Abstract
Objectives
Fecal calprotectin (FC) is widely used to monitor the activity of inflammatory bowel disease (IBD) and to tailor medical treatment to disease activity. Laboratory testing of fecal samples may have a turnaround time of 1–2 weeks, whereas FC home testing allows results within hours and thus enables a rapid response to clinical deterioration.
Design and methods
Fifty-five stool samples were analyzed by the IBDoc® Calprotectin Home Testing kit and the BÜHLMANN fCAL® turbo assay on a Roche Cobas 6000 c501. The correlation between the assays was assessed using Spearman's Rho correlation coefficient and the intermediate imprecision of both assays was calculated.
Results
We found a strong correlation coefficient of 0.887 between FC measured on IBDoc® and the laboratory assay BÜHLMANN fCAL® turbo. The coefficients of variation (CVs) at three different FC levels were in the range 2.3–5.5% (BÜHLMANN fCAL® turbo) and in the range of 4.8–26.6% (IBDoc®).
Conclusions
This study suggests that IBDoc® is a suitable alternative for the assessment of disease activity in IBD patients.
Abbreviations: FC, Fecal calprotectin; IBD, Inflammatory bowel disease; CRP, C-reactive protein; UC, Ulcerative colitis; CD, Crohn's disease
Keywords: IBDoc, Inflammatory bowel disease, IBD, Calprotectin
1. Introduction
Ulcerative colitis (UC) and Crohn's disease (CD) are the two most prevalent forms of inflammatory bowel disease (IBD), with the highest prevalence and incidence in Europe and North America. UC and CD can in most cases be distinguished by differences in clinical presentation, endoscopic and histological appearance, risk factors and genetic predisposition [1]. Continuous monitoring of IBD is crucial to tailor treatment to active disease, maintain remission and reduce the risk of relapse. Recent research has shown that apart from steroid-free remission and symptom control alone, achieving endoscopic and maybe even histological mucosal healing has a major impact on disease control and progression. Achieving mucosal healing minimizes hospital admissions and surgery, lowers the risk for relapse and intensification of medical therapy and improves quality of life [2]. Endoscopic procedures are unpleasant for the patient, time-consuming and bear the risk of intestinal perforation. Thus noninvasive monitoring of disease activity is preferable, though no ideal biomarker has been identified. FC is more sensitive than serum C-reactive protein (CRP) in reflecting disease activity in IBD [3], and can be used to identify patients at risk of relapse and predict both endoscopic and histological mucosal healing [4].
Calprotectin is a heterodimer of the two calcium-binding proteins, S100A8 and S100A9, and is elevated in serum in several inflammatory conditions [5]. Calprotectin is primarily released by activated neutrophils at sites of inflammation, and when measured in feces serves as an indirect estimate of the neutrophil infiltrate in the gastrointestinal tract [6]. Laboratory testing of fecal samples may require transportation from outpatients to the laboratory, and may have a turnaround time of 1–2 weeks, which hampers fast medical treatment response to relapses and increase the risk of clinical deterioration before treatment commences. In the capital region of Denmark fecal samples from outpatients are transported by the normal postal service, and at Copenhagen University Hospital Hvidovre 16% of the fecal samples received are either discarded because of prolonged transport (> 5 days) or results are released from the laboratory with caution, which decreases the clinical utility of the lab results and is costly. Home testing of FC achieves a faster turnaround time (hours) and enables patients and clinicians to act on FC results without delay. The aim of this study was to investigate the correlation between FC measured by the BÜHLMANN fCAL® turbo assay on a Roche Cobas 6000 c501, and the BÜHLMANN home test kit, IBDoc® using the CalApp® for data transfer.
2. Material and methods
2.1. Sample collection, preparation and storage
All stool samples were collected in plastic tubes by the patients and sent in by ordinary mail. The samples were immediately frozen at −20 °C upon receipt and analyzed within 1–2 days. A total of fifty-five stool samples were analyzed for direct comparison of IBDoc® and the BÜHLMANN fCAL® turbo methods. The selection of samples was based on volume (visual evaluation to ensure enough sampling material for testing) and stool consistency (hard lumps were excluded). Stool samples older than 5 days were excluded. Samples with FC concentrations > 1000 µg/g or < 30 µg/g (BÜHLMANN fCAL® turbo) were excluded, as the measuring range on IBDoc® is limited to 30–1000 µg/g. Three fecal samples with different FC levels were analyzed 10 times to determine the intermediate imprecision (coefficient of variation [CV], %) of the assays. The levels analyzed were low (FC < 50 µg/g), medium (50 µg/g > FC > 200 µg/g,) and high (FC > 200 µg/g). respectively, as it is well established that the normal range for FC is considered to be < 50 µg/g [7] and clinical studies have proposed that FC concentrations in the range 150–250 µg/g can be used to distinguish between active disease and mucosal healing [7], [8]. Clinical data of the patients whose samples were studied were not collected.
2.2. Biochemical measurements
FC was measured using the immunoturbidimetric method BÜHLMANN fCAL® turbo with the CALEX® Cap extraction device from BÜHLMANN Laboratories AG (Schönenbuch, Switzerland) on a Cobas 6000 c501 analyzer (Roche Diagnostics A/S, Mannheim, Germany), according to the instructions of the manufacturer. The assay is standardized against the BÜHLMANN fCAL® ELISA by the manufacturer and the measuring range is 20–8000 µg/g. The intermediate imprecision (CV %) was 7% (at level 74.79 µg/g) and 1.4% (at level 252.14 µg/g). The assay is subject to external quality control through participation in the EQUALIS external quality control scheme (EQUALIS, Uppsala, Sweden), with a deviation from the method mean (own output group) of 5.5% (mean value own group 316 µg/g) and −1.5% (mean value own group 1138 µg/g) respectively in the latest (June 2017) EQA distribution.
FC was measured in parallel using the IBDoc® Calprotectin Home Testing kit from BÜHLMANN Laboratories AG according to the instructions of the manufacturer. IBDoc® is a lateral flow-based calprotectin test and the kit consists of a stool extraction device, the CALEX® Valve, and a test cassette. An iPhone 5c was used to install the CalApp®, enabling the phone to read the test cassette and calculate a quantitative calprotectin concentration. IBDoc® is not subject to external quality control.
2.3. Statistics
The correlation between FC (BÜHLMANN fCAL® turbo) and FC (IBDoc®) was assessed by using Spearman's Rho correlation coefficient. The intermediate imprecision of both assays was calculated.
Statistical analyses and graphics were performed using IBM SPSS Statistics version 22 (IBM, Armonk, NY, USA).
3. Results
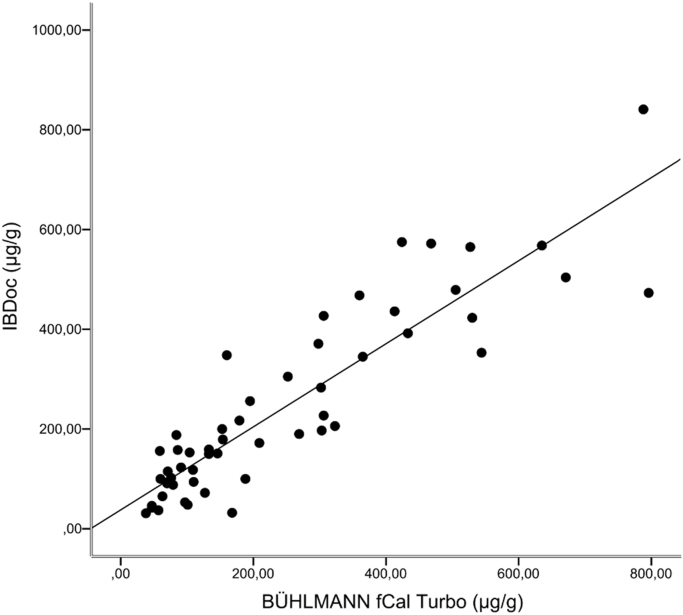
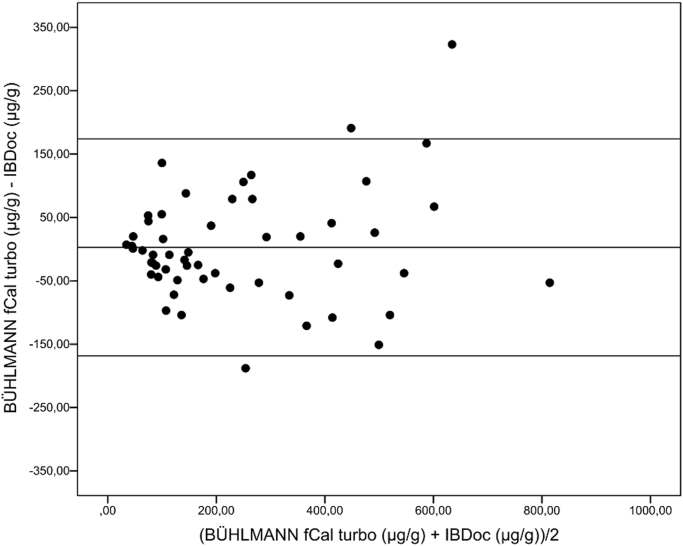
Fifty-five samples were included in this study, covering clinically relevant concentrations in the range 38 –796 µg/g (BÜHLMANN fCal ® turbo). Fig. 1 shows the correlation between FC (BÜHLMANN fCAL® turbo) and FC (IBDoc®), with a Spearman rank correlation coefficient of 0.887. Over FC concentrations close to clinically relevant cut off values (range 30–300 µg/g), the Spearman rank correlation coefficient was 0.689 (n = 36, minimum = 38 µg/g, maximum: 298 µg/g). The Bland-Altman plot (Fig. 2) shows no fixed bias.
Fig. 1.
The relationship between FC readings on BÜHLMANN fCal® turbo and IBDoc® (n = 55), rs = 0.887. y = 0.8329x + 37.758.
Fig. 2.
Bland-Altman plot showing the difference between IBDoc® and BÜHLMANN fCal® turbo in relation to the average values of the assays. Lines shown are linear regression and 95% prediction intervals (n = 55).
Ten extractions of three fecal samples with low (< 50 µg/g), medium (50–200 µg/g) and high (> 200 µg/g) FC concentrations were performed in each assay, showing an intermediate imprecision in the range of 2.3–5.5% (BÜHLMANN fCAL® turbo) and in the range of 4.8–26.6% (IBDoc®) as shown in Table 1. As the measuring range on IBDoc® is limited to 30–1000 µg/g, the intermediate imprecision of IBDoc® (L) is only calculated on four test results, as the other six was < 30 µg/g and thus excluded. The difference in the sample means may suggest bias, but the Bland Altman plot shows no fixed bias. The difference in the sample means probably reflects heterogeneity in the specimens.
Table 1.
Intermediate imprecision of BÜHLMANN Fcal turbo and IBDoc®. Three fecal samples with different FC levels were analyzed n times for each assay. L: FC < 50 µg/g, M: 50 µg/g > FC > 200 µg/g, H: FC > 200 µg/g.
| n | Mean FC conc (µg/g) | SD (µg/g) | CV (%) | |
|---|---|---|---|---|
| BÜHLMANN fCal® turbo (L) | 10 | 52.7 | 2.9 | 5.5 |
| BÜHLMANN fCal® turbo (M) | 10 | 105.1 | 6.3 | 6.0 |
| BÜHLMANN fCal® turbo (H) | 10 | 1057 | 24.2 | 2.3 |
| IBDoc (L) | 4 | 31.3 | 1.5 | 4.8 |
| IBDoc (M) | 10 | 125.1 | 19.1 | 15.3 |
| IBDoc (H) | 10 | 668.4 | 177.8 | 26.6 |
4. Discussion
To the best of our knowledge, this is the first study comparing the performance of the IBDoc® with immunoturbidimetry. In a recent study by Heida et al. a similar correlation coefficient of 0.85 was found when comparing IBDoc® and ELISA readings (BÜHLMANN fCAL ELISA, BÜHLMANN Laboratories AG), and in a study by Bello et al. a correlation coefficient of 0.88 was found comparing IBDoc® and ELISA readings (BÜHLMANN fCAL® ELISA, BÜHLMANN Laboratories) [9], [10]. The intermediate imprecision of IBDoc® was substantially higher than the intermediate imprecision of BÜHLMANN fCal® turbo in our study, especially at FC concentrations greater than 200 µg/g. Lasson et al. found a median intra-individual variation of 40.8% in fecal samples collected on two consecutive days – most pronounced in patients with high levels of FC [11]. Thus considering the finding of a significant intra-individual day-to-day (and even within-day) variability of FC in IBD patients, the CV% in IBDoc® seems less concerning [11], [12]. Discriminating between active disease and mucosal healing is probably the most clinical relevant issue regarding the use of FC. Different levels of cut-off values to distinguish between active disease and mucosal healing have been proposed, most often in the range of 150–250 µg/g [7], [8]. A high intermediate imprecision at the cut-off range is of greatest concern as it increases the risk of false negatives. In this study we found the highest imprecision of 26.6% at samples with FC > 200 µg/g (mean 668.4 µg/g) which we do not view as clinically relevant. The EQUALIS external quality control scheme for FC on BÜHLMANN fCal® turbo showed a deviation from our output group of 5.5% (mean value 316 µg/g) and −1.5% (mean value 1138 µg/g) respectively. From all output groups the deviation was 37.1% and 21.6% respectively. In general there is poor standardization between FC assays, making it difficult to define a uniform threshold to discriminate between active disease and remission [13].
In this study the tests was performed by skilled laboratory technicians, but in a clinical setting, the test is performed by patients themselves. The results obtained with the CalApp® are automatically transferred to the IBDoc portal and the patient's doctor is notified, thus allowing rapid response to test results. Heida et al. investigated the self-reported usability of the IBDoc®, with 87% of the patient reporting that the test was not difficult to perform and 97% of the patients interested in using the home test in the future. A limitation to this finding though, was that the study participants were a priori interested in home testing [10]. Bello et al. found that female sex and less severe disease was associated with higher usability of the IBDoc®, and that younger people especially thought they would use the tool in the future [9]. Elkjaer et al. found that a web-guided “constant care” approach for UC patients – a web based program where patients are educated in UC, in recognizing relapse and in starting correct treatment guided by the e-health program and a web-doctor – increases patient's compliance, quality of life, empowerment to self-initiated treatment and reduces healthcare costs [14].
5. Conclusions
This study suggests that IBDoc® is a suitable alternative for the assessment of disease activity in IBD patients. Point of care testing would reduce the turnaround time significantly and potentially improve the quality of treatment by enabling rapid responses to relapses. In addition, home testing may be the only viable alternative in situations where visits to outpatient clinics are difficult or mailing is precluded because of the poor stability of FC.
Conflicts of interest
None.
Funding sources
This study was supported by BÜHLMANN Laboratories AG. BÜHLMANN did not play a role in the study design, execution, analysis or interpretation of the data.
Author contributions
Julie Løye Hejl: writing the manuscript, clinical supervision.
Klaus Theede: critical revision of the manuscript for important intellectual content.
Brian Møllgren: study concept and design, technical support.
Kirsten Vikkelsø Madsen: study concept and design.
Ashraf Heidari: study concept and design, acquisition of data.
Anna á Steig: study concept and design, acquisition of data.
Mogens Fenger: study concept and design, clinical supervision.
All authors have read and approved the manuscript.
Contributor Information
Julie Hejl, Email: jhej0006@regionh.dk.
Klaus Theede, Email: Klaus.Theede@regionh.dk.
Brian Møllgren, Email: brian.soeren.moellgren@regionh.dk.
Kirsten Vikkelsø Madsen, Email: kima@phmetropol.dk.
Ashraf Heidari, Email: ashi_ssa@yahoo.com.
Anna á Steig, Email: annasteig91@gmail.com.
Mogens Fenger, Email: Mogens.Fenger@regionh.dk.
References
- 1.Ordás I., Eckmann L., Talamini M., Baumgart D.C., Sandborn W.J. Ulcerative colitis. Lancet. 2012;380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 2.Neurath M.F., Travis S.P.L. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–1635. doi: 10.1136/gutjnl-2012-302830. [DOI] [PubMed] [Google Scholar]
- 3.Mosli M.H., Zou G., Garg S.K., Feagan S.G., MacDonald J.K., Chande N. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am. J. Gastroenterol. 2015;110:802–819. doi: 10.1038/ajg.2015.120. [DOI] [PubMed] [Google Scholar]
- 4.Theede K., Holck S., Ibsen P., Kallemose T., Nordgaard-Lassen I., Nielsen A.M. Fecal calprotectin predicts relapse and histological mucosal healing in ulcerative colitis. Inflamm. Bowel Dis. 2016;22:1042–1048. doi: 10.1097/MIB.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 5.Pruenster M., Vogl T., Roth J., Sperandio M. S100A8/A9: from basic science to clinical application. Pharmacol. Ther. 2016;167:120–131. doi: 10.1016/j.pharmthera.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Barnes E.L., Burakoff R. New biomarkers for diagnosing inflammatory bowel disease and assessing treatment outcomes. Inflamm. Bowel Dis. 2016;22:2956–2965. doi: 10.1097/MIB.0000000000000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Angelo F., Felley C., Frossard J.L. Calprotectin in daily practice: where do we stand in 2017. Digestion. 2017:293–301. doi: 10.1159/000476062. [DOI] [PubMed] [Google Scholar]
- 8.Theede K., Holck S., Ibsen P., Ladelund S., Nordgaard-Lassen I., Nielsen A.M. Level of fecal calprotectin correlates with endoscopic and histologic inflammation and identifies patients with mucosal healing in ulcerative colitis. Clin. Gastroenterol. Hepatol. 2015;13:1929–1936.e1. doi: 10.1016/j.cgh.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 9.Bello C., Roseth A., Guardiola J., Reenaers C., Ruiz-Cerulla A., Kemseke C. Van. Usability of a home-based test for the measurement of fecal calprotectin in asymptomatic IBD patients. Dig. Liver Dis. 2017 doi: 10.1016/j.dld.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Heida A., Knol M., Kobold A.M., Bootsman J., Dijkstra G., van Rheenen P.F. Agreement between home-based measurement of stool calprotectin and ELISA results for monitoring inflammatory bowel disease activity. Clin. Gastroenterol. Hepatol. 2017 doi: 10.1016/j.cgh.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Lasson A., Stotzer P.-O.-O., Ohman L., Isaksson S., Sapnara M., Strid H., Öhman L. The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J. Crohn's. Colitis. 2014;9:26–32. doi: 10.1016/j.crohns.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Moum B., Jahnsen J., Bernklev T. Fecal calprotectin variability in Crohn's disease. Inflamm. Bowel Dis. 2010;16:1091–1092. doi: 10.1002/ibd.21136. [DOI] [PubMed] [Google Scholar]
- 13.Amcoff K., Stridsberg M., Lampinen M., Magnuson A., Carlson M., Halfvarson J. Clinical implications of assay specific differences in f-calprotectin when monitoring inflammatory bowel disease activity over time. Scand. J. Gastroenterol. 2017;52:344–350. doi: 10.1080/00365521.2016.1256424. [DOI] [PubMed] [Google Scholar]
- 14.Elkjaer M., Shuhaibar M., Burisch J., Bailey Y., Scherfig H., Laugesen B. E-health empowers patients with ulcerative colitis: a randomised controlled trial of the web-guided “Constant-care” approach. Gut. 2010;59:1652–1661. doi: 10.1136/gut.2010.220160. [DOI] [PubMed] [Google Scholar]