Summary
The T‐cell receptor (TCR)–CD3 complex, expressed on T cells, determines the outcome of a T‐cell response. It consists of the TCR‐αβ heterodimer and the non‐covalently associated signalling dimers of CD3εγ, CD3εδ and CD3ζζ. TCR‐αβ binds specifically to a cognate peptide antigen bound to an MHC molecule, whereas the CD3 subunits transmit the signal into the cytosol to activate signalling events. Recruitment of proteins to specialized localizations is one mechanism to regulate activation and termination of signalling. In the last 25 years a large number of signalling molecules recruited to the TCR–CD3 complex upon antigen binding to TCR‐αβ have been described. Here, we review knowledge about five of those interaction partners: Lck, ZAP‐70, Nck, WASP and Numb. Some of these proteins have been targeted in the development of immunomodulatory drugs aiming to treat patients with autoimmune diseases and organ transplants.
Keywords: protein–protein interaction, signal transduction, T‐cell activation, T‐cell receptor–CD3 complex
Abbreviations
- CAR
chimeric antigen receptor
- ERK
extracellular signal‐regulated kinase
- ITAMs
immunoreceptor tyrosine‐based activation motifs
- LAT
linker for the activation of T cells
- Lck
lymphocyte‐specific protein tyrosine kinase
- Nck
non‐catalytic region of tyrosine kinase
- PRS
proline‐rich sequence
- SH
Src‐homology
- SLP‐76
SH2‐domain‐containing leucocyte protein of 76 000 MW
- TCR
T‐cell receptor
- TSAd
T‐cell specific adaptor protein
- VCA
verprolin homology domain‐cofilin homology domain‐acidic region
- WASP
Wiskott–Aldrich syndrome protein
- WAS
Wiskott–Aldrich syndrome
- ZAP‐70
ζ chain‐associated protein kinase of 70 000 MW
Introduction
Immune responses to infectious pathogens serve to maintain body homeostasis. Among various immune cells, T cells play an important role to fulfil this critical function. A T‐cell response to foreign antigen is initiated by the binding of the T‐cell receptor (TCR)–CD3 complex to a foreign peptide bound to an MHC molecule presented on an antigen‐presenting cell. Information of this binding is transmitted into the cytosol to activate many signalling proteins.1, 2 The final targets are transcription factors, to alter the gene expression profile, metabolic enzymes, to change metabolic activity,3 and cytoskeletal rearrangement. Together this leads to cell proliferation and effector molecule production and secretion, which are crucial for T‐cell‐mediated immune responses.4
The TCR–CD3 complex is a multisubunit protein complex. It is composed of an antigen‐binding TCRαβ heterodimer non‐covalently associated with the non‐variable signal transduction subunits; the CD3 heterodimers CD3εγ and CD3εδ as well as the CD3ζζ homodimers.5, 6, 7 The cytoplasmic tails of CD3ε, CDδ, and CD3γ each contain one immunoreceptor tyrosine‐based activation motif (ITAM) and that of CD3ζ contains three ITAMs, hence one TCR–CD3 complex comprises 10 ITAMs. The conserved amino acid sequence of the ITAMs is D/ExYxxLx(6–8)YxxL. Antigen binding to TCR‐αβ results in phosphorylation of the ITAM residues, leading to recruitment and activation of multiple downstream signalling molecules including enzymes and adaptor proteins.1, 4 As there is a myriad of signalling molecules, regulated protein–protein interactions are one of the critical mechanisms for regulating specificity in signal transduction. Over the past decades a large number of proteins have been reported to be recruited to the TCR–CD3 complex. Here, we review recent data on five (direct or indirect) interaction partners of the TCR–CD3 complex, including the lymphocyte‐specific protein tyrosine kinase (Lck), CD3ζ‐associated protein kinase of 70 000 MW (ZAP‐70), non‐catalytic region of tyrosine kinase (Nck), Wiskott–Aldrich syndrome protein (WASP), and the inhibitor of Notch‐1 signalling Numb (Table 1). Other proteins associated with the TCR–CD3 complex have been discussed elsewhere and they are not covered in this review.8, 9, 10, 11, 12, 13 The effects of some mutations of these proteins on TCR signalling is shown in Table 2.
Table 1.
Selected proteins interacting with the T‐cell receptor (TCR) –CD3 complex
| Proteins associated with TCR–CD3 | Binding domain of the associated protein | Binding motif of the TCR–CD3 | Effect on TCR signalling | References |
|---|---|---|---|---|
| Lck | SH2 | Phospho‐ITAM | Enhancement | 38 |
| ZAP‐70 | SH2 | Phospho‐ITAM | Enhancement | 86 |
| Nck | SH3.1 and SH2 | Proline‐rich sequence (PRS) and Phospho‐ITAM within CD3ε | Enhancement | 40, 65 |
| WASP | SH3 domain bind to Nck | Indirect via Nck | Unknown | 79 |
| Numb | Phosphotyrosine binding (PTB) domain | NPDY motif within CD3ε | Decrease | 84 |
Table 2.
Mutations of T‐cell receptor (TCR) ‐CD3 binding proteins with their effects on TCR signalling
| TCR–CD3 binding proteins | Mutations | Effects on TCR signalling | References |
|---|---|---|---|
| Lck | R154K (SH2 mutant) | Inhibits Lck association with ZAP‐70 and CD3ζ | 38 |
| Y192F | Inhibits Lck association with TSAd, Itk, Pyk2 and SHP‐1 and enhances tyrosine‐phosphorylated proteins | 87 | |
| Y394F | Closed conformation with decreased kinase activity | 35, 88 | |
| Y505F | Open conformation with increased enzymatic activity | 35, 88 | |
| Y505F, K273R | Open conformation but lacking kinase activity | 35 | |
| ZAP70 | Y315F | Inhibition of Vav–ZAP‐70 interaction and reduction of tyrosine phosphorylation | 89 |
| Y319F | Impairment of Ca2+ mobilization, Ras activation and activation of phospholipase Cγ1 | 54 | |
| W131A | Increases kinase activity of ZAP‐70 | 90 | |
| Y315, 319A | Open conformation with increased kinase activity of ZAP‐70 | 51 | |
| Y315, 319F | Closed conformation with ZAP‐70 kinase inactive | 51 | |
| D461N | Inactivates the kinase domain known as ‘kinase dead’ | 50 | |
| Y493F | Inactivates ZAP‐70 catalytic activity | 91 | |
| Nck | Nck1(W38K) (SH3.1 mutant) | Impairs the binding of Nck1 to CD3ε and decreases ERK activation | 65 |
| Nck1(W143K) (SH3.2 mutant) | Impairs the binding of Nck1 to Cbl | 92 | |
| Nck1(W229K) (SH3.3 mutant) | Impairs the expression of CD69 expression and ERK phosphorylation | 63 | |
| Nck1(R308K) (SH2 mutant) |
Impairs the binding of Nck1 to CD3ε
Abrogates the binding of Nck1 to ADAP |
65, 93 | |
| WASP | WASPΔC (deletion of amino acids 444–502 at C terminus) | Inhibits actin polymerization but enhances the activation of NFAT transcription factor and ERK phosphorylation in human T cells | 94 |
| L46P (WH1 mutant) | Impairs the chemotactic migration of human T cells and actin cytoskeleton reorganization | 95 | |
| A47D (WH1 mutant) | Impairs the chemotactic migration of human T cells and actin cytoskeleton reorganization | 95 | |
| Numb | ΔNumb (condition deletion of Numb) | Normal CD3ζ phosphorylation in murine T cells | 96 |
T cells develop in the thymus where self‐reactive T cells are deleted by a process called negative selection, which is based on a strong signal elicited by high‐affinity binding to the self‐peptide MHC.14, 15 A lack of, or mutation in, the critical proteins involved in TCR–CD3 signalling, such as ZAP‐70 and WASP, causes a reduction of the TCR–CD3 signalling strength that allows autoreactive T cells to escape from negative selection and reach peripheral tissues.16, 17, 18, 19, 20 These autoreactive T cells can be activated in response to self‐peptide, which consequently leads to tissue injury known as autoimmune disease.15, 21 Hence, chemical agents blocking specifically the T‐cell activation process are promising therapeutic interventions for the treatment of T‐cell‐driven diseases. Here, we cover the information on some inhibitors that target the signalling proteins at the TCR–CD3 as they may have a potential to be used for the treatment of autoimmune disorders and in organ transplantations.
Lck
Members of the Src family of protein tyrosine kinases modulate signal transduction downstream of transmembrane receptors in most, if not all, cell types. In T cells, Lck is a member of the Src family of 56 000 MW. TCR–CD3 engagement with an antigenic peptide MHC triggers the phosphorylation of the ITAM tyrosines by Lck.22 Phosphorylated ITAMs then become a docking site for ZAP‐70, which is also activated by Lck upon binding to the ITAMs.23 Subsequently, ZAP‐70, together with Lck, phosphorylates downstream signalling molecules, to activate TCR–CD3‐controlled signalling cascades.
Lck contains an N‐terminal membrane anchor region (SH4 domain), a unique domain, an Src‐homology 3 (SH3) domain, an SH2 domain, a catalytic kinase domain and a short C‐terminal tail (Fig. 1a). The SH4 domain is post‐translationally modified by the addition of lipids, including myristoylation and palmitoylation, which allows the attachment of Lck to the plasma membrane. A serine 59 residue in a unique domain of Lck can be phosphorylated by the extracellular signal‐regulated kinase (ERK)24 and phosphorylation of this residue inhibits Lck activity.25 In addition, Lck activity is tightly regulated by a conformational state mainly depending on the phosphorylation and dephosphorylation of two tyrosine residues (Y394 and Y505) on the catalytic kinase domain and the C‐terminal tail, respectively.26 Phosphorylation of Y505 by the C‐terminal Src kinase mediates an intramolecular interaction with the SH2 domain, resulting in an inactive or closed conformation of Lck. When Y505 is dephosphorylated by the phosphatase CD45 or SHP‐1, the SH2 domain detaches from Y505, so promoting an opened conformation. The opened conformation allows phosphorylation of Y394 by Lck trans‐autophosphorylation.26, 27 However, doubly phosphorylated tyrosines Y394 and Y505 might also exist and confer a dominant effect of kinase activity over the inhibitory Y505.28
Figure 1.
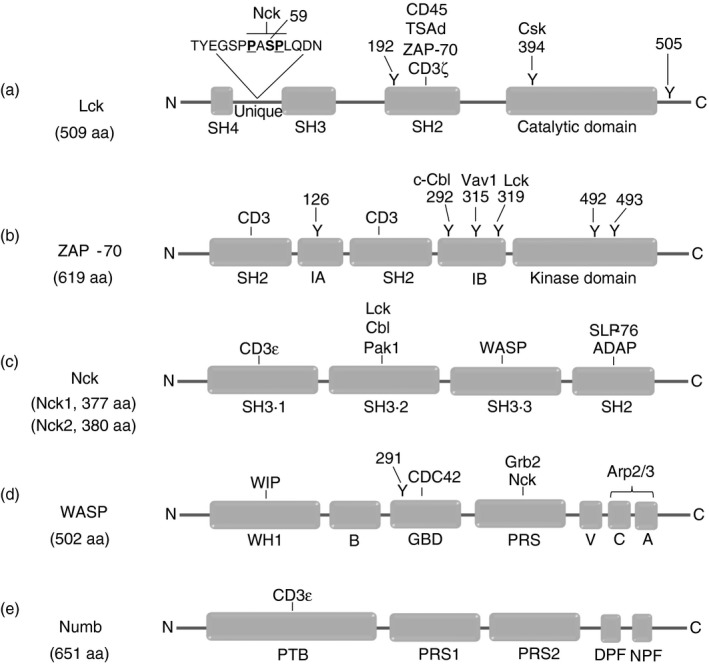
Modular composition of proteins associated with the T‐cell receptor (TCR) –CD3 complex. (a) Lck consists of an Src homology 4 (SH4) domain, a unique domain, an SH3 and SH2 domain, the catalytic domain and a C‐terminal region. The serine (S) and tyrosines (Y) depicted can be phosphorylated upon TCR–CD3 ligation. (b) ZAP‐70 contains an N‐terminal SH2 domain, an interdomain A (IA), a C‐terminal SH2 domain, an interdomain B (IB) and the kinase domain. The tyrosine residues indicated can be phosphorylated upon TCR–CD3 triggering. (c) The Nck family has two members, Nck1 and Nck2, both being composed of three SH3 domains and a C‐terminal SH2 domain. (d) WASP consists of a WH1 (WASP homology 1) and basic domain, followed by a GTPase‐binding domain (GBD), a proline‐rich sequence (PRS) and verprolin homology domain–cofilin homology domain‐acidic region domains (VCA). (e) Numb contains a phospho‐tyrosine binding (PTB) domain, two PRSs and DPF (Asp‐Pro‐Phe) and NPF (Asn‐Pro‐Phe) tri‐peptide motifs at the C‐terminus.
Different pools of Lck have been identified, including Lck in the cytoplasm, Lck anchored to the plasma membrane and Lck associated with the co‐receptors CD4 and CD8.29 Approximately 40% of Lck is already active (phosphorylated at Y394) in resting T cells and upon TCR engagement the amount of active Lck increases, as seen by Forster resonance energy transfer.30, 31, 32 In addition, the distribution of Lck to its correct destination may regulate the function of Lck in phosphorylating its substrates.26 Several lines of evidence have also suggested that initial phosphorylation of the CD3's ITAMs is mediated by free Lck, whereas the co‐receptor‐associated Lck acts as an adaptor molecule to bring the CD4 or CD8 molecule to the phosphorylated TCR–CD3 complex in a later step.33, 34 Furthermore, it has been suggested that the conformation of Lck determines its distribution. Lck in the opened conformation might allow clusters of Lck, whereas the closed conformation inhibits the clustering. TCR triggering induces the clustering of Lck with the phosphorylated TCR–CD3, suggesting that conformation‐driven Lck clustering may determine its localization to perform its activity.35 These findings suggest that Lck recruitment to the TCR–CD3 complex induces the phosphorylation of the ITAMs.
Lck can be co‐immunoprecipitated with the TCR–CD3 complex upon TCR–CD3 ligation, suggesting that these two proteins can interact directly or indirectly with each other.36 An indirect interaction might be mediated by RhoH, a haematopoietic‐specific Rho GTPase.37 The direct interaction might be mediated by the SH2 domain of Lck and phosphorylated ITAMs.38, 39 In addition, the SH2 domain of Lck can also interact with lipid within the plasma membrane upon TCR activation. This binding might be crucial for a lateral diffusion of Lck to interact with the triggered TCR–CD3 complex.39 These data indicate that localization of Lck to TCR–CD3s that are phosphorylated on few tyrosines facilitates the phosphorylation of the other ITAM tyrosines within CD3.
Our own data have suggested that the resting TCR–CD3 is in a closed conformation, in which the ITAM tyrosines are not exposed, but hidden within the quartenary structure of the TCR–CD3 complex.40, 41 Upon peptide–MHC binding to TCR‐αβ an open CD3 conformation is stabilized, that allows access of Lck to the ITAM tyrosine.42 This might be one explanation of how peptide–MHC binding to the TCR–CD3 complex causes CD3 phosphorylation by Lck.
As Lck expression is found only in T cells and natural killer cells, selective inhibitors that target Lck would potentially provide a safe treatment of diseases mediated by over‐activation of T cells such as rheumatoid arthritis, inflammatory bowel disease, psoriasis and organ graft rejection.43 A large number of compounds have been reported that selectively inhibit Lck activity by binding to the ATP pocket of Lck's kinase domain.44 Some of those inhibitors prevent the allograft rejection in mouse models,45, 46 and one inhibits the hind paw swelling in an adjuvant‐induced rat arthritis model.47
ZAP‐70
ZAP‐70 is a cytoplasmic tyrosine kinase expressed predominantly in T and natural killer cells. The importance of ZAP‐70 in humans has been demonstrated as a lack of ZAP‐70 causes a profound combined immunodeficiency, which is characterized by an absence of CD8 T cells and a defective function of CD4 T cells.17, 18 Combined mutations of R192W and R360P in ZAP‐70 cause an autoimmune syndrome. The former mutation results in decreased binding to phospho‐CD3, whereas the latter mutation reduces an autoinhibitory mechanism.48 These mutations that alter TCR signalling thresholds cause autoimmune diseases as phenotypically demonstrated by uncontrollable bullous pemphigoid, colitis and proteinuria.48
ZAP‐70 is structurally composed of two SH2 domains separated by a so‐called interdomain A. Following the tandem SH2 domains is the interdomain B and the kinase domain49 (Fig. 1b). There are several tyrosine residues on the interdomain B and kinase domain that can be phosphorylated after TCR stimulation. These tyrosines have various functions including regulation of the catalytic activity of ZAP‐70 and interaction with other signalling molecules. Tyrosine 292 (Y292), Y315 and Y319 are located within the interdomain B, whereas Y492 and Y493 are located in the kinase domain. In resting T cells, ZAP‐70 is in an autoinhibited conformation mediated by the intramolecular interaction of Y315 and Y319 with the kinase domain.50 Upon TCR engagement, the tandem SH2 domains of ZAP‐70 are recruited to doubly phosphorylated ITAMs of the CD3 subunits. Binding to the CD3 subunits changes ZAP‐70 conformation to an opened conformation with the release of Y315 and Y319 from the kinase domain. This facilitates the phosphorylation of Y315 and Y319 by either Lck51, 52 or by trans‐autophosphorylation.53 Likewise, the conformational change also gives rise to a more flexible kinase domain, resulting in phosphorylation of Y493, which is located within the activation loop of the kinase domain, by either Lck or by trans‐autophosphorylation.51 Phosphorylation of Y493 allows ZAP‐70 to be catalytically active. Lck can bind with its SH2 domain to phospho‐Y319 of ZAP‐70 and is required to mediate the phosphorylation of various tyrosine residues on ZAP‐70.54 Mutation of ZAP‐70's Y31953 or Lck's SH2 domain54 abrogates the Lck–ZAP‐70 interaction and consequently impairs downstream signalling. Taken together, the activation of ZAP‐70 relies on two steps: first binding of the tandem SH2 domains of ZAP‐70 to doubly phosphorylated tyrosines within the ITAMs of CD3, causing a conformational change, and second the Lck‐ and ZAP‐70‐mediated phosphorylation of Y315, Y319 and Y493 resulting in full ZAP‐70 activation.49, 50, 51
By comparing the different ITAMs among the CD3 subunits (CD3ζ, CD3δ, CD3ε and CD3γ), it is likely that ZAP‐70 preferentially binds to fully phosphorylated CD3ζ.9 Recently, a ‘catch‐and‐release’ model for ZAP‐70 activation has been proposed by Katz et al.55 After recruitment of ZAP‐70 to the phosphorylated TCR–CD3 complexes and ZAP‐70 phosphorylation by Lck, activated ZAP‐70 is released from the TCR–CD3 complexes into the plane of the plasma membrane. The association of ZAP‐70 with the membrane might be mediated by the binding of the SH2 domains to lipids or of phosphotyrosines to other membrane‐associated proteins. Consequently, empty phospho‐TCR–CD3 complexes allow the recruitment of additional ZAP‐70 molecules to the TCR–CD3 for activation of additional ZAP‐70. The released ZAP‐70 translocates within the membrane into adjacent protein islands to mediate phosphorylation of its substrates including the linker for the activation of T cells (LAT) and the SH2‐domain‐containing leucocyte protein of 76 000 MW (SLP‐76).55 Phosphorylated LAT and SLP‐76 adaptor proteins have various interacting partners such as the phospholipase C‐γ1, which is recruited to these two proteins to form the LAT/SLP‐76 signalosome.56 Forming of this signalosome results in T‐cell activation, proliferation and differentiation.
As ZAP‐70 is required to initiate T‐cell activation, inhibition of ZAP‐70 from interacting with the TCR–CD3 by small molecules may be used to treat patients with autoimmune diseases and organ transplants. High‐throughput screening of a library of 132 842 compounds has been conducted to find inhibitors that would disrupt the interaction of ZAP‐70 with CD3ζ.57 A series of pyrimidine derivatives that can inhibit ZAP‐70 activity have been identified and patented by researchers and Novartis companies.58
In recent years, chimeric antigen receptor (CAR)‐expressing T cells have been used for tumour immunotherapy. CARs consist of an extracellular anti‐tumour antigen single Fv fragment, a transmembrane region and the cytoplasmic tail of CD3ζ. CAR signalling relies on tumour antigen‐binding‐induced CD3ζ tail phosphorylation. An in silico model has suggested that the sensitivity of TCR signalling is modulated by the differential affinities of ZAP‐70 to the ITAMs of CD3ζ, and sequential phosphorylation of these ITAMs leading to a ‘switch‐like’ response of TCR signalling.59 Cytokine production by T cells could occur without phosphorylation of the CD3ζ, CD3δ, CD3γ chains when there are intact CD3ε chains.60 It has been suggested that no matter which ITAMs are phosphorylated, the number of ITAMs to be phosphorylated would determine the outcome of the T‐cell response.61 Hence, to obtain effective CAR‐T cells with a strong anti‐tumoral cytotoxic function but without producing too much cytokine, preventing the so‐called cytokine storm, one may optimize CD3ζ signalling by titrating the number of ITAMs to be phosphorylated and by using other CD3 chains than CD3ζ.
Nck
Nck is a 47 000 MW cytosolic adapter protein that is composed of three SH3 domains (SH3.1, SH3.2 and SH3.3) and one SH2 domain (Fig. 1c). In humans, two Nck isoforms exist; Nck1/Nckα and Nck2/Nckβ, which share 68% amino acid sequence similarity.62 Although redundant roles of Nck1 and Nck2 have been reported, our previous work has shown that Nck1 and Nck2 molecules are functionally non‐redundant in T‐cell activation.63 In response to TCR triggering, Nck is recruited to SLP‐76 to mediate actin rearrangement, which is essential for immunological synapse formation, T‐cell activation and cell movement.64 Nck is doing so by binding to WASP.
In addition, inducible direct association of Nck to the TCR–CD3 complex occurs when the latter is triggered. For this association, Nck simultaneously uses its SH3.1 and SH2 domains.65 The SH3.1 domain directly interacts with the PxxPxxDY sequence located within the proline‐rich sequence (PRS) of the CD3ε.40 For this association to occur the TCR needs to be in its Active CD3 conformation and the tyrosine needs to be in the non‐phosphorylated state.40, 66 The SH2 domain interacts with the second tyrosine of the CD3ε ITAM, when this tyrosine is phosphorylated.65 The functions of Nck–CD3 interaction is not well understood. A knock‐in mouse strain was generated in which the CD3ε PRS was replaced with another sequence, abolishing the binding to the SH3.1 domain of Nck, but most likely also to Numb (see below).67 The fact that Nck is a positive and Numb a negative regulator of signalling, might explain why the phenotype of the mutant T cells was mild. To only block the Nck–CD3 interaction, another knock‐in mouse line with point mutations of the two central prolines of the PxxP motif of CD3ε PRS to alanine has been generated.68 Indeed, T cells from these knock‐in mice do not recruit Nck to the TCR upon stimulation. In addition, this mutation is accompanied with impaired CD3ζ phosphorylation and decreased ZAP‐70 recruitment to the TCR–CD3 complex, as well as impaired ZAP‐70 phosphorylation.68 Moreover, the SH3.2 domain of Nck can bind to a proline motif in the unique domain of Lck.69 Recently, another adaptor protein called the T‐cell specific adaptor protein (TSAd) was identified that interacts with the Src family of proteins including Lck and promotes actin polymerization via interaction with Nck.70 Nck and Lck contain multiple binding sites on TSAd. The Nck SH2 interacts with phospho‐TSAd whereas the Nck SH3.1 and SH3.3 interact with TSAd PRS. The SH2 Lck binds to phospho‐TSAd and the Lck SH3 binds to the TSAd PRS. Taken together, Nck recruitment to the TCR–CD3 complex may also bring Lck to TCR.
Interestingly, the importance of the Nck–CD3 interaction might depend on the antigen quality, as this interaction was critical for stimulation of T cells with weak (low‐affinity) antigens, but not with strong (high‐affinity) antigens.71 Foreign antigens are often of high affinity and self antigen of low affinity.72 Hence, the requirement of Nck recruitment for T‐cell activation only by low (and not by high) affinity antigens has raised the possibility for inhibition of the Nck–CD3 interaction as a target for treatment of autoimmune diseases caused by self‐reactive T cells. Borroto et al.73 have chemically generated a low‐molecular‐weight inhibitor targeting a non‐canonical pocket within the Nck SH3.1 domain. As expected, this inhibitor prevented the binding of Nck to the TCR–CD3 complex. T‐cell activation in response to low‐affinity antigens was strongly inhibited by this inhibitor, as seen in mouse models for psoriasis, asthma and multiple sclerosis. Interestingly, the T‐cell response to a mouse pathogen acting as a strong high‐affinity peptide was normal after treatment with this inhibitor. Altogether, these results indicate that this synthetic inhibitor could be a candidate to be evaluated in clinical trials to treat various T‐cell‐mediated autoimmune diseases.73
WASP
WASP belongs to the WASP family of proteins consisting of WASP, N‐WASP and WAVE/SCAR molecules.74 Mutation of WASP or lack of WASP expression causes the Wiskott–Aldrich syndrome (WAS), which is characterized by thrombocytopenia, eczema, increased susceptibility to infection and increased risk to develop autoimmune disease.20, 75 WASP contains a WASP homology 1 domain, a basic domain, a PRS, a GTPase‐binding domain and a verprolin homology domain–cofilin homology domain‐acidic region (VCA) domain (Fig. 1d). These domains are required for binding to different cytoskeleton‐regulating proteins. For instance, the GTPase‐binding domain binds CDC42,76 whereas the PRS acts as a binding site for various SH3‐containing proteins such as Nck.77 The function of WASP at the SLP‐76 signalosome in regulating actin skeleton dynamics is well described.78
As WASP is the binding partner of Nck,77 we tested whether recruitment of Nck to the TCR–CD3 complex may also bring WASP to the TCR–CD3. We found that WASP is co‐immunoprecipitated with the TCR–CD3 complex after T‐cell activation.79 However, whether this was mediated by Nck is not known. Although the function of WASP recruitment to the TCR–CD3 complex has not been investigated, these results suggest that there would be an alternative pathway of WASP (besides the SLP‐76 signalosome) to regulate actin reorganization in the vicinity of the TCR–CD3 complex.
Numb
Numb is an adaptor protein that regulates receptor internalization. Numb is up‐regulated in the active phase of multiple sclerosis80 and type 1 diabetes.81 Two homologues of Numb including Numb and Numb‐like have been identified in mammals.82 Numb is composed of a phosphotyrosine binding domain, several proline‐rich regions at the centre of the molecule and two tri‐peptide motifs (Fig 1e).82 Numb is involved in the development of murine thymocytes by regulating pre‐TCR signalling.83 In addition, Numb may control TCR signalling in mature T cells. Constitutive expression of CD69 and interferon‐γ, as well as constitutively phosphorylated ERK, are found in the CD4+ T cells from dominant negative Numb transgenic mice. Upon stimulation, CD4+ T cells from these mice exhibit higher ERK, ZAP‐70 and Akt phosphorylation than those of the wild‐type mice, indicating that Numb may be required for a negative control of TCR‐mediated signal transduction.84 It was suggested that Numb plays a role in TCR degradation by simultaneously binding to both Cbl and a site within CD3ε that overlaps with the Nck binding site, thus mediating TCR degradation.84
Numb can bind with its phosphotyrosine binding domain to the cytoplasmic tail of CD3ε within the PRS to the sequence NPDY.84 Indeed, an endocytosis motif in CD3ε in this region has been identified,85 suggesting that Numb might be involved in TCR–CD3 endocytosis. Interestingly, Numb was suggested to constitutively associate with CD3ε. So far, not much is known about the order of binding of the TCR–CD3 binding partners. Here, we propose that in resting T cells, CD3ε is occupied with Numb that impedes TCR signalling. Upon TCR ligation, a conformational change of the CD3ε may result in the release of Numb and exposure of the CD3ε PRS, which is the site that interacts with Nck. Recruitment of Nck to the TCR also brings Lck to the TCR to facilitate ITAM phosphorylation. Full ITAM phosphorylation then releases Nck so that ZAP‐70 can bind. Once the TCR signal is transmitted, ZAP‐70 is replaced by Numb to mediate TCR degradation and these cause a deviation of T‐cell activation. However, further studies are required to elucidate the mechanism underlying Numb‐regulated TCR signalling and the related TCR degradation pathways.
Conclusion
TCR–CD3 complex is the key molecule to initiate biochemical events in T‐cell activation and differentiation that can lead to different outcomes, depending on the quantity and quality of the stimulus. Nevertheless, how stimulation of the TCR–CD3 complex can give rise to distinct outcomes still remains unclear. Based on the recent findings, we propose that distinct outcomes may be due to the different interaction partners to be recruited to the TCR–CD3 complex upon TCR–CD3 engagement (Fig. 2). These protein partners are involved in both enhance and decrease of TCR signalling and in different downstream signalling pathways. Lck can interact directly or indirectly with the TCR–CD3 complex and phosphorylate the ITAMs to initiate signal transduction. ZAP‐70 directly interacts with the TCR–CD3 complex upon CD3 phosphorylation and activates downstream signalling cascades. Nck is recruited to CD3ε and might co‐recruit Lck and WASP to the TCR–CD3 complex. TCR–CD3‐recruited WASP might control actin reorganization at TCR–CD3. Numb is a new binding partner of the TCR–CD3 complex and participates in TCR degradation to lessen TCR signalling after T‐cell stimulation. However, the exact molecular mechanisms underlying the dynamic distributions of these proteins into and out of the TCR–CD3 complex still need further clarification.
Figure 2.
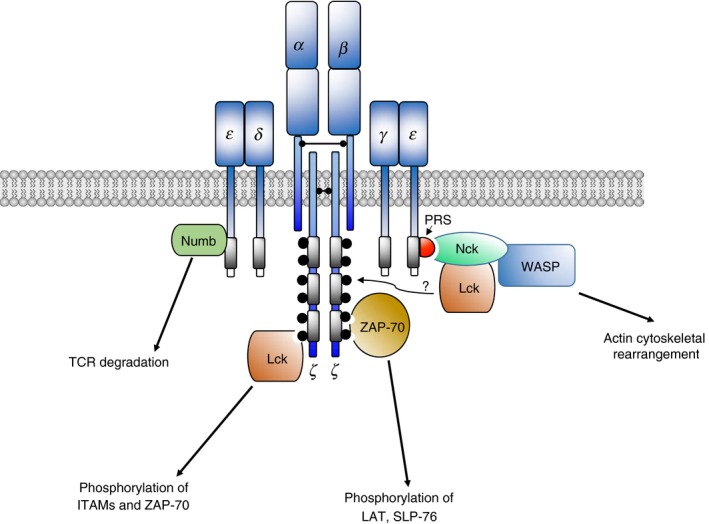
Selected signalling proteins at TCR–CD3 complex. TCR–CD3 ligation induces a conformational change of CD3ε, leading to the exposure of its proline‐rich sequence (PRS). Nck is then recruited to the PRS within the cytoplasmic tail of the CD3ε. Subsequently, Lck is associated with Nck upon TCR activation. Hence, Nck recruitment to TCR may also bring Lck to TCR–CD3 complex to mediate phosphorylation of the ITAM motif. In addition, Lck can directly interact with phospho‐ITAM. When the second tyrosine of the CD3ε ITAM is phosphorylated, Nck can bind with CD3ε using its SH3.1 and SH2 domains in a co‐operative manner. In proximity to the TCR–CD3 complex, Lck phosphorylates tyrosines in each ITAM of the CD3 chains. ZAP‐70 is then recruited to bind to the phospho‐ITAMs, where ZAP‐70 itself is phosphorylated by Lck. WASP can be associated with Nck upon TCR activation to regulate actin polymerization. Numb can be associated with the CD3ε to regulate in TCR degradation leading to a decrease in TCR signalling.
Disclosures
The authors declare no conflict of interest.
Acknowledgements
JN received research grants from Naresuan University (no. R2559B064) and the Thailand Research Fund (TRG5880030). SP received research grants from Naresuan University (no. R2559B025) and the Thailand Research Fund (RSA5880009). Further, this work was funded by the Deutsche Forschungsgemeinschaft (DFG) through EXC294 (Centre for Biological Signalling Studies, BIOSS) to WWS.
References
- 1. Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nat Rev Immunol 2013; 13:257–69. [DOI] [PubMed] [Google Scholar]
- 2. Acuto O, Di Bartolo V, Michel F. Tailoring T‐cell receptor signals by proximal negative feedback mechanisms. Nat Rev Immunol 2008; 8:699–712. [DOI] [PubMed] [Google Scholar]
- 3. Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 2013; 38:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith‐Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol 2009; 27:591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol 2000; 12:242–9. [DOI] [PubMed] [Google Scholar]
- 6. Jacobs H. Pre‐TCR/CD3 and TCR/CD3 complexes: decamers with differential signalling properties? Immunol Today 1997; 18:565–9. [PubMed] [Google Scholar]
- 7. Alarcón B, Gil D, Delgado P, Schamel WW. Initiation of TCR signalling: regulation within CD3 dimers. Immunol Rev 2003; 191:38–46. [DOI] [PubMed] [Google Scholar]
- 8. Dong G, Kalifa R, Nath PR, Gelkop S, Isakov N. TCR crosslinking promotes Crk adaptor protein binding to tyrosine‐phosphorylated CD3ζ chain. Biochem Biophys Res Commun 2017; 488:541–6. [DOI] [PubMed] [Google Scholar]
- 9. Love PE, Hayes SM. ITAM‐mediated signaling by the T‐cell antigen receptor. Cold Spring Harb Perspect Biol 2010; 2:a002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Aós I, Metzger MH, Exley M, Dahl CE, Misra S, Zheng D et al Tyrosine phosphorylate ion of the CD3‐ε subunit of the T cell antigen receptor mediates enhanced association with phosphatidylinositol 3‐kinase in Jurkat T cells. J Biol Chem 1997; 272:25310–8. [DOI] [PubMed] [Google Scholar]
- 11. DeFord‐Watts LM, Young JA, Pitcher LA, van Oers NS. The membrane‐proximal portion of CD3ε associates with the serine/threonine kinase GRK2. J Biol Chem 2007; 282:16126–34. [DOI] [PubMed] [Google Scholar]
- 12. Delgado P, Cubelos B, Calleja E, Martínez‐Martín N, Ciprés A, Mérida I et al Essential function for the GTPase TC21 in homeostatic antigen receptor signalling. Nat Immunol 2009; 10:880–8. [DOI] [PubMed] [Google Scholar]
- 13. Borrotto A, Abia D, Alarcon B. Crammed signaling motifs in the T‐cell receptor. Immunol Lett 2014; 161:113–7. [DOI] [PubMed] [Google Scholar]
- 14. Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol 2012; 13:121–8. [DOI] [PubMed] [Google Scholar]
- 15. von Boehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol 2010; 11:14–20. [DOI] [PubMed] [Google Scholar]
- 16. Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S et al Altered thymic T‐cell selection due to a mutation of the ZAP‐70 gene causes autoimmune arthritis in mice. Nature 2003; 426:454–60. [DOI] [PubMed] [Google Scholar]
- 17. Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signalling and CD8+ thymic selection in humans lacking zap‐70 kinase. Cell 1994; 76:947–58. [DOI] [PubMed] [Google Scholar]
- 18. Elder ME, Lin D, Clever J, Chan AC, Hope TJ, Weiss A et al Human severe combined immunodeficiency due to a defect in ZAP‐70, a T cell tyrosine kinase. Science 1994; 264:1596–9. [DOI] [PubMed] [Google Scholar]
- 19. Roifman CM, Dadi H, Somech R, Nahum A, Sharfe N. Characterization of ζ‐associated protein, 70 kd (ZAP70)‐deficient human lymphocytes. J Allergy Clin Immunol 2010; 126:1226–33. [DOI] [PubMed] [Google Scholar]
- 20. Wu J, Liu D, Tu W, Song W, Zhao X. T‐cell receptor diversity is selectively skewed in T‐cell populations of patients with Wiskott–Aldrich syndrome. J Allergy Clin Immunol 2015; 135:209–16. [DOI] [PubMed] [Google Scholar]
- 21. Skapenko A, Leipe J, Lipsky PE, Schulze‐Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther 2005; 7(Suppl 2):S4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Oers NS, Killeen N, Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP‐70 in murine thymocytes. J Exp Med 1996; 183:1053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science 1994; 263:1136–9. [DOI] [PubMed] [Google Scholar]
- 24. Schröder AJ, Quehl P, Müller J, Samstag Y. Conversion of p56(lck) to p60(lck) in human peripheral blood T lymphocytes is dependent on co‐stimulation through accessory receptors: involvement of phospholipase C, protein kinase C and MAP‐kinases in vivo. Eur J Immunol 2000; 30:635–43. [DOI] [PubMed] [Google Scholar]
- 25. Joung I, Kim T, Stolz LA, Payne G, Winkler DG, Walsh CT et al Modification of Ser59 in the unique N‐terminal region of tyrosine kinase p56lck regulates specificity of its Src homology 2 domain. Proc Natl Acad Sci USA 1995; 92:5778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rossy J, Williamson DJ, Gaus K. How does the kinase Lck phosphorylate the T cell receptor? Spatial organization as a regulatory mechanism. Front Immunol 2012; 3:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene 2004; 23:7918–27. [DOI] [PubMed] [Google Scholar]
- 28. Filipp D, Ballek O, Manning J. Lck, membrane microdomains, and TCR triggering machinery: defining the new rules of engagement. Front Immunol 2010; 3:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim PW, Sun ZY, Blacklow SC, Wagner G, Eck MJ. A zinc clasp structure tethers Lck to T cell coreceptors CD4 and CD8. Science 2003; 301:1725–8. [DOI] [PubMed] [Google Scholar]
- 30. Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, et al Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 2010; 32:766–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stirnweiss A, Hartig R, Gieseler S, Lindquist JA, Reichardt P, Philipsen L et al T cell activation results in conformational changes in the Src family kinase Lck to induce its activation. Sci Signal 2013; 6:ra13. [DOI] [PubMed] [Google Scholar]
- 32. Philipsen L, Reddycheria AV, Hartig R, Gumz J, Kastle M, Kritikos A et al De novo phosphorylation and conformational opening of the tyrosine kinase Lck act in concert to initiate T cell receptor signaling. Sci Signal 2017; 10:pii: eaaf4736. [DOI] [PubMed] [Google Scholar]
- 33. Xu H, Littman DR. A kinase‐independent function of Lck in potentiating antigen‐specific T cell activation. Cell 1993; 74:633–43. [DOI] [PubMed] [Google Scholar]
- 34. Casas J, Brzostek J, Zarnitsyna VI, Hong JS, Wei Q, Hoerter JA et al Ligand‐engaged TCR is triggered by Lck not associated with CD8 coreceptor. Nat Commun 2014; 5:5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rossy J, Owen DM, Williamson DJ, Yang Z, Gaus K. Conformational states of the kinase Lck regulate clustering in early T cell signalling. Nat Immunol 2013; 14:82–9. [DOI] [PubMed] [Google Scholar]
- 36. Stefanová I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP‐1 negative feedback pathways. Nat Immunol 2003; 4:248–54. [DOI] [PubMed] [Google Scholar]
- 37. Chae HD, Siefring JE, Hildeman DA, Gu Y, Williams DA. RhoH regulates subcellular localization of ZAP‐70 and Lck in T cell receptor signalling. PLoS One 2010; 5:e13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Straus DB, Chan AC, Patai B, Weiss A. SH2 domain function is essential for the role of the Lck tyrosine kinase in T cell receptor signal transduction. J Biol Chem 1996; 271:9976–81. [DOI] [PubMed] [Google Scholar]
- 39. Sheng R, Jung DJ, Silkov A, Kim H, Singaram I, Wang ZG et al Lipids regulate Lck protein activity through their interactions with the Lck Src homology 2 domain. J Biol Chem 2016; 291:17639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gil D, Schamel WW, Montoya M, Sánchez‐Madrid F, Alarcón B. Recruitment of Nck by CD3ε reveals a ligand‐induced conformational change essential for T cell receptor signalling and synapse formation. Cell 2002; 109:901–12. [DOI] [PubMed] [Google Scholar]
- 41. Minguet S, Swamy M, Alarcon B, Luescher IF, Schamel WW. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity 2007; 26:43–54. [DOI] [PubMed] [Google Scholar]
- 42. Swamy M, Beck‐Garcia K, Beck‐Garcia E, Hartl FA, Morath A, Yousefi OS et al A cholesterol‐based allostery model of T cell receptor phosphorylation. Immunity 2016; 44:1091–101. [DOI] [PubMed] [Google Scholar]
- 43. Kamens JS, Ratnofsky SE, Hirst GC. Lck inhibitors as a therapeutic approach to autoimmune disease and transplant rejection. Curr Opin Investig Drugs 2001; 2:1213–9. [PubMed] [Google Scholar]
- 44. Bhagwat SS. Kinase inhibitors for the treatment of inflammatory and autoimmune disorders. Purinergic Signal 2009; 5:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Waegell W, Babineau M, Hart M, Dixon K, McRae B, Wallace C et al A420983, a novel, small molecule inhibitor of LCK prevents allograft rejection. Transplant Proc 2002; 34:1411–7. [DOI] [PubMed] [Google Scholar]
- 46. Burchat A, Borhani DW, Calderwood DJ, Hirst GC, Li B, Stachlewitz RF. Discovery of A‐770041, a src‐family selective orally active lck inhibitor that prevents organ allograft rejection. Bioorg Med Chem Lett 2006; 16:118–22. [DOI] [PubMed] [Google Scholar]
- 47. Maier JA, Brugel TA, Sabat M, Golebiowski A, Laufersweiler MJ, VanRens JC et al Development of N‐4,6‐pyrimidine‐N‐alkyl‐N’‐phenyl ureas as orally active inhibitors of lymphocyte specific tyrosine kinase. Bioorg Med Chem Lett 2006; 16:3646–50. [DOI] [PubMed] [Google Scholar]
- 48. Chan AY, Punwani D, Kadlecek TA, Cowan MJ, Olson JL, Mathes EF et al A novel human autoimmune syndrome caused by combined hypomorphic and activating mutations in ZAP‐70. J Exp Med 2016; 213:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang H, Kadlecek TA, Au‐Yeung BB, Goodfellow HE, Hsu LY, Freedman TS et al ZAP‐70: an essential kinase in T‐cell signalling. Cold Spring Harb Perspect Biol 2010; 2:a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yan Q, Barros T, Visperas PR, Deindl S, Kadlecek TA, Weiss A et al Structural basis for activation of ZAP‐70 by phosphorylation of the SH2‐kinase linker. Mol Cell Biol 2013; 33:2188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brdicka T, Kadlecek TA, Roose JP, Pastuszak AW, Weiss A. Intramolecular regulatory switch in ZAP‐70: analogy with receptor tyrosine kinases. Mol Cell Biol 2005; 25:4924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klammt C, Novotná L, Li DT, Wolf M, Blount A, Zhang K et al T cell receptor dwell times control the kinase activity of Zap70. Nat Immunol 2015; 16:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Di Bartolo V, Mège D, Germain V, Pelosi M, Dufour E, Michel F et al Tyrosine 319, a newly identified phosphorylation site of ZAP‐70, plays a critical role in T cell antigen receptor signalling. J Biol Chem 1999; 274:6285–94. [DOI] [PubMed] [Google Scholar]
- 54. Williams BL, Irvin BJ, Sutor SL, Chini CC, Yacyshyn E, Bubeck Wardenburg J et al Phosphorylation of Tyr319 in ZAP‐70 is required for T‐cell antigen receptor‐dependent phospholipase C‐γ1 and Ras activation. EMBO J 1999; 18:1832–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Katz ZB, Novotná L, Blount A, Lillemeier BF. A cycle of Zap70 kinase activation and release from the TCR amplifies and disperses antigenic stimuli. Nat Immunol 2017; 18:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tomlinson MG, Lin J, Weiss A. Lymphocytes with a complex: adapter proteins in antigen receptor signalling. Immunol Today 2000; 21:584–91. [DOI] [PubMed] [Google Scholar]
- 57. Visperas PR, Wilson CG, Winger JA, Yan Q, Lin K, Arkin MR et al Identification of inhibitors of the association of ZAP‐70 with the T cell receptor by high‐throughput screen. SLAS Discov 2017; 22:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kaur M, Singh M, Silakari O. Insight into the therapeutic aspects of ‘Zeta‐chain associated protein kinase 70 kDa’ inhibitors: a review. Cell Signal 2014; 26:2481–92. [DOI] [PubMed] [Google Scholar]
- 59. Mukhopadhyay H, Cordoba SP, Maini PK, van der Merwe PA, Dushek O. Systems model of T cell receptor proximal signaling reveals emergent ultrasensitivity. PLoS Comput Biol 2013; 9:e1003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Guy CS, Vignali KM, Temirov J, Bettini ML, Overacre AE, Smeltzer M et al Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nat Immunol 2013; 14:262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Holst J, Wang H, Eder KD, Workman CJ, Boyd KL, Baquet Z et al Scalable signaling mediated by T cell antigen receptor‐CD3 ITAMs ensures effective negative selection and prevent autoimmunity. Nat Immunol 2008; 9:658–66. [DOI] [PubMed] [Google Scholar]
- 62. Lettau M, Pieper J, Janssen O. Nck adapter proteins: functional versatility in T cells. Cell Commun Signal 2009; 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ngoenkam J, Paensuwan P, Preechanukul K, Khamsri B, Yiemwattana I, Beck‐García E et al Non‐overlapping functions of Nck1 and Nck2 adaptor proteins in T cell activation. Cell Commun Signal 2014; 12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol 2008; 26:233–59. [DOI] [PubMed] [Google Scholar]
- 65. Paensuwan P, Hartl FA, Yousefi OS, Ngoenkam J, Wipa P, Beck‐Garcia E et al Nck binds to the T cell antigen receptor using its SH3.1 and SH2 domains in a cooperative manner, promoting TCR functioning. J Immunol 2016; 196:448–58. [DOI] [PubMed] [Google Scholar]
- 66. Kesti T, Ruppelt A, Wang JH, Liss M, Wagner R, Tasken K et al Reciprocal regulation of SH3 and SH2 domain binding via tyrosine phosphorylation of a common site in CD3ε . J Immunol 2007; 179:878–85. [DOI] [PubMed] [Google Scholar]
- 67. Mingueneau M, Sansoni A, Gregoire C, Roncagalli R, Aguado E, Weiss A et al The proline‐rich sequence of CD3epsilon controls T cell antigen receptor expression on and signalling potency in preselection CD4+CD8+ thymocytes. Nat Immunol 2008; 9:522–32. [DOI] [PubMed] [Google Scholar]
- 68. Borroto A, Arellano I, Dopfer EP, Prouza M, Suchanek M, Fuentes M et al Nck recruitment to the TCR required for ZAP70 activation during thymic development. J Immunol 2013; 190:1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vazquez ML. Biological consequences of the phosphorylation of serine 59 on the tyrosine kinase Lck. [PhD Thesis]. West Lafayette, IN: Purdue University; 2007. [Google Scholar]
- 70. Hem CD, Sundvold‐Gjerstad V, Granum S, Koll L, Abrahamsen G, Buday L et al T cell specific adaptor protein (TSAd) promotes interaction of Nck with Lck and SLP‐76 in T cells. Cell Commun Signal 2015; 13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tailor P, Tsai S, Shameli A, Serra P, Wang J, Robbins S et al The proline‐rich sequence of CD3epsilon as an amplifier of low‐avidity TCR signalling. J Immunol 2008; 181:243–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol 2012; 13:121–8. [DOI] [PubMed] [Google Scholar]
- 73. Borrotto A, Reyes‐Garau D, Jimenez MA, Carrasco E, Moreno B, Martinez‐Pasamar S et al First‐in‐class inhibitor of the T cell receptor for the treatment of autoimmune diseases. Sci Transl Med 2016; 8:370ra184. [DOI] [PubMed] [Google Scholar]
- 74. Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci 2001; 114:1801–9. [DOI] [PubMed] [Google Scholar]
- 75. Vignesh P, Suri D, Rawat A, Lau YL, Bhatia A, Das A et al Sclerosing cholangitis and intracranial lymphoma in a child with classical Wiskott–Aldrich syndrome. Pediatr Blood Cancer 2017; 64:106–9. [DOI] [PubMed] [Google Scholar]
- 76. Miki H, Miura K, Takenewa T. N‐WASP, a novel actin depolymerization protein, regulates the cortical cytoskeletal rearrangement in a PIP2‐dependent manner downstream of tyrosine kinases. EMBO J 1996; 15:5326–35. [PMC free article] [PubMed] [Google Scholar]
- 77. Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ. Nck and phosphatidylinositol 4,5‐bisphosphate synergistically activate actin polymerization through the N‐WASP‐Arp2/3 pathway. J Biol Chem 2001; 276:26448–52. [DOI] [PubMed] [Google Scholar]
- 78. Zeng R, Cannon JL, Abramham RT, Way M, Billadeau DD, Bubeck‐Wardenberg J et al SLP‐76 coordinates Nck‐dependent Wiskott–Aldrich syndrome protein recruitment with Vav‐1/Cdc42‐dependent Wiskott–Aldrich syndrome protein activation at the T cell‐APC contact site. J Immunol 2003; 171:1360–8. [DOI] [PubMed] [Google Scholar]
- 79. Paensuwan P, Ngoenkam J, Khamsri B, Preechanukul K, Sanguansermsri D, Pongcharoen S. Evidence for inducible recruitment of Wiskott–Aldrich syndrome protein to T cell receptor–CD3 complex in Jurkat T cells. Asian Pac J Allergy Immunol 2015; 33:189–95. [DOI] [PubMed] [Google Scholar]
- 80. Ferrandi C, Richard F, Tavano P, Huaben E, Barbie V, Gotteland JP et al Characterization of immune cell subsets during the active phase of multiple sclerosis reveals disease and c‐Jun N‐terminal kinase pathway biomarkers. Mult Scler 2011; 17:43–56. [DOI] [PubMed] [Google Scholar]
- 81. Yang M, Ye L, Wang B, Gao J, Liu R, Hong J et al Decreased miR‐146 expression in peripheral blood mononuclear cells is correlated with ongoing islet autoimmunity in type 1 diabetes patients 1miR‐146. J Diabetes 2015; 7:158–65. [DOI] [PubMed] [Google Scholar]
- 82. Gulino A, Di Marcotullio L, Screpanti I. The multiple functions of Numb. Exp Cell Res 2010; 316:900–6. [DOI] [PubMed] [Google Scholar]
- 83. Aguado R, Martin‐Blanco N, Caraballo M, Canelles M. The endocytic adaptor Numb regulates thymus size by modulating pre‐TCR signaling during asymmetric division. Blood 2010; 116:1705–14. [DOI] [PubMed] [Google Scholar]
- 84. Martin‐Blanco N, Jimenez Teja D, Bretones G, Borroto A, Caraballo M, Screpanti I et al CD3ε recruits Numb to promote TCR degradation. Int Immunol 2016; 28:127–37. [DOI] [PubMed] [Google Scholar]
- 85. Borroto A, Lama J, Niedergang F, Dautry‐Varsat A, Alarcón B, Alcover A. The CD3epsilon subunit of the TCR contains endocytosis signals. J Immunol 1999; 163:25–31. [PubMed] [Google Scholar]
- 86. Isakov N, Wange RL, Burgess WH, Watts JD, Aebersold R, Samelson LE. ZAP‐70 binding specificity to T cell receptor tyrosine‐based activation motifs: the tandem SH2 domains of ZAP‐70 bind distinct tyrosine‐based activation motifs with varying affinity. J Exp Med 1995; 181:375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Granum S, Sundvold‐Gjerstad V, Gopalakrishnan RP, Berge T, Koll L, Abrahamsen G et al The kinase Itk and the adaptor TSAd change the specificity of the kinase Lck in T cells by promoting the phosphorylation of Tyr192. Sci Signal 2014; 7:ra118. [DOI] [PubMed] [Google Scholar]
- 88. D'Oro U, Sakaguchi K, Appella E, Ashwell JD. Mutational analysis of Lck in CD45‐negative T cells: dominant role of tyrosine 394 phosphorylation in kinase activity. Mol Cell Biol 1996; 16:4996–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wu J, Zhao Q, Kurosaki T, Weiss A. The Vav binding site (Y315) in ZAP‐70 is critical for antigen receptor‐mediated signal transduction. J Exp Med 1997; 185:1877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Deindl S, Kadlecek TA, Cao X, Kuriyan J, Weiss A. Stability of an autoinhibitory interface in the structure of the tyrosine kinase ZAP‐70 impacts T cell receptor response. Proc Natl Acad Sci USA 2009; 106:20699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chan AC, Dalton M, Johnson R, Kong GH, Wang T, Thoma R et al Activation of ZAP‐70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J 1995; 14:2499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Miyoshi‐Akiyama T, Aleman LM, Smith JM, Adler CE, Mayer BJ. Regulation of Cbl phosphorylation by the Abl tyrosine kinase and the Nck SH2/SH3 adaptor. Oncogene 2001; 20:4058–69. [DOI] [PubMed] [Google Scholar]
- 93. Lettau M, Kliche S, Kabelitz D, Janssen O. The adaptor proteins ADAP and Nck cooperate in T cell adhesion. Mol Immunol 2014; 60:72–9. [DOI] [PubMed] [Google Scholar]
- 94. Silvin C, Belisle B, Abo A. A role for Wiskott–Aldrich syndrome protein in T‐cell receptor‐mediated transcriptional activation independent of actin polymerization. J Biol Chem 2001; 276:21450–7. [DOI] [PubMed] [Google Scholar]
- 95. Jain N, Tan JH, Feng S, George B, Thanabalu T. X‐linked thrombocytopenia causing mutations in WASP (L46P and A47D) impair T cell chemotaxis. J Biomed Sci 2014; 21:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Anderson AC, Kitchens EA, Chan SW, St Hill C, Jan YN, Zhong W et al The Notch regulator Numb links the Notch and TCR signaling pathways. J Immunol 2005; 174:890–7. [DOI] [PubMed] [Google Scholar]


