Summary
Peripheral blood mononuclear cells taken from patients with scleroderma express increased levels of interleukin (IL)‐13. Moreover, the expression of matrix metalloproteinase‐1 (MMP‐1) from involved scleroderma skin fibroblasts is refractory to stimulation by tumour necrosis factor (TNF)‐α. To elucidate the mechanism(s) involved, we examined the effect of IL‐13 on TNF‐α‐induced MMP‐1 expression in normal and scleroderma human dermal fibroblast lines and studied the involvement of serine/threonine kinase B/protein kinase B (Akt) in this response. Dermal fibroblast lines were stimulated with TNF‐α in the presence of varying concentrations of IL‐13. Total Akt and pAkt were quantitated using Western blot analyses. Fibroblasts were treated with or without Akt inhibitor VIII in the presence of IL‐13 followed by TNF‐α stimulation. MMP‐1 expression was analysed by real‐time polymerase chain reaction (PCR) and enzyme‐linked immunosorbent assay (ELISA). Statistical analysis was performed using analysis of variance (anova) or Student's t‐test. Upon TNF‐α stimulation, normal dermal fibroblasts secrete more MMP‐1 than systemic sclerosis (SSc) fibroblasts. This increase in MMP‐1 is lost when fibroblasts are co‐incubated with IL‐13 and TNF‐α. IL‐13 induced a significant increase in levels of pAkt in dermal fibroblasts, while Akt inhibitor VIII reversed the suppressive effects of IL‐13 on the response of cultured fibroblasts to TNF‐α, increasing their expression of MMP‐1. We show that IL‐13 suppresses MMP‐1 in TNF‐α‐stimulated normal and scleroderma dermal fibroblast. Akt inhibitor VIII is able to reverse the suppressive effect of IL‐13 on MMP‐1 expression and protein synthesis. Our data suggest that IL‐13 regulates MMP‐1 expression in response to TNF‐α through an Akt‐mediated pathway and may play a role in fibrotic diseases such as scleroderma.
Keywords: dermal fibroblasts, fibrosis, IL‐13, inflammatory response, MMP‐1
Introduction
The development of fibrosis is the end result of aberrant inflammatory and tissue repair response resulting from an imbalance between matrix degradation and synthesis. Relevant considerations in this imbalance are the production of metalloproteinase (MMP)‐1, the enzyme involved in collagen degradation in connective tissues, and the synthesis of collagen in connective tissue 1. MMP‐1, the principal collagenase synthesized by skin fibroblasts, plays an important role in the degradation of extracellular matrix in response to proinflammatory cytokines during wound repair. While the pathophysiology of this imbalance could derive from multiple causalities, interleukin (IL)‐13 [which is a T helper type 2 (Th2) cytokine and a major inducer of fibrosis in many chronic diseases such as asthma, chronic infections and parasitic infections] has been implicated in autoimmune disorders such as scleroderma 2. However, the mechanism for the effect of IL‐13 on fibrosis is not understood.
IL‐13 has been shown to modulate collagen homeostasis in normal and keloid fibroblasts, and stimulate the production and activation of transforming growth factor (TGF)‐β in vivo in mice 3, 4. In‐vitro IL‐13 is a potent stimulator of fibroblast proliferation and collagen production 3. Data from human studies suggest that IL‐13 is a pathogenic cytokine in systemic sclerosis (SSc) 5, 6 in which serum IL‐13 levels are increased. Dermal fibroblasts from SSc patients show a myofibroblast phenotype that expresses higher levels of intracellular IL‐1 receptor antagonist (icIL‐1ra) than normal skin fibroblasts after stimulation with tumour necrosis factor (TNF)‐α 7. Expression of MMP‐1 is reduced in biopsies from involved skin of patients with SSc 8. In our previous study, we showed that peripheral blood mononuclear cells (PBMC) from diffuse cutaneous SSc patients produced significant amounts of IL‐13 in response to stimulation with type 1 collagen compared to peripheral blood mononuclear cells (PBMCs) from control or patients with localized disease. Co‐culturing these supernatants with scleroderma fibroblasts for 21 days suppressed their ability to produce MMP‐1 9. Similarly, prolonged culturing of scleroderma fibroblasts with recombinant IL‐13 inhibited significantly the ability of fibroblasts to produce MMP‐1 9. However, the effect of IL‐13 on MMP‐1 gene regulation is not understood clearly.
One intracellular mechanism that has been shown to be up‐regulated by IL‐13 is the phosphatidylinositol 3‐kinase PI3 kinase/protein kinase B (Akt) pathway 10. The serine/threonine protein kinase B (PKB/AKT) is a major component of an intracellular signalling pathway that mediates the response to inflammatory agents. The Akt family of kinases (Akt 1, 2 and 3) regulates proliferation, survival, metabolic and other pleiotrophic cellular activities in normal and tumour cells 11, 12. In the present study, we hypothesized that IL‐13 would decrease the expression of MMP‐1 induced by TNF‐α in normal fibroblasts, and that one possible mechanism for this inhibition might be the PI3 kinase/Akt pathway. Our data suggest that IL‐13 regulates MMP‐1 expression in response to proinflammatory cytokines in normal fibroblasts through an Akt‐mediated pathway with suppression of MMP‐1 production with higher IL‐13 levels.
Methods and materials
Cell lines and culture
This study was conducted using protocols approved by the Institutional Review Board of our institution. The Declaration of Helsinki protocols were followed; patients gave their written informed consent. Adult patients (aged 18–70 years) met the American College of Rheumatology preliminary criteria for the diagnosis of SSc. Human dermal fibroblast cell lines were obtained from eight normal volunteers and five scleroderma patients. Punch biopsies from diffuse scleroderma (dcSSc) patients were taken from involved scleroderma skin of the volar forearm surface. Dermal fibroblasts were grown from punch biopsies from the volar surface of the forearm of normal volunteers. Fibroblasts were allowed to grow out from explanted skin biopsies for 2–3 weeks in Eagle's minimal essential medium (EMEM) with 9% fetal calf serum (FCS), 100 µ/ml penicillin, 100 µg/ml streptomycin and amphotericin B (1 µg/ml).
The fibroblasts were then cultured and maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), penicillin (100 units/ml), streptomycin (100 µg/ml) and 2 mM L‐glutamine (Invitrogen, Grand Island, NY, USA) at 37°C in a humidified atmosphere of 5% CO2 in air. Fibroblasts between 10 and 16 passages were used for experiments. For short‐term experiments, fibroblasts were maintained in 10% DMEM then changed to serum‐free for 24 h prior to experimentation. Normal and scleroderma fibroblasts were treated with varying concentrations of IL‐13, IL‐4, Akt inhibitor VIII or in combination for 1 h and then stimulated by adding TNF‐α for an additional 24 h before harvesting supernatants. For long‐term studies, culture medium containing 10% FBS alone or with IL‐13 were changed every 3 days for 14 days. At the end of the 14‐day incubation period, fibroblasts were maintained in serum‐free (SF) DMEM for 24 h followed by TNF‐α stimulation for an additional 24 h. The cell layer remaining in the tissue culture plates was placed at −80°C for at least 24 h and then subjected to CyQuant analysis, as described below. Control samples received either phosphate‐buffered saline (PBS) (vehicle for TNF‐α) or 5 μM dimethylsulphoxide (DMSO) (vehicle for Akt inhibitor VIII) as an appropriate buffer control where needed. DMSO at a final concentration of 5 μM was non‐toxic to cultured cells as measured by Cyquant analysis.
Reagents
Human recombinant TNF‐α, IL‐13 and IL‐4 were purchased from R&D Systems (Minneapolis, MN, USA). Antibodies for Akt and pAkt were obtained from Cell Signaling Technology (Boston, MA, USA) (nos 9272 and 9271, Ser 473), and antibodies for β‐actin were obtained from Sigma (A5441; Sigma, St Louis, MO, USA). Akt inhibitor VIII (Akt‐1/2) was purchased from Calbiochem (San Diego, CA, USA; Cat. no. 124018). The CyQuant cell proliferation assay kit was purchased from Invitrogen. FBS was purchased from Atlanta Biologicals (Atlanta, GA, USA). ECF Western blotting reagents were purchased from Amersham Biosciences (Piscataway, NJ, USA). All other reagents were analytical grade.
CyQuant analysis
At the end of the incubation period, culture supernatants were stored at −20°C until analysis by enzyme‐linked immunosorbent assay (ELISA) for MMP‐1 protein, and the cell layer was frozen at −80°C for assay of cell numbers by CyQuant cell proliferation assay. Cells were removed from −80°C and allowed to thaw, at which time cell lysis buffer and dye were placed on the cell layer and allowed to incubate overnight in the dark. Fluorescence excitation/emission was read at 480/520 nm using a SPECTRAMAX 340PC microplate spectrophotometer (Molecular Devices, Sunnyside, CA, USA).
Analysis of gene expression
Total RNA was isolated from cells using Trizol (Ambion, Grand Island, NY, USA) and reverse‐transcribed into cDNA using reverse transcriptase [Life Technologies–Applied Biosystems (ABI), Grand Island, NY, USA] for real time–polymerase chain reaction assay (RT–PCR). A Taqman gene assay was used for MMP‐1 (Cat. no. HS00899658‐m1; Life Technologies–ABI); beta actin (Applied Biosystems, Grand Island, NY, USA) was used as housekeeping gene. The PCR was run using sds version 2·3 software and analysed with RQ manager (Life Technologies–ABI).
Analysis of MMP‐1 protein expression
Supernatants from cultured fibroblasts were analysed for MMP‐1 using human MMP‐1 ELISA kit (R&D Systems) and normalized to cell number as determined by CyQuant assay.
Analysis of issue inhibitor of metalloproteinase‐1 (TIMP‐1) protein expression
Supernatants from cultured fibroblasts were analysed for TIMP‐1 using the human TIMP‐1 ELISA kit (R&D Systems).
Quantitation of Akt and pAkt in dermal fibroblasts
The level and activation of Akt was determined by immunoblotting using antibodies specific for the phosphorylated active form (pAkt) or non‐phosphorylated form (total Akt). To test the involvement of the PKB/Akt pathway in MMP‐1 expression, we used the Akt inhibitor VIII, a cell‐permeable quinoxaline compound that inhibits the phosphorylation steps of Akt 1 and Akt 2 isoforms selectively 13, 14. Fibroblasts were treated with interleukin (IL‐13) for the indicated time intervals, then washed with ice‐cold phosphate‐buffered saline (PBS) and lysed in radioimmunoprecipitation assay (RIPA) buffer. The protein concentrations of the lysates were determined by a Bio‐Rad protein assay, and 50 μg/well were electrophoresed in a 10% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred electrophoretically onto a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated overnight with rabbit anti‐phosphorylated Akt antibody (no. 9271, Ser 473, 1/1000 dilution; Cell Signaling). The same blot was stripped and reprobed with Akt antibody (no. 9272, 1/1000 dilution; Cell Signaling) and then with β‐actin antibody (A5441, 1/1000 dilution; Sigma) on the same blot. Akt control cell extract (no. 9273; Cell Signaling) was used as a positive control. Band densities were measured and normalized based on β‐actin levels.
Effect of Akt inhibitor VIII on MMP‐1 protein expression
To determine the effects of Akt inhibition on the PI3 kinase‐Akt pathway and MMP‐1 expression, a dose–response study using an Akt inhibitor was performed. The Akt inhibitor at 2·5 and 5 μM with and without stimulation with 5 ng/ml TNF‐α for 24 h was found to be non‐toxic to these fibroblasts (data not shown). A concentration of 5 μM was used for the Akt inhibitor experiments. Fibroblasts were pretreated with 5 μM of Akt inhibitor VIII for 1 h followed by incubation with or without IL‐13 2·5 ng/ml for an additional 1 h before stimulation with TNF‐α (5 ng/ml) for 24 h. Supernatants were analysed by ELISA for MMP‐1 after 24 h of TNF‐α stimulation and stored at −20°C until further analysis. As a vehicle control, PBS and 0·1% DMSO (v/v) were added to cell culture medium 1 h before stimulation with TNF‐α.
Statistical analysis
Data are reported as mean ± standard error of the mean (s.e.m.). Statistical analysis was performed using analysis of variance (anova) or Student's t‐test where P < 0·05 was considered significant.
Results
IL‐13 suppression of MMP‐1 expression in human dermal fibroblasts
In order to determine whether IL‐13 suppresses TNF‐α‐mediated induction of MMP‐1, we pretreated normal human fibroblast cell lines with various concentrations of IL‐13 (0·5, 1·0, 2·5 and 5 ng/ml) or IL‐4 (1·0 and 5 ng/ml) in triplicate wells for 1 h prior to stimulation with TNF‐α (5 ng/ml) for an additional 24 h. Cultured supernatants were harvested and analysed for MMP‐1 by ELISA. As IL‐13 is a potent inducer of fibroblast proliferation, fibroblasts were normalized with respect to cell number. Treatment of the fibroblasts with varying doses of IL‐13 showed a dose‐dependent decrease in the levels of MMP‐1 protein. This dose‐dependent decrease was noted at doses > 1·0 ng/ml in response to stimulation with TNF‐α. IL‐13 at 2·5 and 5·0 ng/ml inhibited TNF‐α‐induced MMP‐1 expression significantly (Fig. 1a). Lower doses of IL‐13 augmented TNF‐α‐induced MMP‐1 protein expression at 0·5 ng/ml, suggesting that higher concentrations of IL‐13 (two‐ to fivefold higher) are needed to suppress TNF‐α‐induced MMP‐1 expression (Fig. 1a,b). In a separate experiment, an IL‐13 dose response was carried out on scleroderma fibroblast lines. Similar to normal dermal fibroblasts, an IL‐13 dose of 1 to 2 ng/ml inhibited TNF‐α‐inhibited MMP‐1 expression significantly (Fig. 1d). As the IL‐4 receptor alpha (IL‐4Rα) subunit is shared with the IL‐13 receptor, we examined the effect of IL‐4 on TNF‐α‐induced MMP‐1 expression. Healthy and scleroderma fibroblasts were incubated with varying doses of IL‐4 for 1 h followed by stimulation with TNF‐α (5 ng/ml). A dose‐dependent inhibition of TNF‐α‐induced MMP‐1 expression was seen in healthy fibroblasts with IL‐4 at 1 ng/ml and 5 ng/ml (Fig. 1c) and in scleroderma fibroblasts at doses 1 ng/ml and 10 ng/ml (Fig. 1e). These data show that normal and scleroderma fibroblasts respond similarly to IL‐4 and IL‐13.
Figure 1.
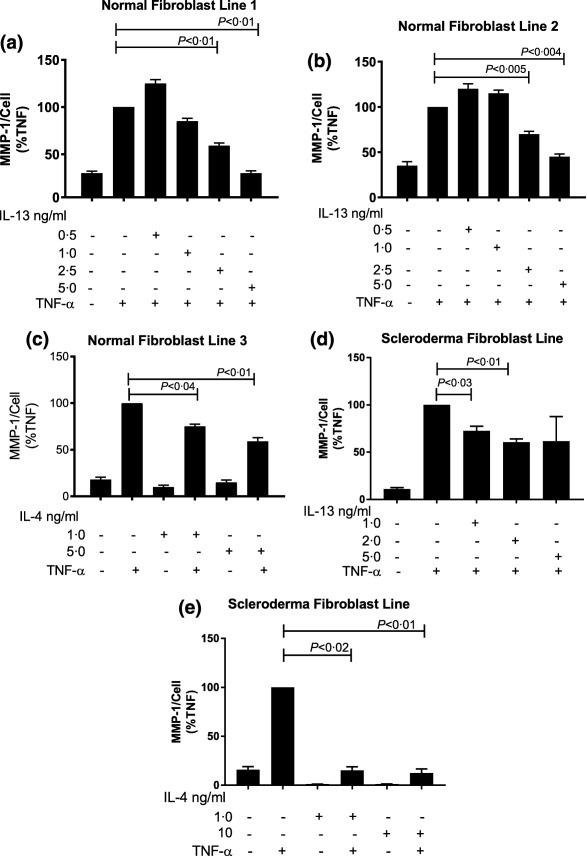
Dose‐dependent effect of interleukin (IL)‐13 on production of matrix metalloproteinase‐1 (MMP‐1) protein in dermal fibroblasts stimulated with tumour necrosis factor (TNF)‐α. Normal fibroblast cell lines: (a,b,c) and scleroderma lines (d,e) were pretreated with various concentrations of IL‐13 or IL‐4 before stimulation with TNF‐α. Data are expressed as the mean ± standard error of the mean (s.e.m.) and values (MMP‐1/cell) are plotted as a percentage of that seen for cultures stimulated with TNF‐ α. P‐values were determined by analysis of variance (anova) or Student's t‐test where P < 0·05 was considered significant. Experiments were repeated to confirm results.
Due to the chronic nature of fibrotic disorders, a study was conducted in normal fibroblasts to assess the effects of chronic exposure of IL‐13 on TNF‐α‐induced MMP‐1 expression. Eight normal dermal fibroblast lines were subjected to long‐term culturing with human recombinant IL‐13. Dermal fibroblasts were incubated with culture medium containing 10% FBS with or without the inclusion of IL‐13 (2·5ng/ml) for 14 days followed by a 24‐h washout period in SF DMEM prior to stimulation with TNF‐α (5 ng/ml), as described previously 9.
Chronic exposure of the normal fibroblasts to IL‐13 suppressed constitutive MMP‐1 protein expression when compared to fibroblasts cultured in vehicle alone (Fig. 2). The suppressive effects of chronic IL‐13 exposure on MMP‐1 protein expression were not overcome when fibroblasts were stimulated later with TNF‐α (Fig. 2). We selected two of these fibroblast lines to analyse the relative expression of MMP‐1 mRNA. The relative expression of MMP‐1 mRNA in response to TNF‐α stimulation was also inhibited by long‐term culture of fibroblasts in the presence of IL‐13 (Fig. 3) in normal fibroblast lines 3 and 6 maintained in medium alone for 14 days. MMP‐1 mRNA measured by RT–PCR was increased 60‐ and 80‐fold in normal fibroblast lines 3 and 6 (Fig. 3a,b, respectively) after stimulation with TNF‐α. In the same normal fibroblast lines cultured for 14 days in the presence of IL‐13 followed by stimulation with TNF‐α, MMP‐1 mRNA was reduced by 30 and 75%, respectively, from the MMP‐1 mRNA levels attained in the same normal fibroblast lines cultured for 14 days with medium and then with TNF‐α.
Figure 2.
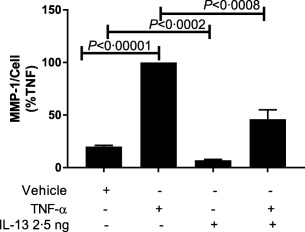
Suppressive effect of long‐term incubation of interleukin (IL)‐13 on production of matrix metalloproteinase‐1 (MMP‐1) protein in response to tumour necrosis factor (TNF)‐α stimulation in normal dermal fibroblasts. Fibroblast lines (n = 8) were treated with vehicle [Dulbecco's modified Eagle's medium (DMEM) + 10% fetal calf serum (FCS) + phosphate‐buffered saline (PBS) with 0·1% bovine serum albumin (BSA) (vehicle for IL‐13 and TNF‐ α)] or IL‐13 2·5 ng/ml for 14 days prior to stimulation with TNF‐α for enzyme‐linked immunosorbent assay (ELISA) analysis of culture supernatants. Experiments were run in triplicate and expressed as percentage of that seen for cultures stimulated with TNF‐α. P‐values determined by Student's t‐test. P < 0·05 represents significance.
Figure 3.
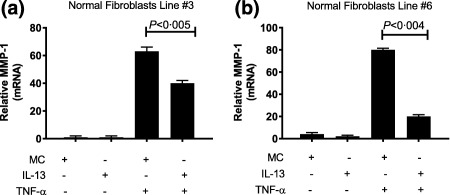
Expression of matrix metalloproteinase‐1 (MMP‐1). Polymerase chain reaction (PCR) analyses of MMP‐1 mRNA in dermal fibroblasts after a 14‐day culture with recombinant interleukin (IL)‐13 followed by stimulation with tumour necrosis factor (TNF)‐α in normal dermal fibroblast lines 3 (a) and 6 (b). Data are expressed as standard error of the mean (s.e.m.) and plotted as relative MMP‐1. P‐values were determined by analysis of variance (anova) and compared with the value in TNF‐α‐treated cells.
Effect of IL‐13 on Akt activation
As protein kinase B/Akt has been shown to be a critical enzyme in the signal transduction pathways of fibrosis 15, we investigated the role that protein kinase B/Akt might have in IL‐13 inhibition of MMP‐1. Protein kinase B/Akt activation in normal fibroblasts was analysed by Western blotting for total Akt and phosphorylated Akt (pAkt) at time‐points 0 h, 20 min, 40 min, 1 h, 4 h and 16 h after stimulation with 2·5 ng/ml of IL‐13 (Fig. 4a). A significant 26% increase in the level of pAkt was detected by Western blot analysis after 20 min of stimulation with IL‐13 (2·5 ng/ml) compared to untreated controls at time 0 (Fig. 4a). The levels of pAkt returned to baseline value at 40 min and decreased thereafter, reaching the lowest level at the 16‐h time‐point (Fig. 4a). In contrast, there was no significant decrease in total Akt level during the 16‐h period of observation (Fig. 4b).
Figure 4.
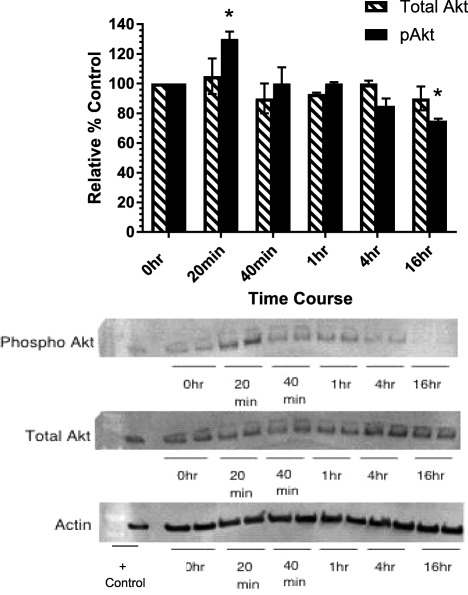
Activation of protein kinase B (Akt) following interleukin (IL)‐13 treatment. Normal dermal fibroblasts were incubated with IL‐13 (2·5 ng/ml) for varying intervals, and the total cell extracts were separated by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) (50 μg protein per lane) (a). The density of phospho‐Akt and total Akt immunoreactive bands were normalized to β‐actin and plotted as percentage of that from unstimulated cells at time 0. (b) Western blot analysis with antibodies to phosphor‐Akt (p‐Akt Ser 473), total Akt (Akt) and β‐actin. *P < 0·05 and **P < 0·01 compared to untreated controls at time 0. Analysis performed by Student's t‐test. Data shown are representative of three experiments using three different cell lines.
Inhibition of TNF‐induced MMP‐1 protein expression by IL‐13 in human normal dermal fibroblasts and SSc from involved skin is mediated by protein kinase B/Akt
To understand further the mechanism of inhibition by which IL‐13 inhibits MMP‐1 protein production and to test the involvement of the PKB/Akt pathway in MMP‐1 expression, we used the Akt inhibitor VIII, a cell‐permeable quinoxaline compound that inhibits the phosphorylation steps of Akt 1 and Akt 2 isoforms selectively 13. We hypothesized that treatment with the Akt inhibitor VIII would be able to restore the response to TNF‐α of the fibroblasts cultured with IL‐13. To analyse our fibroblasts for MMP‐1 protein synthesis, five normal fibroblast lines were pretreated with 5 μM of Akt inhibitor VIII with or without IL‐13 followed by stimulation with TNF‐α (5 ng/ml) for 24 h. Supernatants were analysed by ELISA for MMP‐1 protein synthesis. Three of the five normal fibroblasts lines are shown in Fig. 5. As revealed in Fig. 5, normal fibroblast lines treated with IL‐13 (2·5 ng/ml) before TNF‐α stimulation exhibited significantly suppressed MMP‐1 protein levels compared to fibroblasts stimulated with TNF‐α alone. Treatment with both IL‐13 and Akt inhibitor VIII blocked the suppressive effect of IL‐13 in the response to TNF‐α in fibroblasts and increased MMP‐1 levels significantly (Fig. 5; top row) compared to their levels when stimulated with TNF‐α and IL‐13 in the absence of the Akt inhibitor. The amount of MMP‐1 secreted when incubated with TNF‐α and IL‐13 were restored when the Akt inhibitor was included. The Akt inhibitor alone showed little effect on the TNF‐α response.
Figure 5.
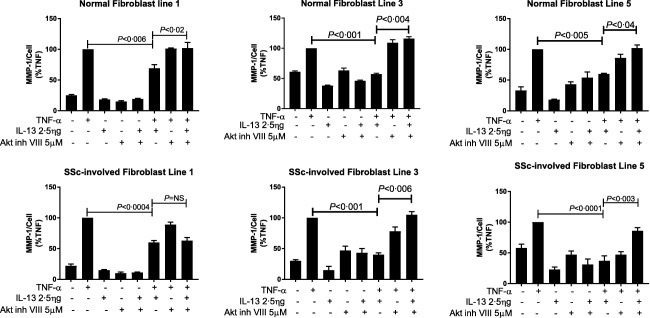
Effect of protein kinase B (Akt) inhibitor VIII on interleukin (IL)‐13‐induced alterations of matrix metalloproteinase‐1 (MMP‐1) protein expression in the response to tumour necrosis factor (TNF)‐α in dermal fibroblasts. Fibroblast lines from normal healthy skin (top row); and involved scleroderma skin (bottom row) were pretreated with 5 μM of Akt inhibitor VIII before stimulation with TNF‐α with or without IL‐13 and analysed for MMP‐1 by enzyme‐linked immunosorbent assay (ELISA). Data are expressed as standard error of the mean (s.e.m.) and plotted as a percentage of the value of the TNF‐treated cells. P‐values were determined by analysis of variance (anova). P < 0·05 compared with the value in TNF‐α and IL‐13‐treated cells.
Next we evaluated the role of Akt in scleroderma. We have shown previously that prolonged culturing of dermal fibroblasts from involved dcSSc skin with rh IL‐13 suppressed protein expression of MMP‐1 9. To ascertain if the Akt inhibitor can block the effect of long‐term culture with IL‐13 on the induction of MMP‐1 in scleroderma fibroblasts after stimulation with TNF‐α we used five different scleroderma fibroblasts lines from SSc‐involved skin. Three of the five lines are shown in Fig. 5; bottom row).
IL‐13 suppressed MMP‐1 production in all SSc lines when compared to TNF‐α alone. The suppressive effect of IL‐13 was lost when these fibroblasts were treated with Akt inhibitor VIII, resulting in a significant increase in MMP‐1 production in two of three lines (Fig. 5; bottom row). Akt inhibitor VIII blocked the suppressive effect of IL‐13 in these fibroblast lines, with more than a twofold increase in the amount of MMP‐1 being observed after TNF‐α stimulation. These data suggest that there may be a subset population in SSc for whom treatment with Akt‐inhibitor may be beneficial in targeting fibrosis.
Protein kinase B/Akt alters the expression of TIMP‐1 in human dermal fibroblasts
We next examined the effect of IL‐13 on fibroblast lines with regard to their expression of TIMP‐1 after stimulation with TNF‐α. IL‐13 and Akt inh VIII alone have varying individual effects on TIMP‐1 protein expression in our fibroblast lines with no significant trends (data not shown). These data suggest that the ability of the Akt inhibitor VIII to block the suppressive effect of IL‐13 on MMP‐1 after TNF‐α stimulation is not associated with an effect on TIMP‐1 in these fibroblast lines.
Quantitative differences in the expression of MMP‐1 in human dermal fibroblasts
We were interested in determining whether there were quantitative differences between normal and scleroderma fibroblast in TNF‐α‐induced expression of MMP‐1 in fibroblast treated with and without Akt inhibitor. The overall expression of MMP‐1 protein is significantly less in SSc‐involved fibroblasts cultured with IL‐13 and TNF‐α when compared to normal fibroblasts (Table 1). In assessing the effect of Akt inhibitor on MMP‐1 protein expression, SSc‐involved fibroblast lines produced less MMP‐1 protein than normal (Table 1). These data suggest quantitative differences in TNF‐α‐induced expression of MMP‐1 between normal and SSc fibroblasts in their response to IL‐13 and Akt inhibitor.
Table 1.
Quantitative differences in the expression of matrix metalloproteinase‐1 (MMP‐1) in human dermal fibroblasts
| Fibroblasts line | Vehicle | IL‐13 2·5 ng + TNF‐α 5 ng/ml | Akt in 5 μM + IL‐13 2·5 ng + TNF‐α 5 ng/ml | P‐value |
|---|---|---|---|---|
| Normal 1 | 1506 ± 56 | 6402 ± 229 | 7222 ± 367 | < 0·03 |
| Normal 2 | 17 275 ± 77 | 19 287 ± 2680 | 43 913 ± 1612 | < 0·003 |
| Normal 3 | 9248 ± 823 | 177 435 ± 12 368 | 246 564 ± 5767 | < 0·009 |
| Normal 4 | 36 018 ± 4298 | 45 085 ± 1604 | 118 215 ± 4014 | < 0·008 |
| Normal 5 | 96 739 ± 2394 | 533 011 ± 176 212 | 1 538 111 ± 352 934 | < 0·005 |
| SSc‐involved 1 | 51 ± 13 | 138 ± 10 | 144 ±17 | P = 0·3 |
| SSc‐involved 2 | 1818 ± 550 | 692 ± 76 | 1517 ± 90 | < 0·003 |
| SSc‐involved 3 | 19 ± 3 | 33 ± 1·5 | 68 ± 7 | < 0·004 |
| SSc‐involved 4 | 18 ± 3·5 | 104 ± 10 | 118 ± 15 | P = 0·2 |
| SSc‐involved 5 | 4·8 ± 0·8 | 52 ± 3 | 30 ± 8 | < 0·008 |
Dermal fibroblasts were cultured with either phosphate‐buffered saline (PBS) (vehicle), interleukin (IL)‐13 2·5 ng/ml + tumour necrosis factor (TNF)‐α 5 ng/ml alone or IL‐13 2·5 ng/ml + TNF‐α 5 ng/ml with the addition of protein kinase B (Akt) inh VIII 5 μM for 24 h. Supernatants were collected and analysed for metalloproteinase (MMP)‐1 production by enzyme‐linked immunosorbent assay (ELISA). Values for MMP‐1 are expressed as pg/ml. P‐values were determined by Student's t‐test by comparing IL‐13 + TNF‐α fibroblasts with fibroblasts treated with Akt inh + IL‐13 + TNF‐α.
In order to compare the magnitude of the quantitative difference in TNF‐α‐induced MMP‐1 expression between normal and scleroderma fibroblasts we controlled for background stimulation by calculating the stimulation index by dividing the quantity of MMP‐1 in treated fibroblasts by the quantity of MMP‐1 in unstimulated fibroblasts. Normal fibroblasts treated with IL‐13 and TNF‐α have a fivefold increase in MMP‐1 compared to IL‐13/TNF‐α treated SSc‐involved fibroblasts. The addition of Akt inhibitor increased MMP‐1 expression threefold in normal fibroblasts and twofold in SSc fibroblasts compared to IL‐13/TNF‐α‐treated fibroblasts (Fig. 6). These data suggest that in both normal and SSc fibroblasts Akt inhibitor can block the suppressive effect of TNF‐α induced IL‐13 inhibition of MMP‐1 effectively, although in SSc there may be a subset population where Akt inhibitor is effective.
Figure 6.
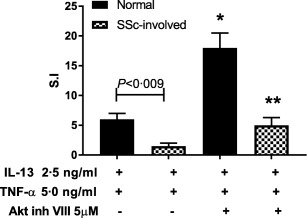
Quantitative effect of interleukin (IL)‐13 and protein kinase B (Akt) inhibitor on the expression of matrix metalloproteinase‐1 (MMP‐1) in normal and scleroderma‐involved dermal fibroblasts in the presence of tumour necrosis factor (TNF)‐α. Three normal fibroblast lines and three scleroderma fibroblasts lines from involved skin were either treated alone with IL‐13 or pretreated with Akt‐inh and IL‐13 followed by stimulation with TNF‐α. Supernatants were analysed by enzyme‐linked immunosorbent assay (ELISA) for MMP‐1 production. Data are expressed as stimulation index by dividing MMP‐1 of treated fibroblast by the MMP‐1 of the vehicle treated fibroblast. P‐values were determined by analysis of variance (anova) where P < 0·05 is significant; *P < 0·05 when compared to normal fibroblasts treated with IL‐13 and TNF‐α; **P < 0·05 when compared to systemic sclerosis (SSc)‐involved fibroblasts treated with IL‐13 and TNF‐α.
Discussion
In a previous study, we showed that type 1 collagen‐stimulated PBMC from patients with diffuse cutaneous systemic sclerosis produced increased IL‐13, and that prolonged cultures of scleroderma dermal fibroblasts with recombinant IL‐13 inhibit significantly their ability to produce MMP‐1 protein after TNF‐α stimulation 9. We hypothesized that IL‐13 might decrease the expression of MMP‐1 induced by an inflammatory cytokine, TNF‐α, in normal and scleroderma fibroblasts, and that a possible mechanism for this inhibition might involve the PI3 kinase/Akt pathway. The inhibition of the expression of collagenase (MMP‐1) could contribute to the accumulation of collagen and may be relevant to this phenomenon for this disease.
In this study, we show that recombinant IL‐13 reduces TNF‐α‐induced MMP‐1 protein and mRNA in short‐ and long‐term cultures of fibroblasts. This inhibition is seen with higher doses of IL‐13. Moreover, Akt inhibitor VIII reverses IL‐13 inhibition of TNF‐α‐induced MMP‐1 in IL‐13‐treated dermal fibroblasts and could inhibit the TNF response by itself. Additionally, we show that there are quantitative differences in fibroblasts’ ability to synthesize MMP‐1 in response to TNF‐α after co‐culturing with IL‐13 and that the addition of Akt inhibitor under these conditions can normalize the response and increase the overall production of MMP‐1. Although TIMP‐1 is very important in regulating homeostasis between wound‐healing and degradation, we show that Akt inhibitor had varying effects on TIMP‐1 protein expression in normal and SSc fibroblasts cultured with IL‐13 and TNF‐α. Taken together, these data suggest IL‐13 may contribute to fibrosis by affecting resistance to MMP‐1 up‐regulation by inflammatory cytokines such as TNF‐α and possibly to other conditions where MMP‐1 plays a role in normal remodelling of the extracellular matrix of the skin.
IL‐13 plays a pivotal role in both physiological and pathological conditions. High‐affinity IL‐13 receptors are reportedly expressed in normal skin fibroblasts 16, 17, and addition of IL‐13 to cultured fibroblasts stimulates transcription of collagen and collagen deposition 18. IL‐13 is known to inhibit the function or production of many proinflammatory cytokines, including TNF‐α, IL‐1β, IL‐6 and IL‐8 in monocytes, macrophages, B cells, natural killer cells and endothelial cells 19, 20, 21, 22. IL‐13 decreases production of proinflammatory cytokines such as TNF‐α and down‐regulates the inflammatory response to IL‐1 through up‐regulation of the level of the IL‐1 receptor antagonist 19, 23. Addition of exogenous IL‐13 to synovial fibroblasts and mononuclear leucocyte cell cultures has been shown to reduce the production of IL‐1β and TNF‐α significantly 24. Inflammatory pathways mediated by these cytokines up‐regulate expression of MMP‐1 3. In the present study, we observed that IL‐13 diminishes the ability of dermal fibroblasts to produce MMP‐1 upon TNF‐α stimulation. Our data relate these findings to studies of the increased collagen deposition in scleroderma patients and highlight the role of IL‐13 as a potent anti‐inflammatory cytokine with respect to MMP‐1 production.
Recently, TNF receptors I and II were found to be up‐regulated on dermal T lymphocytes from patients with diffuse cutaneous systemic sclerosis and supernatants from TNF stimulated lymphocytes produced higher type 1 collagen expression from healthy donors 25. We have shown that stimulation with TNF‐α from healthy dermal fibroblast donors increased the inherent ability of the dermal fibroblast to produce MMP‐1, consistent with our previous findings 26. The effects of IL‐13 are mediated by its ability to regulate a number of downstream genes through multiple signalling pathways such as extracellular‐regulated kinase (ERK1/2), mitogen‐activated protein kinase (MAPK), p38 MAPK and signal transducer and activator of transcription (STAT)‐6 in inflammation and fibrosis 27, 28, 29, 30. Fibroblasts are known to express the IL‐13 R1, IL‐13 R2 and IL‐4 R α‐receptors necessary for signalling 31, 32, 33. We show that IL‐4 can inhibit the MMP‐1 response similarly, probably by engaging the shared receptor, IL‐13Rα1/IL‐4rα. IL‐4 has other fibrogenic effects on human dermal fibroblasts, including stimulating fibroblast chemotaxis and induction of type I and III collagen and fibronectin synthesis 34, 35. One signalling pathway that has been studied in relation to IL‐13 is the PI3‐kinase pathway. Akt is a serine/threonine kinase that has been found to be involved in signal transduction pathways of fibrosis and hypertrophic scarring 36, 37. Akt is activated following its phosphorylation by 3‐phosphoinositide‐dependent (PI3) kinase 1 and mammalian target of rapamycin complex (mTORc2) 12, 38, 39. Akt is activated via phosphorylation of both Thr308 and Ser 473 40, 41.
In the present study, we show that the level of pAkt increases maximally at 20 min, with a steady decrease in pAkt over 24 h in the dermal fibroblast stimulated with IL‐13. Using an Akt inhibitor specific for Akt 1 and 2, we could reverse the IL‐13 inhibition of MMP‐1 in these fibroblasts, supporting the concept that protein kinase B is one pathway involved in IL‐13‐mediated signalling. Other studies have shown the involvement of PI3‐kinase in IL‐13‐mediated expression in dermal fibroblasts. Jinnin et al. 10 showed that tenascin‐C was up‐regulated by IL‐13 in dermal fibroblasts using the PI3 kinase pathway. Ricupero et al. 42 showed that PI3 kinase activation results in increased stabilization of α1 collagen mRNA in fibroblasts. In addition, Lim et al. 43 reported that keloid fibroblasts produced excessive amounts of type I collagen through synchronous activation of both ERK and PI3 kinase/Akt pathways. Relevant to disease, skin fibroblasts from patients with systemic sclerosis show increased activation of the Akt pathway 44. In the present study, we show that activation of Akt by IL‐13 appears to suppress MMP‐1 expression. These results suggest that activation of the PI3 kinase/Akt pathway by TNF‐α may be an endogenous feedback regulator of MMP‐1 expression in skin fibroblasts stimulated by TNF‐α. We saw an increase in MMP‐1 in response to TNF‐α in the presence of the Akt 1/2 inhibitor. This finding may prove helpful in restoration of the normal balance of synthesis and degradation in scleroderma.
Others have also shown the up‐regulation of MMP‐1 by blocking Akt in dermal fibroblasts 41. Using Akt inhibitor, small interfering RNA and dominant‐negative Akt mutant, these authors were able to inhibit basal collagen type 1 expression and up‐regulate Akt 41. Blocking Akt signalling in periostin (–/–) mice dermal fibroblasts inhibited Col1a1 expression 45. In these studies, however, blocking Akt with an inhibitor inhibited collagen and MMP‐1 expression induced by TGF‐β.
Activated Akt may phosphorylate target proteins such as glycogen synthase kinase 3 beta (GSK3 β), cAMP response element binding protein (CREB) and procaspase 9 46, 47, 48. GSK3 has been shown to play a role in regulating various transcription factors, including nuclear factor kappa light‐chain enhancer of activated B cells (NF‐κB), activator protein‐1 (AP1) and CREB, that are critical in regulating pro‐ and anti‐inflammatory cytokine production 49, 50, as well as MMP‐1 induction. Martin et al. 40 showed that inhibition of GSK3 allows CREB to sequester CBP from NF‐κB, thereby decreasing NF‐κB‐dependent inflammatory cytokine responses. Based on these reports, we hypothesize a possible link between the Akt pathway and GSK 3 activity in regulating MMP‐1 gene expression in both normal skin fibroblasts and scleroderma fibroblasts.
TIMP‐1 plays a significant role tissue homeostasis and in the fibrotic diseases balancing collagen formation and degradation. Dermal biopsies from affected SSc skin reveal a significant up‐regulation of TIMP‐1 and decreased MMP‐1 expression when compared to biopsies taken from unaffected skin 8. Monocytes from human control patients incubated with serum from patients with SSc‐activated TIMP‐1 production suggest that TIMP‐1 up‐regulated expression was secreted by the monocytes in SSc patients 51. TIMP‐1 acts to inhibit MMP‐1 function. Fusing a glycosylphosphatidylinositol (GPI) anchor to TIMP‐1 and followed by adding recombinant TIMP‐1 exogenously to fibroblasts reduced the protein excretion of MMP‐1 and reduced the expression of fibrosis‐related genes 52. Exposing human dermal fibroblasts to TNF‐α increased expression of both MMP‐1 and TIMP‐1 53, 54, while IL‐4 and IL‐13 decreased MMP‐1 and increased TIMP‐1 in conjunctival fibroblasts 55. TIMP‐1 over‐expression studies using NIH3T3 cells showed that the p‐Akt pathway is involved in TIMP‐1 expression and blocks the Akt pathway with an inhibitor‐reversed TIMP‐1 over‐expression 56. Our data suggest that IL‐13 suppresses MMP‐1 in human dermal fibroblasts, and this suppressive effect of IL‐13 is regulated through the PI3 kinase/Akt pathway. In the presence of Akt‐inhibitor VIII, the inhibitory effect of IL‐13 on MMP‐1 after TNF‐α stimulation is blocked. We show quantitative differences in MMP‐1 protein synthesis in IL‐13 and TNF‐α co‐cultured fibroblasts between normal and SSc‐involved fibroblasts. The addition of Akt inhibitor in the presence of both IL‐13 and TNF‐α increased MMP‐1 significantly in our fibroblasts. TIMP‐1 protein synthesis varied in our fibroblasts pretreated with Akt inhibitor VIII followed by co‐culturing with IL‐13 and TNF‐α, suggesting that Akt inhibitor's ability to block the suppressive effect of IL‐13 on MMP‐1 is not associated with TIMP regulation. Other regulatory pathways and mechanisms may be recruited in response to IL‐13 suppressive effect of MMP‐1 that may not be related directly to the PKB/Akt pathway. Further studies are needed to understand downstream targets of phospho‐Akt and its effect on MMP‐1 gene regulation and the involvement of downstream‐negative regulators.
Limitations of this study
Our study highlights the potential role of IL‐13 in fibrotic diseases such as scleroderma. A larger number of dermal fibroblasts are needed to differentiate clearly between healthy and scleroderma fibroblast and the role of IL‐13 in fibrotic disorders. Larger patient studies are also needed to determine if resistance to MMP‐1 induced by TNF‐α and IL‐13 modulation are involved in a specific disease subset and are also needed to help differentiate between involved and uninvolved skin from limited cutaneous compared to diffuse cutaneous dcSSc. Although this paper focused on the Akt pathway, other pathways such as GSK‐3, p38 MAPK and ERK1/2 may also play a role in MMP‐1 resistance.
Conclusions
We show that IL‐13 suppresses MMP‐1 in TNF‐α‐stimulated dermal fibroblasts, and quantitative differences are seen in MMP‐1 expression in IL‐13/TNF‐α treated fibroblasts. We show that Akt inhibitor VIII is able to block the suppressive effect of IL‐13 on MMP‐1 expression in fibroblasts and that Akt inhibitor increases MMP‐1 protein synthesis. This inhibition is mediated by the PI3 kinase/Akt pathway, suggestive that this pathway may play a role in IL‐13‐mediated fibrotic diseases such as scleroderma, and may be a target for treatment of fibrotic diseases.
Disclosure
The authors have no financial or commercial conflicts to disclose.
Acknowledgement
This work was supported by the Arthritis Foundation, Scleroderma Foundation and Le Bonheur Children's Research Center grant awarded to M. L. B, US Public Health Service/National Institutes of Health grant AR‐007317‐29 to A. E. P and VA Merit Award to K. A. H.
References
- 1. Kahari VM, Saarialho‐Kere U. Matrix metalloproteinases in skin. Exp Dermatol 1997; 6:199–213. [DOI] [PubMed] [Google Scholar]
- 2. Simms RW, Korn JH. Cytokine directed therapy in scleroderma: rationale, current status, and the future. Curr Opin Rheumatol 2002; 14:717–22. [DOI] [PubMed] [Google Scholar]
- 3. Oriente A et al Interleukin‐13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther 2000; 292:988–94. [PubMed] [Google Scholar]
- 4. Lee CG et al Interleukin‐13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med 2001; 194:809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hasegawa M et al Elevated serum tumor necrosis factor‐alpha levels in patients with systemic sclerosis: association with pulmonary fibrosis. J Rheumatol 1997; 24:663–5. [PubMed] [Google Scholar]
- 6. Kissin EY, Korn JH. Fibrosis in scleroderma. Rheum Dis Clin North Am 2003; 29:351–69. [DOI] [PubMed] [Google Scholar]
- 7. Higgins GC, Wu Y, Postlethwaite AE. Intracellular IL‐1 receptor antagonist is elevated in human dermal fibroblasts that overexpress intracellular precursor IL‐1 alpha. J Immunol 1999; 163:3969–75. [PubMed] [Google Scholar]
- 8. Frost J et al Differential gene expression of MMP‐1, TIMP‐1 and HGF in clinically involved and uninvolved skin in South Africans with SSc. Rheumatology 2012; 51:1049–52. [DOI] [PubMed] [Google Scholar]
- 9. Brown M et al Supernatants from culture of type I collagen‐stimulated PBMC from patients with cutaneous systemic sclerosis versus localized scleroderma demonstrate suppression of MMP‐1 by fibroblasts. Clin Rheumatol 2012; 31:973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jinnin M et al Upregulation of tenascin‐C expression by IL‐13 in human dermal fibroblasts via the phosphoinositide 3‐kinase/Akt and the protein kinase C signaling pathways. J Invest Dermatol 2006; 126:551–60. [DOI] [PubMed] [Google Scholar]
- 11. Stambolic V, Woodgett JR. Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends Cell Biol 2006; 16:461–6. [DOI] [PubMed] [Google Scholar]
- 12. Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide‐regulated kinases: kinase activation by phosphoinositide‐dependent phosphorylation. Annu Rev Biochem 1999; 68:965–1014. [DOI] [PubMed] [Google Scholar]
- 13. Barnett SF et al Identification and characterization of pleckstrin‐homology‐domain‐dependent and isoenzyme‐specific Akt inhibitors. Biochem J 2005; 385:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calleja V et al Role of a novel PH‐kinase domain interface in PKB/Akt regulation: structural mechanism for allosteric inhibition. PLoS Biol 2009; 7:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bujor AM et al Akt inhibition up‐regulates MMP1 through a CCN2‐dependent pathway in human dermal fibroblasts. Exp Dermatol 2010; 19:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zuegg J et al Structural model of human IL‐13 defines the spatial interactions with the IL‐13Ralpha/IL‐4Ralpha receptor. Immunol Cell Biol 2001; 79:332–9. [DOI] [PubMed] [Google Scholar]
- 17. Murata T et al Two different IL‐13 receptor chains are expressed in normal human skin fibroblasts, and IL‐4 and IL‐13 mediate signal transduction through a common pathway. Int Immunol 1998; 10:1103–10. [DOI] [PubMed] [Google Scholar]
- 18. Jinnin M et al Interleukin‐13 stimulates the transcription of the human alpha2(I) collagen gene in human dermal fibroblasts. J Biol Chem 2004; 279:41783–91. [DOI] [PubMed] [Google Scholar]
- 19. Minty A et al Interleukin‐13 is a new human lymphokine regulating inflammatory and immune responses. Nature 1993; 362:248–50. [DOI] [PubMed] [Google Scholar]
- 20. Cleaver CS, Rowan AD, Cawston TE. Interleukin 13 blocks the release of collagen from bovine nasal cartilage treated with proinflammatory cytokines. Ann Rheum Dis 2001; 60:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zurawski G, de Vries JE. Interleukin 13, an interleukin 4‐like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today 1994; 15:19–26. [DOI] [PubMed] [Google Scholar]
- 22. de Waal Malefyt R et al Effects of IL‐13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL‐4 and modulation by IFN‐gamma or IL‐10. J Immunol 1993; 151:6370–81. [PubMed] [Google Scholar]
- 23. Vannier E et al Interleukin‐13 (IL‐13) induces IL‐1 receptor antagonist gene expression and protein synthesis in peripheral blood mononuclear cells: inhibition by an IL‐4 mutant protein. Blood 1996; 87:3307–15. [PubMed] [Google Scholar]
- 24. Isomaki P et al Interleukin‐10 functions as an antiinflammatory cytokine in rheumatoid synovium. Arthritis Rheum 1996; 39:386–95. [DOI] [PubMed] [Google Scholar]
- 25. Hugle T et al Tumor necrosis factor‐costimulated T lymphocytes from patients with systemic sclerosis trigger collagen production in fibroblasts. Arthritis Rheum 2013; 65:481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanangat S et al Novel functions of intracellular IL‐1ra in human dermal fibroblasts: implications in the pathogenesis of fibrosis. J Invest Dermatol 2006; 126:756–65. [DOI] [PubMed] [Google Scholar]
- 27. Kelly‐Welch AE et al Interleukin‐4 and interleukin‐13 signaling connections maps. Science 2003; 300:1527–8. [DOI] [PubMed] [Google Scholar]
- 28. Lee PJ et al ERK1/2 mitogen‐activated protein kinase selectively mediates IL‐13‐induced lung inflammation and remodeling in vivo . J Clin Invest 2006; 116:163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peng Q, Matsuda T, Hirst SJ. Signaling pathways regulating interleukin‐13‐stimulated chemokine release from airway smooth muscle. Am J Respir Crit Care Med 2004; 169:596–603. [DOI] [PubMed] [Google Scholar]
- 30. Yang M et al Interleukin‐13 mediates airways hyperreactivity through the IL‐4 receptor‐alpha chain and STAT‐6 independently of IL‐5 and eotaxin. Am J Respir Cell Mol Biol 2001; 25:522–30. [DOI] [PubMed] [Google Scholar]
- 31. Hershey GK. IL‐13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol 2003; 111:677–90; quiz 691. [DOI] [PubMed] [Google Scholar]
- 32. Keegan AD et al Similarities and differences in signal transduction by interleukin 4 and interleukin 13: analysis of Janus kinase activation. Proc Natl Acad Sci USA 1995; 92:7681–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kruse S, Braun S, Deichmann KA. Distinct signal transduction processes by IL‐4 and IL‐13 and influences from the Q551R variant of the human IL‐4 receptor alpha chain. Respir Res 2002; 3:24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Postlethwaite AE et al Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest 1992; 90:1479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Postlethwaite AE, Seyer JM. Fibroblast chemotaxis induction by human recombinant interleukin‐4. Identification by synthetic peptide analysis of two chemotactic domains residing in amino acid sequences 70–88 and 89–122. J Clin Invest 1991; 87:2147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim S et al Caveolin‐1 increases basal and TGF‐beta1‐induced expression of type I procollagen through PI‐3 kinase/Akt/mTOR pathway in human dermal fibroblasts. Cell Signal 2008; 20:1313–9. [DOI] [PubMed] [Google Scholar]
- 37. Paterno J et al Akt‐mediated mechanotransduction in murine fibroblasts during hypertrophic scar formation. Wound Repair Regen 2011; 19:49–58. [DOI] [PubMed] [Google Scholar]
- 38. Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3‐kinase in innate immunity. Biol Pharm Bull 2007; 30:1617–23. [DOI] [PubMed] [Google Scholar]
- 39. Sarbassov DD et al Phosphorylation and regulation of Akt/PKB by the rictor‐mTOR complex. Science 2005; 307:1098–101. [DOI] [PubMed] [Google Scholar]
- 40. Martin M et al Toll‐like receptor‐mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol 2005; 6:777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bujor AM et al Akt blockade downregulates collagen and upregulates MMP1 in human dermal fibroblasts. J Invest Dermatol 2008; 128:1906–14. [DOI] [PubMed] [Google Scholar]
- 42. Ricupero DA et al Phosphatidylinositol 3‐kinase‐dependent stabilization of alpha1(I) collagen mRNA in human lung fibroblasts. Am J Physiol Cell Physiol 2001; 281:C99–C105. [DOI] [PubMed] [Google Scholar]
- 43. Lim IJ et al Synchronous activation of ERK and phosphatidylinositol 3‐kinase pathways is required for collagen and extracellular matrix production in keloids. J Biol Chem 2003; 278:40851–8. [DOI] [PubMed] [Google Scholar]
- 44. Jun JB et al Scleroderma fibroblasts demonstrate enhanced activation of Akt (protein kinase B) in situ. J Invest Dermatol 2005; 124:298–303. [DOI] [PubMed] [Google Scholar]
- 45. Yang L et al Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS One 2012; 7:e41994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cross DA et al Inhibition of glycogen synthase kinase‐3 by insulin mediated by protein kinase B. Nature 1995; 378:785–9. [DOI] [PubMed] [Google Scholar]
- 47. Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 1998; 273:32377–9. [DOI] [PubMed] [Google Scholar]
- 48. Zhou H et al Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol 2000; 151:483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol 2001; 2:769–76. [DOI] [PubMed] [Google Scholar]
- 50. Dominguez I, Green JB. Missing links in GSK3 regulation. Dev Biol 2001; 235:303–13. [DOI] [PubMed] [Google Scholar]
- 51. Ciechomska M et al Toll‐like receptor‐mediated, enhanced production of profibrotic TIMP‐1 in monocytes from patients with systemic sclerosis: role of serum factors. Ann Rheum Dis 2013; 72:1382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Djafarzadeh R et al Treatment of dermal fibroblasts with GPI‐anchored human TIMP‐1 protein moderates processes linked to scar formation. J Invest Dermatol 2013; 133:803–11. [DOI] [PubMed] [Google Scholar]
- 53. Sato M et al Opposite effects of tumour necrosis factor‐alpha on type I and III collagen gene expression by human dermal fibroblasts in monolayer and three‐dimensional cultures. Br J Dermatol 1998; 138:118–21. [DOI] [PubMed] [Google Scholar]
- 54. Dasu MR et al Matrix metalloproteinase expression in cytokine stimulated human dermal fibroblasts. Burns 2003; 29:527–31. [DOI] [PubMed] [Google Scholar]
- 55. Leonardi A et al Effects of Th2 cytokines on expression of collagen, MMP‐1, and TIMP‐1 in conjunctival fibroblasts. Invest Ophthalmol Vis Sci 2003; 44:183–9. [DOI] [PubMed] [Google Scholar]
- 56. Lu Y et al Tissue inhibitor of metalloproteinase‐1 promotes NIH3T3 fibroblast proliferation by activating p‐Akt and cell cycle progression. Mol Cells 2011; 31:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]


