Abbreviations
- AAV
adeno‐associated virus
- CYP7A1
cholesterol 7α‐hydroxylase
- FGF15
fibroblast growth factor in mice
- FGF19
fibroblast growth factor in humans
- FXR
farnesoid X receptor
- HCC
hepatocellular carcinoma
- HFFCD
high‐fat, high‐fructose, high‐cholesterol diet
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, affecting approximately 20%‐30% of the population in Western countries. Nonalcoholic steatohepatitis (NASH) is the progressive form of NAFLD that can develop into cirrhosis and hepatocellular carcinoma (HCC). However, the underlying mechanism of progression of steatosis to NASH and cirrhosis is poorly understood. Multiple factors, including insulin resistance, inflammation, oxidative stress, lipotoxicity, and bile acid toxicity, have been implicated in NASH progression. The bile acid‐activated receptors farnesoid X receptor (FXR) and G‐protein bile acid receptor‐1 are metabolic integrators that function in the regulation of hepatic metabolism and homeostasis. Bile acids are derived from cholesterol in the liver and are secreted into the gastrointestinal system for nutrient absorption. The gut‐to‐liver axis plays a critical role in the regulation of bile acid synthesis and homeostasis. In the intestine, bile acids activate FXR to induce fibroblast growth factor 15 (FGF15) in mice or FGF19 in humans (Fig. 1). FGF15 and FGF19 are secreted into portal circulation to activate the hepatic FGF receptor 4/β‐Klotho complex, which inhibits cholesterol 7α‐hydroxylase (CYP7A1), the key regulatory enzyme in bile acid synthesis. Activation of FXR by bile acids and FXR agonists has been shown to reduce hepatic lipogenesis and improve glucose tolerance and insulin resistance in diet‐induced obese mouse models.
Figure 1.
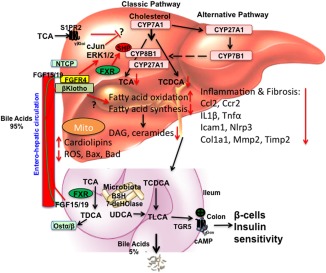
Targeting to bile acid and lipid metabolism in NASH treatment. Bile acids are synthesized from cholesterol in hepatocytes. Cyp7a1 is the first and rate‐limiting enzyme in the classic bile acid synthesis pathway to synthesize CA and CDCA. Cyp8b1 is required for synthesis of CA. Cyp27a1 is involved in steroid side‐chain oxidation. The alternative pathway is initiated by Cyp27a1, followed by Cyp7b1. Bile acids are conjugated to amino acids glycine and taurine for secretion into bile. In mice, CDCA is converted to α‐ and β‐muricholic acids. CDCA activates FXR to induce a negative factor, SHP, which inhibits Cyp7a1 and Cyp8b1 gene transcription. Bile acids are stored in the gallbladder and secreted into the gastrointestinal tract for nutrient absorption. In the intestine, conjugated bile acids are deconjugated by bacterial BSH and bacterial 7‐dehydroxylase activities convert CA and CDCA to DCA and LCA, respectively. In the ileum, FXR induces FGF15 (or human FGF19). Bile acids and FGF15/FGF19 are secreted into portal blood for circulation to the liver. In hepatocytes, FGF15 and FGF19 activate the FGF receptor 4/β‐Klotho complex to activate cJun/ERK1/2 of the MAPK pathway to inhibit Cyp7a1 gene transcription. Conjugated bile acid‐activated S1PR2 may also be involved in inhibiting Cyp7a1. In NAFLD and NASH, hepatic bile acid and lipid metabolisms are impaired to accumulate toxic bile acids and lipids along with cholestatic liver injury. Ceramides and diacylglycerols have been linked to insulin resistance in diabetes. FGF19 and M70 inhibit bile acid synthesis and reduce bile acid hydrophobicity. FGF19 and M70 stimulate fatty acid oxidation and inhibit fatty acid synthesis to reduce lipogenesis, ceramides, and diacylglycerol and to increase unoxidized‐cardiolipins in mitochondria; reduced ROS reduces inflammatory cytokines and chemokines (CCl2, Ccr2, IL‐1β, Tnf‐α), fibrosis markers (Col1a1, Timp2), and apoptosis markers (Bax, Bad). In colon, activation of TGR5 stimulates cAMP and secretion of GLP‐1 to improve insulin sensitivity. Abbreviations: BSH, bile salt hydrolase; CA, cholic acid; cAMP, cyclic adenosine monophosphate; CCl2, (C‐C motif) ligand 2; Ccr2, (C‐C motif) receptor 2; CDCA, chenodeoxycholic acid; Col1a1, collagen, type I, alpha 1; Cyp27a1, mitochondria sterol 27‐hydroxylase; Cyp7a1, cholesterol 7α‐hydroxylase; Cyp7b1, oxysterol 7α‐hydroxylase; Cyp8b1, sterol 12α‐hydroxylase; DAG, dystroglycan; DCA, deoxycholic acid; ERK, extracellular signal‐regulated kinase; GLP‐1, glucagon‐like peptide‐1; Icam1, intercellular cell adhesion molecule 1; IL‐1β, interleukin‐1β; LCA, lithocholic acid; MAPK, mitogen‐activated protein kinase; Mmp2, matrix metalloproteinase 2; Nlrp3, nucleotide‐binding domain and leucine‐rich repeat containing protein 3; NTCP, sodium‐taurocholate cotransporting polypeptide; Ostα/β, organic solute transporter alpha/beta; ROS, reactive oxygen species; S1PR2, sphingosine‐1‐phosphate receptor 2; SHP, small heterodimer partner; TCA, trichloroacetic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid; TGR5, G protein‐coupled bile acid receptor 1; Timp2, tissue inhibitor of metalloproteinase 2; TLCA, taurolithocholic acid; Tnf‐α, tumor necrosis factor alpha; UDCA, ursodeoxycholic acid.
Bile acid derivatives that activate FXR have been developed as therapeutic drugs for treating cholestatic liver diseases and NASH.1, 2 A synthetic bile acid, obeticholic acid, has been approved by the U.S. Food and Drug Administration for primary biliary cirrhosis, and currently patients are being recruited for phase 3 of clinical trials for NASH fibrosis. Synthetic bile acids are safe and devoid of side effects but have inherent problems for bile acids, including pruritus and reducing serum high‐density lipoprotein cholesterol levels.
Nonbile acid‐based drugs, such as FGF19, have been developed for treating chronic liver disease (http://www.clinicaltrials.gov, NCT02443116). FGF19 is a postprandial insulin‐independent activator of hepatic protein and glycogen synthesis.3 The hepatic response to FGF19 is impaired in NAFLD patients with insulin resistance,4 and fasting FGF19 levels are reduced in NAFLD patients.5 FGF19 has been shown to stimulate energy metabolism and reduces steatosis by reducing fatty acid synthesis, increasing fatty acid oxidation, and stimulating adipose tissue browning. However, the underlying mechanism of how FGF19 protects against bile acid‐induced liver injury and inflammation is not clear and requires further study.
As a growth factor, FGF19 has tumorigenic activity. FGF19 transgenic mice have been shown to develop HCC. To eliminate the tumorigenic activity, an FGF19 variant, M70, has been shown to inhibit CYP7A1 expression without causing liver injury in cholestatic mouse models.6 In this issue of Hepatology Communications, Zhou et al.7 reported a detailed study of FGF19 and M70 in reducing bile acid toxicity and lipotoxicity to resolve NASH in mice. A high‐fat (40% Kcal), high‐fructose (20% Kcal), and high‐cholesterol (2%) diet (HFFCD) was used to induce NASH in mice. This high‐fat diet has been widely used to rapidly induce steatosis in 1 month, NASH‐like steatohepatitis with fibrosis in 8 months, and advanced NASH in 20 months. Adeno‐associated virus‐mediated transduction of FGF19 (AAV‐FGF19) and AAV‐M70 suppressed Cyp7a1 messenger RNA expression in HFFCD‐fed mice and significantly reduced all bile acids in hepatocytes and the total bile acid pool size. Lipidomic analysis showed that AAV‐M70 significantly reduced diacylglycerols, triglycerides, and ceramides along with messenger RNA levels of enzymes involved in fatty acid, diacylglycerol, and ceramide synthesis, which are increased in HFFCD‐fed mice. Elevated levels of diacylglycerols and ceramides in hepatocytes have been linked to steatosis and insulin resistance. M70 increased unoxidized cardiolipins in mitochondria and reduced reactive oxidizing species in these mice. Similarly, AAV‐M70 and AAV‐FGF19 reduced apoptosis factors involved in cell death; reduced inflammatory chemokines, cytokines, and fibrosis markers; and stimulated insulin sensitivity and glucose tolerance in HFFCD‐fed mice. Furthermore, AAV‐FGF19 but not AAV‐M70 induced HCC in diet‐induced NASH mice. In summary, Zhou et al. provide evidence that M70 was effective in reducing bile acid toxicity, lipotoxicity, liver inflammation, and NASH progression and improved insulin and glucose tolerance without causing HCC in a diet‐induced NASH mouse model. However, this study did not unveil the underlying mechanisms of FGF19/M70 in reducing lipogenesis and improving glucose and insulin tolerance. Activation of the FGF receptor 4/β‐Klotho complex may activate the c‐Jun N‐terminal kinase/c‐Jun and extracellular signal‐regulated kinase 1/2 activity of the mitogen‐activated protein kinase pathways, and the small heterodimer partner may also be involved to inhibit Cyp7a1 (Fig. 1). Conjugated bile acid‐activated sphingosine‐1‐phosphate receptor 2 signaling may also be involved in inhibiting Cyp7a1. The underlying mechanism of these signaling pathways in inhibiting bile acid synthesis and lipogenesis remains to be further studied. A recent study reported that activation of intestinal FXR induced all enzymes involved in ceramide synthesis.8 It is not clear how FGF19/M70 reduces ceramide synthesis in hepatocytes. Ceramide is synthesized from serine and palmitoyl‐coenzyme A, and increasing hepatic fatty acids may stimulate ceramide and sphingolipid synthesis. Adiponectin stimulates ceramide clearance by activating ceramidase, which converts ceramides to sphingosines. Thus, a decrease in adiponectin reduces ceramide clearance and increases ceramide accumulation in hepatocytes, which increases endoplasmic reticulum stress and reactive oxygen species in mitochondria.
This report also did not study the role of FGF19/M70 on gut microbiota. Gut microbiota regulate bile acid metabolism, bile acid pool size, hydrophobicity, and enterohepatic circulation of bile acids. Several recent studies reported paradoxical effects of intestinal FXR on obesity and NAFLD. Deficiency of intestinal FXR, antagonism of intestinal FXR by glycine‐muricholic acid, and an antioxidant tempol all alter gut microbiota and increase bile acid synthesis to reduce diet‐induced obesity and insulin resistance in mice.8, 9, 10 Probiotics promote bile acid conjugation and bile salt secretion to increase bile acid synthesis and prevent gut‐related diseases.11 On the other hand, activation of intestinal FXR by the intestine‐restricted FXR agonist fexaramine also improves obesity and insulin resistance by inducing FGF15 in mice. FXR agonists and antagonists may shape gut microbiota differently to exert beneficial effects during diet‐induced obesity and insulin resistance. It would be of interest to analyze the gut microbiome of FGF19/M70‐treated mice to unveil the mechanism by which M70 reduces bile acid and lipotoxicity in diabetes and obesity.
Potential conflict of interest: Nothing to report.
Supported by National Institutes of Health Blueprint for Neuroscience Research awards DK44442 and DK58379.
REFERENCES
- 1. Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al.; NASH Clinical Research Network . Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 2015;385:956‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, et al.; POISE Study Group . A placebo‐controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 2016;375:631‐643. [DOI] [PubMed] [Google Scholar]
- 3. Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino‐Powell K, et al. FGF19 as a postprandial, insulin‐independent activator of hepatic protein and glycogen synthesis. Science 2011;331:1621‐1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schreuder TC, Marsman HA, Lenicek M, van Werven JR, Nederveen AJ, Jansen PL, et al. The hepatic response to FGF19 is impaired in patients with nonalcoholic fatty liver disease and insulin resistance. Am J Physiol Gastrointest Liver Physiol 2010;298:G440‐G445. [DOI] [PubMed] [Google Scholar]
- 5. Wojcik M, Janus D, Dolezal‐Oltarzewska K, Kalicka‐Kasperczyk A, Poplawska K, Drozdz D, et al. A decrease in fasting FGF19 levels is associated with the development of non‐alcoholic fatty liver disease in obese adolescents. J Pediatr Endocrinol Metab 2012;25:1089‐1093. [DOI] [PubMed] [Google Scholar]
- 6. Luo Y, Tall AR. Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J Clin Invest 2000;105:513‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou M, Learned RM, Rossi SJ, DePaoli AM, Tian H, Ling L. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol Commun 2017;1:1024‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie C, Jiang C, Shi J, Gao X, Sun D, Sun L, et al. An intestinal farnesoid X receptor‐ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 2017;66:613‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 2013;4:2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest 2015;125:386‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr‐Fgf15 axis in mice. Cell Rep 2014;7:12‐18. [DOI] [PubMed] [Google Scholar]


