Abstract
Objectives: To evaluate the efficacy of tegafur–uracil (UFT), a prodrug of 5-fluorouracil, plus cisplatin and dexamethasone in patients with docetaxel-refractory prostate cancers.
Methods: Twenty-five patients with docetaxel-refractory prostate cancer were administered oral UFT plus intravenous cisplatin (UFT-P therapy) and dexamethasone. Treatment responses were assessed monthly via prostate-specific antigen (PSA) level measurements. Treatment-related adverse events and overall survival were also assessed.
Results: UFT-P therapy resulted in decreased PSA levels in 14 (56%) patients and increased PSA levels in 11 (44%). In patients with increased PSA levels, 7 (64%) of the 11 patients displayed decreased PSA doubling times. The UFT-P therapy response rate was 84% (21/25 patients). Imaging studies revealed that tumor shrinkage during UFT-P therapy occurred in 1 patient in whom bilateral hydronephrosis caused by lymph node metastasis improved. The median survival time from docetaxel initiation was 36 months. In UFT-P-treated patients, the median PSA progression and overall survival times were 6 and 14 months, respectively. UFT-P treatment-related adverse events were mild diarrhea, general fatigue, and anorexia. Treatment was not discontinued for any of the patients. UFT-P therapy did not cause serious hepatic or renal dysfunction or pancytopenia.
Conclusions: UFT-P therapy is a safe and effective treatment for patients with docetaxel-refractory prostate cancer, although large-scale, multicenter, prospective studies are needed to validate these findings.
Keywords: prostate cancer, chemotherapy, tegafur–uracil (UFT), cisplatin, dexamethason
Introduction
In Japan, prostate cancer is a common malignant tumor, and the global prevalence of prostate cancer is increasing. Forecasts for 2020, predict a 2.8-fold rise in prostate cancer mortality rates, compared with those in 20001). The mainstay treatments for prostate cancer include surgery or radiotherapy. However, patients with advanced metastatic cancer, or those with metastases or recurrence after surgery or radiotherapy, often undergo androgen deprivation therapy (ADT). ADT is effective in such patients, and its use has increased worldwide2, 3). However, patients undergoing ADT eventually develop castration-resistant prostate cancer (CRPC)4). The efficacy of docetaxel-based systemic chemotherapy for CRPC was recently reported5, 6). However, nearly all patients with CRPC develop docetaxel-refractory prostate cancer, characterized by increasing serum PSA levels. Reports on drug therapies for such patients are scarce, and effective treatment protocols have not been established.
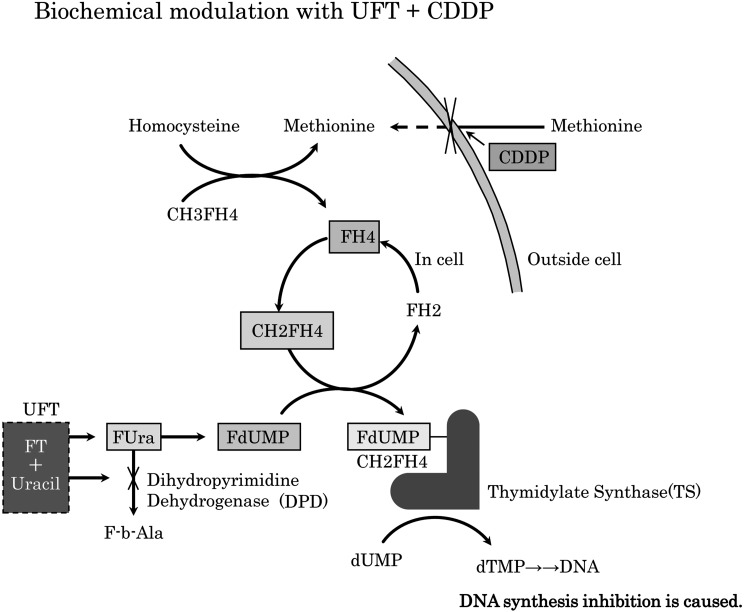
Tegafur–uracil (UFT) is a prodrug combination, in which tegafur is converted to cytotoxic 5-fluorouracil (5-FU) in vivo. In this combination, uracil potentiates the antitumor activity of tegafur7, 8) by enhancing the bioavailability of 5-FU via competitive inhibition of dihydropyrimidine dehydrogenase-mediated catabolism of 5-FU. 5-FU is phosphorylated in a stepwise manner and converted into 5-fluoro-2’-deoxyuridine 5’-monophosphate which forms a ternary complex with the folate derivative 5,10-methylene-tetrahydrofolate and thymidylate synthase, leading to defective DNA synthesis and repair9). This mechanism underlies the antitumor activity of UFT10,11,12,13). Conversely, cisplatin has direct cytotoxic activity, but also indirect activity via inhibition of cellular methionine influx14). In methionine-deficient cells, methyltetrahydrofolic acid converts homocysteine to methionine, liberating tetrahydrofolic acid, which is converted into activated 5,10-methylene-tetrahydrofolate that contributes to the ternary complex described above9). This mechanism is presented in Figure 1.
Figure 1.
When uptake of methionine into the cell is obstructed by cisplatin, methionine synthetase is induced and folic acid metabolism is accelerated. Consequently, the number of deoxidizing folic acids (FH4, CH2FH4) increases, the production life of the ternary complex (FdUMP+CH2FH4+TS) increases, and the cytotoxicity reaction of UFT (DNA synthesis inhibition) is reinforced. FH4; tetrahydrofolate, CH2FH4; methylenetetrahydrofolate, TS; thymidylate synthase, FdUMP; fluorodeoxyuridine monophosphate, UFT; tegafur–uracil.
Several studies have demonstrated the synergistic antitumor activity of cisplatin plus 5-FU15, 16), with particularly favorable efficacy in gastrointestinal and head and neck cancers17, 18). Recently, the efficacy of UFT plus cisplatin (UFT-P therapy) for CRPC has been reported19). The present study evaluated the safety and effectiveness of systemic UFT-P therapy for the treatment of patients with docetaxel-refractory prostate cancer.
Methods
Patients
Twenty-five patients with docetaxel-refractory prostate cancer who were treated at Nagoya City West Medical Center Johoku Hospital, Nagoya City East Medical Center Higashi City Hospital, and The Aichi Prefectural Federation of Agricultural Cooperatives for Health and Welfare Konan and Kainan Hospitals, were enrolled. These were all castrated patients who had a histologic diagnosis of prostate adenocarcinoma and an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–3. All patients underwent a needle biopsy of the prostate before deciding upon the initial treatment. Patients in whom serum PSA levels increased after ADT were confirmed as not developing antiandrogen withdrawal syndrome (AWS). Patients without AWS underwent combined drug therapy (ethinylestradiol, steroids, and estramustine phosphate sodium). In this study, all these drugs were administered before docetaxel treatment. Docetaxel was administered at 75 mg/m2 every 3 weeks (n = 8 patients) or 40 mg/patient every 2 weeks (n = 17 patients). Low-dose dexamethasone (1 mg/day) was administered in combination with docetaxel. Patients with PSA level increases over ≥ 3 consecutive measurements, despite undergoing systemic chemotherapy with docetaxel, were defined as having docetaxel-refractory prostate cancer.
Study design
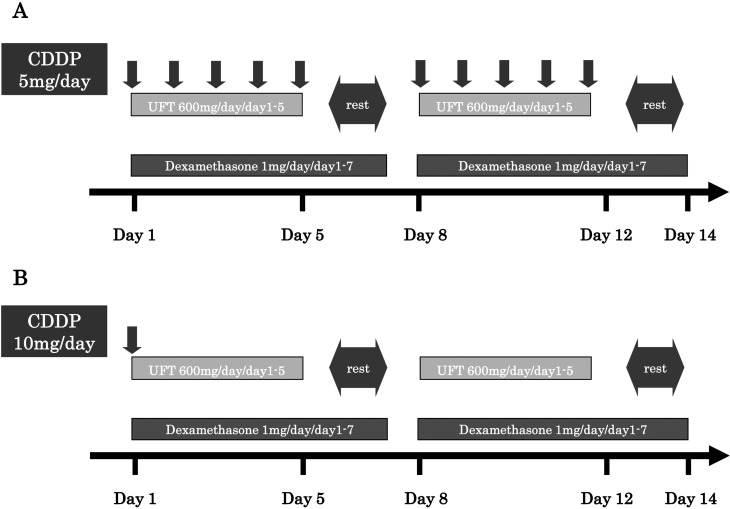
Hospitalization was required for the initiation of systemic chemotherapy. A route for peripheral intravenous access was secured to facilitate drug infusion. Cisplatin (5 mg) was dissolved in physiological saline solution (500 mL) and infused intravenously for 2 hours. Oral UFT (200 mg) was administered 3 times daily with food (600 mg/day). Cisplatin and UFT were administered in cycles of 5 days per week for 3 weeks, followed by a 1-week rest period. Oral dexamethasone (1 mg/day) was continued after docetaxel therapy. After one chemotherapy cycle, serum PSA levels were measured to assess treatment response. Patients with PSA level decreases were switched to maintenance therapy, administered on an outpatient basis, consisting of oral UFT (600 mg/day) administered 5 days/week, intravenous cisplatin (10 mg/day), administered every 2 weeks, and oral dexamethasone (1 mg/day) administered on consecutive days (Figure 2). Thereafter, treatment responses according to PSA measurements were evaluated monthly.
Figure 2.
A: Induction therapy schedule. Intravenous cisplatin (5 mg/day) was administered 5 times a week, oral UFT (600 mg/day) was administered 5 times a week, and oral dexamethasone (1 mg/day) was administered on consecutive days. This regimen was delivered 4 times as 1 treatment cycle. B: Maintenance therapy schedule. Intravenous cisplatin (10 mg/day) was administered once every 2 weeks, oral UFT (600 mg/day) was administered 5 times a week, and oral dexamethasone (1 mg/day) was administered on consecutive days. This regimen was delivered twice as 1 treatment cycle. Treatment was continued unless serious adverse events occurred.
If in-hospital treatment was not possible, patients underwent maintenance therapy on an outpatient basis, without induction therapy. In patients with increased PSA levels, PSA doubling times (PSADTs) were calculated for the periods of 3 months prior to UFT-P therapy initiation (during treatment with docetaxel) and 3 months after UFT-P therapy initiation. Progression-free survival was defined as the period until PSA levels rose by ≥ 50%. Overall survival was defined as the duration from the date of UFT-P initiation to the date of death from any cause. In addition, median survival times from the dates of docetaxel and UFT-P therapy initiation were evaluated. All adverse events were graded according to the National Cancer Institute Common Terminology Criteria for adverse events (version 3.0).
Statistical analyses
This study was approved by the Institutional Ethics Review Committees of all participating centers. For statistical analysis, overall survival was analyzed using JMP® software version 8 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Twenty-five patients were enrolled between January 2008 and June 2012. The patient demographic and clinical characteristics are presented in Table 1. Tumor staging revealed bone metastases in 20 patients and lymph node metastases in 7 patients.
Table 1. Demographic characteristics of patients.
| UFT-P therapy (n = 25) | ||
|---|---|---|
| Mean age (years) (Range) | 74.2 (66–88) | |
| Mean initial PSA (ng/ml) (Range) | 935.1 (1.84–9700) | |
| Gleason | ||
| 6 | 1 | |
| 7 | 5 | |
| 8 | 6 | |
| 9 | 11 | |
| 10 | 2 | |
| PS | ||
| 0 | 8 | |
| 1 | 8 | |
| 2 | 4 | |
| 3 | 5 | |
| Total prostatectomy | ||
| Yes | 2 | |
| No | 23 | |
| Radiotherapy | ||
| Yes | 8 | |
| No | 17 | |
| Metastatic sites at presentation | ||
| Bone | 20 | |
| Lymph node | 7 | |
| Lung | 2 | |
Table 2 presents the factors representative of the response to UFT-P therapy.
Table 2. Factors representative of response to UFT-P therapy.
| UFT-P therapyTotal (n = 25) | |
|---|---|
| Duration of response to ADT* (median) | 23 |
| (Range) | (6–83) |
| Minimum PSA value after start of ADT (median) | 5.8 |
| (Range) | (0.01–45) |
| Interval required for PSA to reach minimum value after start of ADT (median) | 12.9 |
| (Range) | (3–35) |
| Duration of treatment with docetaxel (median) | 16.6 |
| (Range) | (1–42) |
| Minimum PSA value after start of docetaxel (median) | 28.5 |
| (Range) | (0.01–145.8) |
| Interval required for PSA to reach minimum value after start of docetaxel (median) | 5.9 |
| (Range) | (1–21) |
| Total dose of docetaxel (median) | 1222 |
| (Range) | (80–4700) |
*UFT-P denotes UFT and cisplatin; ADT denotes androgen deprivation therapy; PSA denotes prostate-specific antigen.
Antitumor effect of UFT-P in patients with docetaxel-refractory prostate cancer
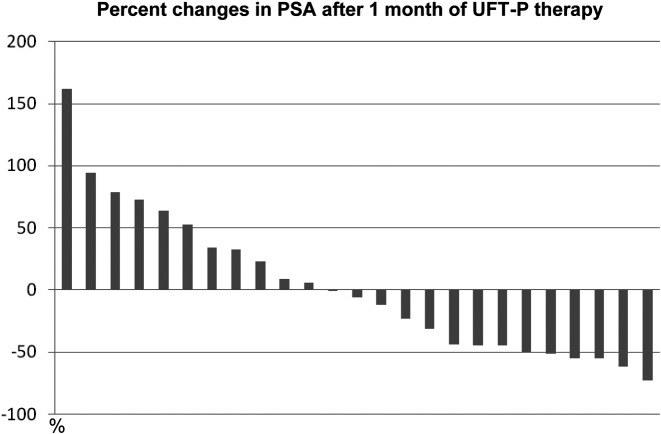
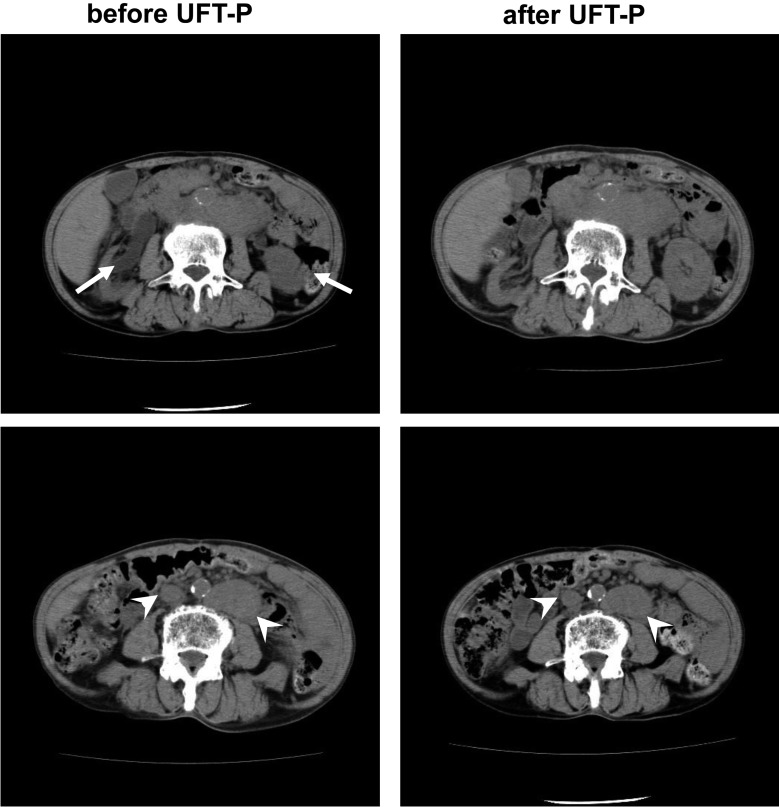
UFT-P therapy caused a decrease in PSA levels in 14 (56%) patients and an increase in PSA levels in 11 (44%; Figure 3). In those with PSA level increases, 7 of 11 patients displayed decreased PSADTs. The response rate was 84% (21/25 patients). However, upon imaging studies, UFT-P therapy was associated with a reduction in tumor volume in only one patient. In that patient, bilateral hydronephrosis due to lymph node metastasis was present before UFT-P therapy, but improved after UFT-P therapy in association with shrinkage of metastatic lymph nodes (Figure 4).
Figure 3.
Each bar indicates a patient who received UFT-P therapy. The percent change in PSA levels was calculated using the following formula: ([PSA levels after 1 month of UFT-P therapy] – [PSA levels before UFT-P therapy])/PSA levels before UFT-P therapy.
Figure 4.
Computed tomography scans, indicating that bilateral hydronephrosis resolved in association with shrinkage of lymph node metastases after UFT-P therapy. The arrows indicate hydronephrosis and the arrowheads indicate lymph node metastasis.
Time to PSA progression and overall survival
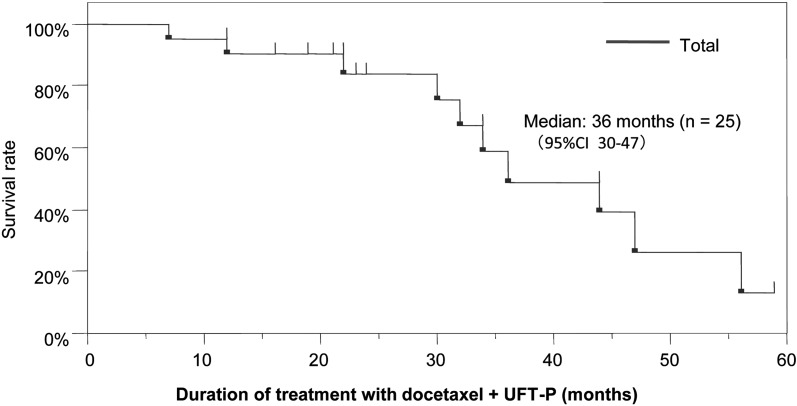
For those that underwent UFT-P therapy, the time to PSA progression was 6 months and the overall survival time was 14 months. The overall survival time following docetaxel plus UFT-P was 36 months (Figure 5).
Figure 5.
Overall survival after treatment with docetaxel plus UFT-P therapy.
Treatment-related adverse events
Overall, UFT-P therapy was well-tolerated. Adverse events included grade 1 diarrhea (20%, 5/25), anorexia (44%, 11/25), and general fatigue (100%, 25/25). There were no incidences of pancytopenia or hepatic or renal dysfunctions. No patients discontinued treatment (Table 3).
Table 3. Adverse events.
| UFT-P therapy responders (n = 21) | UFT-P therapy non-responders (n = 4) | UFT-P therapy total (n = 25) | ||
|---|---|---|---|---|
| Patients who discontinued treatment | 0 | 0 | 0 | |
| Diarrhea | ||||
| Grade 1 | 3 | 2 | 5 | |
| Anorexia | ||||
| Grade 1 | 9 | 2 | 11 | |
| Pancytopenia | 0 | 0 | 0 | |
| Liver/renal dysfunction | 0 | 0 | 0 | |
| General fatigue | ||||
| Grade 1 | 21 | 4 | 25 | |
Discussion
In the present study, UFT-P therapy demonstrated substantial antitumor activity in patients with CRPC who had been administered docetaxel chemotherapy previously. In addition, treatment-related adverse events were mild and treatment discontinuation was not necessary in any patient. These findings suggest that UFT-P might be a safe and effective treatment for patients with docetaxel-refractory CRPC.
Previous studies in patients with docetaxel-refractory CRPC have indicated that cabazitaxel, MDV3100, and abiraterone were effective treatments for CRPC20,21,22). Cabazitaxel is a tubulin-binding taxane that has demonstrated antitumor activity in docetaxel-refractory cell lines23, 24). The TROPIC study demonstrated that cabazitaxel plus prednisone improved overall survival compared with mitoxantrone plus prednisone in patients with docetaxel-refractory CRPC. In the cabazitaxel group, the median overall survival time was 15.1 months and the median time to disease progression was 2.8 months20). Cabazitaxel indicated similar efficacy, in terms of survival, to UFT-P in the present study; however, the time to progression was superior in the present study (> 6 months).
MDV3100 targets multiple steps in the androgen receptor-signaling pathway. It has greater affinity for the androgen receptor, and inhibits nuclear translocation of the androgen receptor, DNA binding, and coactivator recruitment21, 25). The AFFIRM study revealed that patients with metastatic CRPC treated with MDV3100 had a median overall survival time of 18.4 months, and the median time to PSA progression was 8.3 months21). These values are similar, although slightly superior, to those presented in the present study for UFT-P therapy.
Abiraterone is a selective inhibitor of androgen biosynthesis that potently blocks cytochrome P450 c17, a critical enzyme in testosterone synthesis, thereby blocking androgen synthesis by the adrenal glands and testes and within the prostate tumor26). In a previous study, in patients with docetaxel-refractory CRPC treated with abiraterone, the median overall survival time was 15.8 months, and the median time to PSA progression was 8.5 months22). These results indicate a similar overall survival time to the present study, although the time to progression is superior. However, the definition of docetaxel-resistance in the previous study was unclear, making it difficult to compare these studies.
Overall, it appears that treatment methods for docetaxel-refractory CRPC could be divided into three drug classes: immunotherapeutics (sipuleucel-T), endocrinotherapy (MDV3100 and abiraterone), and systemic chemotherapy (cabazitaxel and UFT-P). Generally, endocrinotherapy results in few treatment-related adverse events27). Therefore, it might be assumed that it would be the first choice of treatment for docetaxel-refractory CRPC. However, some patients do not respond to or are ineligible for endocrinotherapy. Therefore, systemic chemotherapy might be the most appropriate for such patients.
In the present study, systemic chemotherapy with UFT-P was administered using the principles of metronomic chemotherapy. Metronomic therapy refers to the therapeutic concept of long-term, continuous exposure to anticancer drugs, with short rest periods, resembling the rhythm of a metronome28). High doses of anticancer drugs cannot be administered continuously on a long-term basis because of unwanted toxicity; therefore, low doses with relatively low toxicity are employed.
It was reported that the side effects of cabazitaxel occured at high frequency, although the characteristics of the anti-cancer drug were similar to docetaxel. In the TROPIC study that was conducted across 26 countries (excluding Japan), in patients with docetaxel-refractory prostate cancer, cabazitaxel was associated with a PSA response rate of 39.2% and a prolonged overall survival of 15.1 months20). Based on these results, cabazitaxel is now widely used in Europe and the United States. In the TROPIC study, the most common nonhematological toxicities were diarrhea, fatigue, back pain, and nausea. No grade ≥ 3 toxicities were reported; however, hematologic toxicities of neutropenia, leukopenia, anemia, and thrombocytopenia were observed, resulting in the need for growth factors administration29). Although the overall survival time in the present study was similar to that in the TROPIC study, the PSA response rate observed here was far superior (84%). In addition, in the present study, UFT-P therapy administered in a metronomic fashion was associated only with grade 1 diarrhea, anorexia, and fatigue, and additional treatments or treatment discontinuations were not necessary, suggesting the superior safety of UFT-P compared with cabazitaxel. Of note, unlike cabazitaxel, UFT-P therapy did not cause hematological toxicities.
Furthermore, in the present study, UFT-P therapy was based on the idea of biochemical modulation. Previous studies in patients with prostate cancer have reported response rates of UFT alone or cisplatin alone as 18.2% and 19%, respectively10, 30). In the present study, strong anticancer effects were noted using these treatments in combination. In a previous study investigating combination therapy of UFT plus cisplatin for CRPC patients, the median overall survival time was 19 months, and the median time to PSA progression was 11 months19). Our study demonstrated for the first time the efficacy of UFT plus cisplatin for docetaxel-refractory CRPC.
In conclusion, UFT-P therapy is a safe and effective regimen for the treatment of docetaxel-refractory CRPC. In addition, because CRPC occurs largely in elderly men, the low-dose nature of the metronomic UFT-P regimen would be especially useful and could be administered continuously. Because the present study was small, large-scale studies with longer follow-up are required.
References
- 1.The Japanese Urological Association, editor. Guidelines for the diagnosis and treatment of prostate cancer 2006 edition. Kanehara Publishing, 2006.
- 2.Akaza H. Future prospects for luteinizing hormone-releasing hormone analogues in prostate cancer treatment. Pharmacology 2010; 85: 110–120. doi: 10.1159/000274486 [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Hinotsu S, Namiki M. Trans-Pacific variation in outcomes for men treated with primary androgen-deprivation therapy (ADT) for prostate cancer. BJU Int 2016; 117: 102–109. doi: 10.1111/bju.12937 [DOI] [PubMed] [Google Scholar]
- 4.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer 2001; 1: 34–45. doi: 10.1038/35094009 [DOI] [PubMed] [Google Scholar]
- 5.Petrylak DP, Tangen CM, Hussain MH. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–1520. doi: 10.1056/NEJMoa041318 [DOI] [PubMed] [Google Scholar]
- 6.Tannock IF, de Wit R, Berry WR. TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512. doi: 10.1056/NEJMoa040720 [DOI] [PubMed] [Google Scholar]
- 7.Fujii S, Kitano S, Ikenaka K. Effect of coadministration of uracil or cytosine on the anti-tumor activity of clinical doses of 1-(2-tetrahydrofuryl)-5-fluorouracil and level of 5-fluorouracil in rodents. Gan 1979; 70: 209–214(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 8.Taguchi T, Nakano Y, Jikuya K. Effect of uracial on the antitumor activity of FUTRAFUL. Gan To Kagaku Ryoho 1978; 5: 1161–1165(in Japanese, Abstract in English). [Google Scholar]
- 9.Shirasaka T, Shimamoto Y, Ohshimo H. Mechanism for synergistic antitumor effect in the combination of 5-fluorouracil with cisplatin in vivo tumor models: from the view of biochemical modulation of 5-fluorouracil. Gan To Kagaku Ryoho 1991; 18: 403–409(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 10.Naito K, Hisazumi H, Misaki T. Clinical effect of UFT on prostatic cancer. Gan To Kagaku Ryoho 1985; 12: 1440–1444(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 11.Nakashima T, Matsumura Y, Nomura Y. Antitumor effect of UFT against malignant tumors of maxillary sinusclinical and biochemical study. Gan To Kagaku Ryoho 1982; 9: 1729–1734(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 12.Futatsuki K, Komita T, Kamano T. Tokyo Cancer Chemotherapy Cooperative Study Group. Clinical results of treatment with the UFT fine granule preparation under cooperative study. Gan To Kagaku Ryoho 1987; 14: 1274–1280(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 13.Kato H, Ichinose Y, Ohta M. Japan Lung Cancer Research Group on Postsurgical Adjuvant Chemotherapy. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 2004; 350: 1713–1721. doi: 10.1056/NEJMoa032792 [DOI] [PubMed] [Google Scholar]
- 14.Shirasaka T, Shimamoto Y, Ohshimo H. Metabolic basis of the synergistic antitumor activities of 5-fluorouracil and cisplatin in rodent tumor models in vivo. Cancer Chemother Pharmacol 1993; 32: 167–172. doi: 10.1007/BF00685830 [DOI] [PubMed] [Google Scholar]
- 15.Schabel FM, Jr, Trader MW, Laster WR., Jr cis-Dichlorodiammineplatinum(II): combination chemotherapy and cross-resistance studies with tumors of mice. Cancer Treat Rep 1979; 63: 1459–1473. [PubMed] [Google Scholar]
- 16.Scanlon KJ, Newman EM, Lu Y. Biochemical basis for cisplatin and 5-fluorouracil synergism in human ovarian carcinoma cells. Proc Natl Acad Sci USA 1986; 83: 8923–8925. doi: 10.1073/pnas.83.23.8923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohtsu A, Shimada Y, Yoshida S. Phase II study of protracted infusional 5-fluorouracil combined with cisplatinum for advanced gastric cancer: report from the Japan Clinical Oncology Group (JCOG). Eur J Cancer 1994; 30A: 2091–2093. doi: 10.1016/0959-8049(94)00350-E [DOI] [PubMed] [Google Scholar]
- 18.Greenberg B, Ahmann F, Garewal H. Neoadjuvant therapy for advanced head and neck cancer with allopurinol-modulated high dose 5-fluorouracil and cisplatin. A phase I-II study. Cancer 1987; 59: 1860–1865. doi: [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi T, Mayama I, Iwabuchi I. Chemo-endocrine therapy with low-dose cisplatin, UFT, and dexamethasone for hormone-refractory prostate cancer patients. Gan To Kagaku Ryoho 2010; 37: 1921–1925(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 20.de Bono JS, Oudard S, Ozguroglu M. TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–1154. doi: 10.1016/S0140-6736(10)61389-X [DOI] [PubMed] [Google Scholar]
- 21.Scher HI, Fizazi K, Saad F. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197. doi: 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 22.Fizazi K, Scher HI, Molina A. COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13: 983–992. doi: 10.1016/S1470-2045(12)70379-0 [DOI] [PubMed] [Google Scholar]
- 23.Aller AW, Kraus LA, Bissery MC. In vitro activity of TXD258 in chemotherapeutic resistant tumor cell lines. Proc Am Assoc Cancer Res 2000; 41: 303 (abstr 1923). [Google Scholar]
- 24.Bissery MC, Bouchard H, Riou JF.Preclinical evaluation of TX 258, a new taxiod. Proc Am Assoc Cancer Res 2000; 41: 214 (abstr 1364). [Google Scholar]
- 25.Tran C, Ouk S, Clegg NJ. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009; 324: 787–790. doi: 10.1126/science.1168175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Bono JS, Logothetis CJ, Molina A.Abiraterone and increased survival metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klotz L, Schellhammer P, Carroll K. A re-assessment of the role of combined androgen blockade for advanced prostate cancer. BJU Int 2004; 93: 1177–1182. doi: 10.1111/j.1464-410x.2004.04803.x [DOI] [PubMed] [Google Scholar]
- 28.Mutsaers AJ. Metronomic chemotherapy. Top Companion Anim Med 2009; 24: 137–143. doi: 10.1053/j.tcam.2009.03.004 [DOI] [PubMed] [Google Scholar]
- 29.Sartor O, Halstead M, Katz L. Improving outcomes with recent advances in chemotherapy for castrate-resistant prostate cancer. Clin Genitourin Cancer 2010; 8: 23–28. doi: 10.3816/CGC.2010.n.004 [DOI] [PubMed] [Google Scholar]
- 30.Rossof AH, Talley RW, Stephens R. Phase II evaluation of cis-dichlorodiammineplatinum(II) in advanced malignancies of the genitourinary and gynecologic organs: a Southwest Oncology Group Study. Cancer Treat Rep 1979; 63: 1557–1564. [PubMed] [Google Scholar]