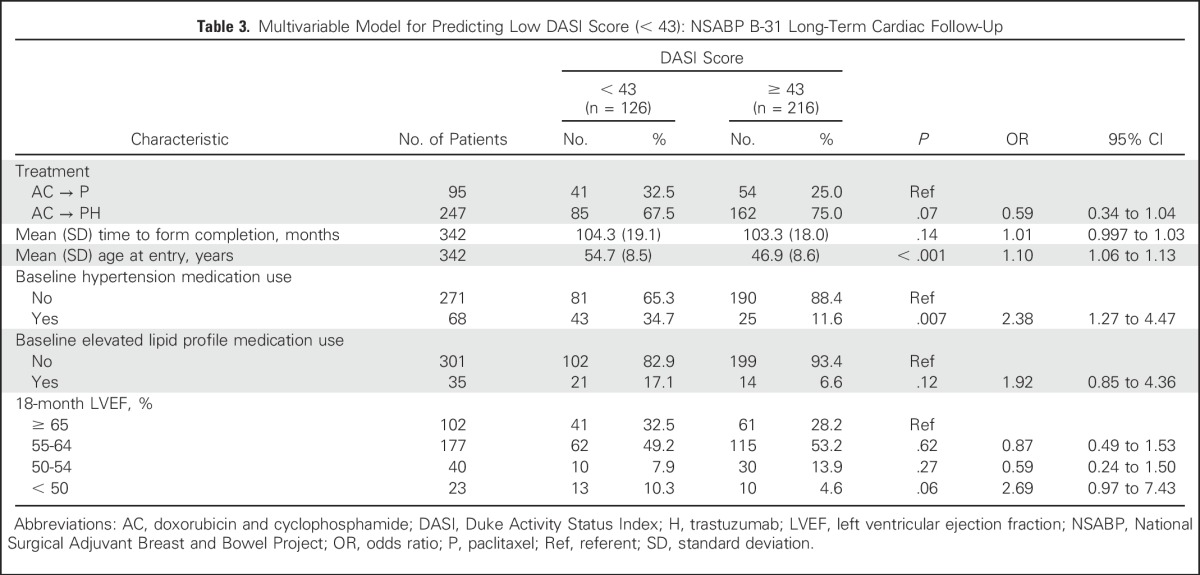
We performed multivariable logistic regression analyses to examine which variables were associated with the lowest tertile DASI scores at the LTF visit. Variables included were treatment assignment and those that were significant (P < .10) in the bivariable analyses. We also included time from random assignment to the completion of the PRO questionnaire because of the large range of completion times. Results of the model are listed in Table 3. Variables significantly associated with poorer cardiac function at the LTF assessment were older age at protocol entry and baseline antihypertensive medication use. Treatment assignment was not associated with poorer cardiac function and was in fact numerically better in the trastuzumab group. An 18-month LVEF < 50% showed a statistically insignificant trend with an increased odds ratio of 2.69 (95% CI, 0.97 to 7.43; P = .06).
Table 3.
Multivariable Model for Predicting Low DASI Score (< 43): NSABP B-31 Long-Term Cardiac Follow-Up
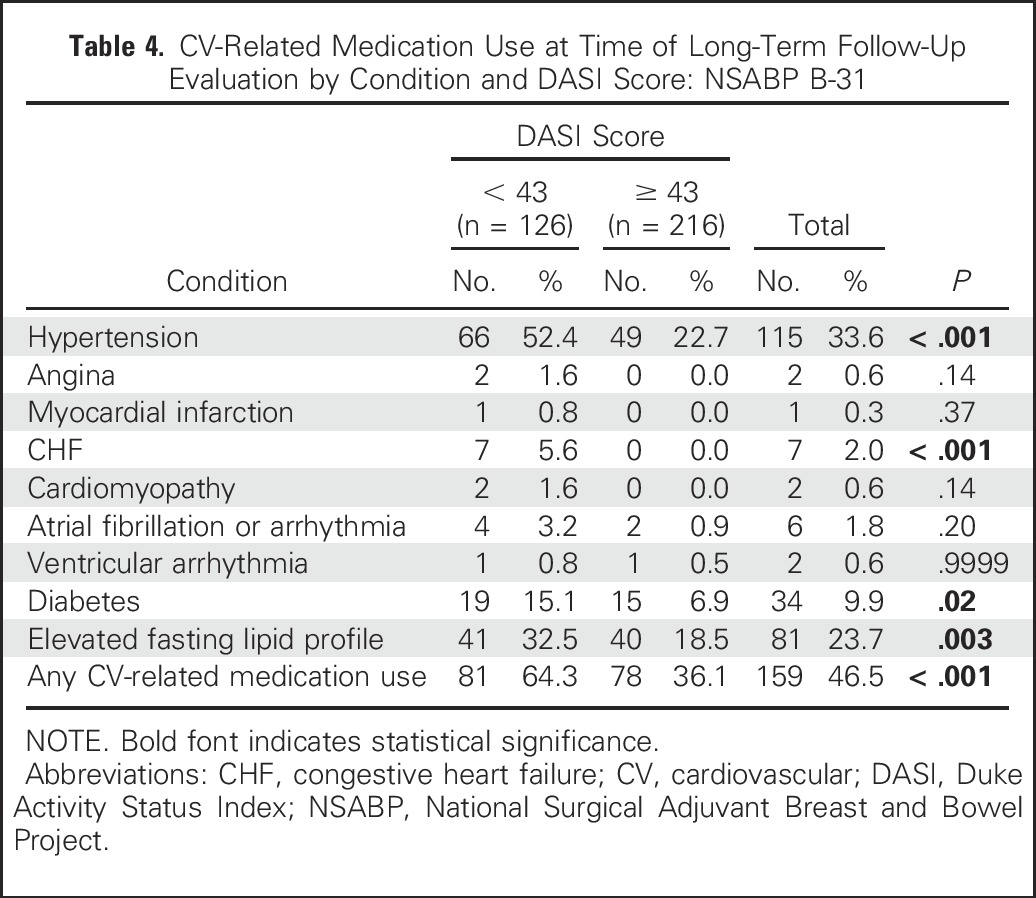
We also examined cardiovascular medication use at LTF assessment according to DASI score (Table 4) and found additional support for the validity of the DASI score in that patients in the lowest tertile were more likely to be taking medication for hypertension (52.4% v 22.7%), CHF (5.6% v 0.0%), as well as any cardiac-related medication (64.3% v 36.1%). Thus, the self-reported cardiac function on the DASI questionnaire seemed to accurately reflect the degree of cardiac morbidity in these long-term survivors of breast cancer.
Table 4.
CV-Related Medication Use at Time of Long-Term Follow-Up Evaluation by Condition and DASI Score: NSABP B-31
DISCUSSION
Cardiac toxicity has been a central consideration in clinical trials evaluating HER2-targeted therapies. Although these studies have carefully documented the cumulative incidence of severe and less severe cardiac dysfunction, long-term assessments of LVEF, HR-QoL, and PRO reporting of cardiac symptoms have been unavailable. To our knowledge, this study is the first to provide LTF measurements of all three of these elements in a well-characterized cohort of HER2-positive breast cancer survivors who remain alive and free of breast cancer recurrence at a median of 8.8 years after treatment initiation. It is also one of the largest long-term studies of cardiac outcomes in patients treated with anthracyclines with or without trastuzumab.12 A limitation is that this LTF study does not provide data from all eligible B-31 patients. Nevertheless, the patients who participated had baseline clinical characteristics similar to those of the overall eligible cohort. In addition, their baseline and 18-month LVEF data were no different from those of the eligible women who did not participate in the LTF study.
Our study demonstrated no detrimental effect on LVEF from the addition of trastuzumab to AC plus paclitaxel chemotherapy among B-31 LTF participants. There was no difference between the baseline and 18-month LVEF in LTF participants and nonparticipants, and the LTF participants showed no late decline in LVEF. With a median follow-up of 8.8 years, 95.3% of patients randomly assigned to receive trastuzumab had ejection fractions ≥ 50%, and only two (0.6%) of 297 had LVEF ≤ 40%. These results are consistent with prior observations that cardiac effects of trastuzumab are generally reversible and qualitatively different from anthracycline-induced cardiomyopathy.15 It is important to emphasize, however, that the median age at study entry for patients in this trial was 50 years, and rules for temporary holding or discontinuation of trastuzumab based on careful cardiac monitoring were strictly observed. Also, although echocardiogram provides more sensitive assessment of cardiac function, MUGA scans were used for this LTF study because of the primary end point and because baseline and 18-month measurements of LVEF were conducted with this method in all patients.
We have also demonstrated the potential value of including PROs along with physiologic measures of cardiac function in follow-up of breast cancer survivors who have received cardiotoxic treatments. Baseline characteristics of older age and use of antihypertensive medications were predictors of lower DASI score at LTF assessment, consistent with their reported association with cardiac events in this clinical trial and other studies.4,16,17 We also provide evidence for the validity of the DASI questionnaire in this patient population, because women with scores in the lowest tertile were more likely to require cardiac-related medications at LTF assessment. Serial follow-up of patients with this 12-item questionnaire could be used to identify patient-perceived changes in cardiac performance more frequently than imaging studies. Prospective studies are warranted to evaluate use of the DASI questionnaire to identify declines in function, which could trigger formal cardiac function assessments.
Population-based, retrospective, and observational studies have raised concerns that trastuzumab-related cardiac toxicity in routine practice may be greater than that seen in clinical trials, especially in older women even without anthracycline use.18,19 However, these studies do not provide information regarding long-term cardiac outcomes or HR-QoL measures in patients who survive without recurrence, underscoring the importance of long-term cardiac follow-up of women enrolled in trials. Our report provides encouraging data from the B-31 study and indicates that in breast cancer survivors without a pre-existing cardiac history, the addition of trastuzumab to adjuvant anthracycline-based chemotherapy does not result in long-term worsening of cardiac function, cardiac symptoms, or HR-QoL.
Appendix
Table A1.
LVEF Measurements and CV-Related Medication Use for Participants With LVEF Decline ≥ 10% From Baseline to LTF and Long-Term LVEF < 50%
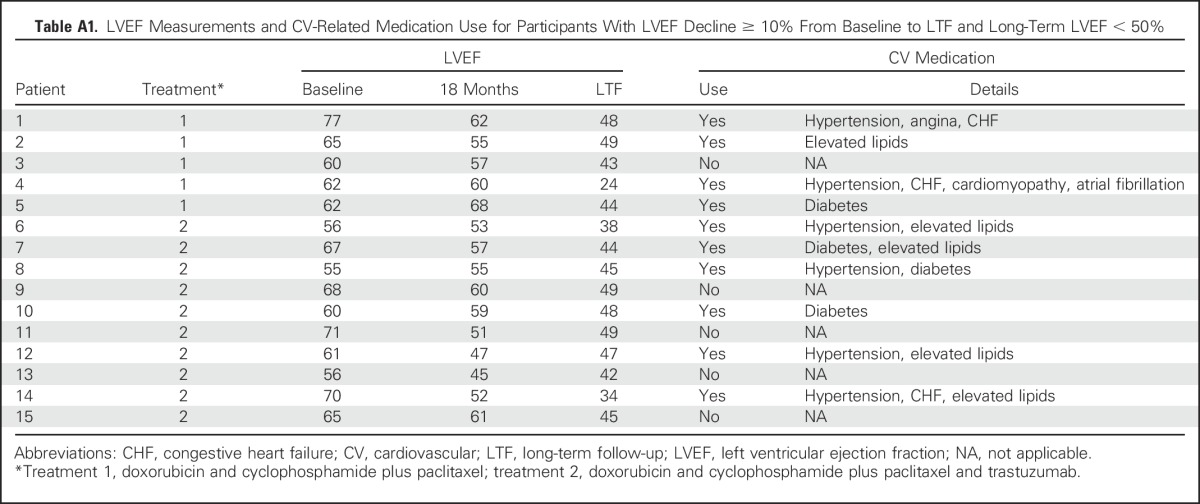
Table A2.
CV-Related Medication Use at Time of LTF Evaluation by Condition and Treatment Group
Footnotes
Supported by Grants No. U10CA-180868, U10CA-180822, and UG1CA-189867 from the National Cancer Institute, Department of Health and Human Services, Public Health Service; the Breast Cancer Research Foundation (P.A.G.); Susan G. Komen for the Cure (E.P.M.); and Genentech.
Clinical trial information: NCT00004067.
AUTHOR CONTRIBUTIONS
Conception and design: Patricia A. Ganz, Edward H. Romond, Priya Rastogi, Charles E. Geyer Jr, Sandra M. Swain, Jong-Hyeon Jeong, Patrick J. Flynn, Eleftherios P. Mamounas, Norman Wolmark
Provision of study materials or patients: Edward H. Romond, Louis Fehrenbacher, Howard M. Gross, Adam M. Brufsky, Thomas E. Seay, James L. Wade III, Eleftherios P. Mamounas
Collection and assembly of data: Reena S. Cecchini, Jong-Hyeon Jeong, Louis Fehrenbacher, Howard M. Gross, Adam M. Brufsky, Patrick J. Flynn, Tanya A. Wahl, Thomas E. Seay, James L. Wade III, David D. Biggs, James N. Atkins, Jonathan Polikoff, John L. Zapas
Data analysis and interpretation: Patricia A. Ganz, Edward H. Romond, Reena S. Cecchini, Priya Rastogi, Charles E. Geyer Jr, Sandra M. Swain, Louis Fehrenbacher, Adam M. Brufsky, Eleftherios P. Mamounas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Follow-Up of Cardiac Function and Quality of Life for Patients in NSABP Protocol B-31/NRG Oncology: A Randomized Trial Comparing the Safety and Efficacy of Doxorubicin and Cyclophosphamide (AC) Followed by Paclitaxel With AC Followed by Paclitaxel and Trastuzumab in Patients With Node-Positive Breast Cancer With Tumors Overexpressing Human Epidermal Growth Factor Receptor 2
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Patricia A. Ganz
Leadership: Intrinsic LifeSciences (I)
Stock or Other Ownership: Xenon Pharma (I), Intrinsic LifeSciences (I), Silarus Therapeutics (I), Merganser Biotech (I), TEVA Pharmaceuticals Industries, Novartis, Merck, Johnson & Johnson, Pfizer, GlaxoSmithKline, Abbott Laboratories
Honoraria: Biogen (I), Keryx (I), Merganser Biotech (I), Silarus Therapeutics (I), InformedDNA, Vifor Pharma (I), Eli Lilly, La Jolla Pharma (I)
Research Funding: Keryx (I)
Patents, Royalties, Other Intellectual Property: Related to iron metabolism and the anemia of chronic disease (I); Up-to-Date royalties for section editor on survivorship
Travel, Accommodations, Expenses: Intrinsic LifeSciences (I), Keryx (I)
Edward H. Romond
Research Funding: Genentech
Reena S. Cecchini
No relationship to disclose
Priya Rastogi
No relationship to disclose
Charles E. Geyer Jr
Stock or Other Ownership: Radius Health, Pieris Pharmaceuticals, ConforMIS
Consulting or Advisory Role: Myriad Genetics, Celgene
Research Funding: Incyte, Merck
Travel, Accommodations, Expenses: AstraZeneca, AbbVie, Genentech
Sandra M. Swain
Honoraria: Roche, Clinigen Group, Pfizer, Novartis, AstraZeneca
Consulting or Advisory Role: Genentech, Clinigen Group, Eli Lilly, Pieris Pharmaceuticals, Inivata
Research Funding: Puma Biotechnology (Inst), Roche (Inst), Genentech (Inst), Pfizer (Inst), Merrimack Pharmaceuticals, Eli Lilly (Inst)
Travel, Accommodations, Expenses: Genentech
Jong-Hyeon Jeong
No relationship to disclose
Louis Fehrenbacher
Research Funding: Genentech (Inst)
Howard M. Gross
No relationship to disclose
Adam M. Brufsky
Consulting or Advisory Role: Pfizer, Genentech, Agendia, Celgene, Novartis, Bayer HealthCare Pharmaceuticals, Eli Lilly, bioTheranostics, NanoString Technologies, Genomic Health, Puma Biotechnology
Patrick J. Flynn
Employment: ARIAD Pharmaceuticals (I), Sanofi (I)
Stock or Other Ownership: ARIAD Pharmaceuticals (I), Sanofi (I)
Consulting or Advisory Role: Shire
Speakers’ Bureau: Eli Lilly, Novartis
Tanya A. Wahl
No relationship to disclose
Thomas E. Seay
No relationship to disclose
James L. Wade III
Employment: Johnson & Johnson (I)
Stock or Other Ownership: Seattle Genetics, Celgene
David D. Biggs
Employment: AstraZeneca (I)
Stock or Other Ownership: AstraZeneca (I)
Consulting or Advisory Role: Genomic Health (Inst)
Travel, Accommodations, Expenses: AstraZeneca (I)
James N. Atkins
No relationship to disclose
Jonathan Polikoff
Travel, Accommodations, Expenses: Genentech
John L. Zapas
No relationship to disclose
Eleftherios P. Mamounas
Honoraria: Genentech, Genomic Health
Consulting or Advisory Role: Genomic Health, Pfizer, bioTheranostics, Celcuity, GRAIL, Macrogenics
Speakers’ Bureau: Genomic Health, Genentech
Travel, Accommodations, Expenses: Genomic Health, Genentech
Norman Wolmark
No relationship to disclose
REFERENCES
- 1.Perez EA, Romond EH, Suman VJ, et al. : Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32:3744-3752, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, et al. : Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783-792, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Seidman A, Hudis C, Pierri MK, et al. : Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20:1215-1221, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Romond EH, Jeong J-H, Rastogi P, et al. : Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2–positive breast cancer. J Clin Oncol 30:3792-3799, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McHorney CA, Ware JE, Jr, Raczek AE: The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 31:247-263, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Ware JEJ, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA, The Health Institute, 1994 [Google Scholar]
- 7.Bairey Merz CN, Olson M, McGorray S, et al. : Physical activity and functional capacity measurement in women: A report from the NHLBI-sponsored WISE study. J Womens Health Gend Based Med 9:769-777, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Hlatky MA, Boineau RE, Higginbotham MB, et al. : A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 64:651-654, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Mark DB, Anstrom KJ, Sheng S, et al. : Quality-of-life outcomes with anatomic versus functional diagnostic testing strategies in symptomatic patients with suspected coronary artery disease: Results from the PROMISE randomized trial. Circulation 133:1995-2007, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sangha O, Stucki G, Liang MH, et al. : The self-administered comorbidity questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Rheum 49:156-163, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Ganz PA, Day R, Ware JE, Jr, et al. : Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst 87:1372-1382, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Ganz PA, Hussey MA, Moinpour CM, et al. : Late cardiac effects of adjuvant chemotherapy in breast cancer survivors treated on Southwest Oncology Group Protocol s8897. J Clin Oncol 26:1223-1230, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Ganz PA, Land SR, Antonio C, et al. : Cancer survivorship research: The challenge of recruiting adult long term cancer survivors from a cooperative clinical trials group. J Cancer Surviv 3:137-147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan-Chiu E, Yothers G, Romond E, et al. : Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2–overexpressing breast cancer: NSABP B-31. J Clin Oncol 23:7811-7819, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Ewer MS, Vooletich MT, Durand J-B, et al. : Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol 23:7820-7826, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Perez EA, Suman VJ, Davidson NE, et al. : Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol 26:1231-1238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinder MC, Duan Z, Goodwin JS, et al. : Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 25:3808-3815, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Bowles EJ, Wellman R, Feigelson HS, et al. : Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. J Natl Cancer Inst 104:1293-1305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thavendiranathan P, Abdel-Qadir H, Fischer HD, et al. : Breast cancer therapy–related cardiac dysfunction in adult women treated in routine clinical practice: A population-based cohort study. J Clin Oncol 34:2239-2246, 2016 [DOI] [PubMed] [Google Scholar]