Summary
Langerhans’ cells (LC) play pivotal roles in skin immune responses, linking innate and adaptive immunity. In aged skin there are fewer LC and migration is impaired compared with young skin. These changes may contribute to declining skin immunity in the elderly, including increased skin infections and skin cancer. Interleukin‐1β (IL‐1β) and tumour necrosis factor‐α (TNF‐α) are mandatory signals for LC migration and previous studies suggest that IL‐1β signalling may be dysregulated in aged skin. Therefore, we sought to explore the mechanisms underlying these phenomena. In skin biopsies of photoprotected young (< 30 years) and aged (> 70 years) human skin ex vivo, we assessed the impact of trauma, and mandatory LC mobilizing signals on LC migration and gene expression. Biopsy‐related trauma induced LC migration from young epidermis, whereas in aged skin, migration was greatly reduced. Interleukin‐1β treatment restored LC migration in aged epidermis whereas TNF‐α was without effect. In uncultured, aged skin IL‐1β gene expression was lower compared with young skin; following culture, IL‐1β mRNA remained lower in aged skin under control and TNF‐α conditions but was elevated after culture with IL‐1β. Interleukin‐1 receptor type 2 (IL1R2) gene expression was significantly increased in aged, but not young skin, after cytokine treatment. Keratinocyte‐derived factors secreted from young and aged primary cells did not restore or inhibit LC migration from aged and young epidermis, respectively. These data suggest that in aged skin, IL‐1β signalling is diminished due to altered expression of IL1B and decoy receptor gene IL1R2.
Keywords: ageing, Langerhans’ cells, skin
Abbreviations
- IL‐1
interleukin‐1
- IL‐1R1
interleukin 1 receptor type 1
- IL‐1R2
interleukin 1 receptor type 2
- IL‐1RA
interleukin 1 receptor antagonist
- IL‐1RAP
interleukin 1 receptor accessory protein
- LC
Langerhans’ cells
- NLRP3
NOD‐like receptor family pyrin domain‐containing 3
- TNFR
tumour necrosis factor receptor
- TNF‐α
tumour necrosis factor‐α
Introduction
Langerhans’ cells (LC) are antigen‐presenting cells that reside within the epidermis forming a protective network. They provide the first line of cellular defence against invading pathogens and other antigenic or inflammatory stimuli and in recent years an additional vital role for LC as regulators of cutaneous tolerogenicity and immune homeostasis has been reported.1, 2 Langerhans’ cells are often described as a bridge between innate and adaptive immunity as they travel from the epithelial barrier to regional lymphoid tissue where they are believed to facilitate adaptive T‐cell immunity and promote tolerance to self‐antigens,3 highlighting their functional complexity within the skin. Studies of skin sensitization in mice have elegantly demonstrated the requirement for two signals, tumour necrosis factor‐α (TNF‐α) and interleukin‐1β (IL‐1β), for the induction of migration and accumulation of LC in lymph nodes.4 Keratinocyte‐derived TNF‐α, stimulated either directly via allergen‐induced inflammation, or by LC‐derived IL‐1β, is believed to signal through the TNF receptor 2 (TNFR2) on LC to stimulate IL‐1β production. The LC‐derived IL‐1β is then thought to act in both an autocrine and paracrine manner to stimulate keratinocytes to produce TNF‐α. This results in a feedback loop that leads to down‐regulation of adhesion molecules such as E‐cadherin, and increased expression of collagenases such as matrix metalloproteinase‐9, that facilitate the passage of LC through the basement membrane.5 This mechanism of action is supported by experiments using neutralizing antibodies, where blocking either one of these cytokine signals inhibits contact‐allergen‐induced LC migration in mice. Studies in healthy human skin indicate that a parallel mechanism is present, as intradermal injection of either IL‐1β or TNF‐α in vivo results in reduced epidermal LC frequency, and IL‐1β injection induces TNF‐α production.6
With increasing age, components of both the innate and adaptive immune systems in the skin decline, and this is associated with an increased susceptibility to cutaneous infections and skin cancer,7 in addition to poor vaccination efficacy.8 Ageing tissues are subject to both immunosenescence and inflammageing, where immune competency deteriorates and chronic low‐grade inflammation ensues.9 In elderly humans and in aged mice, contact hypersensitivity responses are diminished, epidermal LC numbers are reduced, and the ability of LC to migrate in response to TNF‐α is impaired.10, 11 However, we have previously shown that intradermal injection of IL‐1β into aged skin results in a restoration of LC migration equivalent to levels seen in young skin.11, 12 This indicates that age‐related changes in the availability of IL‐1β may contribute to the reduced mobilization of LC observed in the skin of the elderly. In mice, LC are purported to be the sole source of IL‐1β in the epidermis13 whereas in humans, keratinocytes are reported to express this cytokine under stimulatory conditions.14
Studies in ageing mice have implicated both intrinsic changes, and a distorted microenvironment in the age‐associated reduction in dendritic cell trafficking,15 it remains to be determined whether this is also the case in aged human skin. Senescence in ageing is believed to impact on epithelial and immune cells alike. However, the changes that occur within the skin microenvironment during ageing that may impact on LC function are not well characterized.
To gain greater insight into the altered functionality of LC during human skin ageing we employed an ex vivo epidermal model of LC migration.16 We describe the validation of this model for studying LC in aged skin and explore the potential contribution of altered IL‐1β signalling and the epidermal microenvironment to impaired LC migration.
Methods
Participants
Ethical approval was granted by the University of Manchester Research Ethics Committee (ref. 14415) and the study was performed in accordance with the Declaration of Helsinki principles. Healthy young (≤ 30 years old; n = 39) and aged (≥ 70 years old; n = 35) participants were recruited and written informed consent was obtained before inclusion of volunteers in the study. Participants were excluded if they had active skin conditions (e.g. psoriasis or atopic dermatitis), had sunbathed or used a sunbed in the 6 weeks before taking part, or were taking anti‐inflammatory medication such as steroids. A number of volunteers in the aged group were taking daily aspirin (75 mg) and/or medication for hypertension and hypercholesterolaemia. Each volunteer provided four 6‐mm skin punch biopsies from photoprotected buttock skin, taken under local anaesthetic (2% lignocaine).
Generation of primary human keratinocytes and conditioned medium
Skin punch biopsies were transported to the laboratory in Hanks’ buffered salt solution (Sigma Aldrich, Poole, UK) with 100 U/ml penicillin, 100 mg/ml streptomycin and 250 ng/ml amphotericin B (Sigma Aldrich) and keratinocytes were isolated as described previously.17 Basal keratinocytes were grown to passage 2 or 3 in complete EpiLife® (Thermo Fisher, Waltham, MA, USA) medium (60 μm calcium,+ human keratinocyte growth supplement, 100 U/ml penicillin, 100 mg/ml streptomycin and 250 ng/ml amphotericin B). At confluence the medium was refreshed and the cells were cultured for 6 days before conditioned medium was collected and stored at −80° (young n = 7, aged n = 6).
Epidermal explant culture
Epidermal sheets were prepared as described previously.18 One sheet was fixed in ice‐cold acetone immediately (T0) for 20 min before storage in PBS at 4° until staining. The remaining epidermal sheets were then floated onto 500 μl of complete RPMI‐1640 medium (10% fetal calf serum, 100 U/ml penicillin, 100 mg/ml streptomycin and 250 ng/ml amphotericin B) ± either 25 ng/ml (2000 U) recombinant human IL‐1β (R & D Systems, Abingdon, UK) or 250 ng/ml (5000 U) of human recombinant TNF‐α (Peprotech, London, UK) in 24‐well plates for 24 hr (n = 16 per age group for IL‐1β and n = 5 per age group for TNF‐α). In separate experiments epidermal sheets were also treated with 8 ng/ml and 32 ng/ml IL‐1α (R&D Systems). To assess the impact of keratinocyte‐derived factors on LC migration, epidermal sheets were prepared in the same way but were floated on complete EpiLife® medium or keratinocyte conditioned medium (n = 3 per age group) obtained from cells derived from young and aged skin.
Langerhans’ cells staining and enumeration
Following culture, epidermal sheets were fixed in ice‐cold acetone for 20 min and washed in PBS. Cultured and T0 epidermal sheets were then stained with a rat CD207 antibody (clone 929F3; 1/100; Dendritics, Lyon, France) for 1 hr at room temperature and goat anti‐rat Alexafluor 488 (1/200, Thermo Fisher) for 30 min at room temperature. Stained epidermal sheets were then mounted onto glass slides using Vectashield mounting medium (VectorLabs, Peterborough, UK) and the frequency of stained cells was assessed. The % LC migration was calculated using the following equation: [LC per mm2 (non‐cultured; T0) – LC per mm2 (T24)]/ LC/mm2 (T0) × 100.
Skin explant culture
Skin biopsies (6 mm) were collected and one was bisected and placed into RNAlater (Thermo Fisher) immediately (T0). The remaining skin biopsies were placed in RPMI‐1640 (10% fetal calf serum) at 37° for up to 1 hr during transport. Biopsies were then transferred in transwell inserts in 24‐well plates to support the biopsy and maintain an air interface at the epidermis. Biopsies were cultured for 2 hr in complete RPMI‐1640 medium ± 25 ng/ml recombinant human IL‐1β or 250 ng/ml of human recombinant TNF‐α in 24‐well plates. Following culture, biopsies were bisected and placed into RNAlater for up to 48 hr at 4° before longer‐term storage at −80° (n = 6 per age group).
Tissue homogenization, total RNA extraction and cDNA generation
Skin biopsies in RNA later were thawed on ice and minced on an ice cold Petri dish using a scalpel. Tissue was then placed in 1 ml of Trizol (Fisher Scientific, Loughborough, UK) in tubes pre‐filled with Triple Pure High Impact Zirconium beads (3 mm) and homogenized using a Bead Bug Microtube homogenizer (BenchMark Scientific, Sayreville, NJ). The samples were centrifuged at 10 000 g for 15 min at 4° and the supernatant was transferred to a clean RNAase‐free tube before addition of 250 μl of chloroform (Sigma Aldrich). Tubes were shaken by hand for 30 seconds and centrifuged again at 12 000 g for 15 min at 4°. The aqueous top layer was transferred to a clean tube and total RNA extraction was performed using an Ambion RNA mini kit (Fisher Scientific) according to the manufacturer's instructions. RNA was eluted into 50 μl distilled water and genomic DNA was removed using an Ambion DNA‐free™ kit (Fisher Scientific) according to the manufacturer's instructions. Complementary DNA was then generated from 50 ng RNA using an Applied Biosystems High Capacity RNA to cDNA™ kit (Fisher Scientific) according to the manufacturer's instructions in a thermocycler and cDNA was reconstituted in distilled water up to 200 μl.
Real‐time quantitative PCR
Taqman gene expression primer/probe sets for IL1B, IL‐1RAP, IL‐1R2, IL‐1R1, IL‐1RA, CASPASE‐1, TNFA, TNFR1A, TNFR1B, NLRP3 and RPL27 (internal control gene) were purchased from Thermo Fisher and PCR was performed using a Step One Plus Real‐Time PCR machine and Taqman Fast Universal Mastermix No AmpErase® UNG (ThermoFisher) for 40 cycles. All samples were assayed in triplicate and gene expression changes were calculated using the comparative CT method. The CT value of the internal control was subtracted from the sample CT value to obtain ∆CT. Gene expression changes are then expressed as fold change of the ∆∆CT (∆CT relative to the average young T0 ∆CT value for each gene). Where no amplification was detected, a CT value of 40 was substituted to allow the data to be included in the analyses.
Enzyme‐linked immunoassay
Levels of secreted ADAMTS1, IL‐1β and TNF‐α in keratinocyte or explant supernatants were measured by ELISA (R&D Systems) according to the manufacturer's instructions.
Statistics
graphpad prism 7 was used to perform all statistical analyses. Unpaired t‐tests were used for comparison of baseline LC numbers and gene expression differences, and secreted keratinocyte factors between the two age groups. Paired t‐tests with Bonferroni correction or one‐way repeated measures analysis of variance with post‐hoc comparisons were used to compare LC counts between T0 and cultured epidermal sheets within an age group. Langerhans’ cell migration data and gene expression data in stimulated skin were analysed by two‐way repeated measures analysis of variance with post‐hoc comparisons of treatment groups to control groups. Statistically significant results were defined as P < 0·05.
Results
Langerhans’ cell migration in aged skin is impaired during epidermal explant culture and can be restored by addition of IL‐1β
To facilitate a more detailed study of age‐associated changes in LC function in human skin we first wished to determine whether the impaired migration of LC in aged skin could be replicated ex vivo. To this end we employed an epidermal explant model to compare trauma‐induced LC migration, initiated by the skin biopsy procedure itself, from epidermal sheets of young and aged individuals. When grouping data together from all individuals that took part in the study it was clear that LC numbers were significantly lower in aged skin compared with young skin (~ 20% fewer; Fig. 1a) as previously reported.10 Effects on LC migration during culture were determined by subtracting the LC frequency/mm2 in the cultured epidermal sheets (T24) from the frequency/mm2 in non‐cultured (T0) epidermal sheets, dividing this number by the T0 sheet LC frequency and then multiplying by 100. Following 24‐hr culture of epidermal sheets with control medium the per cent LC migration from aged epidermis was only 4% compared with 15% from young epidermis (Fig. 1b), demonstrating that trauma‐induced LC migration is affected in ageing skin.
Figure 1.
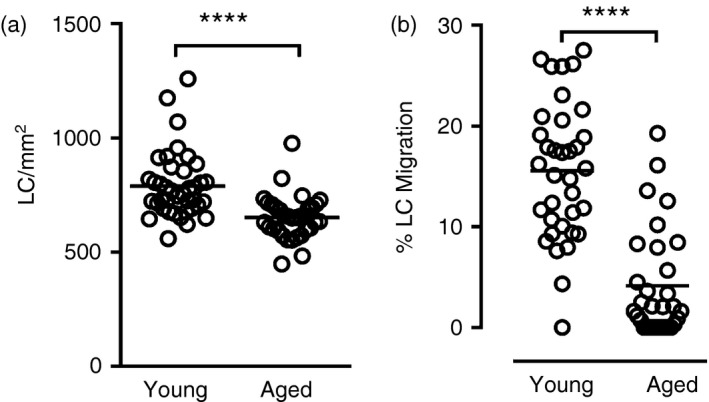
In aged epidermis Langerhans’ cell (LC) frequency is significantly lower than in young epidermis, and LC migration from epidermal explants in response to biopsy trauma and culture is impaired. These graphs present a summary of data collected across a number of experiments presented in this manuscript (a: n = 39 young, n = 35 aged; b: n = 33 young, n = 31 aged). LC frequency in young and aged epidermis was determined in epidermal sheets fixed immediately (T0) after isolation (a) and in epidermal sheets cultured for 24 hr; % LC migration during culture was calculated relative to the T0 sheet (b). Each circle represents a different individual and the horizontal line indicates the mean, **** P < 0·0001.
To determine the impact of LC mobilizing signals, IL‐1β and TNF‐α, on this age‐associated impairment, LC frequency in cultured epidermis was assessed after treatment with recombinant human cytokines. In young epidermis no effect of IL‐1β or TNF‐α was seen compared with control culture and LC migration was maintained at 15–20% (Fig. 2a). In line with in vivo observations, in aged epidermis TNF‐α treatment induced only 7% LC migration and did not improve the response when compared with control cultured epidermal sheets (Fig. 2b). In contrast, treatment with IL‐1β restored LC migration from aged epidermis to 15%, a response equivalent to that seen in young epidermis. This further validated the epidermal explant culture as a useful model for studying the impairment in LC migration in aged skin. No detectable IL‐1β or TNF‐α secreted protein was found in the supernatants of control cultured epidermal explants.
Figure 2.
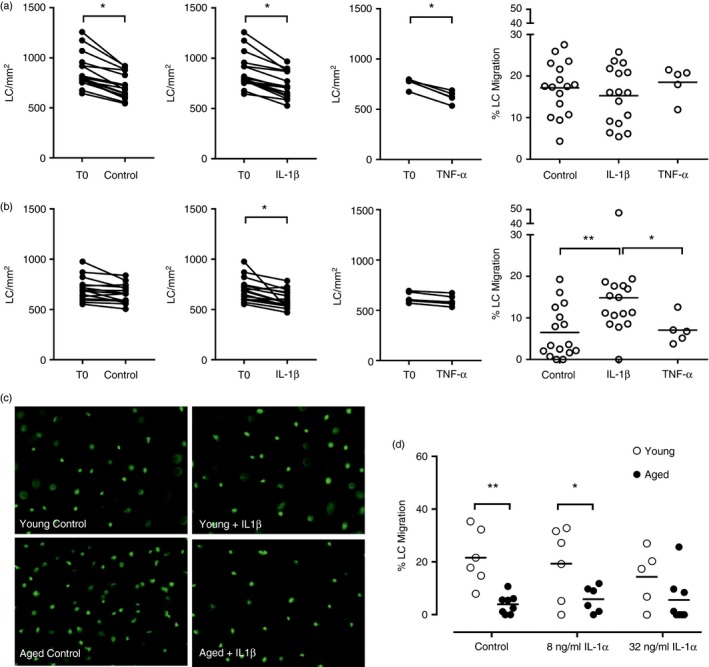
Impaired Langerhans’ cell (LC) migration from aged epidermis in the explant model can be restored by treatment with interleukin‐1β (IL‐1β) but not tumour necrosis factor‐α (TNF‐α). Epidermal sheets from young (a) and aged (b) skin were fixed immediately (T0) or cultured in medium alone (n = 16/age group), or in the presence of 25 ng/ml IL‐1β (n = 16/age group) or 250 ng/ml TNF‐α (n = 5/age group). LC frequency was determined for T0 and cultured epidermal sheets and the % LC migration from young and aged epidermis following each culture condition was calculated relative to the T0 sheet. Experiments were also performed to examine the effect of IL‐1α on LC migration (n = 5–7/age group). Representative photomicrographs for one young and one aged individual's epidermal sheets with CD207+ LC (green) are shown following control culture and culture with IL‐1β. Adjoined circles represent the same individual and the mean % LC migration is indicated by the horizontal line, *P < 0·05, **P < 0·01. [Colour figure can be viewed at wileyonlinelibrary.com]
In a smaller number of individuals, experiments were also performed to assess the potential of IL‐1α to restore LC migration (Fig. 2d). The concentrations of IL‐1α were chosen based on relative biological activity to IL‐1β, and were determined in the same in vitro cell‐based assay by the manufacturer. Interleukin‐1α was not effective at increasing LC migration from aged epidermis at either concentration used with only 6% migration compared with 4% migration under control culture conditions. In young epidermis the mean % LC migration was ~20% in the control culture and this was maintained at 19% and 14% after treatment with 8 ng/ml and 32 ng/ml of IL‐1α, respectively.
Expression of IL1B mRNA is lower in unstimulated aged human skin compared with young skin
Restoration of LC migration from aged epidermis by exogenous IL‐1β suggests that cells are only fully functional when in receipt of the necessary signals from the surrounding microenvironment. Hence, we sought to determine whether the properties and function of skin microenvironment are altered or disrupted in ageing, particularly in relation to IL‐1β signalling. To explore this we assessed the expression of genes implicated in the IL‐1 and TNF pathways in baseline (T0) whole skin samples of young and aged donors. The mean fold change in IL1B mRNA was found to be significantly lower in aged skin compared with young skin (Fig. 3). Expression of ILR1, IL1RA, IL1R2, IL1RAP, CASPASE‐1, NLRP3, TNFA, TNFRSF1A (TNF‐R1) and TNFRSF1B (TNF‐R2) was also measured and no differences were found in these transcripts between young and aged skin.
Figure 3.
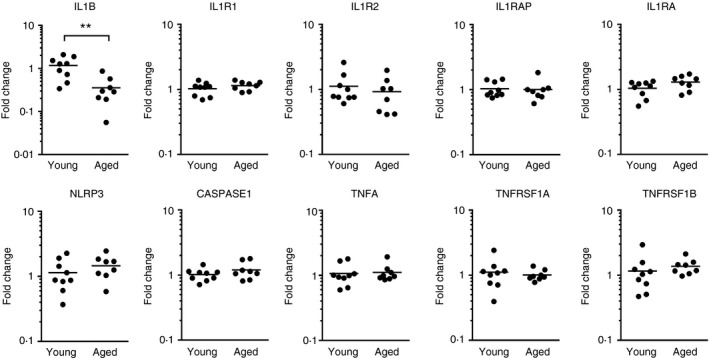
Interleukin‐1B (IL1B) gene expression is lower in aged skin than in young skin. Quantitative PCR was used to assess mRNA expression of genes involved in IL‐1 and tumour necrosis factor‐α (TNF‐α) signalling in young (n = 9) and aged (n = 8) unstimulated human skin. Results are presented for each individual as the fold change in gene expression relative to the average gene expression in the young group. Each circle represents a different individual and the horizontal line indicates the mean, **P < 0·01.
Langerhans’ cell migration from young and aged epidermis is not affected by keratinocyte‐derived factors ex vivo
As the reduced expression of IL1B in resting aged skin indicates a dysregulated cutaneous immunological environment we wanted to determine the impact of ageing on keratinocytes, and how this may influence LC migration. We have previously found that keratinocytes derived from uninvolved psoriatic skin maintain a disease‐like phenotype in culture and secrete factors that inhibit LC migration from healthy epidermis (Eaton, Mellody, Pilkington, Dearman, Kimber, Griffiths unpublished data). Therefore, to explore the impact of keratinocyte‐derived factors on LC migration in ageing, proliferating keratinocytes were derived from young and aged skin as they were for psoriasis skin, and epidermal sheets of young and aged skin were cultured on conditioned medium generated from these human primary keratinocytes. This method facilitates the generation of sufficient quantities of cells from small amounts of skin, and allows generation of enough supernatant for multiple analyses. The capacity of each keratinocyte donor's LC to migrate was predetermined using the epidermal explant assay to ensure that supernatants were generated from individuals with the typical age‐associated migratory phenotype.
In the first instance we sought to test the hypothesis that keratinocytes in aged skin secrete a factor that inhibits LC migration. Epidermal sheets from young skin were cultured with control medium and a significant reduction in LC frequency after 24 hr was observed compared with the T0 epidermis (equivalent to 13% migration; Fig. 4a). Epidermal sheets from the same young individuals were cultured with conditioned medium from young keratinocytes to control for accumulation of potentially toxic factors in the 6‐day conditioned medium and no significant impact on LC frequency was found. Culture of young epidermis with conditioned medium from aged keratinocytes also had no effect on LC migration (Fig. 4a).
Figure 4.
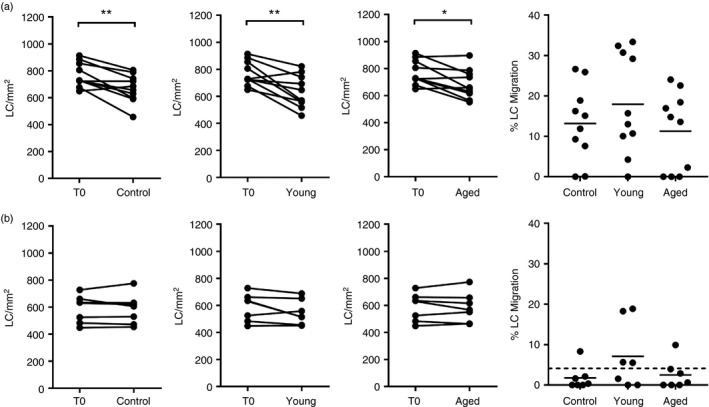
Secreted factors of primary human keratinocytes do not impact on Langerhans’ cell (LC) migration from young or aged epidermis. LC frequency in young (a) and aged (b) epidermis was determined in T0 sheets and sheets were that cultured for 24 hr in control medium or with keratinocyte conditioned medium derived from cells of young and aged donors. The % LC migration from young and aged epidermis following culture under each condition was calculated relative to the corresponding T0 sheet. The dotted line (b) indicates the average % LC migration from aged epidermis shown in Figure 1(a). Adjoined circles represent the same individual and the mean % LC migration is indicated by the horizontal line *P < 0·05, **P < 0·01, (n = 10 young; n = 7 aged).
As no inhibitory effect of aged keratinocyte supernatants was observed we went on to explore the possibility that keratinocytes in aged skin may lack expression of factors required for LC mobilization. Hence, we evaluated the potential of young keratinocyte conditioned medium to promote LC migration from aged epidermis (Fig. 4b). After 24‐hr culture with control medium no change in the LC number in aged epidermis was observed as expected (~2% migration). Furthermore, following culture with young and aged keratinocyte conditioned medium no consistent effect on LC migration was found (7% and 2% LC migration, respectively). The keratinocytes derived from young and aged donors used in this study were in a resting, unstimulated state but showed evidence that an age‐associated phenotype was maintained in culture with elevated secretion of ADAMTS1 from aged cells (Fig. 5). This protease has previously been identified to be expressed in human skin19 and is associated with inflammation,20 senescence21 and ageing.22
Figure 5.
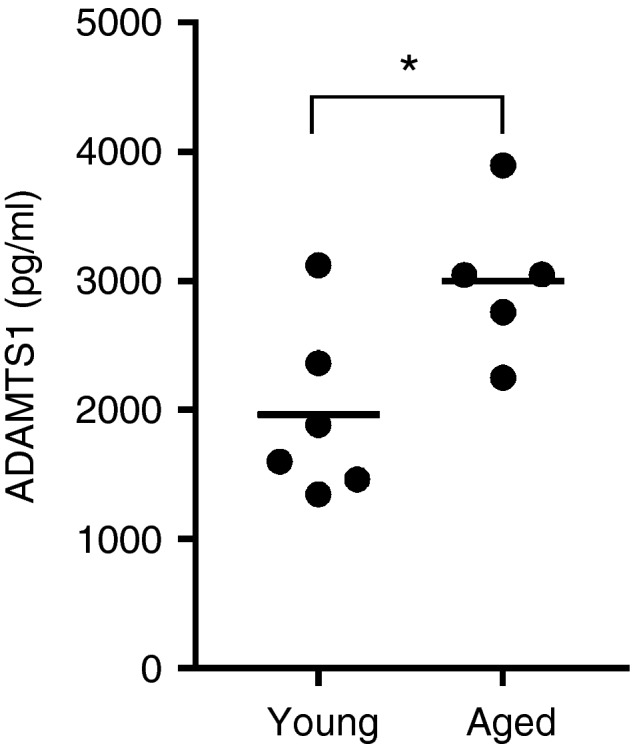
ADAMTS1 concentration in conditioned medium generated from proliferating primary human keratinocytes derived from young (n = 6) and aged (n = 5) skin. Each circle represents a different individual and the horizontal line indicates the mean, *P < 0·05.
IL1B and IL1R2 are differentially expressed between age groups following cytokine stimulation
As conditioned medium was generated from resting keratinocytes and the secreted factors had little impact on LC migration, we went on to examine whether age‐associated differences exist in the expression of IL‐1 and TNF family genes in skin under inflammatory conditions. To test the hypothesis that impaired cytokine or receptor gene expression could be the reason for age‐related impaired LC migration in response to trauma and TNF‐α we cultured whole skin biopsies for 2 hr in the absence and presence of LC mobilization signals, IL‐1β and TNF‐α, and the effect on gene expression was examined.
Trauma‐induced inflammation (control culture) resulted in a ~20‐fold increase in IL1B gene expression in young skin relative to T0, but only a 10‐fold increase in aged skin (Fig. 6). Interleukin‐1β treatment significantly increased IL1B expression in both young (40‐fold) and aged (30‐fold) skin, whereas TNF‐α treatment significantly induced IL1B expression in young skin only. This suggests that aged skin may have a diminished ability to up‐regulate IL1B in response to trauma and TNF‐α. Additionally, in young skin, IL‐1β and TNF‐α treatments demonstrated equivalent abilities to induce the expression of IL1B. No effect of culture, cytokine stimulation or age was seen on CASPASE1, or NLRP3 (data not shown). IL‐1 and TNF receptor‐associated genes IL1R1, IL1RAP, TNFRSF1A and TNFRSF1B were only minimally increased in response to culture alone (~two‐ threefold), and no further impact of cytokine treatment or age was observed. TNFA expression was significantly increased in response to both control culture and both cytokine treatments, although a greater magnitude of response to IL‐1β was observed. Overall, TNFA expression was lower relative to IL1B under all conditions and was consistent between the age groups. Expression of IL1R2, the gene encoding the IL‐1 decoy receptor, which inhibits IL‐1 signalling, was significantly elevated in response to both IL‐1β and TNF‐α compared with the control culture in aged skin but not in young skin.
Figure 6.
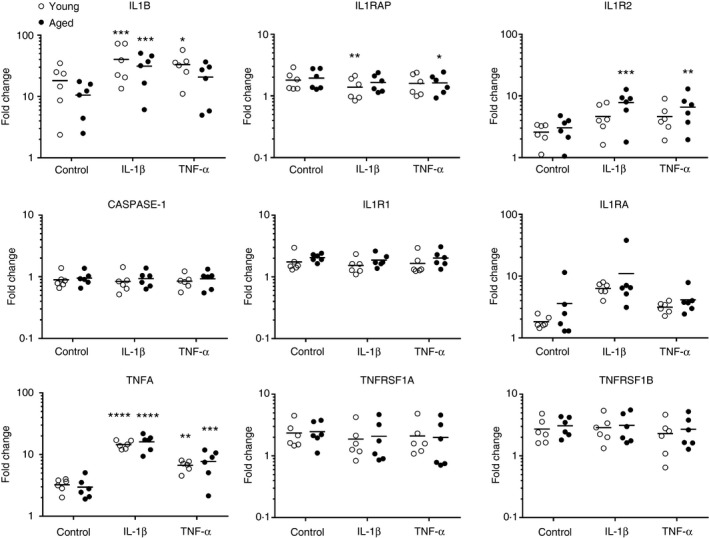
IL1B gene expression is not significantly up‐regulated in aged skin following tumour necrosis factor‐α (TNF‐α) treatment. Quantitative PCR was used to assess mRNA expression of genes involved in interleukin‐1 (IL‐1) and TNF‐α signalling in young and aged human skin (n = 6/ age group) following 24‐hr culture with control medium, 25 ng/ml IL‐1β and 250 ng/ml TNF‐α. Results are presented as the fold change in gene expression for each individual relative to the average gene expression in the young unstimulated (T0) group. Each circle represents a different individual and the horizontal line indicates the mean, ****P < 0·0001, ***P < 0·001, **P < 0·01, *P < 0·05.
Discussion
As the proportion of the population who are elderly continues to grow it is becoming ever more important to develop strategies to promote healthy ageing. Age‐related senescence of the skin immune system is associated with increased susceptibility to infections,8 and skin morbidities in the elderly represent a great economic burden. Langerhans’ cells are regarded as sentinels of the immune system; as such a decline in their numbers and function could have significant consequences for skin health in the aged. To enable further study of defective LC behaviour in aged skin we employed an epidermal explant model that we have previously used to examine the defective migration of LC in uninvolved psoriasis skin.16 Using this model we provide evidence that lower levels of IL‐1β in ageing skin contribute to impaired LC migration.
The epidermal explant model uses trauma‐induced signals, resulting from the biopsy procedure, to study spontaneous LC migration in skin ex vivo. In healthy young skin approximately 15–20% of LC migrate from the epidermis during culture. However, in ageing skin, we found the LC migratory response to trauma to be significantly impaired. This inability of LC to migrate efficiently in aged skin could impact on the efficacy of T‐cell stimulation.23, 24 Langerhans’ cells are reported to promote both cell‐mediated immunity and tolerance by activation of different T‐cell subsets,3 therefore, decline in LC function could contribute to the detrimental changes observed in skin immune competency in the elderly.
Following addition of IL‐1β, LC migration in aged skin was restored to levels seen in young epidermis; in contrast, no response to TNF‐α was observed. These findings of a differential impact of IL‐1β and TNF‐α support results obtained from previous studies performed in human skin in vivo,10, 12 confirming the epidermal explant model to be a suitable system for studying LC migratory responses in ageing. As both cytokines are required for LC mobilization,4, 6 these results strengthen the case for deficient IL‐1 signalling in elderly skin. This notion is further supported by the lower level of IL1B gene expression found in unstimulated aged skin. Induction of IL‐1β expression is an early event in signal cascades that are initiated in skin during activation of innate immune responses to pathogens, contact allergens and other stimuli including ultraviolet radiation.14, 25, 26 A lower baseline IL‐1β status in aged skin may result in a delayed or diminished up‐regulation of IL‐1 cascades following inflammatory challenge. In addition, IL‐1 is believed to be important for homeostatic cell‐to‐cell communication27 and therefore reduced levels of IL‐1β are likely to be detrimental even in the absence of inflammatory conditions. Combined with poor dendritic cell responses this deficiency may partially explain why infection rates are often increased and contact hypersensitivity responses are decreased in elderly skin.
Interestingly, stimulation with IL‐1α did not significantly restore LC migration in aged skin in the explant model. This was unexpected as both IL‐1α and IL‐1β signal through the same receptor, IL‐1R1. However, multiple studies have also found the two IL‐1 isoforms to behave differently. Boraschi et al. showed that IL‐1α and IL‐1β exhibited an equal ability to stimulate thymocyte proliferation and fibroblast prostaglandin E2 synthesis in vitro, but in vivo only IL‐1β showed immunostimulatory activity by increasing the number of antigen‐specific antibody‐producing cells in the spleens of immunized mice.28 During sterile inflammation the cellular sources, intracellular location and kinetics of IL‐1α and IL‐1β expression are reported to differ; IL‐1α is thought to be released by necrotic cells in the early phase of tissue injury and is associated with infiltration of neutrophils, whereas IL‐1β correlated with the later recruitment and retention of macrophages.29 Furthermore, IL‐1α binds the inhibitory decoy receptor IL‐1R2 less efficiently than does IL‐1β,30 and anti‐IL‐1α does not impair LC migration in response to oxazalone or TNF‐α‐treated mice as anti‐IL‐1β does.4
The responsiveness of LC to IL‐1β in the aged suggested that these cells were inherently functionally normal and we have previously found that CD14+ monocytes of young and aged individuals were equally able to generate LC‐like cells and that these cells were functionally comparable in terms of their response to IL‐1β, TNF‐α and migratory chemokines.31 This suggested that systemic immunosenescence associated with ageing was not directly responsible for altered activity of LC in aged skin and led us to hypothesize that a dysregulated skin microenvironment may be a contributing factor. Primary human keratinocytes derived from skin of elderly individuals exhibited an age‐associated phenotype with regard to differential expression of markers including protease ADAMTS1, known to be involved in keratinocyte differentiation19 and inflammation.20 However, the conditioned medium derived from these cells demonstrated no consistent inhibitory or stimulatory effect on LC migration in either young or aged epidermal explant cultures. As the conditioned medium was generated from keratinocytes under resting conditions it may be the case that insufficient quantities of LC mobilizing or retaining factors were present. This was not a concern initially due to the differences we observed in IL1B gene expression in resting aged skin and also because previous experiments with conditioned medium from keratinocytes derived from uninvolved psoriasis skin showed an inhibitory effect on LC migration from healthy skin (Eaton, Mellody, Pilkington, Dearman, Kimber, Griffiths unpublished data). However, the mechanisms underlying impaired LC migration in psoriasis skin are thought to occur through IL‐1β‐independent mechanisms, and the level of keratinocyte involvement in impaired LC function in ageing and psoriasis are probably very different.
Subsequent investigations of the response of the skin to biopsy trauma and stimulatory culture revealed further differences in IL‐1 gene expression between the age groups. Whole skin was cultured immediately after biopsy for 2 hr, which was deemed sufficient time to allow sterile inflammatory processes to begin without loss of significant numbers of LC through migration into the culture medium. TNF‐α and IL‐1β each proved capable of inducing gene expression of the other. While the ability of IL‐1β to induce TNF‐α mRNA has previously been described in murine skin,32, 33 to our knowledge this is the first time TNF‐α has been shown to induce IL1B mRNA in human skin. In comparison with young skin, aged skin showed a less robust induction of IL1B mRNA following both control culture and TNF‐α stimulation. This may be due to the lower baseline levels of IL1B mRNA in aged skin, but more likely represents a diminished capability of aged skin to up‐regulate IL1B gene expression following the TNF‐α signal. Classical induction of IL‐1β expression is by nuclear factor‐κB following activation of toll‐like receptors or IL1R1, but nuclear factor‐κB is also responsible for TNF‐α expression, and both cytokines can activate nuclear factor‐κB.26 Therefore, it seems unlikely that the differences in IL1B gene expression between the age groups are due to decreased nuclear factor‐κB activity.
The lack of any difference in expression of TNFR, IL1R1 and the accessory protein IL‐1 receptor accessory protein (RAP) between young and aged skin suggests that the observed LC defect in ageing is probably not due to a deficiency in one of these receptors. Although aged skin did show a greater induction of IL1R2 gene expression in response to both IL‐1β and TNF‐α, suggesting that under inflammatory conditions IL‐1β signalling may be further suppressed due to greater availability of the IL‐1R2 decoy receptor, which reduces IL‐1β binding to IL‐1R1 and sequesters IL‐1RAP.34 In aged skin the addition of recombinant IL‐1β protein may provide a large enough signal to induce IL1B gene expression in the target cell, and/or may bypass the need for de novo IL‐1β synthesis. Increased availability of IL‐1β may also overcome the suppressive actions of IL‐1R2. Hence, when IL‐1β is available this could support initiation of the feedback loop in IL‐1 signalling that allows LC migration to occur.
Interleukin‐1 in the context of ageing is usually discussed with relation to increased systemic inflammation and autoimmunity, which is often associated with the ageing process.35 However, in the healthy aged, such as those included in these studies who do not suffer from chronic inflammatory disease, IL‐1β signalling appears to be reduced in the skin in association with impaired LC function. It remains to be determined which cell type or types are responsible for the deficient IL1B gene expression in baseline aged skin, and if this translates into reduced active IL‐1β protein. In the past the ability of keratinocytes to produce mature IL‐1β has been controversial.36 Although now it is believed that keratinocytes can readily produce mature IL‐1β under inflammatory conditions where free cytosolic calcium is available for inflammasome activation.14
Studies in mice report LC to be the primary source of constitutive IL‐1β in the epidermis13, 32, 37 and this fits with the proposed sequence of events involved in LC mobilization. In mice, injection of IL‐1β results in delayed LC migration and dendritic cell accumulation in the lymph node when compared with TNF‐α, indicating that LC‐derived IL‐1β may be required to act on keratinocytes to stimulate TNF‐α, which then provides the second mandatory signal for LC migration.4 This is supported by studies in aged mice where dendritic cells showed a reduced capability for inflammasome activation and IL‐1β production in response to influenza.38 Interestingly, Agrawal et al. found evidence of reduced activation of the phosphoinositide 3‐kinase (PI3K) pathway in monocyte‐derived myeloid dendritic cells from elderly individuals and suggested that this may contribute to the reduced migratory capacity and function of these cells.39 As TNF‐α can induce IL‐1β production via the PI3K‐Akt signalling pathway,40 and given the important role of this pathway in myeloid dendritic cell development and function,41 further interrogation of this pathway in LC in aged skin is warranted.
Therefore, in aged skin we propose that the capability of LC to express IL‐1β is diminished, potentially due to cellular senescence of LC in situ and/or as a result of influences within the age‐associated microenvironment. If under inflammatory conditions LC in aged skin are less able to effectively up‐regulate IL‐1β gene expression even in the presence of TNF‐α, perhaps other pathways such as PI3K signalling are affected. Further work is required to determine the contribution of LC to IL‐1 deficiency in ageing human skin, including mechanisms that may impact upon inflammasome activation. A greater understanding of these processes could support the development of therapeutic interventions to improve skin health and vaccination efficacy in the elderly.
Disclosures
The authors report no conflicts of interest.
Acknowledgements
SMP, CEM, IK and RJD designed the study, SMP and SO performed the experiments, LE provided technical assistance and intellectual input. SMP wrote the paper and CEM, IK, RJD, SO and LE edited the paper. We thank Walgreens Boots Alliance for funding this research and all the volunteers who took part in the study.
Contributor Information
Suzanne M. Pilkington, Email: suzanne.pilkington@manchester.ac.uk.
Christopher E. M. Griffiths, Email: Christopher.griffiths@manchester.ac.uk
References
- 1. Lutz MB, Dohler A, Azukizawa H. Revisiting the tolerogenicity of epidermal Langerhans cells. Immunol Cell Biol 2010; 88:381–6. [DOI] [PubMed] [Google Scholar]
- 2. Seneschal J, Clark RA, Gehad A, Baecher‐Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 2012; 36:873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mutyambizi K, Berger CL, Edelson RL. The balance between immunity and tolerance: the role of Langerhans cells. Cell Mol Life Sci 2009; 66:831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells require signals from both tumour necrosis factor‐α and interleukin‐1β for migration. Immunology 1997; 92:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ratzinger G, Stoitzner P, Ebner S, Lutz MB, Layton GT, Rainer C et al Matrix metalloproteinases 9 and 2 are necessary for the migration of Langerhans cells and dermal dendritic cells from human and murine skin. J Immunol 2002; 168:4361–71. [DOI] [PubMed] [Google Scholar]
- 6. Cumberbatch M, Bhushan M, Dearman RJ, Kimber I, Griffiths CE. IL‐1β‐induced Langerhans’ cell migration and TNF‐α production in human skin: regulation by lactoferrin. Clin Exp Immunol 2003; 132:352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fulop T, Larbi A, Witkowski JM, Kotb R, Hirokawa K, Pawelec G. Immunosenescence and cancer. Crit Rev Oncog 2013; 18:489–513. [DOI] [PubMed] [Google Scholar]
- 8. Pera A, Campos C, Lopez N, Hassouneh F, Alonso C, Tarazona R et al Immunosenescence: implications for response to infection and vaccination in older people. Maturitas 2015; 82:50–5. [DOI] [PubMed] [Google Scholar]
- 9. Cevenini E, Monti D, Franceschi C. Inflamm‐ageing. Curr Opin Clin Nutr Metab Care 2013; 16:14–20. [DOI] [PubMed] [Google Scholar]
- 10. Bhushan M, Cumberbatch M, Dearman RJ, Andrew SM, Kimber I, Griffiths CE. Tumour necrosis factor‐α‐induced migration of human Langerhans cells: the influence of ageing. Br J Dermatol 2002; 146:32–40. [DOI] [PubMed] [Google Scholar]
- 11. Cumberbatch M, Dearman RJ, Kimber I. Influence of ageing on Langerhans cell migration in mice: identification of a putative deficiency of epidermal interleukin‐1β . Immunology 2002; 105:466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhushan M, Cumberbatch M, Dearman RJ, Kimber I, Griffiths CE. Exogenous interleukin‐1β restores impaired Langerhans cell migration in aged skin. Br J Dermatol 2004; 150:1217–18. [DOI] [PubMed] [Google Scholar]
- 13. Matsue H, Cruz PD Jr, Bergstresser PR, Takashima A. Langerhans cells are the major source of mRNA for IL‐1β and MIP‐1α among unstimulated mouse epidermal cells. J Invest Dermatol 1992; 99:537–41. [DOI] [PubMed] [Google Scholar]
- 14. Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD. The inflammasome mediates UVB‐induced activation and secretion of interleukin‐1β by keratinocytes. Curr Biol 2007; 17:1140–5. [DOI] [PubMed] [Google Scholar]
- 15. Linton PJ, Li SP, Zhang Y, Bautista B, Huynh Q, Trinh T. Intrinsic versus environmental influences on T‐cell responses in aging. Immunol Rev 2005; 205:207–19. [DOI] [PubMed] [Google Scholar]
- 16. Shaw FL, Mellody KT, Ogden S, Dearman RJ, Kimber I, Griffiths CEM. Treatment‐related restoration of Langerhans cell migration in psoriasis. J Invest Dermatol 2014; 134:268–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aasen T, Izpisua Belmonte JC. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat Protoc 2010; 5:371–82. [DOI] [PubMed] [Google Scholar]
- 18. Cumberbatch M, Singh M, Dearman RJ, Young HS, Kimber I, Griffiths CE. Impaired Langerhans cell migration in psoriasis. J Exp Med 2006; 203:953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krampert M, Kuenzle S, Thai SN, Lee N, Iruela‐Arispe ML, Werner S. ADAMTS1 proteinase is up‐regulated in wounded skin and regulates migration of fibroblasts and endothelial cells. J Biol Chem 2005; 280:23844–52. [DOI] [PubMed] [Google Scholar]
- 20. Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase‐disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem 1997; 272:556–62. [DOI] [PubMed] [Google Scholar]
- 21. Dean JP, Nelson PS. Profiling influences of senescent and aged fibroblasts on prostate carcinogenesis. Br J Cancer 2008; 98:245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toba H, de Castro Bras LE, Baicu CF, Zile MR, Lindsey ML, Bradshaw AD. Increased ADAMTS1 mediates SPARC‐dependent collagen deposition in the aging myocardium. Am J Physiol Endocrinol Metab 2016; 310:E1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Plowden J, Renshaw‐Hoelscher M, Gangappa S, Engleman C, Katz JM, Sambhara S. Impaired antigen‐induced CD8+ T cell clonal expansion in aging is due to defects in antigen presenting cell function. Cell Immunol 2004; 229:86–92. [DOI] [PubMed] [Google Scholar]
- 24. Donnini A, Argentati K, Mancini R, Smorlesi A, Bartozzi B, Bernardini G et al Phenotype, antigen‐presenting capacity, and migration of antigen‐presenting cells in young and old age. Exp Gerontol 2002; 37:1097–112. [DOI] [PubMed] [Google Scholar]
- 25. Murphy JE, Robert C, Kupper TS. Interleukin‐1 and cutaneous inflammation: a crucial link between innate and acquired immunity. J Invest Dermatol 2000; 114:602–8. [DOI] [PubMed] [Google Scholar]
- 26. Feldmeyer L, Werner S, French LE, Beer H‐D. Interleukin‐1, inflammasomes and the skin. Eur J Cell Biol 2010; 89:638–44. [DOI] [PubMed] [Google Scholar]
- 27. Dinarello CA. Interleukin‐1, interleukin‐1 receptors and interleukin‐1 receptor antagonist. Int Rev Immunol 1998; 16:457–99. [DOI] [PubMed] [Google Scholar]
- 28. Boraschi D, Villa L, Volpini G, Bossu P, Censini S, Ghiar P et al Differential activity of interleukin 1α and interleukin 1β in the stimulation of the immune response in vivo . Eur J Immunol 1990; 20:317–21. [DOI] [PubMed] [Google Scholar]
- 29. Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E et al IL‐1α and IL‐1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol 2011; 187:4835–43. [DOI] [PubMed] [Google Scholar]
- 30. Boraschi D, Tagliabue A. The interleukin‐1 receptor family. Vitam Horm 2006; 74:229–54. [DOI] [PubMed] [Google Scholar]
- 31. Ogden S, Dearman RJ, Kimber I, Griffiths CE. The effect of ageing on phenotype and function of monocyte‐derived Langerhans cells. Br J Dermatol 2011; 165:184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Enk AH, Angeloni VL, Udey MC, Katz SI. An essential role for Langerhans cell‐derived IL‐1 beta in the initiation of primary immune responses in skin. J Immunol 1993; 150:3698–704. [PubMed] [Google Scholar]
- 33. Flint MS, Dearman RJ, Kimber I, Hotchkiss SAM. Production and in situ localisation of cutaneous tumour necrosis factor α (TNF α) and interleukin 6 (IL‐6) following skin sensitisation. Cytokine 1998; 10:213–19. [DOI] [PubMed] [Google Scholar]
- 34. Peters VA, Joesting JJ, Freund GG. IL‐1 receptor 2 (IL‐1R2) and its role in immune regulation. Brain Behav Immun 2013; 32:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr 2006; 83:447S–55S. [DOI] [PubMed] [Google Scholar]
- 36. Mizutani H, Black R, Kupper TS. Human keratinocytes produce but do not process pro‐interleukin‐1 (IL‐1) β. Different strategies of IL‐1 production and processing in monocytes and keratinocytes. J Clin Investig 1991; 87:1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heufler C, Topar G, Koch F, Trockenbacher B, Kampgen E, Romani N et al Cytokine gene expression in murine epidermal cell suspensions: interleukin 1β and macrophage inflammatory protein 1α are selectively expressed in Langerhans cells but are differentially regulated in culture. J Exp Med 1992; 176:1221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stout‐Delgado HW, Vaughan SE, Shirali AC, Jaramillo RJ, Harrod KS. Impaired NLRP3 inflammasome function in elderly mice during influenza infection is rescued by treatment with Nigericin® . J Immunol 2012; 188:2815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Agrawal A, Agrawal S, Cao J‐N, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3‐kinase‐signaling pathway. J Immunol 2007; 178:6912–22. [DOI] [PubMed] [Google Scholar]
- 40. Tian J, Chen JW, Gao JS, Li L, Xie X. Resveratrol inhibits TNF‐α‐induced IL‐1β, MMP‐3 production in human rheumatoid arthritis fibroblast‐like synoviocytes via modulation of PI3kinase/Akt pathway. Rheumatol Int 2013; 33:1829–35. [DOI] [PubMed] [Google Scholar]
- 41. van de Laar L, Buitenhuis M, Wensveen FM, Janssen HL, Coffer PJ, Woltman AM. Human CD34‐derived myeloid dendritic cell development requires intact phosphatidylinositol 3‐kinase‐protein kinase B‐mammalian target of rapamycin signaling. J Immunol 2010; 184:6600–11. [DOI] [PubMed] [Google Scholar]


