Summary
The complement system activation is involved in the development of anti‐neutrophil cytoplasmic antibody‐associated vasculitis (AAV). The study aimed to investigate the expression of complement regulatory proteins (CRPs) CD46, CD55 and CD59 in kidneys of 51 AVV patients. The expression of CD46, CD55 and CD59 in kidneys was detected by immunohistochemistry and double immunofluorescence staining. The immunohistochemical examination revealed that expression of the three CRPs could be detected in the glomeruli and tubules of both AAV patients and normal controls. The expression levels of the three CRPs in glomeruli of patients with AAV were significantly lower than those of normal controls. The scores of CD46 and CD55 expression in the tubules of AAV patients were significantly lower than those of normal controls, while there was no significant difference between the scores of CD59 expression in tubules of AAV patients and those of normal controls. Among AAV patients, the expression level of CD46 in glomeruli correlated inversely with the proportion of normal glomeruli, while it correlated with tubular atrophy in renal interstitium (r = –0·305, P = 0·026; r = 0·330, P = 0·023, respectively). The expression levels of CD55 and CD59 in glomeruli correlated with the proportion of total crescents (r = 0·384, P = 0·006; r = 0·351, P = 0·011, respectively). Double immunofluorescence staining indicated that all three CRPs were expressed on endothelial cells, podocytes and mesangial cells in glomeruli. The expression levels of the three CRPs were dysregulated in kidneys of patients with AAV. The expression levels of CD46, CD55 and CD59 were associated with the severity of renal injury of AAV patients.
Keywords: anti‐neutrophil cytoplasmic antibody, complement regulate proteins, pathology
Introduction
Anti‐neutrophil cytoplasmic antibodies (ANCAs) are directed predominantly against neutrophil cytoplasmic constituents, in particular myeloperoxidase (MPO) or proteinase 3 (PR3) 1, 2, and are associated with systemic small vessel necrotizing vasculitis, termed collectively ANCA‐associated vasculitis (AAV). AAV comprises granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA) 3.
Increasing evidence has confirmed that complement activation plays a key role in the pathogenesis of AAV 4, 5, 6, 7. Xiao et al. 4 first reported that complement activation was involved in an animal study of experimental vasculitis. In humans, Chen et al. 5 showed C3c deposition in one‐third of renal biopsies from 112 AAV patients. Our previous study found that systemic activation of complement occurred in human AAV 6. Complement activation products – Bb, C3d and C5b‐9 – were detected along the glomerular capillary wall and mesangial area of glomeruli of patients with AAV in a granular pattern 7. However, there was no correlation between the level of complement activation products deposited in renal specimens and of corresponding complement activation products in circulation, which suggested that the circulating complement activation products were not the only sources for complement activation products in kidney. There remained the possibility of the complement system activation occurring in kidney 6.
In order to avoid uncontrolled activation and an autologous immune reaction, complement activation is regulated tightly in the normal state by complement regulatory proteins (CRPs). Complement regulatory proteins exist as soluble proteins or as membrane‐bound CRPs (mCRPs). The mCRPs presenting on the host cell membranes play a braking role in local complement activation 8. Abnormal expression of the mCRPs may affect the complement activation in kidneys of patients with nephritis. The involvement of mCRPs CD46, CD55 and CD59 in immune responses of various autoimmune disease, such as systemic lupus erythematosus, rheumatoid arthritis and some immune complex‐mediated glomerulonephritis 9, 10, 11, have been proven. However, so far the expression of mCRPs in kidneys of patients with AAV has been unclear. Therefore, we investigated the expression level of mCRPs CD46, CD55 and CD59 in kidneys of patients with AAV, and analysed the relationship between the mCRPs and clinical parameters.
Materials and methods
Patients and samples
From January 2011 to June 2015, we collected renal biopsy specimens retrospectively from 51 AAV patients from West China Hospital, all of whom were tested positive for p‐ANCA or c‐ANCA by indirect immunofluorescence, and positive for MPO‐ANCA or PR3‐ANCA by antigen‐specific enzyme‐linked immunosorbent assay (ELISA). They all met the Chapel Hill Consensus Conference (CHCC) definition of AAV with complete clinical and pathological data. However, patients with secondary vasculitis or co‐existence of other renal diseases were excluded. For normal controls, renal tissues from normal parts of 11 nephrectomized kidneys were obtained and confirmed as normal controls using light microscopy, immunofluorescence and electron microscopy. The ethics committee of West China Hospital of Sichuan University approved the study and each participant provided written informed consent.
Renal histology
We evaluated the renal histology of patients with AAV according to previous standardized protocol 12, 13, 14. The presence of glomerular lesions, including fibrinoid necrosis, crescents and glomerulosclerosis, were calculated as the percentage of the total number of glomeruli in a biopsy. Interstitial and tubular lesions were scored semiquantitatively on the basis of the percentage of the tubulointerstitial compartment that was affected: interstitial infiltrate (scores 0, 1, 2, 3 for 0, 0–20, 20–50 and > 50%, respectively), interstitial fibrosis (scores 0, 1, 2 for 0, 0–50 and > 50, respectively) and tubular atrophy (scores 0, 1, 2 for 0, 0–50 and > 50%, respectively).
Detection of complement regulatory proteins expression in kidney by immunohistochemistry
Immunohistochemical staining was performed for CD46, CD55 and CD59 on 4‐μm deparaffinized sections of formaldehyde‐fixed renal tissue using rabbit anti‐human CD46 polyclonal antibodies (Abcam, Cambridge, UK; ab108307), mouse anti‐human CD55 monoclonal antibodies (Abcam; ab1422) and rabbit anti‐human CD59 polyclonal antibodies (Sigma‐Aldrich, St Louis, MO, USA; HPA026494) as primary antibodies. All the primary antibodies were diluted 1 : 1000 with 1% bovine serum albumin (BSA). After deparaffinization in xylene‐ethanol at room temperature and rehydration in phosphate‐buffered saline (PBS), sections were immersed in freshly prepared 3% hydrogen peroxide for 30 min at room temperature to quench endogenous peroxidase activity. For antigen retrieval, the sections for CD46 and CD55 were heated in ethylenediamine tetraacetic acid (EDTA) buffer (pH 8.0) in an 800 W microwave oven for 2 min and then at 200 W for 9 min. The sections for CD59 were heated in a 200 W microwave oven for 9 min and then at 800 W for 2 min. Following antigen retrieval, tissue sections were blocked in PBS containing 3% normal bovine serum and incubated overnight at 4°C in primary antibody. After washing with PBS, secondary antibodies (goat anti‐mouse and goat anti‐rabbit; Jackson ImmunoResearch, West Grove, PA, USA) were added and incubated for 30 min at 37°C. After washing by PBS, the sections were developed in fresh hydrogen peroxide plus 3‐3′‐diaminobenzidine tetrahydrochloride solution for 50 s. Finally, the sections were counterstained with haematoxylin and dehydrated through alcohol and xylene. Renal tissues from the normal part of nephrectomized kidneys due to renal carcinoma were used as normal controls. Lymph node slides were used as positive controls for CD46 and CD55. As negative controls, the primary antibodies were substituted by normal rabbit immunoglobulin (Ig)G or normal mouse IgG. The sections were examined by light microscopy.
Evaluation of immunohistochemical staining
All the glomeruli and all the tubules in a section at ×400 and at ×200, respectively, and at least 10 fields of tubulointerstitial vision per kidney were observed blindly for a semiquantitive assessment of immunohistochemical staining. Image Pro Plus analysis software version 6.0 was employed for evaluating glomerular staining of CD46, CD55 and CD59. Positive signals were quantified as the mean optical density (integrated option density/area). Staining of three mCRPs on the tubules was scored separately by two researchers unaware of the clinical pathology of the cases. An immunoreactivity scoring system was applied using the extensional standard, as follows 15: (a) a fraction of positively stained tubules of 5% or less scored 0, 6–25% scored 1, 26–50% scored 2, 51–75% scored 3 and above 75% scored 4, and (b) for staining intensity, no staining scored 0, buff scored 1, yellow scored 2 and brown scored 3. According to the proportion and intensity of positively stained tubules, the final staining score was (a) and (b) multiplied together. If the scores by the two researchers had a disparity greater than 3, specimens were rescored.
Detection of CD46, CD55, CD59 expression on glomerular intrinsic cells
In order to determine on which cell types of glomeruli CD46, CD55 and CD59 were expressed, we performed double staining of the three mCRPs and specific markers of glomerular intrinsic cells by immunofluorescence, including endothelial cells identified by staining with primary antibodies against CD31 (R&D Systems, Minneapolis, MN, USA; AF3628) 16 and mesangial cells identified by staining with primary antibodies against α‐smooth muscle actin (α‐SMA; Abcam; ab5694) 17 and podocytes identified by staining with primary antibodies against podocalixin (R&D Systems; MAB1658) 18.
Renal specimens of AAV patients were embedded in optimum cutting temperature (OCT) compound, frozen in acetone dry ice mixture and cut into 3–5‐μm sections on a cryostat and stored at −80°C until use. Non‐specific binding sites were blocked with PBS containing 5% bovine serum for 1 h at room temperature. For double staining, we incubated the specimens overnight with the first primary antibody at 4°C. After washing with PBS, the corresponding secondary antibody was applied for 1 h. Sections were then incubated overnight with the second primary antibody at 4°C. After washing with PBS, the corresponding secondary antibody was applied for 1 h. The samples were washed with PBS, stained with 4′,6‐diamidino‐2‐phenylindole (DAPI) (Zhongshan Golden Bridge Biotechnology, Beijing, China) and mounted with cover clips. In negative controls, primary antibodies were replaced by PBS. Secondary antibodies (Jackson ImmunoResearch; no. 115‐605‐003, 115‐025‐003, 111‐605‐045 and 305‐605‐003) matched with a corresponding primary antibody were used to display fluorescent signals (1 : 200 dilution). Confocal images were captured with a Zeiss LSM 710 confocal microscope (Zeiss, Jena, Germany). Images were exported from the ZEN 2012 (blue edition) microscopy software.
Statistical analysis
For normally distributed data, we used a t‐test to assess differences of quantitative parameters between groups, and present descriptive statistics for these data as mean ± standard deviation. For non‐normally distributed data, we used the non‐parametric test to assess differences of quantitative parameters between groups, and present descriptive statistics for these data as median and interquartile range (IQR). We analysed the association between two continuous variables using Pearson's correlation (for two parametric variables) or Spearman's rank correlation (for two non‐parametric variables, or one non‐parametric variable with one parametric variable). Spearman's correlation was used to analyse the association between two categorical variables. A P‐value of 0·05 or less was considered statistically significant. Analysis was performed with spss version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Demographic and general data
Among the 51 patients in active stage of AAV, 23 were male and 28 were female. According to the Chapel Hill Consensus Conference definition 3, 15 of these patients were classified as GPA and the remaining 36 patients were classified as MPA. The level of Birmingham Vasculitis Activity Scores (BVAS) 19 was 17·2 ± 6·3 (range 3–32). The clinical and histopathological data are listed in Table 1.
Table 1.
Clinical and histopathological data of 52 patients with ANCA‐associated vasculitis
| Parameters | Number |
|---|---|
| Total number of patients | 51 |
| Male/female | 23/28 |
| Average age at disease onset (years) | 48·6 ± 15·4 |
| Serum creatinine (μmol/l) | |
| Median (IQR) | 343·0 (187·0–438·0) |
| Range | 58·7–1748 |
| MPO‐ANCA positive patients | 45 |
| PR3‐ANCA positive patients | 8 |
| Renal insufficiency at diagnosis | 46 (88%) |
| Urinary protein (g/24 h) | |
| Median, IQR | 2·0 (1·0–3·6) |
| Range | 0·3–10·2 |
| Skin rash | 4 (7·6%) |
| Arthralgia | 8 (15·4%) |
| Muscle pain | 7 (13·4%) |
| Pulmonary | 29 (55·7%) |
| ENT | 3 (5·8%) |
| Ophthalmic involvement | 3 (5·8%) |
| Gastrointestinal involvement | 1 (1·9%) |
| Nervous system | 1 (1·9%) |
| BVAS | 17·2 ± 6·3 |
| Average glomeruli per biopsy | 14·8 ± 7·1 |
| Glomerular lesions (%) | |
| Total crescents | 32·5 ± 29·1 |
| Tubulointerstitial lesions | |
| Interstitial infiltration (–/+/++/+++) | 0/23/15/13 |
| Interstitial fibrosis (–/+/++) | 20/30/1 |
| Tubular atrophy (–/+/++) | 8/30/13 |
ANCA = anti‐neutrophil cytoplasmic antibody; MPO = myeloperoxidase; PR3 = proteinase 3; BVAS = Birmingham Vasculitis Activity Scores; ENT = ear, nose and throat; IQR = interquartile range; s.d. = standard deviation.
Immunohistochemistry for CD46, CD55 and CD59
In renal specimens, immunohistochemical examination revealed high expression of CD46 and CD55 in the glomeruli of normal controls, while CD46 and CD55 were detected at low levels or with scanty expression in glomeruli of AAV patients (Fig. 1a,b,d,e). The mean optical densities of CD46 and CD55 in glomeruli of AAV patients were significantly lower than those of normal controls [0·0159 (0·0024–0·0292) versus 0·0281 (0·0232–0·0330), P < 0·05; 0·0141 (0·0002–0·0282) versus 0·0403 (0·0135–0·0671), P < 0·05; respectively] (Fig. 1g,h). The CD46 and CD55 were also detected in the proximal tubules, distal tubules and collecting ducts of both normal controls and AAV patients (Fig. 2a,b,d,e). The scores of CD46 and CD55 in the tubules of AAV patients were significantly lower than those of normal controls (P < 0·001 and P < 0·001). In addition, the CD46 and CD55 were detected on inflammatory cells in the interstitial compartments (Fig. 3a,b).
Figure 1.
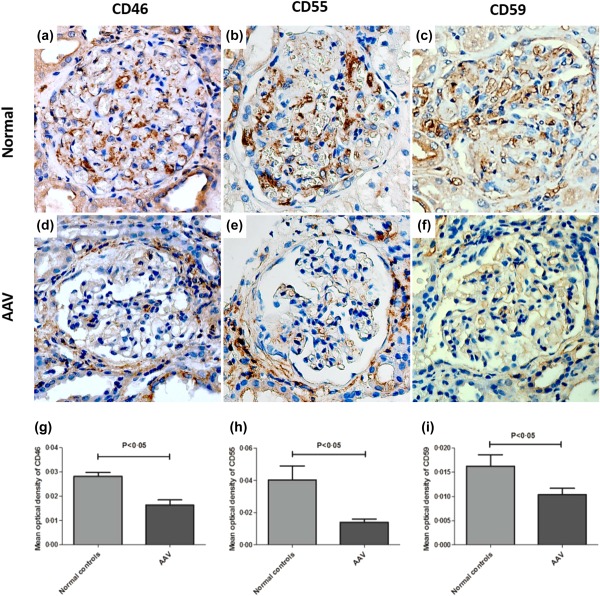
Immunohistochemistry staining for CD46, CD55 and CD59 in glomeruli of renal specimens. (a–c) Immunohistochemical staining of CD46, CD55 and CD59 in glomeruli of a normal control. (d–f) Immunohistochemical staining of CD46, CD55 and CD59 in glomeruli of patient with anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis. (g–i) Comparison of the mean optical density of CD46, CD55 and CD59 in glomeruli of patients with ANCA‐associated vasculitis to that of normal controls. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
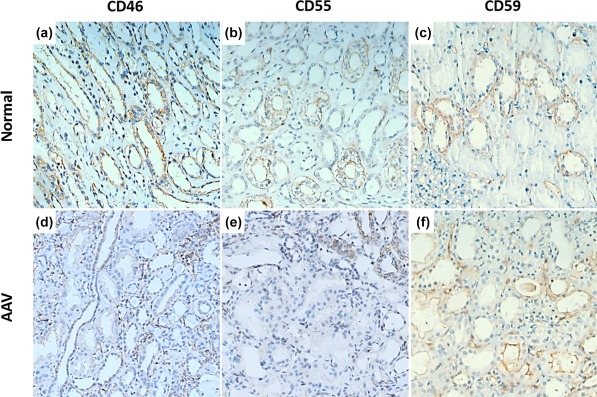
Immunohistochemistry staining for CD46, CD55 and CD59 in the tubulointerstitial compartment of renal specimens. (a–c) Immunohistochemical staining of CD46, CD55 and CD59 in the tubulointerstitial compartment of a normal control. (d–f) Immunohistochemical staining of CD46, CD55 and CD59 in the tubulointerstitial compartment of patient with anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
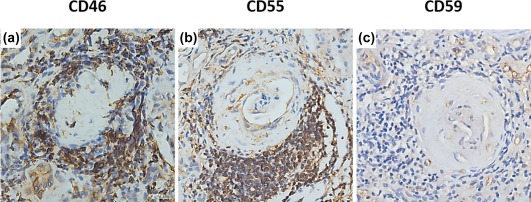
Immunohistochemical staining of CD46, CD55 and CD59 on inflammatory cells in renal specimen of a patient with anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis. (a) Immunohistochemical staining of CD46 on inflammatory cells in kidney of anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis. (b) Immunohistochemical staining of CD55 on inflammatory cells in kidney of anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis. (c) Immunohistochemical staining of CD59 on inflammatory cells in kidney of anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis. [Colour figure can be viewed at wileyonlinelibrary.com]
In renal specimens, CD59 was detected mainly along the glomerular capillary wall in the glomeruli and in small vessels of normal controls and AVV patients (Fig. 1c,f). The mean optical density of CD59 in glomeruli of AAV patients was significantly lower than that of normal controls [0·0102 (0·0006–0·0196) versus 0·0162 (0·0083–0·0241), P < 0·05] (Fig. 1i). CD59 expression was also detected in tubules of AAV patients and normal controls, especially in the proximal and distal tubules (Fig. 2c,f). There was no significant difference between the scores of CD59 expression in tubules of AAV patients and those of normal controls. In renal specimens of AAV patients, CD59 was scanty on inflammatory cells (Fig. 3c).
Relationship between renal expression of the three mCRPs (CD46, CD55, CD59) and clinical and pathological parameters of AAV patients
The mean optical density of CD46 in glomeruli was correlated inversely with the proportion of normal glomeruli (r = –0·305, P = 0·026), and the expression score of CD46 in tubules was correlated with tubular atrophy in renal interstitium (r = 0·330, P = 0·023) (Fig. 4a). The mean optical density of CD55 in glomeruli was correlated with the proportion of total crescents (r = 0·0384, P = 0·006) (Fig. 4b). The mean optical density of CD59 in glomeruli was correlated with the proportion of total crescents (r = 0·0351, P = 0·011) (Fig. 4c).
Figure 4.
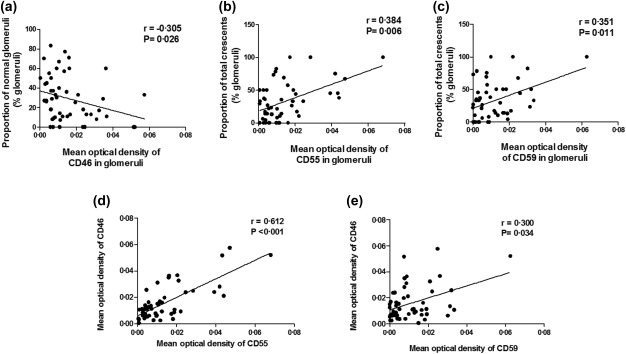
Association between the mean optical density of CD46, CD55 and CD59 in the glomeruli and clinicopathological parameters of patients with anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis. (a) Association between the mean optical density of CD46 in the glomeruli and the proportion of normal glomeruli. (b) Association between the mean optical density of CD55 in the glomeruli and the proportion of total crescents in renal specimens. (c) Association between the mean optical density of CD59 in the glomeruli and the proportion of total crescents in renal specimens. (d) Association between the mean optical density of CD46 and that of CD55 in the glomeruli. (e) Association between the mean optical density of CD46 and that of CD59 in the glomeruli.
The expression of CD46, CD55 and CD59 in renal specimens of AAV patients was analysed with regard to clinical parameters such as age, Birmingham Vasculitis Activity Score (BVAS), serum creatinine, eGFR, 24 h proteinuria level, blood pressure and haematuria. Analysis showed that the expression level of CD59 of AAV patients with haematuria was significantly lower than that of AAV patients without haematuria [0·0062 (0·000–0·0143) versus 0·0137 (0·0006–0·0267, P < 0·5)].
In addition, we found that the mean optical density of CD46 in glomeruli correlated with the mean optical density of CD55 and CD59 in glomeruli (r = 0·612, P < 0·001; r = 0·300, P = 0·034, respectively) (Fig. 4d,e).
Co‐localization of mCRPs and specific markers of three renal intrinsic cells
Immunohistochemical assay revealed that all three mCRPs (CD46, CD55 and CD59) were detected in glomeruli of normal controls and AAV patients. We performed double staining to determine further which type of glomerular intrinsic cells expressed the three mCRPs. We found that CD46, CD55 and CD59 could co‐localize, to varying degrees, with CD31, α‐SMA and podocalyxin in glomeruli (Fig. 5), suggesting that all three mCRPs were expressed on endothelial cells, mesangial cells and podocytes. In addition, in glomeruli of normal controls, CD46 co‐localized equally with CD31, α‐SMA and podocalyxin (Fig. 5a–c), whereas CD55 and CD59 co‐localized mainly with CD31 and podocalyxin (Fig. 5d,f,g,i).
Figure 5.
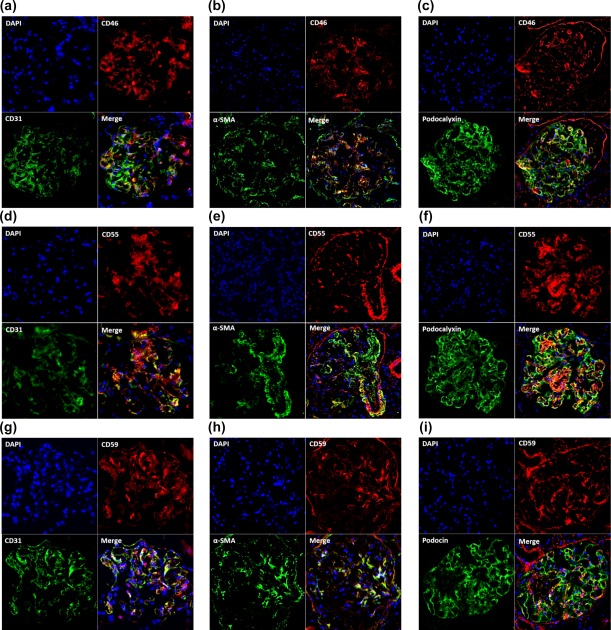
Double immunofluorescence staining of CD46, CD55, CD59 and markers of three glomerular intrinsic cells in renal specimens of normal controls. (a–c) Co‐localization of CD46 (red) with CD31 (green), α‐smooth muscle actin (α‐SMA) (green) and podocalyxin (green) in glomeruli. (d–f) Co‐localization of CD55 (red) with CD31 (green), α‐SMA (green) and podocalyxin (green) in glomeruli. (g–i) Co‐localization of CD59 (red) with CD31 (green), α‐SMA (green) and podocalyxin (green) in glomeruli. The specific co‐staining for CD31, α‐SMA, podocalyxin and the three complement regulatory proteins (CRPs) was apparent in the merged picture as yellow/orange in glomeruli. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
Considerable evidence has confirmed that activation of the complement system is involved in the pathogenesis of AAV 7, 20, 21. The complement activation products were detected in kidneys of patients with AAV, even in ANCA‐negative AAV patients 4, 5, 6, 7, 22. The complement system was regulated strictly in the normal state. CRPs play an important role in inhibiting the amplification of complement activation via targeting on different components. As key mCRPs, CD46 and CD55 functioned to reduce C3/C5 convertase activity 23, 24, while CD59 suppressed the formation of C5b‐9 25, 26. Until now, the expression of mCRPs in kidneys of patients with AAV has not been clear. Therefore, in the present study, the expression of mCRPs CD46, CD55 and CD59 in kidneys of AAV patients was investigated, and their relationships with clinical parameters were analysed further.
We observed that the expression levels of the three mCRPs CD46, CD55 and CD59 of AAV patients were remarkably lower than those of normal controls. Complement activation‐mediated injury might occur if regulatory proteins were down‐regulated and then could not protect host cell surfaces adequately. Complement activation was regulated mainly at two stages in the pathways: formation of the C3/C5 convertase and assembly of the C5b‐9 8. Decreased expression of CD46 and CD55 could lead to C3/C5 convertase activation and the increase of complement activation fragments, such as Bb, Ba, C3a, C3d and C5a, while a decrease of CD59 could lead to the formation of C5b‐9. Our results matched our previous study 6, which indicated the deposition of Bb, C3d and C5b‐9 in glomeruli of AAV patients. The decreased expression of mCRPs in glomeruli might contribute to local complement activation.
Among AAV patients, we found that the expression level of CD46 in glomeruli was correlated inversely with the proportion of normal glomeruli, and the expression level of CD46 on tubules was correlated with tubular atrophy. In addition, the expression levels of CD55 and CD59 in glomeruli were correlated with the proportion of total crescents. We speculated that the mCRPs could up‐regulate as feedback once the complement system was over‐activated, which might be a protective response. On the basis of the severity of AAV, these results suggest that CRPs could be regulated adaptively and partly reflect the severity of renal histopathological injury in AAV.
The study by Kusano and Takano 27 showed that haematuria was associated with damage to endothelial cells. In the present study, double immunofluorescence staining showed that CD59 was expressed mainly on endothelial cells. In addition, expression of CD59 in AAV patients with haematuria was lower than that in AAV patients without haematuria. These findings indicate that a decrease of CD59, the main regulatory factor for the formation of C5b‐9, might be associated with endothelial injury in AAV.
In summary, expression levels of CD46, CD55 and CD59 were dysregulated in kidneys of patients with AAV. Expression levels of CD46, CD55 and CD59 were associated with the severity of renal injury of AAV patients.
Disclosure
All authors declare no competing interests.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (no. 81500524).
References
- 1. Segelmark M, Wieslander J. IgG subclasses of antineutrophil cytoplasm autoantibodies (ANCA). Nephrol Dial Transplant 1993; 8:696–702. [DOI] [PubMed] [Google Scholar]
- 2. Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti‐neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro . Proc Natl Acad Sci USA 1990; 87:4115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jennette JC, Falk RJ, Bacon PA et al 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum 2013; 65:1–11. [DOI] [PubMed] [Google Scholar]
- 4. Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti‐neutrophil cytoplasmic autoantibodies. Am J Pathol 2007; 170:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen M, Xing GQ, Yu F, Liu G, Zhao MH. Complement deposition in renal histopathology of patients with ANCA‐associated pauci‐immune glomerulonephritis. Nephrol Dial Transplant 2009; 24:1247–52. [DOI] [PubMed] [Google Scholar]
- 6. Gou SJ, Yuan J, Chen M, Yu F, Zhao MH. Circulating complement activation in patients with anti‐neutrophil cytoplasmic antibody associated vasculitis. Kidney Int 2013; 83:129–37. [DOI] [PubMed] [Google Scholar]
- 7. Gou SJ, Yuan J, Wang C, Zhao MH, Chen M. Alternative complement pathway activation products in urine and kidneys of patients with ANCA‐associated GN. Clin J Am Soc Nephrol 2013; 8:1884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thurman JM, Renner B. Dynamic control of the complement system by modulated expression of regulatory proteins. Lab Invest 2011; 91:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Endoh M, Yamashina M, Ohi H et al Immunohistochemical demonstration of membrane cofactor protein (MCP) of complement in normal and diseased kidney tissues. Clin Exp Immunol 1993; 94:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cosio FG, Sedmak DD, Mahan JD, Nahman NS Jr. Localization of decay accelerating factor in normal and diseased kidneys. Kidney Int 1989; 36:100–7. [DOI] [PubMed] [Google Scholar]
- 11. Tamai H, Matsuo S, Fukatsu A et al Localization of 20‐kD homologous restriction factor (HRF20) in diseased human glomeruli. An immunofluorescence study. Clin Exp Immunol 1991; 84:256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bajema IM, Hagen EC, Hermans J et al Kidney biopsy as a predictor for renal outcome in ANCA‐associated necrotizing glomerulonephritis. Kidney Int 1999; 56:1751–8. [DOI] [PubMed] [Google Scholar]
- 13. Bajema IM, Hagen EC, Hansen BE et al The renal histopathology in systemic vasculitis: an international survey study of inter‐ and intra‐observer agreement. Nephrol Dial Transplant 1996; 11:1989–95. [DOI] [PubMed] [Google Scholar]
- 14. Hauer HA, Bajema IM, van Houwelingen HC et al European Vasculitis Study Group (EUVAS). Renal histology in ANCA‐associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int 2002; 61:80–9. [DOI] [PubMed] [Google Scholar]
- 15. Shang Y, Chai N, Gu Y et al Systematic immunohistochemical analysis of the expression of CD46, CD55 and CD59 in colon cancer. Arch Pathol Lab Med 2014; 138:910–9. [DOI] [PubMed] [Google Scholar]
- 16. Winiarski BK, Acheson N, Gutowski NJ et al An improved and reliable method for isolation of microvascular endothelial cells from human omentum. Microcirculation 2011; 18:635–45. [DOI] [PubMed] [Google Scholar]
- 17. Sarrab RM, Lennon R, Ni L et al Establishment of conditionally immortalized human glomerular mesangial cells in culture, with unique migratory properties. Am J Physiol Renal Physiol 2011; 301:F1131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nielsen JS, McNagny KM. The role of podocalyxin in health and disease. J Am Soc Nephrol 2009; 20:1669–76. [DOI] [PubMed] [Google Scholar]
- 19. Luqmani RA, Bacon PA, Moots RJ et al Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Q J Med 1994; 87:671–8. [PubMed] [Google Scholar]
- 20. Hilhorst M, van Paassen P, van Rie H et al Complement in ANCA‐associated glomerulonephritis. Nephrol Dial Transplant 2015; 13:gfv288. [DOI] [PubMed] [Google Scholar]
- 21. Xing GQ, Chen M, Liu G et al Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci‐immune vasculitis. J Clin Immunol 2009; 29:282–91. [DOI] [PubMed] [Google Scholar]
- 22. Sethi S, Zand L, De Vriese AS et al Complement activation in pauci‐immune necrotizing and crescentic glomerulonephritis: results of a proteomic analysis. Nephrol Dial Transplant 2017; 32:i139–45. [DOI] [PubMed] [Google Scholar]
- 23. Oglesby TJ, Allen CJ, Liszewski MK et al Membrane cofactor protein (CD46) protects cells from complement‐mediated attack by an intrinsic mechanism. J Exp Med 1992; 175:1547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nicholson‐Weller A, Wang C. Structure and function of decay accelerating factor CD55. J Lab Clin Med 1994; 123:485–91. [PubMed] [Google Scholar]
- 25. Morgan BP. Isolation and characterization of th complement‐inhibiting protein CD59 antigen from platelet membranes. Biochem J 1992; 282:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davies A, Lachmann PJ. Membrane defence against complement lysis: the structure and biological properties of CD59. Immunol Res 1993; 12:258–75. [DOI] [PubMed] [Google Scholar]
- 27. Kusano T, Takano H. Endothelial cell injury in acute and chronic glomerular lesions in patients with IgA nephropathy. Hum Pathol 2016; 49:135–44. [DOI] [PubMed] [Google Scholar]


