Summary
CD4+ CD25+ Foxp3+ regulatory T (Treg) cells play an important role in maintaining immune homeostasis. Interleukin‐10 (IL‐10), a cytokine with anti‐inflammatory capacities, also has a critical role in controlling immune responses. In addition, it is well known that production of IL‐10 is one of the suppression mechanisms of Treg cells. However, the action of IL‐10 on Treg cells themselves remains insufficiently understood. In this study, by using a Schistosoma japonicum‐infected murine model, we show that the elevated IL‐10 contributed to Treg cell induction but impaired their immunosuppressive function. Our investigations further suggest that this may relate to the up‐regulation of serum transforming growth factor (TGF‐β) level but the decrease in membrane‐bound TGF‐β of Treg cells by IL‐10 during S. japonicum infection. In addition, similar IL‐10‐mediated regulation on Treg cells was also confirmed in the murine model of asthma. In general, our findings identify a previously unrecognized opposing regulation of IL‐10 on Treg cells and provide a deep insight into the precise regulation in immune responses.
Keywords: CD4+CD25+ regulatory T cells, IL‐10, regulation
Abbreviations
- APC
allophycocyanin
- BALF
bronchoalveolar lavage fluid
- EGFP‐Tg
enhanced green fluorescent protein transgenic
- FCM
flow cytometry
- IL‐10
interleukin‐10
- IL‐10R
IL‐10 receptor
- i.p.
intraperitoneally
- mTGF‐β
membrane‐bound TGF‐β
- OVA
ovalbumin
- PE
phycoerythrin
- PerCP
peridinin chlorophyll protein
- rmIL‐10
recombinant murine IL‐10
- S. japonicum
Schistosoma japonicum
- SEA
soluble egg antigens
- TGF‐β
transforming growth factor‐β
- Treg
regulatory T
Introduction
Hosts infected with helminths (e.g. schistosomes) develop various immune responses against invading pathogens, which may also lead to damage to host organs. Consequently, helminths have evolved numerous immune subversive ways to down‐regulate host immune defences and limit collateral immunopathology in host organs, allowing the long‐term survival of both the hosts and the parasites.1, 2, 3 Interestingly, epidemiological data and animal experiments indicate that chronic infections with helminths are linked to the decreased prevalence of allergy and autoimmune diseases, including type 1 diabetes, multiple sclerosis, Crohn's disease, rheumatoid arthritis and asthma.4, 5
A heterogeneous population of regulatory T (Treg) cells plays crucial roles in maintaining immune homeostasis and limiting excessive immune responses to infection. To date, multiple Treg cell subsets (e.g. nTreg, pTreg, Tr1, Th3 and CD8+ Treg cells) have been found to exert negative regulation on various immune cells by diverse mechanisms.6 Many studies have centred on CD4+ CD25+ Foxp3+ Treg cells, which are dominantly induced during schistosome infection and play an important down‐regulatory role in limiting protective immunity and immunopathological injury and protecting against immune‐mediated diseases.5, 7, 8
The secretion of high amounts of interleukin‐10 (IL‐10) is one of the important mechanisms of Treg‐mediated suppression.6 Meanwhile, IL‐10 is a pleiotropic cytokine that is highly produced and important in immune regulation during schistosome infection.9 It is well known that IL‐10 mediates its immunosuppressive action on multiple types of cells.10 It can affect many important functions of monocytes, macrophages and dendritic cells by inhibiting the expression of co‐stimulatory molecules, pro‐inflammatory cytokines and chemokines. Also, IL‐10 can directly inhibit helper T‐cell function and proliferation.10, 11, 12, 13, 14 However, the action of IL‐10 on Treg cells themselves is less characterized and seems paradoxical. Previous studies suggest that IL‐10 is important for potentiating Treg cell induction and maintaining their suppressive function,15, 16, 17 whereas the regulation of IL‐10 on Treg cells is currently not fully understood.
In this study, by using a murine model with Schistosoma japonica infection we investigated whether and how IL‐10 regulates Treg cells. Our results showed that the elevated IL‐10 increased Treg cells but impaired their immunosuppressive activity. We further showed that IL‐10 increased the levels of serum transforming growth factor‐β (TGF‐β) but decreased the membrane‐bound TGF‐β on Treg cells; and finally, IL‐10‐mediated regulation on Treg cells was also proved in a murine model of asthma. Hence, our data document that IL‐10 shows opposite regulation on Treg cells, providing an insight into the mechanism of precise control of immune regulation.
Materials and methods
Ethics statement
All animal experiments were performed in accordance with the Chinese laws for animal protection and experimental guidelines. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University for the use of laboratory animals.
Mice, parasites, animal infection and antigen preparation
Specific pathogen‐free 7‐ to 8‐week‐old female BALB/c mice, wild‐type and enhanced green fluorescent protein transgenic (EGFP‐Tg) C57BL/6 mice were purchased from SLAC Laboratory (Shanghai, China) and bred in a specific pathogen‐free animal facility.
Oncomelania hupensis harbouring S. japonicum (Chinese mainland strain) cercariae were purchased from the Jiangsu Institute of Parasitic Diseases (Wuxi, China). Each mouse was infected percutaneously by exposure of the abdominal skin for 20 min to 12 cercariae of S. japonicum.
Soluble egg antigen (SEA) was prepared as previously described.18 Protein concentrations were determined using the DC protein assay (Bio‐Rad, Hercules, CA).
Cytokine and antibodies
Recombinant murine IL‐10 (rmIL‐10) was purchased from PeproTech (Rocky Hill, NJ). The rat anti‐mouse IL‐10 neutralizing antibody (JES5‐2A5), rat anti‐mouse IL‐10 receptor (IL‐10R) blocking antibody (1B1.3a) and purified rat IgG1 isotype control antibody were purchased from BioLegend (San Diego, CA). Hamster anti‐mouse CD3 antibody (145‐2C11) and Alexa Fluor‐conjugated anti‐Ki‐67 were purchased from BD Pharmingen (San Jose, CA). Peridinin chlorophyll protein‐conjugated (PerCP‐Cy5.5‐) anti‐CD3, FITC‐conjugated anti‐CD4, allophycocyanin‐conjugated (APC‐) anti‐CD25, phycoerythrin‐conjugated (PE‐) Cy7‐anti‐CD25, PerCP‐Cy5.5‐anti‐Foxp3, PE‐anti‐Foxp3, PE‐Cy7‐anti‐CD44, PerCP‐Cy5.5‐anti‐CD62L, PE‐anti‐CTLA‐4, APC‐anti‐ICOS, PE‐anti‐TGF‐β, PE‐anti‐IL‐10R and the Mouse Regulatory T Cell Staining Kit were purchased from eBioscience (San Diego, CA).
Cytokine or antibody administration and ovalbumin sensitization murine models
Detailed experimental designs of murine models are shown in the Supplementary material (Fig. S2).
For rmIL‐10 administration, each mouse was injected intraperitoneally (i.p.) with PBS as control or 100 ng of rmIL‐10 three times per day (once every 8 hr) for 5 consecutive days. For antibody administration, each mouse was administered i.p. with 500 μg of anti‐IL‐10, anti‐IL‐10R or IgG1 isotype control antibodies once a week, beginning 8 weeks after S. japonicum infection and continuing through the following 4 weeks.19
Briefly, mice were sensitized i.p. with 50 μg of Grade VII chicken ovalbumin (OVA, Sigma‐Aldrich, St Louis, MO) adsorbed to 9% potassium alum (Sigma‐Aldrich) as previously described,20 and boosted with the same antigen on days 7, 14 and 21. Mice were then challenged with aerosolized OVA (5 mg/ml) by the intratracheal route for 30 min once daily for 5 consecutive days (days 28–32). All mice were killed 24 hr after final airway challenge to assess airway inflammation.
Bronchoalveolar lavage fluid cell counts
As previously described,20 after 24 hr of final challenge, mice were terminally anaesthetized, their tracheas were cannulated, and the internal airspaces were lavaged twice with 500 μl PBS. Fluids were centrifuged and pellets were recovered for cell counts. Cytospins were prepared by spinning 5 × 105 cells onto poly‐l‐lysine‐coated slides (BDH Laboratory Supplies, Poole, UK) followed by Diff Quick (Boehringer Mannheim, Mannheim, Germany) staining. Differential cell counts were performed on a minimum of 200 cells at a magnification of 100 ×.
Histopathology
After 24 hr of final challenge, lungs were excised, fixed in formalin and embedded in paraffin for histopathological analysis.21 The degree of peribronchial inflammation was evaluated using a semi‐quantitative system that takes into account extent and severity of inflammation on a scale from 0 to 4, as previously described.21 Experiments were performed in a double‐blinded fashion.
Cell isolation
Single‐cell suspensions were prepared from mouse spleens and used to isolate CD4+ CD25+ and CD4+ CD25− T cells by using a mouse Treg cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and a magnetic activated cell sorter (MACS, Miltenyi Biotec) according to the manufacturer's instructions, achieving > 96% purity as determined by flow cytometry (FCM) analysis.
Antigen‐presenting cells were prepared from single‐cell suspensions by negative selection using CD90.2 magnetic microbeads (Miltenyi Biotec) to deplete T cells and were then irradiated with 30 Gy at 2·7 Gy/min using a 137Cs source (Gammacell 1000 Elite; Nordion International Inc., Kanata, Ontario, Canada).
In vitro treatment of cells
Spleen cells or purified CD4+ CD25+ Treg cells from S. japonicum‐infected mice were pre‐treated with 20 μg/ml of anti‐IL‐10, anti‐IL‐10R, isotype IgG1 antibodies or medium alone for 30 min in complete PRMI‐1640 (containing 10% fetal calf serum, 100 U/ml of penicillin plus 100 μg/ml streptomycin) in a 24‐well plate. Subsequently, SEA (20 μg/ml), anti‐CD3 (2 μg/ml) or rmIL‐10 (20 ng/ml) were added to the cultures. After 72 hr, cells were collected for FCM analysis and/or supernatants were collected for cytokine detection by ELISA.
Suppression assay
Antigen‐presenting cells (1 × 105/well) and CD4+ CD25− T cells (1 × 105/well) were plated in 96‐well round‐bottomed plates in triplicate and activated with soluble anti‐CD3 (2 μg/ml) and/or SEA (20 μg/ml) in the presence or absence CD4+ CD25+ Treg cells (1 × 105/well) pre‐treated with 20 μg/ml anti‐IL‐10, anti‐IL‐10R, isotype rat‐IgG1 antibodies or medium alone. Cultures were incubated for 72 hr and pulsed with 3H‐thymidine (0·5 μCi/well) for the last 16 hr. Cells were then collected and incorporation of 3H‐thymidine was measured with a liquid scintillation counter.
CD4+ CD25− T cells from EGFP‐Tg mice and CD4+ CD25+ Treg cells from wild‐type mice were co‐cultured as described above. The suppressive activity of Treg cells on CD4+ CD25− T cells was measured by analysing the expression of Ki‐67 in EGFP+ cells (CD4+ CD25− T cells).
Flow cytometry analysis
To analyse CD4+ CD25+ Foxp3+ Treg cells and their surface effector phenotype, the Mouse Regulatory T Cell Staining Kit (eBioscience) was used according to the manufacturer's recommendations. In brief, 1 × 106 spleen cells or cells collected from co‐cultures were stained with anti‐CD3‐PerCP‐Cy5.5, anti‐CD4‐FITC, anti‐CD25‐APC and fluorescent antibodies to surface phenotype markers (anti‐CD62L‐PerCP‐Cy5.5, anti‐CD44‐PE‐Cy7, anti‐CTLA‐4‐PE or anti‐ICOS‐APC), subsequently permeabilized with cold Fix/Perm Buffer and blocked with anti‐mouse CD16/32. The anti‐Foxp3‐PE was then added.
For analysing Ki‐67 expression, EGFP+ cells (CD4+ CD25− T cells) collected from co‐cultures were labelled with anti‐Ki‐67‐AlexaFluor by using Foxp3 fixation/permeabilization buffers (eBioscience).
For detection of the expression of membrane‐bound TGF‐β (mTGF‐β) or IL‐10R on CD4+ CD25+ Treg cells, 1 × 106 spleen cells or cells collected from co‐cultures were stained with anti‐CD3‐PerCP‐Cy5.5, anti‐CD4‐FITC, anti‐CD25‐APC and anti‐TGF‐β‐PE/anti‐IL‐10R‐PE.
Following immunofluorescence staining, cells were examined by FCM using a FACS Calibur or Verse instrument (BD Bioscience) and analysed using cellquest (BD Bioscience) or flowjo (Tree Star, Ashland, OR; version 10.0.7).
Adoptive transfer
Freshly isolated CD4+ CD25+ Treg cells (5 × 105 cells per mouse) from S. japonicum‐infected mice (8 weeks post‐infection) were adoptively transferred intravenously into OVA‐sensitized mice as indicated in the Supplementary material (Fig. S2c).22 The recipient OVA‐sensitized mice were immediately injected i.p. with rmIL‐10 or PBS starting on day 28 after the first OVA sensitization, then subjected to OVA challenge. All mice were killed 24 hr after the final airway challenge.
Detection of cytokines
ELISA kits (Bender MedSystems, Vienna, Austria) were used for measurements of IL‐10 and TGF‐β, according to the manufacturer's instructions.
Statistical analysis
The statistical analysis was performed using spss version 10.1 for Windows (SPSS, Inc., Chicago, IL). The Kolmogorov–Smirnov test was used to evaluate the normality of the distribution of the examined quantitative variables. Where normality tests were passed, a Student's t‐test was used for analysing the differences between two groups. Otherwise, a Mann‐Whitney's U‐test was used. The differences between more than two groups were analysed with one‐way analysis of variance with a least squares difference post hoc test. P < 0·05 was considered statistically significant.
Results
Schistosoma japonicum infection increased serum IL‐10 levels and Treg cell frequency but decreased immunosuppressive activity of Treg cells in mice
Results in Fig. 1(a–c) showed that both the serum levels of IL‐10 and the frequency of CD4+ CD25+ Foxp3+ Treg cells were significantly increased in S. japonicum‐infected mice compared with normal mice. However, the S. japonicum infection did not change the expression levels of Foxp3 by Treg cells (Fig. 1d,e). Intriguingly, by a classical [3H]thymidine incorporation assay, the immunosuppressive activity of CD4+ CD25+ Treg cells from S. japonicum‐infected mice was shown to be significantly impaired compared with those derived from normal mice (Fig. 1f). Similar results were obtained by the Ki‐67‐based assay (Fig. 1g,h). These results illustrate that schistosome infection elevates the Treg cell frequency and serum IL‐10 level but decreases the immunosuppressive activity of Treg cells.
Figure 1.
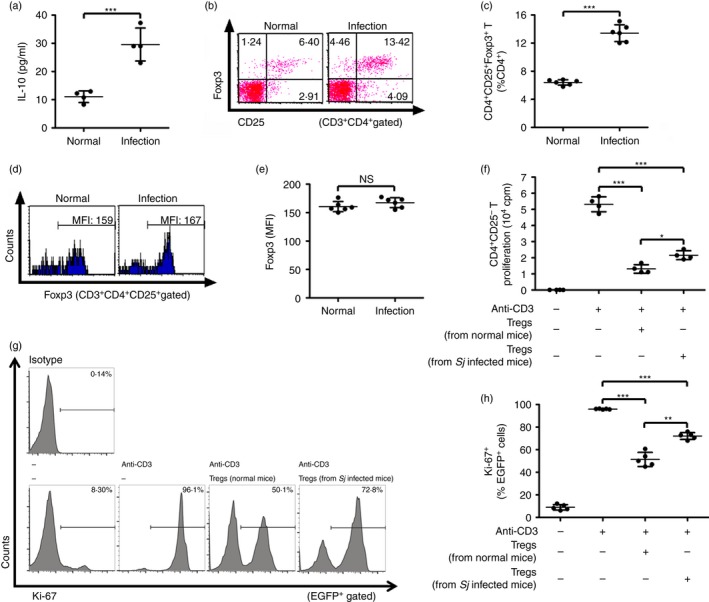
Schistosoma japonicum infection increased serum interleukin‐10 (IL‐10) levels and T regulatory (Treg) cell frequency but decreased immunosuppressive activity of Treg cells in mice. (a) Eight weeks after infection, serum IL‐10 of S. japonicum‐infected mice was measured by ELISA (n = 4 per group). (b, c) Eight weeks after infection, splenocytes from infected or normal control mice (n = 6 per group) were isolated and stained with PerCP‐Cy5.5 anti‐CD3, FITC anti‐CD4, APC anti‐CD25, PE anti‐Foxp3. Flow cytometry dot plots of CD25+ Foxp3+ Treg cells gated on CD3+ CD4+ T cells are shown. Scatter plot shows the average percentages of CD4+ CD25+ Foxp3+ Treg cells in CD4+ T cells. (d, e) Histogram profiles of Foxp3 gated on CD3+ CD4+ CD25+ T cells are shown. Scatter plot shows respectively the average MFI of Foxp3 expression in CD3+ CD4+ CD25+ T cells (n = 6 per group). (f) CD4+ CD25− T cells (1 × 105/well) from normal control mice were cultured in triplicate wells with normal mouse‐derived antigen‐presenting cells (1 × 105/well), 2 μg/ml of soluble anti‐CD3 and CD4+ CD25+ T cells (1 × 105/well) purified from either S. japonicum‐infected or normal control mice at 37° for 72 hr (n = 4 per group). Proliferation was determined by 3H‐thymidine incorporation. (g and h) CD4+ CD25− T cells from EGFP‐Tg mice and CD4+ CD25+ Treg cells from either S. japonicum‐infected or normal control mice were co‐cultured as described in (f). The suppressive activity of Treg cells on CD4+ CD25− T cells was measured by analysing the expression of Ki‐67 in EGFP + cells (CD4+ CD25− T cells) (n = 5 per group). Histogram profiles of Ki‐67 gated on EGFP + cells are shown. Scatter plot shows the average percentages of Ki‐67+ cells in EGFP + cells. Each assay has been performed three times on independent cell samples. FCM dot plots or histograms are representative of three independent experiments. Data (means ± SD) are representative of three independent experiments. *P < 0·05, **P < 0·01, ***P < 0·001, n.s., not significant. [Colour figure can be viewed at wileyonlinelibrary.com]
IL‐10 administration increased Treg cells but decreased their immunosuppressive activity in S. japonicum‐infected mice
To demonstrate the possible effect of IL‐10 on Treg cells during schistosome infection, we first detected IL‐10R expression on CD4+ CD25+ Treg cells. As shown in the Supplementary material (Fig. S1), we observed a doubled frequency of IL‐10R+ CD4+ CD25+ Treg cells in S. japonicum‐infected mice compared with that in normal mice. The S. japonicum‐infected mice were next injected i.p. with rmIL‐10 to further increase IL‐10 levels in vivo (see Supplementary material, Fig. S2a). Twenty‐four hours after the last injection, the frequency of CD4+ CD25+ Foxp3+ Treg cells in the spleen was determined by FCM. Results showed that when compared with untreated or PBS‐injected control mice, rmIL‐10 administration resulted in a greater increase of Treg cells (Fig. 2a,b) in S. japonicum‐infected mice, but did not increase the levels of Foxp3 in these Treg cells (Fig. 2c,d). In addition, both [3H]thymidine incorporation and Ki‐67‐based assays showed that rmIL‐10 injection of S. japonicum‐infected mice resulted in a significant impairment of the immunosuppressive activity of Treg cells compared with PBS or blank controls (Fig. 2e–g). These findings demonstrate that a higher level of IL‐10 contributes to an increased frequency but decreased immunosuppressive activity of Treg cells during S. japonicum infection.
Figure 2.
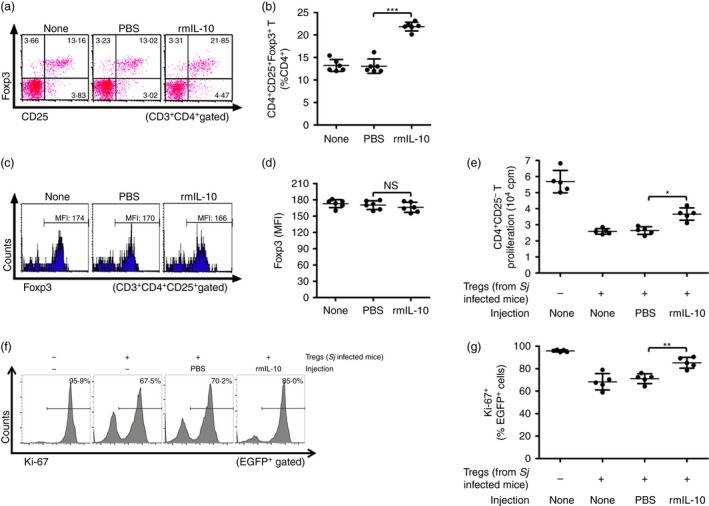
Interleukin‐10 (IL‐10) administration increased T regulatory (Treg) cells but decreased their immunosuppression activity in Schistosoma japonicum‐infected mice. (a, b) S. japonicum‐infected mice (n = 6 per group) were injected intraperitoneally with PBS (control) or recombinant mouse IL‐10 (rmIL‐10). At 24 hr after the last injection, single cell suspensions of splenocytes were prepared from mouse spleens and stained with PerCP‐Cy5.5 anti‐CD3, FITC anti‐CD4, APC anti‐CD25, and PE anti‐Foxp3. Flow cytometry dot plots of CD25+ Foxp3+ Treg cells gated on CD3+ CD4+ T cells are shown. Scatter plot shows, respectively, the average percentages of CD4+ CD25+ Foxp3+ Treg cells in CD4+ T cells (c, d) Histogram profiles of Foxp3 gated on CD3+ CD4+ CD25+ T cells are displayed. Scatter plot shows the average MFI of Foxp3 expression in CD3+ CD4+ CD25+ T cells (n = 6 per group). (e) CD4+ CD25− T cells (1 × 105/well) from normal control mice were cultured in triplicate wells at 37° for 72 hr with normal mouse‐derived antigen‐presenting cells (1 × 105/well), 2 μg/ml of soluble anti‐CD3 and CD4+ CD25+ T cells (1 × 105/well) purified from S. japonicum‐infected mice injected with either PBS or rmIL‐10 (n = 5 per group). Proliferation was determined by [3H]thymidine incorporation. (f, g) CD4+ CD25− T cells from EGFP‐Tg mice and CD4+ CD25+ Treg cells from S. japonicum‐infected mice injected with either PBS or rmIL‐10 were co‐cultured as described in (e) (n = 5 per group). The suppressive activity of Treg cells on CD4+ CD25− T cells was measured by analysing the expression of Ki‐67 in EGFP + cells (CD4+ CD25− T cells). Histogram profiles of Ki‐67 gated on EGFP + cells are shown. Scatter plot shows the average percentages of Ki‐67+ cells in EGFP + cells. Each assay has been performed three times on independent cell samples. Flow cytometry dot plots or histograms are representative of three independent experiments. Data (mean ± SD) are representative of three independent experiments. *P < 0·05, **P < 0·01, ***P < 0·001, n.s., not significant. [Colour figure can be viewed at wileyonlinelibrary.com]
In vitro IL‐10 treatment enhanced Treg cell induction by SEA but impaired their immunosuppressive activity
Previous reports, including our own, showed that SEAs are responsible for Treg cell induction in vitro and in vivo.23, 24 Therefore, we further investigated the effect of IL‐10 on Treg cells in an in vitro SEA induction system. The SEA in vitro stimulation alone induced significantly higher frequencies of Treg cells in spleen cells from S. japonicum‐infected mice compared with untreated control mice (Fig. 3a,b). The addition of rmIL‐10 in vitro further increased the frequency of SEA‐induced Treg cells (Fig. 3a,b). Subsequently, suppression assays were performed to further examine the in vitro effect of rmIL‐10 on the immunosuppressive activity of Treg cells. As shown in Fig. 3(c–e), the in vitro treatment with rmIL‐10 resulted in significant impairment of suppressive activity of Treg cells. Hence, these in vitro data further indicate that IL‐10 contributes to increase the frequency but decrease the immunosuppressive activity of Treg cells.
Figure 3.
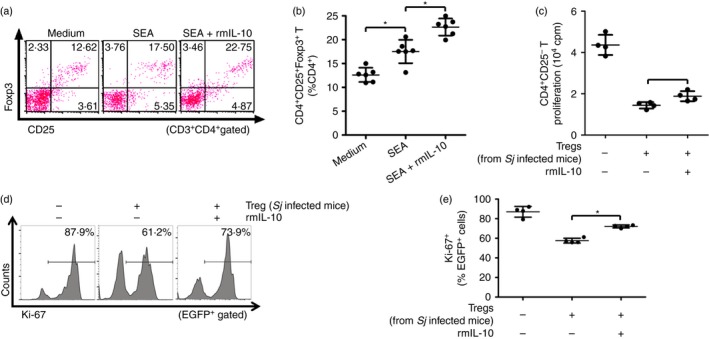
In vitro interleukin‐10 (IL‐10) treatment enhanced regulatory T (Treg) induction by soluble egg antigen (SEA) but impaired their immunosuppressive activity. (a) Splenocytes (2 × 107 cells/well) from infected mice were stimulated with SEA (20 μg/ml) in the presence or absence of recombinant murine (rm) IL‐10 (20 ng/ml) in triplicate wells in a 24‐well plate at 37° for 72 hr (n = 6 per group). Cells were stained with PerCP‐Cy5.5 anti‐CD3, FITC anti‐CD4, APC anti‐CD25, and PE anti‐Foxp3. Flow cytometry dot plots of CD25+ Foxp3+ Treg cells gated on CD3+ CD4+ T cells are shown. (b) Scatter plot shows the average percentages of CD4+ CD25+ Foxp3+ Treg cells in CD4+ T cells. (c) CD4+ CD25+ T cells were purified from Schistosoma japonicum‐infected mice and pre‐incubated with or without rmIL‐10 (20 ng/ml) for 30 min as described in the Materials and methods (n = 4 per group). The cells (1 × 105 cells/well) were then cultured in triplicate wells in a 96‐well U‐bottom plate with normal mouse‐derived CD4+ CD25− T cells and antigen‐presenting cells at a ratio of 1 : 1 : 1, in the presence of 2 μg/ml anti‐CD3 for 72 hr at 37°. The proliferation was measured by incorporation of [3H]thymidine. (d, e) CD4+ CD25− T cells from EGFP‐Tg mice and CD4+ CD25+ Treg cells from S. japonicum‐infected mice pre‐incubated with or without rmIL‐10 were co‐cultured as described in (c) (n = 4 per group). The suppressive activity of Treg cells on CD4+ CD25− T cells was measured by analysing the expression of Ki‐67 in EGFP + cells (CD4+ CD25− T cells). Histogram profiles of Ki‐67 gated on EGFP + cells are shown. Scatter plot shows the average percentages of Ki‐67+ cells in EGFP + cells. Each assay has been performed three times on independent cell samples. Flow cytometry dot plots or histograms are representative of three independent experiments. Data (mean ± SD) are representative of three independent experiments. *P < 0·05. [Colour figure can be viewed at wileyonlinelibrary.com]
Injection of anti‐IL‐10 or anti‐IL‐10R antibody decreased the frequencies but enhanced immunosuppressive activity of Treg cells in S. japonicum‐infected mice
The activity of IL‐10 is mediated by binding of its specific cell surface receptor IL‐10R.10 Therefore, we blocked IL‐10 signalling by i.p. injection of S. japonicum‐infected mice with anti‐IL‐10 or anti‐IL‐10R antibody to further investigate the regulatory effects of IL‐10 on Treg cells (see Supplementary material, Fig. S2b). As shown in Fig. 4(a,b), injection with either anti‐IL‐10 or anti‐IL‐10R antibody decreased the frequencies of Treg cells. Moreover, ex vivo suppression assays showed that Treg cells from S. japonicum‐infected mice injected with either anti‐IL‐10 or anti‐IL‐10R antibody possessed enhanced suppressive activity compared with isotype control group (Fig. 4c–e). These results, once again implicate the regulatory role of IL‐10 on Treg cell frequencies and suppressive activity.
Figure 4.
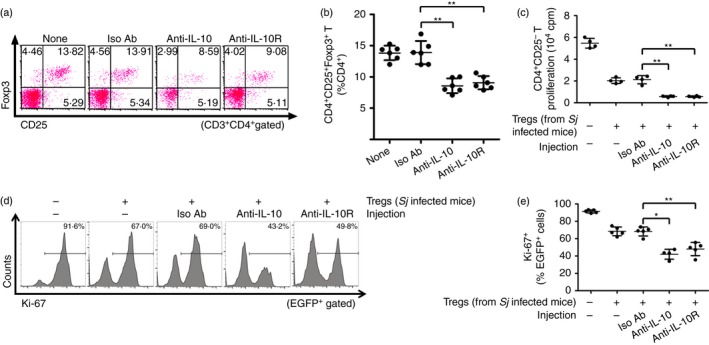
Injection of anti‐interleukin‐10 (IL‐10) or anti‐interleukin‐10 receptor (IL‐10R) decreased the frequencies but enhanced the immunosuppressive activity of regulatory T (Treg) cells in Schistosoma japonicum‐infected mice. (a) S. japonicum‐infected mice (n = 6 per group) were injected intraperitoneally with anti‐IL‐10, anti‐IL‐10R or rat IgG1 isotype control antibody as described in the Materials and methods. Splenocytes were then isolated and stained with PerCP‐Cy5.5 anti‐CD3, FITC anti‐CD4, APC anti‐CD25, and PE anti‐Foxp3. FCM dot plots of CD25+ Foxp3+ Treg cells gated on CD3+ CD4+ T cells are shown. (b) Scatter plot shows the average percentages of CD4+ CD25+ Foxp3+ Treg cells in CD4+ T cells. (c) Normal mice derived CD4+ CD25− T cells (1 × 105 cells/well) and antigen‐presenting cells (1 × 105 cells/well) were stimulated with anti‐CD3 (2 μg/ml) in the absence or presence of CD4+ CD25+ Treg cells (1 × 105 cells/well) from anti‐IL‐10, anti‐IL‐10R or isotype control antibodies injected into S. japonicum‐infected mice at 37° or 72 hr (n = 4 per group). The proliferation was measured by incorporation of [3H]thymidine. (d, e) CD4+ CD25− T cells from EGFP‐Tg mice and CD4+ CD25+ Treg cells from S. japonicum‐infected mice injected with anti‐IL‐10, anti‐IL‐10R or isotype control antibodies were co‐cultured as described in (c) (n = 4~5 per group). The suppressive activity of Treg cells on CD4+ CD25− T cells was measured by analysing the expression of Ki‐67 in EGFP + cells (CD4+ CD25− T cells). Histogram profiles of Ki‐67 gated on EGFP + cells are shown. Scatter plot shows the average percentages of Ki‐67+ cells in EGFP + cells. Each assay has been performed three times on independent cell samples. Flow cytometry dot plots or histograms are representative of three independent experiments. Data (mean ± SD) are representative of three independent experiments. *P < 0·05, **P < 0·01. [Colour figure can be viewed at wileyonlinelibrary.com]
Anti‐IL‐10 or anti‐IL‐10R antibody treatment in vitro diminished SEA‐mediated induction of Treg cells and restored their immunosuppressive activity
Anti‐IL‐10 or anti‐IL‐10R antibody was used to further confirm that IL‐10/IL‐10R interaction is critical to the regulation of Treg cell frequencies and suppressive activity. The FCM analysis showed that in vitro treatment with either anti‐IL‐10 or anti‐IL‐10R antibody in the culture significantly impaired Treg cell generation induced by SEA and IL‐10 in vitro stimulation (Fig 5a,b). In addition, the in vitro suppression assays showed that treatment with either anti‐IL‐10 or anti‐IL‐10R antibody restored the immunosuppressive activity of Treg cells (Fig. 5c–e). Taken together, these in vitro results further indicate the regulatory role of IL‐10 on Treg frequencies and suppressive activity.
Figure 5.
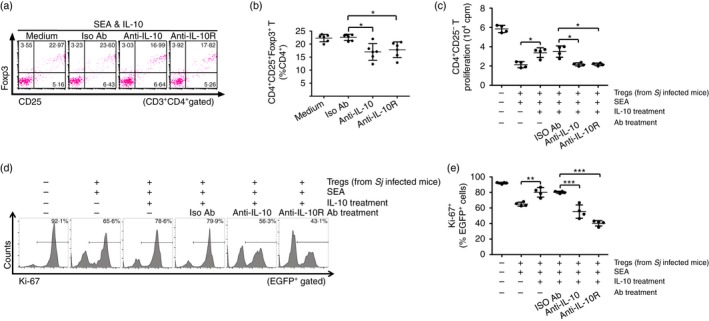
Anti‐interleukin‐10 (IL‐10) or anti‐interleukin‐10 receptor (IL‐10R) antibody in vitro treatment diminished soluble egg antigen (SEA) ‐mediated induction of regulatory T (Treg) cells and restored their suppression activity. (a) Splenocytes isolated from S. japonicum‐infected mice were pre‐treated with 20 μg/ml of anti‐IL‐10, anti‐IL‐10R or rat IgG1 isotype control antibody for 30 min and then followed by 20 ng/ml of rmIL‐10 treatment in the presence of SEA for a further 72 hr (n = 6 per group), as described in the Materials and methods. Cells were stained with PerCP‐Cy5.5 anti‐CD3, FITC anti‐CD4, APC anti‐CD25, and PE anti‐Foxp3. FCM dot plots of CD25+ Foxp3+ Treg cells gated on CD3+ CD4+ T cells are shown. (b) Scatter plot shows the average percentages of CD4+ CD25+ Foxp3+ Treg cells in CD4+ T cells. (c) Anti‐IL‐10 or anti‐IL‐10R pre‐treated CD4+ CD25+ Treg cells (1 × 105/well) were co‐cultured in triplicate wells with normal mouse‐derived CD4+ CD25− T cells and antigen‐presenting cells at a ratio of 1 : 1 : 1 in the presence of anti‐CD3 (2 μg/ml) at 37° for 72 hr (n = 4 per group). The proliferation was measured by incorporation of [3H]thymidine. Each assay has been performed three times on independent cell samples. (d, e) CD4+ CD25− T cells from EGFP‐Tg mice and CD4+ CD25+ Treg cells from S. japonicum‐infected mice pre‐treated with anti‐IL‐10, anti‐IL‐10R or isotype control antibodies were co‐cultured as described in (c) (n = 4 per group). The suppressive activity of Treg cells on CD4+ CD25− T cells was measured by analysing the expression of Ki‐67 in EGFP + cells (CD4+ CD25− T cells). Histogram profiles of Ki‐67 gated on EGFP + cells are shown. Scatter plot shows the average percentages of Ki‐67+ cells in EGFP + cells. Flow cytometry dot plots or histograms are representative of three independent experiments. Data (mean ± SD) are representative of three independent experiments. *P < 0·05, **P < 0·01, ***P < 0·001. [Colour figure can be viewed at wileyonlinelibrary.com]
IL‐10 administration attenuated Treg‐mediated suppression of asthma airway inflammation
To further confirm the above findings, the OVA‐sensitized asthma murine model was adopted and the Supplementary material (Fig. S2c) shows the experimental designs. Treg cells were purified from S. japonicum‐infected mice and adoptively transferred into mice with asthma. Meanwhile the recipient mice were injected immediately with rmIL‐10 or PBS as control. As shown in Fig. 6, either adoptively transferring of Treg cells alone or rmIL‐10 injection alone in mice with asthma significantly limited the lung pathology (Fig. 6a), decreased average histological scores (Fig. 6b), and infiltrating inflammatory cells in the airways (Fig. 6c). However, injection of rmIL‐10 in mice with asthma and transferred Treg cells impaired Treg‐mediated immunosuppression of airway immunopathology (Fig. 6a,b) and inflammatory cell infiltration (Fig. 6c). Taken together, these in vivo data suggest that the rmIL‐10 attenuated the immunosuppression on airway inflammation in Treg‐transferred mice with asthma.
Figure 6.
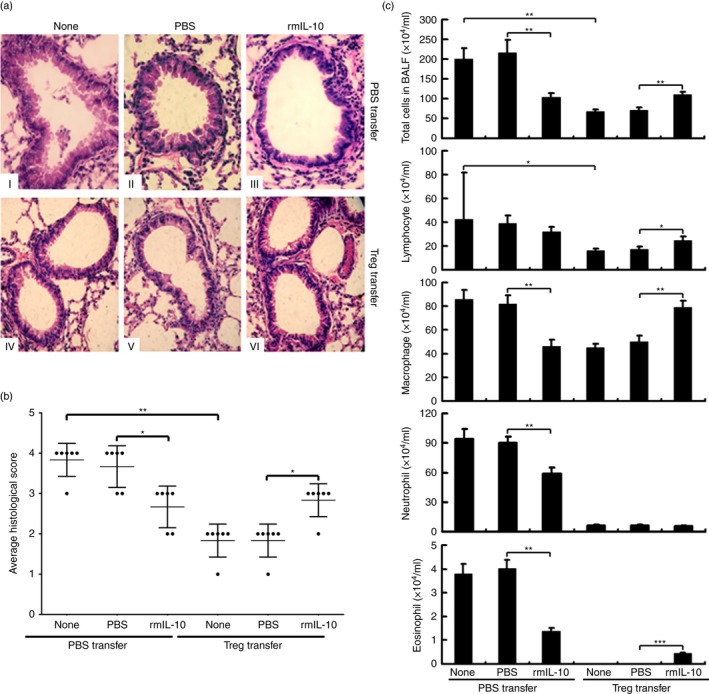
Interleukin‐10 (IL‐10) administration attenuated regulatory T (Treg) cell‐mediated suppression of asthma airway inflammation. (a) At 12 weeks after infection, CD4+ CD25+ Treg cells (5 × 105 cells/200 μl PBS/mouse) were purified from Schistosoma japonicum‐infected mice and adoptively transferred into ovalbumin (OVA) ‐sensitized asthma mice by caudal vein injection. Recipient mice were immediately injected intraperitoneally with regulatory mouse (rm) IL‐10 or PBS for five consecutive days of the OVA airway challenge stage (as seen in the Supplementary material, Fig. S2c; n = 6 per group). All mice were killed 24 hr after the final airway challenge to assess respiratory inflammation. Haematoxylin & eosin‐stained lung sections (40×) were analysed for leucocyte infiltrate and epithelial hypertrophy. (b) The degree of inflammation and the extent of leucocyte infiltration, epithelial cell hypertrophy and mucus production in the periodic acid–Schiff and haematoxylin analyses were assessed. A semi‐quantitative rating scale ranged from 0 (none) to 4 (maximal). Each average score per category was multiplied by the extent of appearance in the lungs (also rated 0–4) to yield an overall inflammation index. (c) Differential cell counts of bronchoalveolar lavage fluid were performed. Images are representative and data (mean ± SD) are representative of two independent experiments. *P < 0·05, **P < 0·01, ***P < 0·001.
IL‐10 administration increases serum TGF‐β but decreases membrane TGF‐β on Treg cells in S. japonicum‐infected mice
The binding of soluble TGF‐β to TGF‐β receptors on CD4+ T cells is critical for the induction of peripheral Treg cells.25, 26 Next we detected the TGF‐β levels in serum of infected mice with or without rmIL‐10 injection (see Supplementary material, Fig. S2a). Result showed that serum TGF‐β levels were significantly elevated in S. japonicum‐infected mice, and rmIL‐10 injection resulted in a further increase of serum TGF‐β levels (Fig. 7a).
Figure 7.
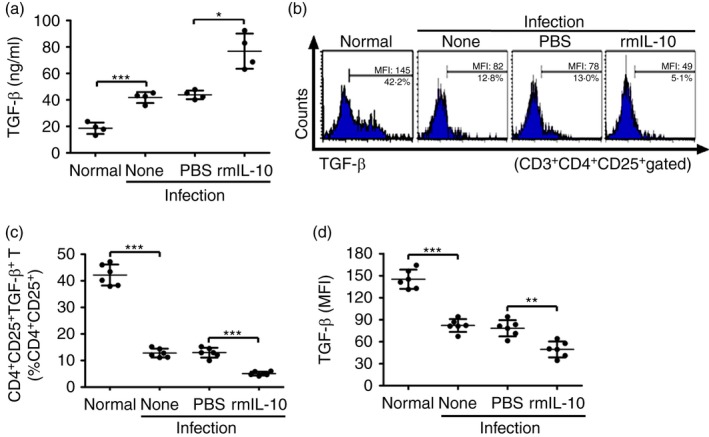
Interleukin‐10 (IL‐10) administration increases serum transforming growth factor‐β (TGF‐β) but decreases membrane TGF‐β on regulatory T (Treg) cells in Schistosoma japonicum‐infected mice. (a) S. japonicum‐infected mice were injected intraperitoneally with PBS or recombinant murine (rm) IL‐10 (n = 4 per group). At 24 hr after the last injection, mouse serum samples were collected and TGF‐β was measured by ELISA. (b) Splenocytes were isolated and surface stained with PerCP‐Cy5.5 anti‐CD3, FITC anti‐CD4, APC anti‐CD25, and PE anti‐TGF‐β. Histogram profiles of mTGF‐β gated on CD3+ CD4+ CD25+ Treg cells are shown. (c, d) Scatter plots show respectively the average percentages of CD4+ CD25+ mTGF‐β + Treg cells in CD3+ CD4+ CD25+ Treg cells and the average MFI of mTGF‐β expression in CD3+ CD4+ CD25+ Treg cells (n = 6 per group). Data (means ± SD) are representative of two independent experiments. *P < 0·05, **P < 0·01, ***P < 0·001. [Colour figure can be viewed at wileyonlinelibrary.com]
Membrane‐bound TGF‐β (mTGF‐β) based cell–cell contact has been considered one of the primary mechanisms of suppression by Treg cells.27, 28 Our result showed that S. japonicum infection decreased both the frequencies of mTGF‐β‐expressing Treg cells and their surface expression levels of mTGF‐β (Fig. 7b–d). Injection of rmIL‐10 in S. japonicum‐infected mice further reduced both the frequencies of mTGF‐β‐expressing Treg cells and their surface expression levels of mTGF‐β (Fig. 7b–d).
In addition, we also analyse the activation phenotype of Treg cells by FCM. Compared with PBS‐injected mice, rmIL‐10 administration induced an increase of resting (CD62Lhi CD44low) Treg cells (rTreg) and a decrease of activated (CD62Llow CD44hi) Treg cells (aTreg) in S. japonicum‐infected mice (see Supplementary material, Fig. S3a), but did not significantly change the expression level of CTLA‐4 or ICOS on these Treg cells (see Supplementary material, Fig. S3b and S3c).
Therefore, these results imply that IL‐10‐mediated regulation of the levels of soluble TGF‐β and mTGF‐β on Treg cells may be responsible for the increase of the frequency but impairment of the immunosuppressive activity of Treg cells in S. japonicum‐infected mice, although the mechanisms are currently unknown and need further extensive investigation in future.
IL‐10 in vitro treatment regulated mTGF‐β expression on surface of Treg cells
To further confirm the regulatory effects of IL‐10 on mTGF‐β expression on Treg cells in vitro, spleen cells from S. japonicum‐infected mice were cultured with SEA in the presence or absence of rmIL‐10 and then analysed by FCM. Consistent with in vivo data above, SEA in vitro treatment decreased both the frequencies of mTGF‐β‐expressing Treg cells and their surface expression levels of mTGF‐β (Fig. 8a–c). In addition, rmIL‐10 in vitro treatment further significantly reduced the frequencies of TGF‐β‐expressing Treg cells as well as their TGF‐β expression levels (Fig. 8a–c). Therefore, these in vitro results indicate that IL‐10 regulates the expression of surface mTGF‐β on Treg cells.
Figure 8.
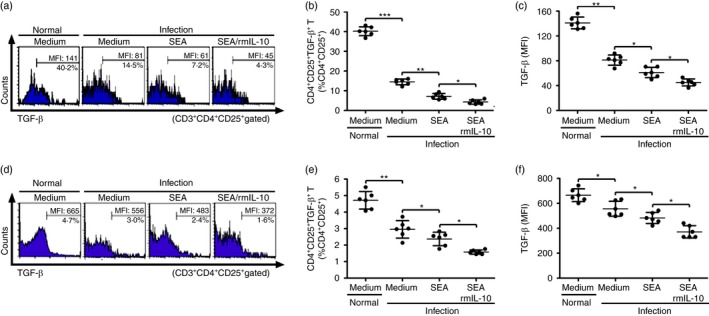
Interleukin‐10 (IL‐10) in vitro treatment regulated murine transforming growth factor‐β (mTGF‐β) expression on surface of regulatory T (Treg) cells. (a) Splenocytes (1 × 107/well) from Schistosoma japonicum‐infected mice were stimulated in triplicate wells with SEA (20 μg/ml) in the presence or absence of rmIL‐10 (20 ng/ml) in a 24‐well‐plate at 37° for 72 hr (n = 6 per group). Splenocytes were then collected and stained with PerCP‐Cy5.5 anti‐CD3, FITC anti‐CD4, APC anti‐CD25 and anti‐TGF‐β. Histogram profiles of mTGF‐β gated on CD3+ CD4+ CD25+ Treg cells are shown. (b, c) Scatter plots show respectively the average percentages of CD4+ CD25+ mTGF‐β + Treg cells in CD3+ CD4+ CD25+ Treg cells and the average MFI of mTGF‐β expression in CD3+ CD4+ CD25+ Treg cells. (d) Purified CD4+ CD25+ Treg cells (1 × 106/well) from S. japonicum‐infected mice were cultured in triplicate wells with or without recombinant murine (rm) IL‐10 (20 ng/ml) under soluble egg antigen (SEA) stimulation (20 μg/ml) for 72 hr (n = 6 per group). CD4+ CD25+ Treg cells from normal mice served as a control. Histogram profiles of mTGF‐β gated on CD3+ CD4+ CD25+ Treg cells are shown. (e, f) Scatter plots show respectively the average percentages of CD4+ CD25+ mTGF‐β + Treg cells in CD3+ CD4+ CD25+ Treg cells and the average MFI of mTGF‐β expression in CD3+ CD4+ CD25+ Treg cells. Data (means ± SD) of 12 mice are representative of two independent experiments. *P < 0·05, **P < 0·01, ***P < 0·001. [Colour figure can be viewed at wileyonlinelibrary.com]
To further investigate the possible direct regulation of IL‐10 on Treg cells, CD4+ CD25+ Treg cells were purified from normal or S. japonicum‐infected mice and treated with IL‐10 and in vitro stimulation with or without SEA. As shown in Fig. 8(d–f), S. japonicum infection decreased both frequencies of mTGF‐β‐expressing Treg cells and their surface expression levels of mTGF‐β (Fig. 8d–f). SEA in vitro treatment further reduced both the frequencies of TGF‐β‐expressing Treg cells and their mTGF‐β expression levels (Fig. 8d–f). Furthermore, IL‐10 treatment of Treg cells further enhanced SEA‐mediated reduction of the frequencies of mTGF‐β‐expressing Treg cells and their mTGF‐β expression levels (Fig. 8d–f).
Discussion
Precisely controlled immune regulation is important in hosts with chronic infections, including schistosomiasis. Such balanced immune regulation is necessary both for the invading pathogens to escape or attenuate the host immune responses and for the hosts to minimize pathology.3, 29 Studies have highlighted the critical roles of Treg cells and IL‐10 in regulating immune responses, for example, both of which are highly induced and essential regulators for controlling the immune response during chronic schistosome infections.9, 30, 31, 32, 33 However, the regulatory links between these two critical regulators have remained unclear. Here, our results proved that IL‐10 mediated opposite regulation on Treg cells by using murine models of schistosomiasis japinica and asthma.
Consistent with previous reports, our results showed that the frequencies of Treg cells and the serum levels of IL‐10 increased in parallel in mice after S. japonicum infection.9, 33, 34, 35, 36 Previous studies have suggested that IL‐10 may contribute to Treg cell induction;37 however, in this study there was a surprising result that the immunosuppressive activities of Treg cells from mice with S. japonicum infection were significantly lower compared with those from uninfected mice. It is well established that IL‐10, an important negative regulator, inhibits the activities of multiple cell types, including helper T cells, dendritic cells, macrophagesand natural killer cells.10, 38, 39 The function of IL‐10 is mediated through activation of IL‐10R on target cells.10 A previous study has observed higher expression of IL‐10R on CD4+ Foxp3+ Treg cells than on CD4+ Foxp3− conventional T cells.40 In our study, we further observed that S. japonicum infection doubled the expression of IL‐10R on CD4+ Foxp3+ Treg cells. Therefore, it is likely that IL‐10 may provide a negative regulatory loop and conversely suppress the immunosuppressive activity of Treg cells.
In this study, by using S. japonicum‐infected or OVA‐sensitized asthma murine models, both our in vivo and in vitro data proved that IL‐10 has opposite regulation on Treg cells, increasing the frequencies while reducing the immunosuppressive activities of Treg cells. This finding provides the evidence to suggest that Treg cells, as essential regulators of the immune system, can be positively and negatively regulated simultaneously by another critical regulator of IL‐10. In another words, the regulatory effects of IL‐10 on immune responses can be bidirectional. Our results indicate that at least partially mediated by IL‐10, the quantity and immunoregulatory activity of Treg cells are fine‐tuned to maintain an appropriate balance, e.g. between protective immune responses and suppression of harmful immunopathology, caused by effector T‐cell responses.
Studies suggest that IL‐10 plays a key role in maintaining Foxp3 expression in Treg cells, which is the most crucial transcription factor for conferring their development and suppressive function.17, 41, 42, 43 We show here, however, that the further increase of mouse serum IL‐10 by rmIL‐10 injection in S. japonicum‐infected mice did not further increase Foxp3 expression in Treg cells. One possibility is that the basal levels of IL‐10 in mice may already be sufficient to maintain Foxp3 expression.
Our result showed that in parallel with impaired Treg cell suppressive activity, IL‐10 treatment also led to the reduction of mTGF‐β expression on Treg cells, which is thought to be an important mechanism of Treg‐mediated cell–cell contact immunosuppression.27, 28 Hence, this might suggest a mechanism for IL‐10‐mediated regulation on Treg cell function, but this requires more experiments for further clarification. In addition, due to functional plasticity,44 Treg cells may adapt to environmental elevated IL‐10 and consequently manifest functional deterioration by reprogramming into an effector‐like phenotype. However, in addition to mTGF‐β, our study still cannot rule out the possibility that there are other unknown factors that may relate to regulation of Treg cells by IL‐10 during S. japonicum infection, which needs intensive study in the future.
Studies have shown that CD4+ CD25+ Treg cells can be induced by helminth infection to down‐regulate the host's immune response.5, 7, 8 Binding of soluble TGF‐β to its receptor was proved to play an essential role in conversion of naive peripheral CD+ CD25− T cells into Foxp3+ Treg cells.25 Here, we showed that IL‐10 contributed to the increase of serum TGF‐β in S. japonicum‐infected mice. These results suggest that IL‐10 may contribute to the increase of Treg cells during S. japonicum infection by up‐regulating serum TGF‐β level. In addition, we observed that the proportion of aTreg within total Treg cells decreased while that of rTreg cells increased in IL‐10‐injected mice. Given that aTreg cells are more prone to apoptosis and that rTreg cells are highly proliferative upon stimulation,45 it is possible that the IL‐10‐induced rTreg/aTreg ratio change could be involved in the increase of Treg cells during S. japonicum infection. However, whether and how this change in rTreg/aTreg ratio affects the overall immunosuppressive function of Treg cells is not yet clear. Although our current findings may help to explain the contradictory results that IL‐10 increases Treg cells while impairing their immunosuppressive activities, further evidence and detailed mechanisms for these findings require future study.
In conclusion, our study present evidence in S. japonicum‐infected and OVA‐sensitized asthma mouse models that IL‐10 has bidirectional regulatory roles in Treg cells, increasing Treg cells while impairing their immunosuppressive activities. Our findings provide new insight into the mechanisms involved in regulating the homeostasis of Treg cells to prevent overwhelming immunosuppression and suggest a potential intervention target for treatment of chronic infection, cancer, allergy, autoimmune disease and transplantation.
Disclosures
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Supporting information
Figure S1. Interleukin‐10 receptor (IL‐10R) expression on CD4+ CD25+ regulatory T (Treg) cells. Splenocytes from infected (8 weeks post‐infection) or normal control mice were isolated and stained with anti‐CD3‐PerCP‐Cy5.5, anti‐CD4‐FITC, anti‐CD25‐APC and anti‐IL‐10R‐PE (n = 4 per group). Representative flow cytometry dot plots gated on CD3+ CD4+ CD25+ Treg cells (a) and the average percentages of IL‐10R+ CD4+ CD25+ Treg cells (b) are shown.
Figure S2. Experimental designs of murine models.
Figure S3. Phenotypic analysis of regulatory T (Treg) cells in Schistosoma japonicum‐infected mice with recombinant murine interleukin‐10 (rmIL‐10) injection. The S. japonicum‐infected mice were injected intraperitoneally with PBS or rmIL‐10. (a) At 24 hr after the last injection, splenocytes were isolated and stained for CD62Lhi CD44low resting Treg cells and CD62Llow CD44hi activated Treg cells (n = 4 per group). Representative flow cytometry dot plots gated on CD4+ CD25+ Foxp3+ T cells and the average percentages of rTreg and aTreg cells within total Treg cells are shown. (b, c) Expression of CTLA‐4 (b) and ICOS (c) on Treg cells (n = 4 per group). Representative histograms gated on CD4+ CD25+ Foxp3+ T cells and the average percentages of CTLA‐4+ Treg cells (b) and ICOS+ Treg cells (c) within total Treg cells are shown. **P < 0·01 ***P < 0·001.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (No. 81430052) to Chuan Su, the National Natural Science Foundation of China (No. 81501766) and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (15KJB310007) to Sha Zhou, and the Priority Academic Programme for Development of Jiangsu Higher Education Institutions (PAPD).
Contributor Information
Xiaojun Chen, Email: chenxiaojun0201@126.com.
Chuan Su, Email: chuansu@njmu.edu.cn.
References
- 1. Grencis RK. Immunity to helminths: resistance, regulation, and susceptibility to gastrointestinal nematodes. Annu Rev Immunol 2014; 33:201–25. [DOI] [PubMed] [Google Scholar]
- 2. Taylor MD, van der Werf N, Maizels RM. T cells in helminth infection: the regulators and the regulated. Trends Immunol 2012; 33:181–9. [DOI] [PubMed] [Google Scholar]
- 3. Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol 2011; 11:375–88. [DOI] [PubMed] [Google Scholar]
- 4. Weinstock JV, Elliott DE. Helminth infections decrease host susceptibility to immune‐mediated diseases. J Immunol 2014; 193:3239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finlay CM, Walsh KP, Mills KH. Induction of regulatory cells by helminth parasites: exploitation for the treatment of inflammatory diseases. Immunol Rev 2014; 259:206–30. [DOI] [PubMed] [Google Scholar]
- 6. Shevach EM. Mechanisms of foxp3+ T regulatory cell‐mediated suppression. Immunity 2009; 30:636–45. [DOI] [PubMed] [Google Scholar]
- 7. Dunne DW, Cooke A. A worm's eye view of the immune system: consequences for evolution of human autoimmune disease. Nat Rev Immunol 2005; 5:420–6. [DOI] [PubMed] [Google Scholar]
- 8. Erb KJ. Can helminths or helminth‐derived products be used in humans to prevent or treat allergic diseases? Trends Immunol 2009; 30:75–82. [DOI] [PubMed] [Google Scholar]
- 9. Redpath SA, Fonseca NM, Perona‐Wright G. Protection and pathology during parasite infection: IL‐10 strikes the balance. Parasite Immunol 2014; 36:233–52. [DOI] [PubMed] [Google Scholar]
- 10. Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin‐10 and the interleukin‐10 receptor. Annu Rev Immunol 2001; 19:683–765. [DOI] [PubMed] [Google Scholar]
- 11. Banchereau J, Pascual V, O'Garra A. From IL‐2 to IL‐37: the expanding spectrum of anti‐inflammatory cytokines. Nat Immunol 2012; 13:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL‐10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL‐2 production and proliferation. J Immunol 1993; 150:4754–65. [PubMed] [Google Scholar]
- 13. Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin‐10 induces a long‐term antigen‐specific anergic state in human CD4+ T cells. J Exp Med 1996; 184:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taga K, Mostowski H, Tosato G. Human interleukin‐10 can directly inhibit T‐cell growth. Blood 1993; 81:2964–71. [PubMed] [Google Scholar]
- 15. Hsu P, Santner‐Nanan B, Hu M, Skarratt K, Lee CH, Stormon M et al IL‐10 potentiates differentiation of human induced regulatory T cells via STAT3 and Foxo1. J Immunol 2015; 195:3665–74. [DOI] [PubMed] [Google Scholar]
- 16. Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM et al Interleukin‐10 signaling in regulatory T cells is required for suppression of Th17 cell‐mediated inflammation. Immunity 2011; 34:566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H et al Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol 2009; 10:1178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wynn TA, Cheever AW, Jankovic D, Poindexter RW, Caspar P, Lewis FA et al An IL‐12‐based vaccination method for preventing fibrosis induced by schistosome infection. Nature 1995; 376:594–6. [DOI] [PubMed] [Google Scholar]
- 19. Gangi E, Vasu C, Cheatem D, Prabhakar BS. IL‐10‐producing CD4+ CD25+ regulatory T cells play a critical role in granulocyte‐macrophage colony‐stimulating factor‐induced suppression of experimental autoimmune thyroiditis. J Immunol 2005; 174:7006–13. [DOI] [PubMed] [Google Scholar]
- 20. Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth‐induced regulatory T cells. J Exp Med 2005; 202:1199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Emo J, Meednu N, Chapman TJ, Rezaee F, Balys M, Randall T et al Lpa2 is a negative regulator of both dendritic cell activation and murine models of allergic lung inflammation. J Immunol 2012; 188:3784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McKee AS, Pearce EJ. CD25+ CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol 2004; 173:1224–31. [DOI] [PubMed] [Google Scholar]
- 23. Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol 2009; 39:1098–107. [DOI] [PubMed] [Google Scholar]
- 24. Yang J, Zhao J, Yang Y, Zhang L, Yang X, Zhu X et al Schistosoma japonicum egg antigens stimulate CD4 CD25 T cells and modulate airway inflammation in a murine model of asthma. Immunology 2007; 120:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N et al Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF‐β induction of transcription factor Foxp3. J Exp Med 2003; 198:1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol 2005; 6:1219–27. [DOI] [PubMed] [Google Scholar]
- 27. Nakamura K, Kitani A, Strober W. Cell contact‐dependent immunosuppression by CD4+ CD25+ regulatory T cells is mediated by cell surface‐bound transforming growth factor β . J Exp Med 2001; 194:629–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+ CD25+ T regulatory cells control anti‐islet CD8+ T cells through TGF‐β‐TGF‐β receptor interactions in type 1 diabetes. Proc Natl Acad Sci U S A 2003; 100:10878–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med 2009; 206:2059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sakaguchi S. Regulatory T cells: mediating compromises between host and parasite. Nat Immunol 2003; 4:10–1. [DOI] [PubMed] [Google Scholar]
- 31. Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol 2007; 7:875–88. [DOI] [PubMed] [Google Scholar]
- 32. Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol 2006; 176:5839–47. [DOI] [PubMed] [Google Scholar]
- 33. Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink‐Kane M, Leusink M et al The pathogenesis of schistosomiasis is controlled by cooperating IL‐10‐producing innate effector and regulatory T cells. J Immunol 2004; 172:3157–66. [DOI] [PubMed] [Google Scholar]
- 34. Chuah C, Jones MK, Burke ML, McManus DP, Gobert GN. Cellular and chemokine‐mediated regulation in schistosome‐induced hepatic pathology. Trends Parasitol 2014; 30:141–50. [DOI] [PubMed] [Google Scholar]
- 35. van der Vlugt LE, Labuda LA, Ozir‐Fazalalikhan A, Lievers E, Gloudemans AK, Liu KY et al Schistosomes induce regulatory features in human and mouse CD1dhi B cells: inhibition of allergic inflammation by IL‐10 and regulatory T cells. PLoS ONE 2012; 7:e30883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baumgart M, Tompkins F, Leng J, Hesse M. Naturally occurring CD4+ Foxp3+ regulatory T cells are an essential, IL‐10‐independent part of the immunoregulatory network in Schistosoma mansoni egg‐induced inflammation. J Immunol 2006; 176:5374–87. [DOI] [PubMed] [Google Scholar]
- 37. Boks MA, Kager‐Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A. IL‐10‐generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction—a comparative study of human clinical‐applicable DC. Clin Immunol 2012; 142:332–42. [DOI] [PubMed] [Google Scholar]
- 38. Saraiva M, O'Garra A. The regulation of IL‐10 production by immune cells. Nat Rev Immunol 2010; 10:170–81. [DOI] [PubMed] [Google Scholar]
- 39. Couper KN, Blount DG, Riley EM. IL‐10: the master regulator of immunity to infection. J Immunol 2008; 180:5771–7. [DOI] [PubMed] [Google Scholar]
- 40. Wilhelm AJ, Rhoads JP, Wade NS, Major AS. Dysregulated CD4+ T cells from SLE‐susceptible mice are sufficient to accelerate atherosclerosis in LDLr−/− mice. Ann Rheum Dis 2015; 74:778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133:775–87. [DOI] [PubMed] [Google Scholar]
- 42. Ramsdell F, Ziegler SF. FOXP3 and scurfy: how it all began. Nat Rev Immunol 2014; 14:343–9. [DOI] [PubMed] [Google Scholar]
- 43. Tanoue T, Atarashi K, Honda K. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol 2016; 16:295–309. [DOI] [PubMed] [Google Scholar]
- 44. da Silva MM, Piccirillo CA. Functional stability of Foxp3+ regulatory T cells. Trends Mol Med 2012; 18:454–62. [DOI] [PubMed] [Google Scholar]
- 45. Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R et al Only the CD45RA+ subpopulation of CD4+ CD25high T cells gives rise to homogeneous regulatory T‐cell lines upon in vitro expansion. Blood 2006; 108:4260–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Interleukin‐10 receptor (IL‐10R) expression on CD4+ CD25+ regulatory T (Treg) cells. Splenocytes from infected (8 weeks post‐infection) or normal control mice were isolated and stained with anti‐CD3‐PerCP‐Cy5.5, anti‐CD4‐FITC, anti‐CD25‐APC and anti‐IL‐10R‐PE (n = 4 per group). Representative flow cytometry dot plots gated on CD3+ CD4+ CD25+ Treg cells (a) and the average percentages of IL‐10R+ CD4+ CD25+ Treg cells (b) are shown.
Figure S2. Experimental designs of murine models.
Figure S3. Phenotypic analysis of regulatory T (Treg) cells in Schistosoma japonicum‐infected mice with recombinant murine interleukin‐10 (rmIL‐10) injection. The S. japonicum‐infected mice were injected intraperitoneally with PBS or rmIL‐10. (a) At 24 hr after the last injection, splenocytes were isolated and stained for CD62Lhi CD44low resting Treg cells and CD62Llow CD44hi activated Treg cells (n = 4 per group). Representative flow cytometry dot plots gated on CD4+ CD25+ Foxp3+ T cells and the average percentages of rTreg and aTreg cells within total Treg cells are shown. (b, c) Expression of CTLA‐4 (b) and ICOS (c) on Treg cells (n = 4 per group). Representative histograms gated on CD4+ CD25+ Foxp3+ T cells and the average percentages of CTLA‐4+ Treg cells (b) and ICOS+ Treg cells (c) within total Treg cells are shown. **P < 0·01 ***P < 0·001.


