Abstract
Ischiofemoral impingement (IFI) is a rare cause of hip pain defined by a narrowing of the space between the lateral aspect of the os ischium and the lesser trochanter of the femur. Several underlying anatomic, functional and iatrogenic pathologies have been identified for symptomatic IFI in native hip joints and after total hip arthroplasty. Clinical symptoms vary but most commonly consist of pain of the lower buttock and groin including the inner thigh, and a snapping or clunking phenomenon is often reported. Symptoms may be provoked by a combined extension, adduction and external rotation during physical examination and during long-stride walking. Radiographs of the pelvis and an axial or false-profile-view of the hip as well as magnetic resonance imaging (MRI)-scans should be obtained to strengthen the diagnosis. On MRI, the quadratus femoris muscle signal and the space confined by the anatomic structures surrounding the muscle, the quadratus femoris space, are to be assessed. Targeted infiltration of the muscle can be helpful both diagnostically and therapeutically. The literature on differential diagnoses and treatment options for IFI is limited; therapeutic suggestions are offered only in case reports and series. With this work, we aim to give a systematic approach to the non-surgical and surgical treatment options for IFI based upon the current literature and the authors’ personal experience.
INTRODUCTION
Ischiofemoral impingement (IFI) is a rare cause of hip pain first described in three patients after total hip arthroplasty and proximal femoral osteotomy [1]. More recently, IFI affecting native hip joints—even in childhood—has been described, along with a variety of underlying pathologies [2–4]. This has led to considerable interest not only in the causative anatomy and pathophysiology, but also in the improvement of diagnostic and treatment options [5–8]. Clinically, symptomatic ischiofemoral impingement is a rather rare entity. However, typical changes in magnetic resonance tomography are common. So far, some small case series have been published in addition to reports of single cases. Additionally, insight on conservative treatment regimens and surgical techniques has been offered. In the following, we aim to provide a concise overview of the available literature, and to suggest a viable treatment algorithm on the basis of these publications and the authors’ experience with the management of ischiofemoral impingement.
DEFINITION AND PATHOLOGICAL ANATOMY
IFI is defined as a narrowing of the space between the lateral aspect of the os ischium and the lesser trochanter of the femur (Fig. 1, Supplementary Video S1) with entrapment of soft tissue structures [9]. A mechanical constriction of the soft tissues between them, particularly the quadratus femoris muscle, has been put forward as a cause of symptomatic IFI [2, 3, 9].
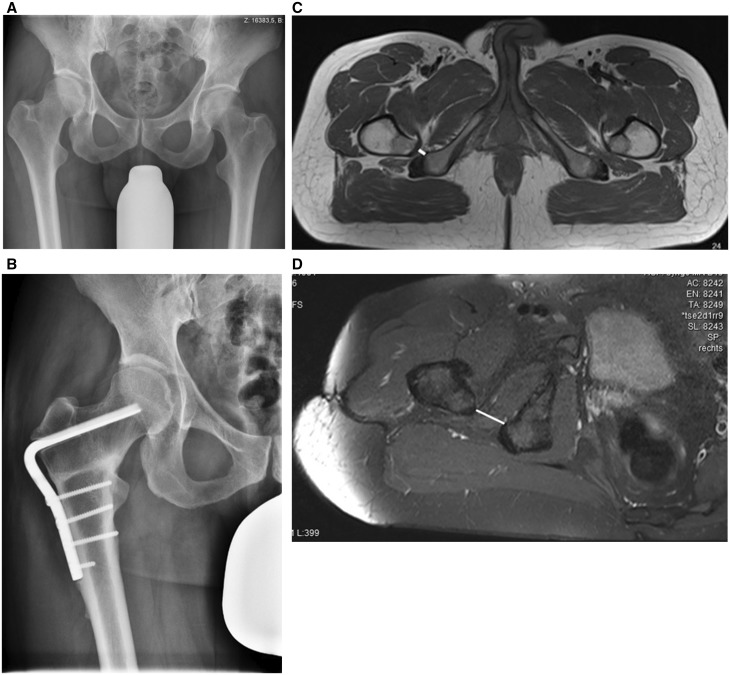
Fig. 1.
(A) Anteroposterior pelvic view exhibiting a reduced offset of the right hip on the grounds of coxa valga, suggesting possible IFI. (B) Frontal view radiograph of the pelvis, status post proximal femur osteotomy for coxa valga causing IFI. Note increased distance between the lesser trochanter and the os ischium. (C) Ischiofemoral space in a patient with IFI due to bilateral coxa valga. The right side is symptomatic. Solid line: Ischiofemoral space (IFS). (D) MRI of the same patient post proximal femur osteotomy and hardware removal, now with normal IFS (solid line).
There are several reasons for a narrowing of the ischiofemoral space (IFS), which is defined as the shortest distance between the bony margins of the ischium and the femur as measured on axial magnetic resonance imaging (MRI) sequences (Fig. 1c and d). Congenital anatomic conditions such as Coxa valga, developmental dysplasia of the hip (DDH), acquired deformities as in osteoarthritis with superomedial migration of the femur, sequelae of Morbus Perthes, proximal femoral fractures, osteochondroma of the proximal femur or pelvis, iatrogenic causes including total hip arthroplasty or valgus proximal femoral osteotomy and positional or functional factors such as increased hip adduction resulting from abductor insufficiency, predispose for mechanical conflict between the lesser trochanter and the ischium [1, 4, 10–13]. Also, a narrowing of the quadratus femoris space (QFS) between the superolateral aspect of the hamstring muscles and the posteromedial aspect of the psoas tendon (Fig. 2) can be caused by pathologies of these bordering soft tissues, such as hamstring enthesopathy [3, 14].
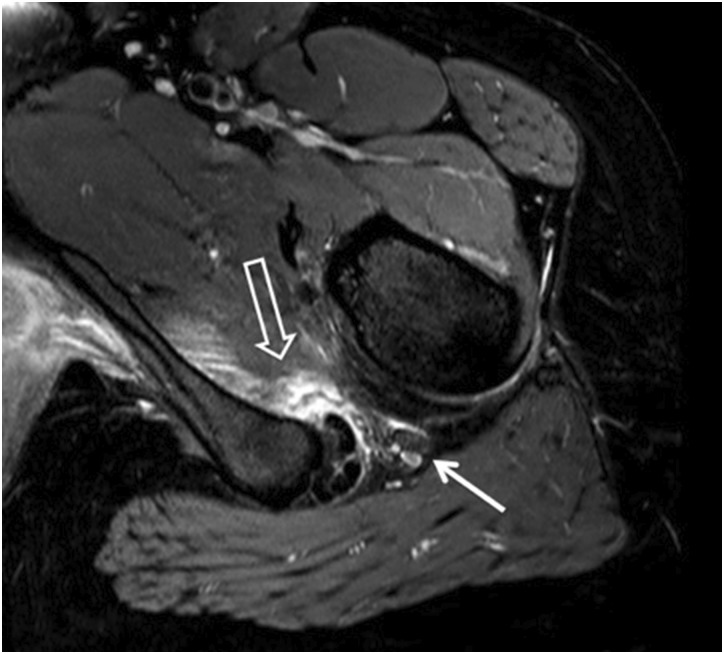
Fig. 2.
MRI T2 sequence of a patient with IFI of the left hip. Outlined arrow: quadratus femoris muscle belly oedema, solid arrow: Sciatic nerve.
The majority of patients described in the literature are female [3, 10, 14, 15]. A bilateral narrowing of the IFS with quadratus femoris abnormalities was reported to occur in 25% of the patients examined by Torriani et al., but bilateral narrowing does not seem to be necessarily accompanied by bilateral muscle abnormalities or symptoms of IFI [3, 16–18].
Ali et al. hypothesized on gait abnormality on the grounds of gluteal tendon enthesopathy or rupture causing or furthering the pathology [14]. They also mention the possibility that degeneration or atrophy of the quadratus femoris could be the cause, instead of the consequence, of ischiofemoral impingement, by a muscular imbalance [14]. What originally causes the atrophy in this theory is, however, unclear. An underlying neurological or muscular disorder cannot be excluded.
CLINICAL SYMPTOMS
Load-dependent pain of the lower buttock and/or the groin and the inside of the thigh is uniformly described as the main symptom [2, 3, 19]. The pain can radiate towards the knee [3]. In most reports, symptoms are chronic for several months or years, often increasing in intensity [16, 17, 20–22]. Also, radiation of the pain mimicking sciatica can occur, likely due to the close anatomic relation of the quadratus femoris muscle and the sciatic nerve [2, 18]. Palpating the ischium to cause pain during passive provocative movement can point towards IFI.
A snapping, clunking or locking sensation of the hip joint is often described by patients during long-stride walking caused by the lesser trochanter forcefully bypassing the os ischium (Supplementary audio S1) [2, 3, 14, 16, 17, 23]. Ganz et al. attributed a sense of instability in some of their patients to a contrecoup mechanism which, in combination with inadequate acetabular coverage, could subluxate the hip when the lesser trochanter impinges on the ischium [10].
Patients usually describe worsening of symptoms or snapping during pronounced extension of the symptomatic hip, e.g. during running or taking larger steps.
Physical examination findings are not always specifically suggestive of IFI. The provocation of symptoms by passively extending, adducting and externally rotating the hip, first described by Johnson, is most commonly mentioned as the test of choice [1, 21, 22]. A passive flexion and internal rotation was suggested by Tosun et al. to provoke pain by stretching the impaired quadratus femoris muscle [23].
Gómez-Hoyos et al. described and validated two tests for IFI: The long stride walking test consists of pain evoked by the patient taking large steps. It has a sensitivity of 92% and a specificity of 82%. The IFI test is performed by passively extending and adducting the patient’s leg. The resulting pain is not seen in extension and abduction. For this test, the authors state a sensitivity of 82% and a specificity of 85% [24].
Painless patients might exhibit functional leg length discrepancy due to compensatory abduction of the leg in order to increase the distance between the lesser trochanter and the ischium [10].
RADIOLOGICAL FINDINGS
Conventional radiographs
Imaging should include and begin with conventional radiographs of the pelvis and an axial view of the hip to evaluate hip configuration and assess concomitant bony pathologies [25]. In patients with history of hip surgery, special attention must be paid to changes in hip offset.
Magnetic resonance imaging
MRI-scans are the method of choice in order to assess IFS, QFS and possible soft tissue affection. Apart from the distance of the lesser trochanter and ischium, femoral neck anteversion and lesser trochanteric retroversion, signal alterations of the quadratus femoris, surrounding muscles and tendons as well as intraarticular pathologies should be evaluated [26].
Patients characteristically present with a reduced IFS and QFS with concomitant quadratus femoris muscle belly oedema, partial tear or atrophy [3, 14, 27, 28]. These changes are not always located at the narrowest part of the QFS [29]. Tosun et al. report some degree of fatty degeneration of the quadratus femoris muscle in 94% of the patients examined in their study [28].
Interestingly, in a recent publication by Özdemir et al., signal alterations in the quadratus femoris muscle were seen in 9.1% of asymptomatic subjects. However, these alterations were always associated with a narrow IFS and QFS [29]. After studying a population with multiple hereditary exostoses causing IFI, Yoong et al. reported that signal alterations in the quadratus femoris were associated with a reduced IFS in most cases and patients exhibiting alterations were always symptomatic [12].
Ali et al. reported a case where after trauma, no pathologies where seen on an initial MRI scan. However, subsequent MRIs showed incremental narrowing of the IFS and an abnormal signal in the quadratus femoris muscle [16]. In chronic cases resistant to conservative therapy, a tumour mass has to be taken into account, as well [30]. Thus, in cases of suggestive symptoms with no initial visible pathology, repeat MRI studies may be warranted.
After total hip arthroplasty, the use of metal-artefact reduced sequences of MRI (MARS-MRI) or single photon emission computed tomography (SPECT) can be helpful to evaluate the ischiofemoral distance and adjacent soft tissues (Fig. 3).
Fig. 3.
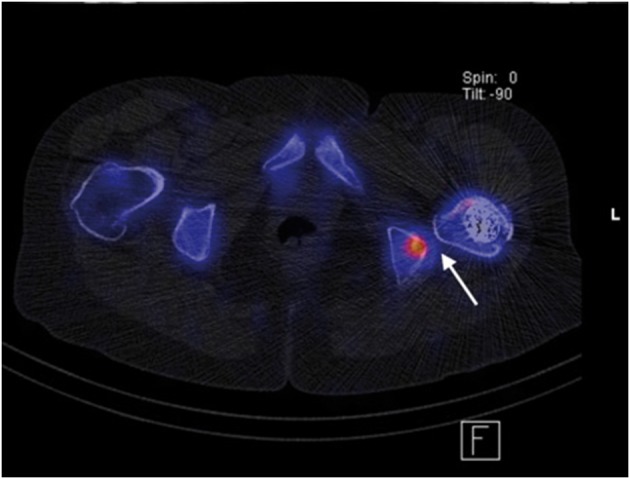
SPECT of patient with IFI after THA. Altered bone metabolism due to the impingement (arrow).
IFS and quadratus femoris space
In the first publication on the issue, a normal IFS was established as 20 mm wide, without clarifying the origin of this statement [1]. Several radiological and cadaveric studies measuring normal and pathological IFS and other spatial parameters have since been conducted [3, 29, 31, 32]. In these, the IFS in a healthy population is reported to be between 18 and 26 mm [3, 29, 31, 32]. The quadratus femoris space was measured at 12 mm in women by Torriani et al., while Sussmann et al. postulated 20.4 mm in a cadaveric study [3, 31].
These ranges are due to numerous influences both anatomical and dependent on the measurement technique. Some authors measured the ischiofemoral distance using CT-scans, others using MRI. Secondly, depending on departmental protocols, the scans are performed with the legs in a relaxed (therefore, most likely externally rotated), a neutral or an internally rotated position [3, 32]. Average distance between the lesser trochanter and ischium differs in neutral rotation, 40° internal rotation, and 60° external rotation with 2.8, 4.3 and 1.4 cm (SD 0.7), respectively [33].
In addition, gender-specific differences in pelvic anatomy affecting the IFS are discussed [3, 32]. The intertuber distance, which is wider in females, is reported to be associated with a reduced ischiofemoral distance [29].
In a recent study, the ischiofemoral distance was shown to decrease with age and with reduction of hip offset [32]. Furthermore, the IFS exhibits a broad intraindividual range [29].
In a recent meta-analysis, Singer et al. showed that a cut-off IFS of ≤ 15 mm yielded a sensitivity of 74.9% and a specificity of 81%, while a cut-off QFS ≤10 mm resulted in a sensitivity of 78.7% and a specificity of 74.1% [34].
Other findings
Concomitant pathologies are consistently reported and should be thoroughly analysed before treatment to establish the most appropriate therapy. Intraarticular concomitant pathologies of the hip were reported in three out of five patients examined by Hatem et al. [27]. With this respect, Ricciardi et al. hypothesized that extra-articular impingement may cause a contrecoup mechanism which is finally leading to intraarticular damage [35].
Extra-articular soft tissue entities reducing the QFS include—among others—acute and chronic psoas and hamstring tendinopathy/avulsion and neobursa formation in the vicinity of the impinging structures [3, 14, 15]. Sometimes, sclerotic areas or cysts within these structures are seen [2, 22]. Extensive muscle belly oedema in the quadratus femoris is associated with a greater likelihood of oedema in the hamstring or psoas [3]. Measurement of the proximal hamstring insertion area revealed significantly larger areas in symptomatic patients [28]. There seem to be interrelations not only between the IFS limited by bony landmarks and the QFS limited by the adjacent muscles, but also among these adjacent soft tissues themselves.
In summary, the combination of the aforementioned symptoms and typical changes seen on MRI points towards the correct diagnosis. In uncertain cases, CT- or ultrasound-guided infiltration of the quadratus femoris muscle (Fig. 4) with corticosteroids and local anaesthetics is helpful in ruling out differential diagnoses [1, 16]. Also, the use of fluoroscopy or 3D animation of CT images (Supplementary Video S1) to visualise the actual impingement upon provoking manoeuvres is recommended [27, 36]. The case for dynamic imaging has recently been made by Johnson et al. and by Bardakos [5, 37].
Fig. 4.
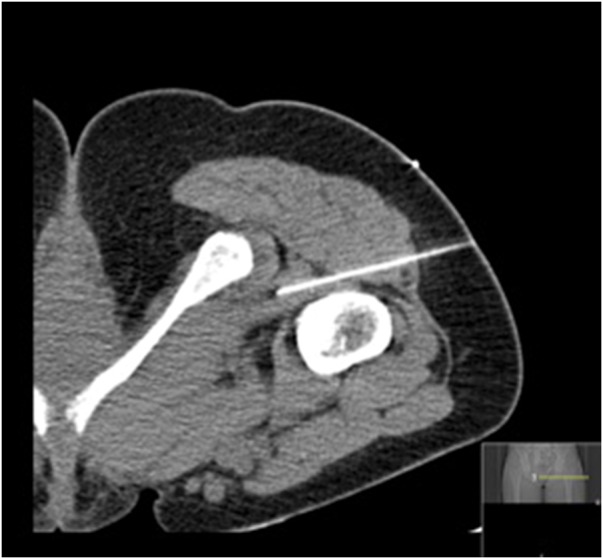
CT-guided injection of the quadratus femoris muscle. The patient is in prone position.
THERAPY
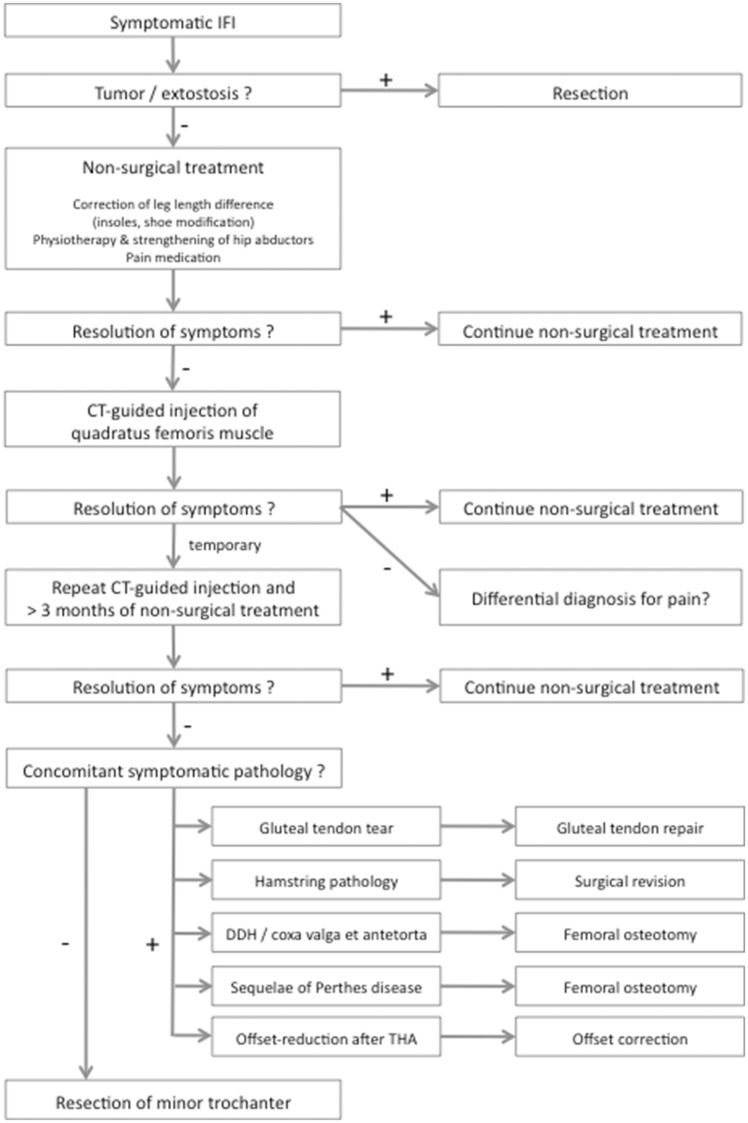
So far, clinical publications on therapeutic strategies in IFI have been limited to case reports and small cohorts. To provide a systematic approach, the following therapeutic algorithm is proposed (Fig. 5) based on the available literature and the first author’s personal experience.
Fig. 5.
Treatment algorithm for symptomatic IFI including concomitant pathologies that commonly cause IFI.
It is essential to first assess concomitant pathologies that entail symptomatic IFI. Eliminating the underlying concomitant causes will often also resolve symptomatic IFI.
While specific conservative measures for most of these entities exist and should remain the treatment of choice, they lend themselves to surgical correction in cases where conservative therapy falls short.
Causes for secondary ischiofemoral impingement
Pathologies can be divided into functional and structural pathologies reducing IFS.
Structural pathologies often include coxa valga and DDH which can be addressed by strengthening of the abductor mechanism and in cases of failure by varus and/or lateralizing proximal femoral osteotomy to restore physiological anatomical conditions (Fig. 1a–d) [38]. By the same principle, corrective osteotomy is to be considered for fracture sequelae. Gomez-Hoyos et al. were able to show the connection between femoral neck anteversion, but not lesser trochanteric version, on IFI [26].
An insufficiency of the hip abductor mechanism can occur in native hip joints and after total hip arthroplasty and cause a functional decrease of IFS [39]. When gait abnormality and abductor weakness persist after a regimen of muscle strengthening and stretching physiotherapy and analgesia, surgery is indicated [40]. Several procedures have been described ranging from open or endoscopic transosseous repair to tendon transfer techniques [41, 42].
Leg length discrepancy is associated with numerous musculoskeletal disorders and results in functional reduction of the IFS at the longer leg. A common iatrogenic cause is total hip arthroplasty. Its treatment is primarily conservative, using shoe inserts or modifications [43].
Hamstring enthesopathy or rupture is commonly encountered in athletes and can contribute to both a functional and structural IFS narrowing [24]. The first line of treatment consists of activity modification, physiotherapy and non-steroidal anti-inflammatory drugs (NSAIDs), with optional additive ultrasound, shock wave and electrical stimulation therapy [44, 45]. Surgery is recommended for injuries to more than two tendons, tendon avulsion of more than 2 cm or after failure of a non-surgical treatment attempt. In general, transosseous tendon reinsertion or scar tissue resection and side-to-side muscle adaptation can be performed [44–46].
Regarding reduced offset after total hip arthroplasty (THA), Johnson resolved symptoms by resecting the lesser trochanter [1]. The common principles of hip arthroplasty component positioning and its influence on range of motion and offset should be considered during revision surgery [47, 48]. As an exchange of a well-fixed component might not be warranted for IFI, the use of head–neck adapters and/or lateralizing inserts might present a less invasive method of offset correction [49].
In eight patients with complex extra-articular hip impingement most likely including IFI on the grounds of Perthes’ disease, Ganz et al. proposed a distalization of the lesser trochanter as part of the surgical strategy normalizing ischiofemoral anatomy [10]. The eight patients described were without recurring impingement at 3.5 years follow-up.
Exostoses compressing the quadratus femoris space can be resected to successfully relieve symptoms, as was reported by Viala et al. and Yoong et al. [12, 13].
For a summary of reported outcomes after non-surgical and surgical therapy, see Table I.
Table I.
Overview of the treatment methods, duration of follow-up and outcome in the available literature
| Authors | Year of publication | Study | No. of patients | Age | Gender | Treatment | Duration of follow-up | Outcome |
|---|---|---|---|---|---|---|---|---|
| Patti et al. | 2008 | Case report | 1 | 43 | f | NSAIDs | 3 m | Resolution of pain |
| Ali et al. | 2011 | Case report | 1 | 17 | f | Open subperiosteal iliopsoas release and reattachment | 10 w | Asymptomatic |
| Viala et al. | 2012 | Case report | 1 | 37 | f | Open resection of exostosis | 6 m | ‘Pain improved’ |
| Tosun et al. | 2012 | Case report | 1 | 11 | f | NSAIDs, rest | n/a | ‘Successfully treated’ |
| López-Sánchez et al. | 2013 | Case report | 1 | 16 | n/a | NSAIDs, analgesia, rest, progressive reintroduction of sports activities | n/a | ‘Marked improvement’ |
| Lee et al. | 2013 | Case report | 1 | 48 | f | NSAIDs, gabapentin, physiotherapy | 6 w | VAS reduced 7–8/10 to 2–3/10, full range of motion |
| Ganz et al. | 2013 | Case series | 14 | 11–63 | 10 f | Open distalization of lesser trochanter | 3.5 y (2–12) | No impingement, full flexion strength (8 patients with Perthes' disease sequelae) |
| 4 m | ||||||||
| Kim et al. | 2014 | Case series | 2 | 24; 23 | m | Proliferation therapy of QFM | 6 and 7 m | VAS reduced 9 − 10/10 to 1–2/10 and |
| VAS reduced 9–10/10 to 0–1/10 | ||||||||
| QFM oedema reduced | ||||||||
| Safran, Ryu | 2014 | Case report | 1 | 19 | f | Endoscopic iliopsoas detachment and resection of lesser trochanter | 2 y | No pain |
| Hip flexion strength 5−/5 | ||||||||
| iHOT: 85 (from 32) | ||||||||
| Backer et al. | 2014 | Case–control | 20 (I: 7, C: 13) | I: 47 (15–66) | I: 7 f; | Steroid, ultra-sound-guided | 2 w | Pain reduction I: 1.7/10 (1 − 2/10) versus C: 0.8 (0 − 2/10) |
| C: 42 (16–62) | C: 12 f | |||||||
| Klinkert et al. | 2015 | Case report | 1 | 30 | f | Steroid, ultra-sound-guided | 4 m | Asymptomatic |
| Truong et al. | 2015 | Case report | 1 | 14 | f | Open resection of lateral 50% of ischial tuberosity | 12 w | Pain-free walking without crutches |
| Hatem et al. | 2015 | Case series | 5 | 33.9 (16–59) | 3 f | Endoscopic partial resection of lesser trochanter | 2.3 y (2–2.5) | Improvement mHHS: 43 points (26–66) |
| 2 m | VAS reduced 6.6/10 (6–7.3/10) to 1/10 (0–4/10) | |||||||
| Jo, O'Donnell | 2015 | Case report | 1 | 17 | f | Endoscopic resection of lesser trochanter | 4 m | Asymptomatic |
NSAID, Non-steroidal anti-inflammatory drugs; VAS, Visual analogue scale; QFM, Quadratus femoris muscle; iHOT, International hip outcome tool; I, Injection group; C, Control group; mHHS, Modified Harris Hip Score.
Idiopathic ischiofemoral impingement
Once concomitant pathologies have been addressed, deemed not to be causing IFI, or excluded, we concur with the majority of publications that the first line of therapeutic efforts for symptomatic IFI should be conservative, and surgery be reserved for cases not responding to conservative treatment [2, 3, 10, 14, 15, 17, 22].
The most commonly proposed therapeutic measures are physiotherapy for strengthening and stretching of the quadratus femoris muscle and other external rotators of the hip, avoiding movements that provoke impingement, and NSAIDs [2, 10, 19, 22, 28]. Safran et al. reported successful treatment of IFI with these measures in more than 20 cases seen in their practice, but do not provide any outcome measures. [22]. Lee et al. suggested adjunct analgesia in the form of gabapentin and added interferential current therapy. They report a normalization of hip range of motion and substantial pain relief in one patient [17].
Additional CT- or ultrasound-guided infiltration of the quadratus femoris muscle with corticosteroids and/or local anaesthetics is described by some authors for diagnostic and therapeutic aims [3, 14–16, 19, 22] (Fig. 4). Backer et al. show superior pain relief with ultrasound-guided cortisone injections over ‘conservative’ treatment. However, they did not describe details of the ‘conservative’ therapy applied in the control group, and their follow-up was limited to 2 weeks [15].
Successful use of proliferation therapy in two patients has been reported by Kim et al. [20].
For cases refractory to non-surgical treatment, several surgical options have been put forward. Of note, a minimum duration for conservative therapy after which it is deemed failed when symptoms persist or increase has not been defined. Because of the paucity of available data, the decision to address IFI surgically remains an individual one.
Johnson et al., in their first description of the pathology, resected the lesser trochanter in an open procedure in three patients [1]. The procedure was successful in all of them. Ali et al. reported using this treatment in a case study, with the patient being asymptomatic 10 weeks postoperatively [16].
Lesser trochanter resection can be performed arthroscopically, as well, as was described by Safran et al. and, in variations, by other authors [18, 19, 22, 27, 50]. Wilson et al. report an improvement in modified Harris Hip Scores from a preoperative average of 43–91 points after 12 months in 7 patients [19]. Hatem et al. report an increase of the modified Harris Hip Score from 51.3 points preoperatively to 94.2 points at a follow-up of 2.4 years in 5 patients. Also, they saw a significant pain reduction from 6.6 to 1 points on the visual analogue scale. In their work, they propagate a solely partial resection of the lesser trochanter intending to avoid a potential weakening of the psoas muscle and thus, hip flexion. Additionally, they hypothesized a lower risk of femoral fracture after partial resection [27].
With the same intention, IFI has also been treated by surgically addressing the ischium. Truong et al. describe the use of open ischioplasty on one patient in a case report, with resolution of the impingement 12 weeks postoperatively [36].
Regardless of the technique employed, surgical treatment of symptomatic IFI in cases not responding to conservative measures is consistently reported to resolve symptomatic impingement. However, as was stated above, the low prevalence of IFI and the small numbers reported forbid generalizable assumptions as of yet.
CONCLUSION
IFI is an important differential diagnosis for gluteal pain, pain radiating along the posterior or medial thigh, and snapping or locking of the hip joint. The diagnosis is confirmed by an accurate physical examination with provocation of symptoms in passive extension, adduction and external rotation and characteristic findings on MRI scans. These typically show a decreased distance between the lesser trochanter and the most lateral aspect of the ischium in conjunction with oedema in the quadratus femoris muscle belly.
In uncertain cases, probationary infiltration of the quadratus femoris or repeat imaging is warranted.
Underlying structural or muscular pathologies must be taken into account when symptomatic IFI has been confirmed. Conditions causing or furthering the impingement should be addressed specifically. If conservative treatment fails, surgical options should be utilized.
In cases with no discernible underlying concomitant pathology, with concomitant diseases present but not furthering impingement, or with remaining symptoms after treatment of the underlying conditions, IFI should be addressed directly. Because of very high success rates after conservative therapy, all available conservative measures should be employed in every case before considering surgery. We suggest a conservative regimen of 3 months of physiotherapy and strengthening of the hip abductors, activity modification and analgesia with additional injection therapy. Cases refractory to this regimen are surgically addressed. When surgical treatment is needed, several techniques are available with favourable outcomes considering pain, function and complication rates, and underlying concomitant pathologies should be corrected.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Hip Preservation Surgery online.
FUNDING
There was no extramural funding for this study.
CONFLICT OF INTEREST
None declared.
Supplementary Material
REFERENCES
- 1. Johnson KA. Impingement of the lesser trochanter on the ischial ramus after total hip arthroplasty. Report of three cases. J Bone Joint Surg Am 1977; 59: 268–9. [PubMed] [Google Scholar]
- 2. Patti JW, Ouellette H, Bredella MA. et al. Impingement of lesser trochanter on ischium as a potential cause for hip pain. Skeletal Radiol 2008; 37:939–41. [DOI] [PubMed] [Google Scholar]
- 3. Torriani M, Souto SC, Thomas BJ. et al. Ischiofemoral impingement syndrome: an entity with hip pain and abnormalities of the quadratus femoris muscle. AJR Am J Roentgenol 2009; 193:186–90. [DOI] [PubMed] [Google Scholar]
- 4. Stenhouse G, Kaiser S, Kelley SP. et al. Ischiofemoral Impingement in children: imaging with clinical correlation. AJR Am J Roentgenol 2016; 206: 426–30. [DOI] [PubMed] [Google Scholar]
- 5. Bardakos NV. Hip impingement: beyond femoroacetabular. J Hip Preserv Surg 2015; 2: 206–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Sa D, Alradwan H, Cargnelli S. et al. Extra-articular hip impingement: a systematic review examining operative treatment of psoas, subspine, ischiofemoral, and greater trochanteric/pelvic impingement. Arthroscopy 30: 1026–41. [DOI] [PubMed] [Google Scholar]
- 7. Gollwitzer H. Ischiofemorales impingement. Arthroskopie 2014; 27: 98–101. [Google Scholar]
- 8. Stafford GH, Villar RN.. Ischiofemoral impingement. J Bone Joint Surg Br Vol 2011; 93-B: 1300–2. [DOI] [PubMed] [Google Scholar]
- 9. Taneja AK, Bredella MA, Torriani M.. Ischiofemoral impingement. Magn Reson Imaging Clin N Am 2013; 21: 65–73. [DOI] [PubMed] [Google Scholar]
- 10. Ganz R, Slongo T, Turchetto L. et al. The lesser trochanter as a cause of hip impingement: pathophysiology and treatment options. Hip Int 2013; 23(Suppl 9): S35–41. [DOI] [PubMed] [Google Scholar]
- 11. Schatteman J, Vanhoenacker FM, Somville J. et al. Ischiofemoral impingement due to a solitary exostosis. JBR-BTR 2015; 98: 39–42. [DOI] [PubMed] [Google Scholar]
- 12. Yoong P, Mansour R, Teh ack.. Multiple hereditary exostoses and ischiofemoral impingement: a case-control study. Skeletal Radiol 2014; 43: 1225–30. [DOI] [PubMed] [Google Scholar]
- 13. Viala P, Vanel D, Larbi A. et al. Bilateral ischiofemoral impingement in a patient with hereditary multiple exostoses. Skeletal Radiol 2012; 41: 1637–40. [DOI] [PubMed] [Google Scholar]
- 14. Ali AM, Teh J, Whitwell D, Ostlere S.. Ischiofemoral impingement: a retrospective analysis of cases in a specialist orthopaedic centre over a four-year period. Hip Int 2013; 23: 263–8. [DOI] [PubMed] [Google Scholar]
- 15. Backer MW, Lee KS, Blankenbaker DG. et al. Correlation of ultrasound-guided corticosteroid injection of the quadratus femoris with MRI findings of ischiofemoral impingement. AJR Am J Roentgenol 2014; 203: 589–93. [DOI] [PubMed] [Google Scholar]
- 16. Ali AM, Whitwell D, Ostlere SJ.. Case report: imaging and surgical treatment of a snapping hip due to ischiofemoral impingement. Skeletal Radiol 2011; 40: 653–6. [DOI] [PubMed] [Google Scholar]
- 17. Lee S, Kim I, Lee SM, Lee J.. Ischiofemoral impingement syndrome. Ann Rehabil Med 2013; 37: 143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howse EA, Mannava S, Tamam C. et al. Ischiofemoral space decompression through posterolateral approach: cutting block technique. Arthrosc Tech 2014; 3: e661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson MD, Keene JS.. Treatment of ischiofemoral impingement: results of diagnostic injections and arthroscopic resection of the lesser trochanter. J Hip Preserv Surg 2016; 3: 146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim WJ, Shin HY, Koo GH. et al. Ultrasound-guided prolotherapy with polydeoxyribonucleotide sodium in ischiofemoral impingement syndrome. Pain Pract 2014; 14: 649–55. [DOI] [PubMed] [Google Scholar]
- 21. López-Sánchez MC, Armesto Pérez V, Montero Furelos LÁ. et al. Pinzamiento isquiofemoral: dolor de cadera de causa poco frecuente. Reumatol Clín 2013; 9: 186–7. [DOI] [PubMed] [Google Scholar]
- 22. Safran M, Ryu J.. Ischiofemoral impingement of the hip: a novel approach to treatment. Knee Surg Sports Traumatol Arthrosc 2014; 22: 781–5. [DOI] [PubMed] [Google Scholar]
- 23. Tosun O, Cay N, Bozkurt M, Arslan H.. Ischiofemoral impingement in an 11-year-old girl. Diagn Interv Radiol 2012; 18: 571–3. [DOI] [PubMed] [Google Scholar]
- 24. Gomez-Hoyos J, Martin RL, Schroder R. et al. Accuracy of 2 clinical tests for ischiofemoral impingement in patients with posterior hip pain and endoscopically confirmed diagnosis. Arthroscopy 2016; 32: 1279–84. [DOI] [PubMed] [Google Scholar]
- 25. Park S, Lee HY, Cuong PM. et al. Supine versus standing radiographs for detecting ischiofemoral impingement: a propensity score-matched analysis. AJR Am J Roentgenol 2016; 206: 1253–63. [DOI] [PubMed] [Google Scholar]
- 26. Gomez-Hoyos J, Schroder R, Reddy M. et al. Femoral neck anteversion and lesser trochanteric retroversion in patients with ischiofemoral impingement: a case–control magnetic resonance imaging study. Arthroscopy 2016; 32: 13–8. [DOI] [PubMed] [Google Scholar]
- 27. Hatem MA, Palmer IJ, Martin HD.. Diagnosis and 2-year outcomes of endoscopic treatment for ischiofemoral impingement. Arthroscopy 31: 239–46. [DOI] [PubMed] [Google Scholar]
- 28. Tosun O, Algin O, Yalcin N. et al. Ischiofemoral impingement: evaluation with new MRI parameters and assessment of their reliability. Skeletal Radiol 2012; 41: 575–87. [DOI] [PubMed] [Google Scholar]
- 29. Maras OZ, Aydingoz U, Gormeli CA. et al. Ischiofemoral space on MRI in an asymptomatic population: normative width measurements and soft tissue signal variations. Eur Radiol 2015; 25: 2246–53. [DOI] [PubMed] [Google Scholar]
- 30. Papoutsi D, Daniels J, Mistry A. et al. Ischiofemoral impingement due to a lipoma of the ischiofemoral space. BMJ Case Rep 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sussman WI, Han E, Schuenke MD.. Quantitative assessment of the ischiofemoral space and evidence of degenerative changes in the quadratus femoris muscle. Surg Radiol Anat 2013; 35: 273–81. [DOI] [PubMed] [Google Scholar]
- 32. Hujazi I, Jones T, Johal S. et al. The normal ischiofemoral distance and its variations. J Hip Preserv Surg 2016; 3: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kivlan BR, Martin RL, Martin HD.. Ischiofemoral impingement: defining the lesser trochanter-ischial space. Knee Surg Sports Traumatol Arthrosc 2017; 25: 72–76. [DOI] [PubMed] [Google Scholar]
- 34. Singer AD, Subhawong TK, Jose J. et al. Ischiofemoral impingement syndrome: a meta-analysis. Skeletal Radiol 2015; 44: 831–7. [DOI] [PubMed] [Google Scholar]
- 35. Ricciardi BF, Fabricant PD, Fields KG. et al. What are the demographic and radiographic characteristics of patients with symptomatic extraarticular femoroacetabular impingement? Clin Orthop Relat Res 2015; 473: 1299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Truong WH, Murnaghan ML, Hopyan S. et al. Ischioplasty for femoroischial impingement. A case report 2012; 2: e51.. [DOI] [PubMed] [Google Scholar]
- 37. Johnson AC, Hollman JH, Howe BM. et al. Variability of ischiofemoral space dimensions with changes in hip flexion: an MRI study. Skeletal Radiol 2017; 46: 59–64. [DOI] [PubMed] [Google Scholar]
- 38. Adler KL, Cook PC, Yen YM. et al. Current concepts in hip preservation surgery: Part I. Sports Health 2015; 7: 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lachiewicz PF. Abductor tendon tears of the hip: evaluation and management. J Am Acad Orthop Surg 2011; 19: 385–91. [DOI] [PubMed] [Google Scholar]
- 40. Chandrasekaran S, Vemula SP, Gui C. et al. Clinical features that predict the need for operative intervention in Gluteus medius tears. Orthop J Sports Med 2015; 3: 2325967115571079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Davies H, Zhaeentan S, Tavakkolizadeh A. et al. Surgical repair of chronic tears of the hip abductor mechanism. Hip Int 2009; 19: 372–6. [DOI] [PubMed] [Google Scholar]
- 42. Whiteside LA. Surgical technique: Gluteus maximus and tensor fascia lata transfer for primary deficiency of the abductors of the hip. Clin Orthop Relat Res 2014; 472: 645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gurney B. Leg length discrepancy. Gait Posture 2002; 15: 195–206. [DOI] [PubMed] [Google Scholar]
- 44. Ahmad CS, Redler LH, Ciccotti MG. et al. Evaluation and management of Hamstring injuries. Am J Sports Med 2013; 41: 2933–47. [DOI] [PubMed] [Google Scholar]
- 45. Lempainen L, Banke IJ, Johansson K. et al. Clinical principles in the management of hamstring injuries. Knee Surg Sports Traumatol Arthrosc 2015; 23: 2449–56. [DOI] [PubMed] [Google Scholar]
- 46. Sonnery-Cottet B, Daggett M, Gardon R. et al. Surgical management of recurrent musculotendinous Hamstring injury in professional athletes. Orthop J Sports Med 2015; 3: 2325967115606393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mirza SB, Dunlop DG, Panesar SS. et al. Basic science considerations in primary total hip replacement arthroplasty. Open Orthop J 2010; 4: 169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Widmer KH. Containment versus impingement: finding a compromise for cup placement in total hip arthroplasty. Int Orthop 2007; 31(Suppl 1): 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Woelfle JV, Fraitzl CR, Reichel H. et al. Significantly reduced leg length discrepancy and increased femoral offset by application of a head–neck adapter in revision total hip arthroplasty. J Arthroplasty 2014; 29: 1301–7. [DOI] [PubMed] [Google Scholar]
- 50. Jo S, O'Donnell JM.. Endoscopic lesser trochanter resection for treatment of ischiofemoral impingement. J Hip Preserv Surg 2015; 2:184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.