Summary
Tolerogenic dendritic cells (tolDCs) are a promising therapeutic tool to restore immune tolerance in autoimmune diseases. The rationale of using tolDCs is that they can specifically target the pathogenic T‐cell response while leaving other, protective, T‐cell responses intact. Several ways of generating therapeutic tolDCs have been described, but whether these tolDCs should be loaded with autoantigen(s), and if so, with which autoantigen(s), remains unclear. Autoimmune diseases, such as rheumatoid arthritis, are not commonly defined by a single, universal, autoantigen. A possible solution is to use surrogate autoantigens for loading of tolDCs. We propose that heat‐shock proteins may be a relevant surrogate antigen, as they are evolutionarily conserved between species, ubiquitously expressed in inflamed tissues and have been shown to induce regulatory T cells, ameliorating disease in various arthritis mouse models. In this review, we provide an overview on how immune tolerance may be restored by tolDCs, the problem of selecting relevant autoantigens for loading of tolDCs, and why heat‐shock proteins could be used as surrogate autoantigens.
Keywords: autoimmune diseases, heat‐shock proteins, regulatory T cells, tolerogenic dendritic cells
Abbreviations
- ACPAs
anti‐citrullinated peptide antibodies
- ER
endoplasmic reticulum
- HSP
heat‐shock proteins
- IDO
indoleamine 2,3‐deoxygenase
- IL‐2
interleukin‐2
- MITAP
minimal information model for tolerogenic antigen‐presenting cells
- PD‐L1
programmed death ligand 1
- RA
rheumatoid arthritis
- TGF‐β
transforming growth factor‐β
- TolAPC
tolerogenic antigen‐presenting cell
- TolDC
tolerogenic dendritic cell
- Treg
regulatory T cell
Restoring immune tolerance to ‘self’ in autoimmune disease: a promising clinical intervention
Immune tolerance is crucial for preventing destructive immune responses to self tissues. In healthy individuals, immune tolerance is maintained at different levels: in the thymus, where T cells that strongly react to self‐antigens are deleted, and in the periphery, where self‐reactive T cells that escaped negative selection in the thymus are kept in check by regulatory cells. A breach in immune tolerance facilitates immune attacks on self‐tissues that, when becoming dysregulated, lead to chronic autoimmune disorders.
Regulatory T (Treg) cells play a pivotal role in maintaining immune tolerance in the periphery. They are a heterogeneous population of cells that can be either derived from the thymus (naturally occurring Treg cells) or induced in the periphery from naive CD4+ T cells (induced Treg cells). They exert their suppressive action on immune effector cells through a number of distinct mechanisms, including inhibition of antigen‐presenting cell function, killing of effector cells, secretion of immunosuppressive cytokines and compounds, and interference with metabolic pathways (reviewed in refs 1, 2).
Treg cells are critical to prevent autoimmune disease. A total loss of functional Treg cells, as seen in patients with IPEX (immunodysregulation polyendocrinopathy enteropathy X‐linked syndrome), leads to severe autoimmunity affecting multiple organs.3 In specific autoimmune diseases, however, it is thought that a more subtle change in the function of Treg cells is involved in the pathogenesis. For example, although patients with type I diabetes have similar numbers of Treg cells to healthy controls, their Treg cells display reduced suppressive activity and defects in interleukin‐2 (IL‐2) signalling.4, 5, 6 In patients with rheumatoid arthritis (RA), Treg cells have reduced ability to suppress inflammatory cytokine production.7 Furthermore, enhanced numbers of Treg cells co‐expressing IL‐17 were found in both the peripheral blood and synovial fluid of patients with RA, suggesting conversion of Treg cells into inflammatory cytokine‐producing effector cells.8
Restoration of Treg cell function is emerging as a promising clinical intervention for autoimmune diseases. One way of achieving this is by replenishing the Treg cell pool in autoimmune patients with functional Treg cells, either by treating patients with drugs that selectively expand Treg cells in vivo, or by generating new Treg cells ex vivo before injecting them into the patient (reviewed in refs 2, 9). However, a downside of this approach is that expanding Treg cells ‘randomly’ may give rise to general suppression of the immune response, thereby increasing the risk of infection, and perhaps even cancer. A preferred approach would be to direct the Treg response to defined and relevant antigens that are being expressed in the target tissue. This would not only limit off‐target immunosuppression, but would most likely also increase the efficacy of the Treg cell therapy, as was shown in mouse models.10, 11 An outstanding issue is, however, how to achieve the expansion of antigen‐specific Treg cells, and how to choose the relevant antigen(s). Here, we propose to use tolerogenic DCs (tolDCs) to induce Treg cells against heat‐shock proteins that are ubiquitously expressed in inflamed target tissues, as outlined below.
Tolerogenic dendritic cells as a therapeutic tool
Dendritic cells (DCs) are a heterogeneous family of professional antigen‐presenting cells that can be classified on the basis of their ontogeny, surface marker expression profile and anatomical location (reviewed in ref. 12). DCs are as important for the induction of effective immunity against invading pathogens as they are for the maintenance of immune tolerance. Patients with primary immunodeficiency with mutations in GATA2 have defective DC function, resulting in enhanced susceptibility not only to infection and cancer, but also to autoimmune conditions, most likely due to a reduction in Treg cells.13
The role of DCs in instigating immunity versus tolerance is largely determined by their maturation status. Under steady‐state conditions, tissue DCs are immature, expressing low levels of MHC class II and co‐stimulatory molecules; their ‘default’ setting is to induce tolerance. These immature DCs can become immunogenic when they sense pathogen‐associated molecular patterns and danger‐associated molecular patterns via pattern recognition receptors. These include Toll‐like receptors, retinoic‐acid‐inducible gene I‐like receptors, and nucleotide‐binding oligomerization domain‐like receptors. Pattern recognition receptor‐mediated signalling plays a central role in the maturation process that DCs need to undergo to acquire potent T‐cell stimulatory properties.14 Fully matured DCs express high levels of MHC class II, co‐stimulatory markers (e.g. CD86) and pro‐inflammatory cytokines (e.g. IL‐12p70, IL‐23, tumour necrosis factor), all required for the efficient induction of T effector cell responses. Furthermore, during DC maturation the expression of chemokine receptors is modulated (e.g. CCR5 is down‐regulated and CCR7 is up‐regulated) enabling DC migration towards lymphoid tissues to present antigen to naive T cells. However, the outcome of maturation of DCs is not always the generation of DCs with immunogenic properties. Certain danger‐associated molecular patterns and immune suppressive compounds have been shown to drive the maturation of DCs with tolerogenic properties (i.e. tolDCs).15, 16, 17, 18 These tolDCs may be phenotypically mature (i.e. high levels of MHC class II and co‐stimulatory molecules), but may express co‐inhibitory molecules [e.g. programmed death ligand 1 (PD‐L1), PD‐L2, immunoglobulin‐like transcript 3], lack expression of pro‐inflammatory cytokines and instead produce immunosuppressive cytokines and compounds [e.g. IL‐10, transforming growth factor‐β (TGF‐β), indoleamine 2,3‐dioxygenase (IDO)]. The maturation status of these DCs has been referred to as ‘semi‐mature’. Hence, there is plasticity with regard to the functional maturation of DC, and the environmental cues that DCs receive during the maturation process determine whether they become immunogenic or tolerogenic.
Dendritic cells are able to mediate tolerance through several mechanisms. They can induce iTreg cells through, for example, membrane‐bound PD‐L1, which blocks the Akt/mTOR pathway to preferentially stimulate naive T cells to become iTreg cells.19 Furthermore, PD‐L1 and PD‐L2 provide inhibitory signals to both CD8+ and CD4+ T cells, which drives the T cell into a state of tolerance.19 Secreted compounds such as IL‐10, IL‐27, TGF‐β, retinoic acid and IDO, can convert naive T cells into iTreg cells. DCs can also promote T‐cell tolerance through T‐cell killing, and the induction of T‐cell hyporesponsiveness (anergy).20, 21
The importance of DCs in maintaining immune tolerance has led to exploring the therapeutic use of DCs. Various ways have been described to create DCs with stable tolerogenic properties (tolDCs). The tolerogenic properties of these in vitro generated tolDCs depend on the specific method used (reviewed in ref. 22). For example, tolDCs generated with the immunosuppressive agents dexamethasone and/or the active form of Vitamin D3 (1α,25‐dihydroxyvitamin D3) are characterized by a semi‐mature phenotype, with high levels of MHC class II, intermediate levels of co‐stimulatory molecules, low levels of pro‐inflammatory cytokines and high levels of the immunosuppressive cytokines IL‐10 and TGF‐β.23, 24, 25, 26, 27 TolDCs can also be genetically engineered, for example through the transduction of immunosuppressive or pro‐apoptotic molecules (e.g. IL‐10, CTLA‐4, FASL) or silencing of immunostimulatory molecules (e.g. CD80/CD86, IL‐12) (reviewed in ref. 28). These different types of tolDCs have been shown to reduce or prevent autoimmune diseases or transplant rejection in animal models, providing important proof of principle evidence that these cells can be applied therapeutically.27, 29, 30, 31, 32, 33 Their therapeutic benefit is associated with a reduction of pro‐inflammatory effector T cells and natural killer cells, and the induction of Treg cells or IL‐10‐producing T cells.27, 29, 34, 35, 36
Efforts have been made to translate these findings from animal studies to the clinical setting. Good Manufacturing Protocols to generate tolDCs from human donor cells have been developed,26, 37 and methods to preserve the tolDCs and reduce the production costs are being explored.29 As there are diverse methods of generating tolDCs and other types of tolerogenic APC (tolAPCs), a minimum information model for tolAPC (MITAP) was generated. MITAP enables researchers to report their data in a standardized and more transparent manner, facilitating data comparison and interpretation, ultimately paving the way for the development of standardized protocols for the production of tolDCs and other tolAPCs for therapeutic application.38 A number of tolDCs have been tested in phase I clinical trials, including for type I diabetes,30 Crohn's disease39 and RA.40, 41 Encouragingly, tolDC therapy in all these studies was found to be feasible and safe, providing rationale to conduct further studies into their efficacy.
The problem of targeting autoantigen(s) – which ones?
One of the main advantages of tolDC therapy is the specific targeting of pathogenic immune responses. Many of the drugs that are currently used to treat autoimmune diseases are non‐antigen‐specific, leading to general immunosuppression. With tolDCs, autoreactive T cells can, theoretically, be exclusively targeted. But how to achieve this is still a debate. A number of studies have provided clear evidence that tolDCs need to be loaded with a disease‐relevant antigen to exert their beneficial immune modulatory action. Loading of tolDCs with type II collagen was required, for example, for antigen‐specific disease remission in the collagen‐induced arthritis model.27, 42, 43 More recent research shows that this is also applicable in other autoimmune diseases.44 Furthermore, when comparing the therapeutic action of unloaded tolDCs and tolDCs loaded with a disease relevant peptide (MOG40–55) in the experimental autoimmune encephalomyelitis model, Mansilla et al.45 showed that although the unloaded tolDCs inhibited disease symptoms, the MOG40–55‐loaded tolDCs diminished disease even more.
In contrast, other studies have shown that disease remission can be established when administering unloaded tolDCs.46, 47 This may suggest that tolDCs are able to take up the relevant antigen in vivo. It has been hypothesized that unloaded tolDCs induce T‐cell anergy rather than promoting Treg cells. These anergic T cells might be capable of suppressing excessive T helper type 17 and type 1 responses.48 Non‐antigen‐pulsed tolDCs might also induce regulatory populations that do not require an antigen. For instance, B cells can be converted into regulatory B cells partly through the production of retinoic acid by the tolDCs.49 However, if these non‐antigen‐pulsed tolDCs are able to take up antigen in vivo, one has to consider the safety of these tolDCs, as it is possible that the non‐antigen‐pulsed tolDCs also take up other antigens that should not be targeted.
Nonetheless, if tolDCs need to be loaded with antigen(s) before infusion, a remaining problem is the question of which antigen to use, and in what form. In many autoimmune diseases, including RA, the knowledge about the relevant autoantigen(s) involved is insufficient. Moreover, even if some of the relevant autoantigens are known, as is the case for multiple sclerosis, the problem of HLA diversity remains.44 Some peptides (e.g. proteolipid protein) that have been shown to be involved in the pathogenesis of multiple sclerosis are restricted to a specific HLA‐class (e.g. HLA‐DQB1*0602), making it more difficult to standardize the peptides used for all patients with multiple sclerosis.50
For RA, no universal autoantigen exists. Several candidate self‐proteins have been described in relation to the pathogenesis of this disease. Epitopes from joint‐derived antigens such as collagen type II and human cartilage‐derived glycoprotein HCgp39 are presented by DCs and macrophages to T cells in inflamed joints of patients with RA.51 Furthermore, the endoplasmic reticulum (ER) stress‐associated protein GRP78/BiP is described as a potential autoantigen. The ER stress response is increased in RA synovial tissue and fluid and the ER chaperone, GRP78, is important for synoviocyte proliferation and angiogenesis, which are substantial indicators of RA.52
Post‐translational modifications may also be important in generating novel epitopes that trigger autoimmunity. Anti‐citrullinated peptide antibodies (ACPAs) are found in the sera of 70–80% of patients with RA.53 Immunogenetic studies have shown that more than 90% of patients with RA share an HLA‐II epitope in the DRB1 chain (HLA‐DRB1 *0101, *0401, *0404). This so‐called shared epitope is also associated with ACPAs; shared epitope‐positive patients are predisposed to having ACPAs.54, 55 Feitsma et al. identified two HLA‐DRB1‐restricted CD4+ T‐cell clones that recognized citrullinated vimentin and were also present in the inflamed joints of patients with RA. This indicates that CD4+ T cells can respond to naturally processed epitopes from an autoantigen.54 The finding that ACPAs were present in the inflamed joints of patients but not in the joints of healthy individuals, together with the discovery that citrullinated autoantigen‐specific CD4+ T cells were only found in the peripheral blood mononuclear cells from patients with RA, suggests that both the ACPAs and these CD4+ T cells play a significant role in the pathogenesis of RA.55, 56 Scally et al. (and others) provide molecular evidence on how CD4+ T cells are able to recognize citrullinated antigens.57, 58, 59 They also showed that in the autoantigen recognizing CD4+ T‐cell population of HLA‐DRB1*04:01 + RA patients, the percentage Treg cells (both activated and resting) was reduced, whereas the populations of naive and effector memory CD4+ T cells were increased compared with healthy subjects.57 This indicates that citrullinated peptides are plausible autoantigens in RA.
To test if citrullinated antigens are good candidates for an immunomodulatory therapy, a phase I clinical trial was performed. In this study autologous in vitro generated tolDCs were exposed to citrullinated autoantigenic epitopes and administered intradermally into patients.40 The trial showed that the DC vaccination was safe and indicated an anti‐inflammatory effect after DC administration. However, using citrullinated peptides has the consequence that therapy is limited to patients with HLA‐DRB1 (*0101, *0401, *0404) and it is unknown if the reactivity in these patients is similar. We took a different approach in our recent phase I safety trial in patients with rheumatoid and inflammatory arthritis.41 TolDCs were loaded with autologous synovial fluid; the rationale being that this fluid contains relevant joint‐associated antigens. The downside of this approach is that it is not always possible to obtain sufficient synovial fluid from patients with RA for tolDC loading. Furthermore, as the antigens are unknown, it is difficult to monitor changes in the antigen‐specifc T‐cell response after tolDC administration.
The use of surrogate autoantigens could be a preferred option for the loading of tolDCs. Possible candidates are heat‐shock proteins (HSPs). HSPs are typically intracellular proteins with no peptide leader sequences that can target secretion. However, there is evidence that HSPs can have access to the extracellular milieu, either by passive or active mechanisms. Both the endogenous up‐regulation of HSPs with so‐called HSP co‐inducers and the exogenous administration of (recombinant) HSPs have led to immunomodulatory effects in various models of experimental autoimmunity.60, 61, 62 Therefore, HSPs could be used as surrogate autoantigens not only for RA but also for other autoimmune diseases. This will be discussed in further detail in the next section (Figure 1).
Figure 1.
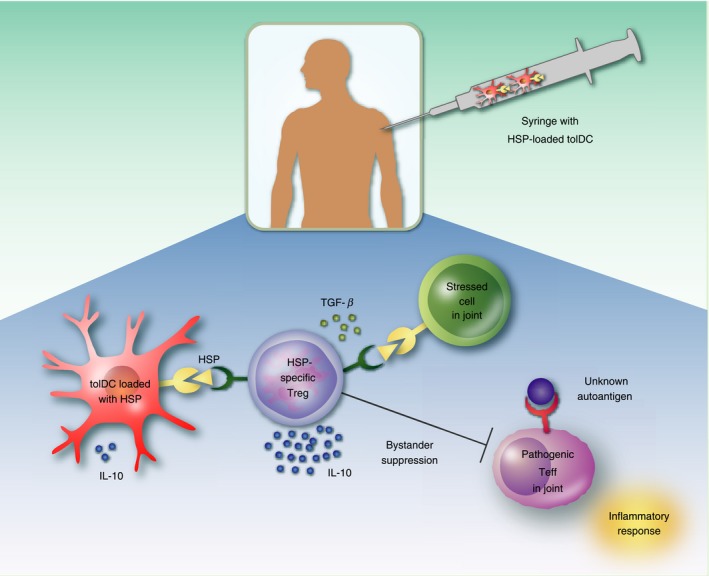
Heat‐shock protein (HSP) loaded tolerogenic dendritic cell (tolDC) vaccination in rheumatoid arthritis (RA). This figure depicts the potential process that takes place in the patient's joint after injection with HSP loaded tolDCs. TolDCs produce anti‐inflammatory cytokines [e.g. interleukin‐10 (IL‐10)] and present epitopes of HSP to naive CD4+ T cells. These CD4+ T cells differentiate into HSP‐specific regulatory T (Treg) cells and suppress stressed (HSP expressing) cells via immunomodulatory cytokines like IL‐10 and transforming growth factor‐β (TGF‐β). Furthermore, bystander suppression could lead to suppression of pathogenic effector T (Teff) cells recognizing the unknown autoantigen, thereby inhibiting inflammatory symptoms. The presence of self HSP in the synovial fluid of RA patients might favour the selection of the generation of Treg cells and their function.
HSPs as surrogate autoantigens for autoimmunity
The main function of HSPs is to support folding and transport of a large variety of (misfolded) proteins as intracellular molecular chaperones. Their expression can be significantly up‐regulated under conditions of stress like fever, viral infection, nutritional deficiency, cold and exposure to the pro‐inflammatory cytokines interferon‐γ and tumour necrosis factor.63, 64, 65 Generally, HSPs can be classified into different families based on their monomeric molecular weight (HSP 10, HSP 20–30, HSP 40, HSP 60, HSP 70, HSP 90 and HSP 100 families). Some HSP family members (e.g. HSP 60 and HSP 70) are highly conserved throughout evolution, resulting in immunological cross‐recognition of certain mammalian and microbial HSP homologues.
Initial observations that ignited studies on the role of HSPs in autoimmunity were made in the mycobacteria‐induced adjuvant arthritis model in rats. Generated mycobacteria‐specific T‐cell lines were shown to have arthritogenic potential66 and it was later discovered that HSP 60 was the antigen recognized by the mycobacteria‐specific T‐cell lines.67 Further studies followed showing that synovial fluid cells and peripheral blood mononuclear cells of patients with chronic inflammatory arthritis could also respond to mycobacterial HSP 60. In contrast, HSP 60 responses were absent in control subjects.68 Moreover, monoclonal antibodies recognizing mammalian HSP 60 were produced and it was found that HSP 60 was expressed in the synovial membranes of patients with chronic arthritis.69, 70 Similar results were found for the HSP family members HSP 40 and HSP 70. Synovial fluid and peripheral blood T cells of patients with RA could recognize a bacterial variant of HSP 40, but those from healthy subjects or disease controls could not.71 In addition, the human homologues of HSP 40 and HSP 70 were found to be over‐expressed in the synovial lining of the joints of patients with RA.72, 73
Interestingly, numerous experimental animal models and even a few clinical trials have shown that treatment with (myco)bacterial HSPs can induce HSP‐specific anti‐inflammatory T‐cell responses. Experimental autoimmune disease models in both rat and mouse showed significantly reduced arthritis severity after prophylactic immunization with mycobacterial HSP 60 or HSP 70.74, 75 Although the exact mechanism for disease amelioration is still not completely understood, suppression of arthritis is probably induced by IL‐10‐producing Treg cells.75, 76, 77, 78 One possible explanation for the propagation and/or induction of a regulatory phenotype in HSP 60/70‐specific T cells lies in the high homology between the bacterial and mammalian variants of the HSPs. Even though HSPs are considered immunogenic – microbial HSP 60, for example, was already known as the so‐called ‘common antigen of Gram negatives’ before its molecular definition79 – the highly conserved parts of the proteins could induce a tolerogenic response as these can be recognized as self‐antigens by the body's own immune system.80 Moreover, since bacterial HSPs are mostly encountered in the tolerizing gut or lung mucosa, conserved and hence repeatedly encountered HSP antigens are more likely to obtain a regulatory phenotype. In addition to conservation and microbial‐self cross‐recognition, HSP 70 family members are directly involved with antigen processing and consequently, HSP 70 fragments were found to be one of the most frequent cytosolic MHC class II natural ligand sources.81, 82, 83 Presentation of HSP 70 peptides may therefore be part of the earlier mentioned default tolerant state of the immune system, where MHC class II presented HSP peptides are part of a continuous and credible target for Treg cells. It is, however, important to keep in mind that in a dysregulated immune system, as is seen in patients with autoimmune diseases, antigens that would normally induce an anti‐inflammatory immune response could now potentially induce a pro‐inflammatory response.
As the HSPs used for these experiments are from bacterial origin and can potentially induce an unwanted anti‐inflammatory response towards these bacteria, a safer form of the HSPs is needed. One way to accomplish this is to use bacterial HSP‐derived peptides that show high homology with the mammalian variant. The high homology to the self‐antigen will prevent unwanted responses towards the bacteria and at the same time ensure cross‐reactivity with the mammalian HSPs presented in the inflamed joint. Indeed, two of the three clinical trials using HSPs as therapy were performed with HSP‐derived peptides (Table 1). A pilot phase II trial using an HSP 40‐derived peptide, dnaJP1; which also contains the ‘shared epitope’,84 was tested in patients with juvenile idiopathic arthritis. After oral administration of the dnaJP1, a change from a pro‐inflammatory to a tolerogenic T‐cell response to dnaJP1 could be observed.85, 86 In a second phase II trial, an HSP 60‐derived peptide, DiaPep277, was used to treat patients with type I diabetes. It was found that DiaPep277 was safe and showed a trend towards a greater preservation of beta‐cell function compared with controls.87, 88 In a third recent trial, a mammalian HSP 70 family member, BiP, was tested in patients with RA. In this case, whole protein was administered intravenously. The results of this phase I/II safety trial showed no serious adverse drug reactions. Moreover, at the higher treatment doses disease remissions were seen in some cases.89
Table 1.
Heat‐shock proteins (HSPs) and peptides associated with therapeutic interventions in chronic inflammatory diseases. dnaJP1 and DiaPep277 were tested in phase II clinical trials in juvenile RA and diabetes (refs. 85, 87). mB29a is now explored for the loading of tolDCs in RA (refs. 83, 92). The peptides are based on human Hsp sequences
| HSP | Peptide | Sequence |
|---|---|---|
| HSP 40 (dnaJB1) | dnaJP1 | QKRAAYDQYGHAAFE |
| HSP 60 (HspD1) | DiaPep277 | VLGGGVALLRVIPALDSLTPANED |
| HSP 70 (HspA9) | mB29a | VLRVINEPTAAALAY |
As discussed earlier, one potential disadvantage of using peptides is HLA diversity in patients. Consequently, HSP peptides need to either (i) be able to bind multiple HLA‐DR molecules, including the RA‐associated HLA‐DRB1 *0101, *0401, *0404 molecules, or (ii) a peptide pool of several HSP peptides able to bind one or more of the RA‐associated HLA‐DR molecules needs to be administered. For HSP 60 and HSP 70 several pan‐DR peptides have been discovered. Kamphuis et al. used a computer algorithm to identify both self and bacterial HSP 60 peptides able to bind a number of distinct HLA‐DR haplotypes. They found several peptides that were able to bind the major RA/juvenile idiopathic arthritis‐associated HLA‐DR molecules and T cells from both juvenile idiopathic arthritis and RA patients were able to respond to five out of eight peptides.90, 91 In addition, de Wolf et al. showed that an HSP 70 peptide, B29, also binds multiple HLA‐DR molecules. They concluded that more than 80% of human individuals can present B29 to their T cells (and among patients with RA possibly even more due to the high presence of HLA‐DRB1 *0401). In subsequent cultures they showed that 10 out of 14 healthy individuals could respond to the peptide.92 The B29 peptide was earlier tested in a mouse model of arthritis and it was found that prophylactic intranasal administration of B29 could suppress disease. Moreover, CD25+ CD4+ T cells from B29 immunized mice could decrease disease severity in recipient arthritic mice, indicating that B29‐specific Treg cells are effective in diminishing autoimmune arthritis.83
Next to the Treg cell inducing potential of B29, bone‐marrow‐derived DCs pulsed with Mycobacterium tuberculosis or mouse HSP 70 induced IL‐10 production in antigen‐specific T cells and suppressed arthritis, showing that HSP 70 loading of DCs by itself is tolerizing.93
In order to make both tolDC therapy and HSP peptide treatment in autoimmune diseases (e.g. RA) as potent as possible, a combination therapy could be the solution. Pulsing tolDCs with HSP peptides could (i) solve the autoantigen problem and (ii) the HSP peptides will be targeted to the HSP‐specific T cells by DCs with stable tolerogenic function, making sure a regulatory response towards the antigen is induced.
Conclusion
The fundamental problem in autoimmune diseases is the failure of the immune system to down‐regulate its own potentially dangerous cells, leading to destruction of tissue expressing the autoantigen. In the case of RA, currently available immunosuppressive therapies offer relief but fail to induce long‐term physiological regulation resulting in medication‐free remission.
As argued here, to restore immune tolerance, autologous tolDCs loaded with an HSP‐derived peptide antigen could be used. Such a therapy could, potentially, both tolerize arthritogenic T cells and induce disease‐suppressive regulatory T cells. Targeting the physiological mechanism of re‐establishing tolerance for self‐antigens offers the opportunity to inhibit joint‐destroying immune responses long‐term.
Disclosure
JMvL has received honoraria from Arthrogen, BMS, Eli Lilly, Janssen, MSD, Pfizer and Roche, and research grants from Astra Zeneca, MSD and Genentech. The other authors have declared no conflicts of interest. WvE has shares in Trajectum Pharma, Inc., a SME that develops HSP peptides for immunotherapy.
Acknowledgements
WvE and CH conceptualized the paper. MJ, WvE, RS and CH wrote the paper. JI, JMvL and FB read and commented on the paper. MJ, RS, WvE and CH revised the paper. We thank the Dutch Reumafonds for their support in the preclinical development of tolDCs loaded with HSP 70 peptides for the induction of tolerance. This work was partly supported by Marie Skłodowska‐Curie individual fellowship project #654882.
Contributor Information
Willem van Eden, Email: w.vaneden@uu.nl.
Catharien M. U. Hilkens, Email: w.vaneden@uu.nl
References
- 1. Sakaguchi S, Wing K, Onishi Y, Prieto‐Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol 2009; 21:1105–11. [DOI] [PubMed] [Google Scholar]
- 2. Bluestone JA, Trotta E, Xu D. The therapeutic potential of regulatory T cells for the treatment of autoimmune disease. Expert Opin Ther Targets 2015; 19:1091–103. [DOI] [PubMed] [Google Scholar]
- 3. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L et al The immune dysregulation, polyendocrinopathy, enteropathy, X‐linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001; 27:20–1. [DOI] [PubMed] [Google Scholar]
- 4. Brusko TM, Wasserfall CH, Clare‐Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T‐cells in type 1 diabetes. Diabetes 2005; 54:1407–14. [DOI] [PubMed] [Google Scholar]
- 5. Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S et al Defects in IL‐2R signaling contribute to diminished maintenance of FOXP3 expression in CD4+ CD25+ regulatory T‐cells of type 1 diabetic subjects. Diabetes 2010; 59:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4+CD25+ T‐cells from patients with type 1 diabetes. Diabetes 2005; 54:92–9. [DOI] [PubMed] [Google Scholar]
- 7. Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA et al Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti‐TNFα therapy. J Exp Med 2004; 200:277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang T, Sun X, Zhao J, Zhang J, Zhu H, Li C et al Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann Rheum Dis 2015; 74:1293–301. [DOI] [PubMed] [Google Scholar]
- 9. Trzonkowski P, Bacchetta R, Battaglia M, Berglund D, Bohnenkamp HR, ten Brinke A et al Hurdles in therapy with regulatory T cells. Sci Transl Med 2015; 7:304 ps18. [DOI] [PubMed] [Google Scholar]
- 10. Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J et al In vitro‐expanded antigen‐specific regulatory T cells suppress autoimmune diabetes. J Exp Med 2004; 199:1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, Bluestone JA. Expansion of functional endogenous antigen‐specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J Immunol 2005; 175:3053–9. [DOI] [PubMed] [Google Scholar]
- 12. Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU et al Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 2014; 14:571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collin M, Bigley V, Haniffa M, Hambleton S. Human dendritic cell deficiency: the missing ID? Nat Rev Immunol 2011; 11:575–83. [DOI] [PubMed] [Google Scholar]
- 14. Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007; 449:419–26. [DOI] [PubMed] [Google Scholar]
- 15. van der Kleij D, Latz E, Brouwers JF, Kruize YC, Schmitz M, Kurt‐Jones EA et al A novel host–parasite lipid cross‐talk. schistosomal lyso‐phosphatidylserine activates toll‐like receptor 2 and affects immune polarization. J Biol Chem 2002; 277:48122–9. [DOI] [PubMed] [Google Scholar]
- 16. Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin‐10‐treated human dendritic cells induce a melanoma‐antigen‐specific anergy in CD8+ T cells resulting in a failure to lyse tumor cells. Blood 1999; 93:1634–42. [PubMed] [Google Scholar]
- 17. Sato K, Yamashita N, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft‐versus‐host disease and leukemia relapse. Immunity 2003; 18:367–79. [DOI] [PubMed] [Google Scholar]
- 18. Lan YY, Wang Z, Raimondi G, Wu W, Colvin BL, de Creus A et al J Immunol 2006; 177:5868–77. [DOI] [PubMed] [Google Scholar]
- 19. Francisco LM, Sage PT, Sharpe AH. The PD‐1 pathway in tolerance and autoimmunity. Immunol Rev 2010; 236:219–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hammer GE, Ma A. Molecular control of steady‐state dendritic cell maturation and immune homeostasis. Annu Rev Immunol 2013; 31:743–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H et al Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med 2009; 206:2131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hilkens CM, Isaacs JD, Thomson AW. Development of dendritic cell‐based immunotherapy for autoimmunity. Int Rev Immunol 2010; 29:156–83. [DOI] [PubMed] [Google Scholar]
- 23. Pedersen AE, Gad M, Walter MR, Claesson MH. Induction of regulatory dendritic cells by dexamethasone and 1α,25‐dihydroxyvitamin D(3). Immunol Lett 2004; 91:63–9. [DOI] [PubMed] [Google Scholar]
- 24. Anderson AE, Sayers BL, Haniffa MA, Swan DJ, Diboll J, Wang XN et al Differential regulation of naive and memory CD4+ T cells by alternatively activated dendritic cells. J Leukoc Biol 2008; 84:124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson AE, Swan DJ, Sayers BL, Harry RA, Patterson AM, von Delwig A et al LPS activation is required for migratory activity and antigen presentation by tolerogenic dendritic cells. J Leukoc Biol 2009; 85:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harry RA, Anderson AE, Isaacs JD, Hilkens CM. Generation and characterisation of therapeutic tolerogenic dendritic cells for rheumatoid arthritis. Ann Rheum Dis 2010; 69:2042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stoop JN, Harry RA, von Delwig A, Isaacs JD, Robinson JH, Hilkens CM. Therapeutic effect of tolerogenic dendritic cells in established collagen‐induced arthritis is associated with a reduction in Th17 responses. Arthritis Rheum 2010; 62:3656–65. [DOI] [PubMed] [Google Scholar]
- 28. Hilkens CM, Isaacs JD. Tolerogenic dendritic cell therapy for rheumatoid arthritis: where are we now? Clin Exp Immunol 2013; 172:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mansilla MJ, Contreras‐Cardone R, Navarro‐Barriuso J, Cools N, Berneman Z, Ramo‐Tello C et al Cryopreserved vitamin D3‐tolerogenic dendritic cells pulsed with autoantigens as a potential therapy for multiple sclerosis patients. J Neuroinflammation 2016; 13:113. 016‐0584‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 2011; 34:2026–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomson AW, Robbins PD. Tolerogenic dendritic cells for autoimmune disease and transplantation. Ann Rheum Dis 2008; 67(Suppl 3):iii90–6. [DOI] [PubMed] [Google Scholar]
- 32. Boks MA, Kager‐Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A. IL‐10‐generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction – a comparative study of human clinical‐applicable DC. Clin Immunol 2012; 142:332–42. [DOI] [PubMed] [Google Scholar]
- 33. Lutz MB. Therapeutic potential of semi‐mature dendritic cells for tolerance induction. Front Immunol 2012; 3:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang L, Fu J, Sheng K, Li Y, Song S, Li P et al Bone marrow CD11b+F4/80+ dendritic cells ameliorate collagen‐induced arthritis through modulating the balance between treg and Th17. Int Immunopharmacol 2015; 25:96–105. [DOI] [PubMed] [Google Scholar]
- 35. Park JE, Jang J, Choi JH, Kang MS, Woo YJ, Seong YR et al DC‐based immunotherapy combined with low‐dose methotrexate effective in the treatment of advanced CIA in mice. J Immunol Res 2015; 2015:834085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li X, Han Y, Zhou Q, Jie H, He Y, Han J et al Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen‐induced arthritis. J Cell Mol Med 2016; 20:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. García‐González P, Morales R, Hoyos L, Maggi J, Campos J, Pesce B et al A short protocol using dexamethasone and monophosphoryl lipid A generates tolerogenic dendritic cells that display a potent migratory capacity to lymphoid chemokines. J Transl Med 2013; 11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lord P, Spiering R, Aguillon JC, Anderson AE, Appel S, Benitez‐Ribas D et al Minimum information about tolerogenic antigen‐presenting cells (MITAP): a first step towards reproducibility and standardisation of cellular therapies. PeerJ 2016; 4:e2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jauregui‐Amezaga A, Cabezón R, Ramírez‐Morros A, España C, Rimola J, Bru C et al Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory Crohn's disease: a phase I study. J Crohns Colitis 2015; 9:1071–8. [DOI] [PubMed] [Google Scholar]
- 40. Benham H, Nel HJ, Law SC, Mehdi AM, Street S, Ramnoruth N et al Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype‐positive rheumatoid arthritis patients. Sci Transl Med 2015; 7:290ra87. [DOI] [PubMed] [Google Scholar]
- 41. Bell GM, Anderson AE, Diboll J, Reece R, Eltherington O, Harry RA et al Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann Rheum Dis 2017; 76:227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Popov I, Li M, Zheng X, San H, Zhang X, Ichim TE et al Preventing autoimmune arthritis using antigen‐specific immature dendritic cells: a novel tolerogenic vaccine. Arthritis Res Ther 2006; 8:R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Duivenvoorde LM, Han WG, Bakker AM, Louis‐Plence P, Charbonnier LM, Apparailly F et al Immunomodulatory dendritic cells inhibit Th1 responses and arthritis via different mechanisms. J Immunol 2007; 179:1506–15. [DOI] [PubMed] [Google Scholar]
- 44. Raiotach‐Regue D, Grau‐Lopez L, Naranjo‐Gomez M, Ramo‐Tello C, Pujol‐Borrell R, Martinez‐Caceres E et al Stable antigen‐specific T‐cell hyporesponsiveness induced by tolerogenic dendritic cells from multiple sclerosis patients. Eur J Immunol 2012; 42:771–82. [DOI] [PubMed] [Google Scholar]
- 45. Mansilla MJ, Sellès‐Moreno C, Fàbregas‐Puig S, Amoedo J, Navarro‐Barriuso J, Teniente‐Serra A et al Beneficial effect of tolerogenic dendritic cells pulsed with MOG autoantigen in experimental autoimmune encephalomyelitis. CNS Neurosci Ther 2015; 21:222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Charbonnier LM, van Duivenvoorde LM, Apparailly F, Cantos C, Han WG, Noël D et al Immature dendritic cells suppress collagen‐induced arthritis by in vivo expansion of CD49b+ regulatory T cells. J Immunol 2006; 177:3806–13. [DOI] [PubMed] [Google Scholar]
- 47. Creusot RJ, Chang P, Healey DG, Tcherepanova IY, Nicolette CA, Fathman CG. A short pulse of IL‐4 delivered by DCs electroporated with modified mRNA can both prevent and treat autoimmune diabetes in NOD mice. Mol Ther 2010; 18:2112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maggi J, Schinnerling K, Pesce B, Hilkens CM, Catalan D, Aguillon JC. Dexamethasone and monophosphoryl lipid A‐modulated dendritic cells promote antigen‐specific tolerogenic properties on naive and memory CD4+ T cells. Front Immunol 2016; 7:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Di Caro V, Phillips B, Engman C, Harnaha J, Trucco M, Giannoukakis N. Retinoic acid‐producing, Ex‐vivo‐generated human tolerogenic dendritic cells induce the proliferation of immunosuppressive B lymphocytes. Clin Exp Immunol 2013; 174:302–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kaushansky N, Altmann DM, David CS, Lassmann H, Ben‐Nun A. DQB1*0602 rather than DRB1*1501 confers susceptibility to multiple sclerosis‐like disease induced by proteolipid protein (PLP). J Neuroinflammation 2012; 9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tsark EC, Wang W, Teng YC, Arkfeld D, Dodge GR, Kovats S. Differential MHC class II‐mediated presentation of rheumatoid arthritis autoantigens by human dendritic cells and macrophages. J Immunol 2002; 169:6625–33. [DOI] [PubMed] [Google Scholar]
- 52. Yoo SA, You S, Yoon HJ, Kim DH, Kim HS, Lee K et al A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J Exp Med 2012; 209:871–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kastbom A, Strandberg G, Lindroos A, Skogh T. Anti‐CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis 2004; 63:1085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Feitsma AL, van der Voort EI, Franken KL, el Bannoudi H, Elferink BG, Drijfhout JW et al Identification of citrullinated vimentin peptides as T cell epitopes in HLA‐DR4‐positive patients with rheumatoid arthritis. Arthritis Rheum 2010; 62:117–25. [DOI] [PubMed] [Google Scholar]
- 55. von Delwig A, Locke J, Robinson JH, Ng WF. Response of Th17 cells to a citrullinated arthritogenic aggrecan peptide in patients with rheumatoid arthritis. Arthritis Rheum 2010; 62:143–9. [DOI] [PubMed] [Google Scholar]
- 56. Snir O, Rieck M, Gebe JA, Yue BB, Rawlings CA, Nepom G et al Identification and functional characterization of T cells reactive to citrullinated vimentin in HLA‐DRB1*0401‐positive humanized mice and rheumatoid arthritis patients. Arthritis Rheum 2011; 63:2873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scally SW, Petersen J, Law SC, Dudek NL, Nel HJ, Loh KL et al A molecular basis for the association of the HLA‐DRB1 locus, citrullination, and rheumatoid arthritis. J Exp Med 2013; 210:2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chemin K, Pollastro S, James E, Ge C, Albrecht I, Herrath J et al A novel HLA‐DRB1*10:01‐restricted T cell epitope from citrullinated type II collagen relevant to rheumatoid arthritis. Arthritis Rheumatol 2016; 68:1124–35. [DOI] [PubMed] [Google Scholar]
- 59. James EA, Rieck M, Pieper J, Gebe JA, Yue BB, Tatum M et al Citrulline‐specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol 2014; 66:1712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Eden W, van Herwijnen M, Wagenaar J, van Kooten P, Broere F, van der Zee R. Stress proteins are used by the immune system for cognate interactions with anti‐inflammatory regulatory T cells. FEBS Lett 2013; 587:1951–8. [DOI] [PubMed] [Google Scholar]
- 61. Kolinski T, Marek‐Trzonkowska N, Trzonkowski P, Siebert J. Heat shock proteins (HSPs) in the homeostasis of regulatory T cells (Tregs). Cent Eur J Immunol 2016; 41:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wieten L, van der Zee R, Spiering R, Wagenaar‐Hilbers J, van Kooten P, Broere F et al A novel heat‐shock protein coinducer boosts stress protein Hsp70 to activate T cell regulation of inflammation in autoimmune arthritis. Arthritis Rheum 2010; 62:1026–35. [DOI] [PubMed] [Google Scholar]
- 63. Kaufmann SH. Heat shock proteins and the immune response. Immunol Today 1990; 11:129–36. [DOI] [PubMed] [Google Scholar]
- 64. Fink AL. Chaperone‐mediated protein folding. Physiol Rev 1999; 79:425–49. [DOI] [PubMed] [Google Scholar]
- 65. Matz JM, Blake MJ, Tatelman HM, Lavoi KP, Holbrook NJ. Characterization and regulation of cold‐induced heat shock protein expression in mouse brown adipose tissue. Am J Physiol 1995; 269:R38–47. [DOI] [PubMed] [Google Scholar]
- 66. Holoshitz J, Naparstek Y, Ben‐Nun A, Cohen IR. Lines of T lymphocytes induce or vaccinate against autoimmune arthritis. Science 1983; 219:56–8. [DOI] [PubMed] [Google Scholar]
- 67. van Eden W, Thole JE, van der Zee R, Noordzij A, van Embden JD, Hensen EJ et al Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature 1988; 331:171–3. [DOI] [PubMed] [Google Scholar]
- 68. Res PC, Schaar CG, Breedveld FC, van Eden W, van Embden JD, Cohen IR et al Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet 1988; 2:478–80. [DOI] [PubMed] [Google Scholar]
- 69. de Graeff‐Meeder ER, Voorhorst M, van Eden W, Schuurman HJ, Huber J, Barkley D et al Antibodies to the mycobacterial 65‐kd heat‐shock protein are reactive with synovial tissue of adjuvant arthritic rats and patients with rheumatoid arthritis and osteoarthritis. Am J Pathol 1990; 137:1013–7. [PMC free article] [PubMed] [Google Scholar]
- 70. Boog CJ, de Graeff‐Meeder ER, Lucassen MA, van der Zee R, Voorhorst‐Ogink MM, van Kooten PJ et al Two monoclonal antibodies generated against human hsp60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J Exp Med 1992; 175:1805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Albani S, Keystone EC, Nelson JL, Ollier WE, La Cava A, Montemayor AC et al Positive selection in autoimmunity: abnormal immune responses to a bacterial dnaJ antigenic determinant in patients with early rheumatoid arthritis. Nat Med 1995; 1:448–52. [DOI] [PubMed] [Google Scholar]
- 72. Kurzik‐Dumke U, Schick C, Rzepka R, Melchers I. Overexpression of human homologs of the bacterial DnaJ chaperone in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum 1999; 42:210–20. [DOI] [PubMed] [Google Scholar]
- 73. Schett G, Redlich K, Xu Q, Bizan P, Gröger M, Tohidast‐Akrad M et al Enhanced expression of heat shock protein 70 (hsp70) and heat shock factor 1 (HSF1) activation in rheumatoid arthritis synovial tissue. differential regulation of hsp70 expression and hsf1 activation in synovial fibroblasts by proinflammatory cytokines, shear stress, and antiinflammatory drugs. J Clin Invest 1998; 102:302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. van Eden W, van der Zee R, Prakken B. Heat‐shock proteins induce T‐cell regulation of chronic inflammation. Nat Rev Immunol 2005; 5:318–30. [DOI] [PubMed] [Google Scholar]
- 75. Wieten L, Berlo SE, Ten Brink CB, van Kooten PJ, Singh M, van der Zee R et al IL‐10 is critically involved in mycobacterial HSP70 induced suppression of proteoglycan‐induced arthritis. PLoS One 2009; 4:e4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Prakken BJ, Wendling U, van der Zee R, Rutten VP, Kuis W, van Eden W. Induction of IL‐10 and inhibition of experimental arthritis are specific features of microbial heat shock proteins that are absent for other evolutionarily conserved immunodominant proteins. J Immunol 2001; 167:4147–53. [DOI] [PubMed] [Google Scholar]
- 77. Prakken BJ, Roord S, van Kooten PJ, Wagenaar JP, van Eden W, Albani S et al Inhibition of adjuvant‐induced arthritis by interleukin‐10‐driven regulatory cells induced via nasal administration of a peptide analog of an arthritis‐related heat‐shock protein 60 T cell epitope. Arthritis Rheum 2002; 46:1937–46. [DOI] [PubMed] [Google Scholar]
- 78. Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL‐10‐producing T cells that cross‐react with the mammalian self‐hsp70 homologue. J Immunol 2000; 164:2711–7. [DOI] [PubMed] [Google Scholar]
- 79. Shinnick TM, Vodkin MH, Williams JC. The Mycobacterium tuberculosis 65‐kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun 1988; 56:446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol 2012; 12:157–67. [DOI] [PubMed] [Google Scholar]
- 81. Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Müller M et al Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci USA 2005; 102:7922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T et al Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 2005; 307:593–6. [DOI] [PubMed] [Google Scholar]
- 83. van Herwijnen MJ, Wieten L, van der Zee R, van Kooten PJ, Wagenaar‐Hilbers JP, Hoek A et al Regulatory T cells that recognize a ubiquitous stress‐inducible self‐antigen are long‐lived suppressors of autoimmune arthritis. Proc Natl Acad Sci USA 2012; 109:14134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. La Cava A, Nelson JL, Ollier WE, MacGregor A, Keystone EC, Thorne JC et al Genetic bias in immune responses to a cassette shared by different microorganisms in patients with rheumatoid arthritis. J Clin Invest 1997; 100:658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Prakken BJ, Samodal R, Le TD, Giannoni F, Yung GP, Scavulli J et al Epitope‐specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci USA 2004; 101:4228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Koffeman EC, Genovese M, Amox D, Keogh E, Santana E, Matteson EL et al Epitope‐specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double‐blind, placebo‐controlled, pilot phase II trial. Arthritis Rheum 2009; 60:3207–16. [DOI] [PubMed] [Google Scholar]
- 87. Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta‐cell function in new‐onset type 1 diabetes and immunomodulation with a heat‐shock protein peptide (DiaPep277): a randomised, double‐blind, phase II trial. Lancet 2001; 358:1749–53. [DOI] [PubMed] [Google Scholar]
- 88. Raz I, Avron A, Tamir M, Metzger M, Symer L, Eldor R et al Treatment of new‐onset type 1 diabetes with peptide DiaPep277 is safe and associated with preserved beta‐cell function: extension of a randomized, double‐blind, phase II trial. Diabetes Metab Res Rev 2007; 23:292–8. [DOI] [PubMed] [Google Scholar]
- 89. Kirkham B, Chaabo K, Hall C, Garrood T, Mant T, Allen E et al Safety and patient response as indicated by biomarker changes to binding immunoglobulin protein in the phase I/IIA RAGULA clinical trial in rheumatoid arthritis. Rheumatology (Oxford) 2016; 55:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kamphuis S, Kuis W, de Jager W, Teklenburg G, Massa M, Gordon G et al Tolerogenic immune responses to novel T‐cell epitopes from heat‐shock protein 60 in juvenile idiopathic arthritis. Lancet 2005; 366:50–6. [DOI] [PubMed] [Google Scholar]
- 91. de Jong H, Lafeber FF, de Jager W, Haverkamp MH, Kuis W, Bijlsma JW et al Pan‐DR‐binding Hsp60 self epitopes induce an interleukin‐10‐mediated immune response in rheumatoid arthritis. Arthritis Rheum 2009; 60:1966–76. [DOI] [PubMed] [Google Scholar]
- 92. de Wolf C, van der Zee R, den Braber I, Glant T, Maillère B, Favry E et al An arthritis‐suppressive and treg cell‐inducing CD4+ T cell epitope is functional in the context of HLA‐restricted T cell responses. Arthritis Rheumatol 2016; 68:639–47. [DOI] [PubMed] [Google Scholar]
- 93. Spiering R, van der Zee R, Wagenaar J, van Eden W, Broere F. Mycobacterial and mouse HSP70 have immuno‐modulatory effects on dendritic cells. Cell Stress Chaperones 2013; 18:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


