Abstract
Background
Lower vitamin D levels have been associated with manifest Parkinson’s disease, prompting the hypothesis that vitamin D insufficiency or deficiency may increase risk for PD.
Objectives
To evaluate vitamin D levels in a population at risk for developing PD.
Methods
Plasma vitamin D levels were measured in the Parkinson Associated Risk Syndrome Study, a cohort of asymptomatic individuals, some of whom are at high risk for PD. Vitamin D levels were compared between subjects at high risk for PD (hyposmia and dopamine transporter scan deficit) versus all others and examined for correlations with dopaminergic system integrity.
Results
Mean vitamin D levels did not differ between groups, with a level of 27.8 ng/mL (standard deviation = 12.0) in the high-risk group versus 24.7 ng/mL (standard deviation = 9.0) in all others (P = 0.09). Vitamin D levels did not associate with putaminal dopamine transporter uptake.
Conclusions
Our data from the asymptomatic Parkinson Associated Risk Syndrome cohort do not support the hypothesis that chronic vitamin D insufficiency threatens dopaminergic system integrity, contributing to PD pathogenesis.
Keywords: vitamin D, Parkinson’s disease, cognition, cohort studies
Vitamin D deficiency is frequent (41.5%) in the United States and has been reported to associate with several chronic conditions. Early cross-sectional studies reported that persons with Parkinson’s disease (PD) are more deficient than the general population,1 and genetic variation in the vitamin D receptor (VDR) gene has been linked to PD risk,2 spurring the hypothesis that vitamin D metabolism is important in the development or progression of PD.3–5 Moreover, animal studies have shown motor impairment and reduced stride length in VDR knockout mice,6 and animal and cell culture studies have demonstrated neuroprotective effects of vitamin D against MPTP and 6- OHDA exposure.7,8
Complications exist, however. For example, the first epidemiological risk study from Finland found lower baseline vitamin D levels in individuals who went on to develop PD compared to those who did not,9 but the more recent prospective Atherosclerosis Risk in Communities (ARIC) study cohort of almost 13,000 subjects found no association between baseline vitamin D levels and risk of PD 17 years later.10 Moreover, whereas studies involving populations with more advanced PD have consistently shown associations between decreased vitamin D and PD,5,11 they face potential confounding attributed to reduced sun exposure, leading to reduced dermal synthesis of Vitamin D, in patients with progressive motor limitations.
To date, no study has explored vitamin D in subjects with premotor PD on the cusp of diagnosis. The Parkinson Associated Risk Syndrome (PARS) study is a well-characterized cohort of subjects without a diagnosis of PD who have undergone olfactory testing and dopamine transporter (DAT) imaging, suggesting that some may be at high risk for PD. This cohort is thus well suited to studying vitamin D levels in “prodromal” PD, preceding the advent of motor limitations that may confound results.
Materials and Methods
Population
Data used in this study came from the Parkinson Associated Risk Syndrome (PARS) Study, an observational study designed to identify subjects at high risk for developing PD.12–14 Briefly, PARS subjects undergo testing with the University of Pennsylvania Smell Identification Test (UPSIT), baseline clinical evaluations, collection of blood and cerebrospinal fluid, and DAT imaging. For this study, subjects were high risk if they displayed hyposmia (<15th percentile for age- and sex-based norms on UPSIT) and <80% age-expected putaminal DAT binding. We based DAT imaging cutoffs on standards used to define subjects without evidence of dopaminergic deficit in large PD clinical trials such as PRECEPT.15 Moreover, a subset of PARS individuals with hyposmia and <65% age-expected DAT binding were considered at highest risk. The latter cutoff is more stringent than one in which we have previously reported sensitivities of >90% for detection of parkinsonian syndromes defined by expert clinical diagnosis16 and has been previously used to define a highest-risk group among PARS study participants.14 Of 303 subjects who completed baseline clinical and imaging evaluations, plasma samples were available for 198, all of which were included in our study.
Institutional Review Board Approval
The study was approved by the Western Institutional Review Board, the Human Research Protection Office at the U.S. Army Medical Research Material and Command, and all local institutional review boards for participating centers. Informed consent was obtained from each participant.
Vitamin D Measurement
Plasma samples were collected at baseline and stored at −80°C until they were assayed using liquid chromatography tandem mass spectrometry (LC/MS/MS), the gold standard for measuring vitamin D.17 The assays were performed by Heartland Assays, LLC (Ames, IA), with the Agilent 1290/6460 Series Triple Quadrupole LC/MS systems. Total vitamin D was measured as 25-hydroxyvitamin D (25(OH)D) and determined by adding 25(OH)D2 and 25(OH)D3 levels.
Statistical Analysis
Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS) statistics (version 22; SPSS, Inc., Chicago, IL). Demographic characteristics, baseline motor features, and vitamin D levels were compared using t tests, the Mann-Whitney U test, or chi-square test. Two groups were defined for comparison: (1) subjects with hyposmia and a reduction in DAT binding (hyposmia + <80% age-expected DAT binding) and (2) all other subjects. The hyposmia + DAT reduction group is considered at high risk for PD based on DAT imaging data from early PD clinical trials.15 A second analysis using a more stringent DAT cutoff (<65%) to define a highest-risk group14 was also performed.
Linear regression was used to determine whether there were differences in vitamin D levels between the two groups after adjusting for age, sex, and season of blood draw. Partial correlations were used to determine whether there was an association between total vitamin D and age-expected putaminal DAT binding while controlling for age, sex, and season of blood draw.
Results
Among 198 participants with available samples, 56 were in the hyposmia and DAT deficit group (high risk for PD) and 142 made up the “all others” group. As shown in Table 1, there were no differences in age or season of blood draw. The mean total UPDRS score was significantly higher in the high-risk group (4.5 [standard deviation {SD} = 5.0]) compared to all others (2.4 [SD = 3.4]; P = 0.001).
TABLE 1.
Baseline characteristics and vitamin D levels of high risk group vs. all others
| DAT Cutoff <80% Age-Expected | DAT Cutoff <65% Age-Expected | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Hyposmia/DAT Reduction (n = 56) | All Others (n = 142) | P Value | Hyposmia/DAT Reduction (n = 22) | All Others (n = 176) | P Value | |
| Age (years) Mean (SD) |
64.9 (8.4) | 66.8 (8.3) | 0.15a | 67.3 (6.9) | 66.1 (8.5) | 0.52a |
| Sex (males) No. (%) |
37 (66.1) | 79 (55.6) | 0.2b | 18 (81.8) | 98 (55.7) | 0.02b |
| Total UPDRS Mean (SD) |
4.5 (5.0) | 2.4 (3.4) | 0.001c | 4.6 (3.3) | 2.8 (4.1) | 0.04a |
| Season of blood draw N (%summer/fall) |
30 (53.6) | 56 (39.4) | 0.08b | 13 (59.1) | 73 (41.5) | 0.2b |
| 25(OH)D total (ng/mL) Mean (SD) |
27.8 (12.0) | 24.7 (9.0) | 0.09a | 26.3 (13.0) | 25.5 (9.7) | 0.80a |
| 25(OH)D2 (ng/mL) Mean (SD) |
1.5 (3.9) | 0.72 (1.8) | 0.45c | 1.1 (2.8) | 0.9 (2.6) | 0.6c |
| 25(OH)D3 (ng/mL) Mean (SD) |
26.4 (12.5) | 24.0 (9.1) | 0.2a | 25.2 (13.1) | 24.6 (9.8) | 0.8a |
| 25(OH)D deficiency N (%) |
15 (26.8) | 44 (31.0) | 0.4b | 8 (36.4) | 51 (29.0) | 0.4b |
| 25(OH)D insufficiency N (%) |
23 (41.1) | 66 (46.5) | 0.4b | 7 (31.8) | 83 (46.6) | 0.4b |
Vitamin D deficiency is defined as total 25(OH)D <20 ng/mL, insufficiency as total 25(OH)D of 20 to 30 ng/mL, and sufficiency as 25(OH)D >30 ng/mL.
P value for t test.
P value for χ2.
P value for Mann-Whitney U test.
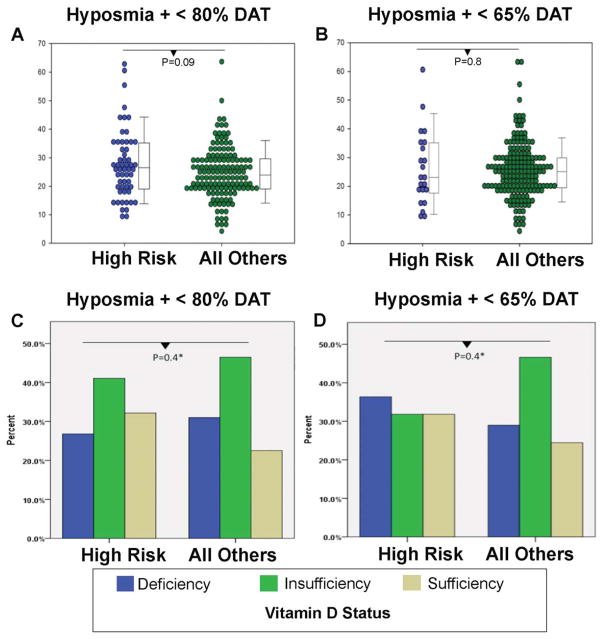
Total plasma vitamin D levels did not differ in PARS subjects at high risk for developing PD (mean = 27.8 ng/mL [SD = 12.0]) versus all other groups (mean = 24.7 ng/mL [SD= 9.0]), with a trend toward higher levels of vitamin D in the high-risk group (P = 0.09 [95% confidence interval for difference between groups = −0.423, 6.638]; Fig. 1). Vitamin D2 and vitamin D3 levels did not differ between the two groups (Table 1). The proportion of PARS participants meeting criteria for vitamin D deficiency (level <20 ng/mL) and insufficiency (level, 20–30 ng)was similar comparing individuals at high risk for PD versus not, with a deficiency rate of 26.8% in the high-risk group versus 31.0%in those not at high risk, and an insufficiency rate of 41.1% in the high-risk group versus 46.5% in those not at high risk (Fig. 1). Using linear regression, there was no difference in vitamin D levels between the two groups after adjusting for age, sex, and season of blood draw (2.87 [–0.255, 5.99] higher in high-risk group; P = 0.07). There was also no association between total vitamin D and putaminal DAT binding when controlling for age, sex, and season of blood draw using partial correlations.
FIG. 1.
Vitamin D levels and status in PARS participants stratified by risk. (A) Swarm plots with box plots of vitamin D levels in the high-risk group (hyposmia + DAT<80%) vs. all others. (B) Swarm plots with box plots of vitamin D levels in the highest-risk group (hyposmia + DAT <65%) vs. all others. (C) Vitamin D deficiency and insufficiency in the high-risk group (hyposmia + DAT <80%) vs. all others. (D) Vitamin D deficiency and insufficiency in the highest-risk group (hyposmia + DAT <65%) vs. all others. Box plots denote median and interquartile range. Vitamin D deficiency is defined as total vitamin D <20 ng/mL, insufficiency as total vitamin D 20 to 30 ng/mL, and sufficiency as total vitamin D >30 ng/mL.
Results were similar when a more stringent cutoff of DAT binding <65% of age-expected norms was used to define the highest-risk group (Table 1).
Discussion
Vitamin D insufficiency is common in advanced PD, just as in the general adult population, but whether this represents a risk factor for dopaminergic neurodegeneration and PD is unclear. Clarity on this topic is important because vitamin D supplementation may be a viable neuroprotective strategy for PD, should strong evidence for an effect emerge from studies that are not at risk for confounding. In this study, we examined plasma vitamin D in asymptomatic subjects at high risk for developing PD from the PARS cohort. We found that vitamin D levels did not differ in the high-risk group compared to age- and sex-matched controls, suggesting that sustained vitamin D insufficiency is not common before a diagnosis of PD.
The major strength of the current study involves investigation of vitamin D levels in a unique, well-characterized population in which blood samples were drawn before a diagnosis of PD. Whereas case-control studies in longstanding PD often show significantly lower vitamin D levels in PD patients, these studies may be confounded by decreased sun exposure, leading to lower levels of dermal vitamin D synthesis, in patients with reduced mobility. Because PARS subjects do not meet criteria for PD at enrollment, decreased sun exposure attributed to limited mobility is less likely to confound results. However, a decline in daily functioning has been demonstrated in prodromal PD 5 or more years before the diagnosis.18 Consistent with these findings, the high-risk group in PARS had a significantly, albeit slightly, increased UPDRS score compared to controls. Nevertheless, this would not explain the current findings given that there was no difference in vitamin D levels between groups (and a trend toward higher levels in the high risk group). Last, we used the gold-standard LC/MS/MS method to measure vitamin D levels.
Several limitations should be noted. First, the sample size was relatively small, with only 56 participants in the high-risk group, limiting power to detect very small differences in vitamin D levels between groups. However, we had 80% power to detect a difference as small as 5 ng/mL—a difference that has been demonstrated in multiple past studies.1,5 In addition, the 95% confidence interval for the true difference between groups in our study ranged from –0.432 to 6.638, meaning that the high-risk group may have had a mean vitamin D level that was up to 6.6 ng/mL greater than controls, or up to 0.43 ng/mL lesser than controls. Given that the direction suggested by past studies is for PD individuals (and individuals at risk for PD) to have lower vitamin D levels, the small magnitude estimated in this direction (0.43 ng/mL) by our data is unlikely to be clinically meaningful.
Second, the high-risk group was defined by hyposmia and a somewhat liberal age-expected putaminal DAT binding cutoff of 80% or less, meaning not all of those in the high-risk group may develop PD. We note in this regard, however, that, among PARS subjects, the high-risk group appeared to have a higher total vitamin D level, although this did not reach statistical significance; our data do not trend in the direction expected if lower vitamin D levels predispose to the development of PD. Moreover, we also repeated the analysis with amore stringent DAT cutoff of<65% age-expected norms, obtaining similar results.
Third, this is a cross-sectional study in which vitamin D levels were measured at one point in time and do not necessarily represent the chronic vitamin D status of the individual participants. Last, vitamin D supplementation status was not known, so it is possible that those in the high-risk group had higher rates of supplementation. However, neither vitamin D2 nor vitamin D3 levels differed between the groups, arguing against higher rates of supplementation in the high-risk group, given that most supplements contain only vitamin D2.
In summary, in the prediagnostic PARS cohort, some of whom are at high risk for developing PD, plasma vitamin D levels were no different in subjects at high risk for developing PD versus those who were not. There were no correlations between DAT binding and vitamin D levels. Our findings are consistent with the most recent prospective study that found no association between serum vitamin D levels and PD risk. Moreover, they do not support the hypothesis that chronic vitamin D insufficiency contributes to the pathogenesis of PD.
Acknowledgments
Funding agencies: Support for this study was provided by the Pechenik Montague Award Fund.
Footnotes
Relevant conflicts of interest/financial disclosures: Nothing to report. Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008;65:1348–1352. doi: 10.1001/archneur.65.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YH, Kim JH, Song GG. Vitamin D receptor polymorphisms and susceptibility to Parkinson’s disease and Alzheimer’s disease: a meta-analysis. Neurol Sci. 2014;35:1947–1953. doi: 10.1007/s10072-014-1868-4. [DOI] [PubMed] [Google Scholar]
- 3.Ding H, Dhima K, Lockhart KC, et al. Unrecognized vitamin D3 deficiency is common in Parkinson disease: Harvard Biomarker Study. Neurology. 2013;81:1531–1537. doi: 10.1212/WNL.0b013e3182a95818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato Y, Honda Y, Iwamoto J. Risedronate and ergocalciferol prevent hip fracture in elderly men with Parkinson disease. Neurology. 2007;68:911–915. doi: 10.1212/01.wnl.0000257089.50476.92. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Sun Y, Ji HF, Shen L. Vitamin D levels in Alzheimer’s and Parkinson’s diseases: a meta-analysis. Nutrition. 2013;29:828–832. doi: 10.1016/j.nut.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Burne TH, McGrath JJ, Eyles DW, Mackay-Sim A. Behavioural characterization of vitamin D receptor knockout mice. Behav Brain Res. 2005;157:299–308. doi: 10.1016/j.bbr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Wang JY, Wu JN, Cherng TL, et al. Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 2001;904:67–75. doi: 10.1016/s0006-8993(01)02450-7. [DOI] [PubMed] [Google Scholar]
- 8.Shinpo K, Kikuchi S, Sasaki H, Moriwaka F, Tashiro K. Effect of 1,25-dihydroxyvitamin D(3) on cultured mesencephalic dopaminergic neurons to the combined toxicity caused by L-buthionine sulfoximine and 1-methyl-4-phenylpyridine. J Neurosci Res. 2000;62:374–382. doi: 10.1002/1097-4547(20001101)62:3<374::AID-JNR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Saaksjarvi K, Heliovaara M. Serum vitamin D and the risk of Parkinson disease. Arch Neurol. 2010;67:808–811. doi: 10.1001/archneurol.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrestha S, Lutsey PL, Alonso A, Huang X, Mosley TH, Jr, Chen H. Serum 25-hydroxyvitamin D concentrations in Mid-adulthood and Parkinson’s disease risk. Mov Disord. 2016;31:972–978. doi: 10.1002/mds.26573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Evatt ML, Maldonado LG, et al. Vitamin D from different sources is inversely associated with Parkinson disease. Mov Disord. 2015;30:560–566. doi: 10.1002/mds.26117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chahine LM, Weintraub D, Hawkins KA, et al. Cognition in individuals at risk for Parkinson’s: Parkinson associated risk syndrome (PARS) study findings. Mov Disord. 2016;31:86–94. doi: 10.1002/mds.26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siderowf A, Jennings D, Eberly S, et al. Impaired olfaction and other prodromal features in the Parkinson At-Risk Syndrome Study. Mov Disord. 2012;27:406–12. doi: 10.1002/mds.24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings D, Siderowf A, Stern M, et al. Imaging prodromal Parkinson disease: the Parkinson Associated Risk Syndrome Study. Neurology. 2014;83:1739–1746. doi: 10.1212/WNL.0000000000000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marek K, Seibyl J, Eberly S, et al. Longitudinal follow-up of SWEDD subjects in the PRECEPT Study. Neurology. 2014;82:1791–1797. doi: 10.1212/WNL.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings DL, Seibyl JP, Oakes D, Eberly S, Murphy J, Marek K. (123I) beta-CIT and single-photon emission computed tomographic imaging vs clinical evaluation in Parkinsonian syndrome: unmasking an early diagnosis. Arch Neurol. 2004;61:1224–1229. doi: 10.1001/archneur.61.8.1224. [DOI] [PubMed] [Google Scholar]
- 17.Jones G. Assay of vitamins D2 and D3, and 25-hydroxyvitamins D2 and D3 in human plasma by high-performance liquid chromatography. Clin Chem. 1978;24:287–298. [PubMed] [Google Scholar]
- 18.Darweesh SK, Verlinden VJ, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of prediagnostic functioning in Parkinson’s disease. Brain. 2017;140:429–441. doi: 10.1093/brain/aww291. [DOI] [PubMed] [Google Scholar]