Abstract
Periostin, a secreted matricellular protein, has been reported to induce epithelial‐mesenchymal transition (EMT), which increases motility and invasiveness in various epithelial cancer cells. Periostin is also overexpressed in intrahepatic cholangiocarcinoma (ICC) and suggested to be a biomarker for tumor progression and poor prognosis; however, its functional role in ICC is not fully understood. Here, we investigated whether periostin influences malignant potential through the induction of EMT in ICC. Analyses of surgical resected ICC specimens revealed that the gene expression of periostin was significantly higher in ICC tumors than in adjacent nontumor liver tissues and was closely correlated with the expression of mesenchymal markers, including N‐cadherin, vimentin, and fibronectin. However, the expression level of periostin varied in each case. Consistently, the expression of periostin in HuH28 (an undifferentiated ICC cell) was markedly higher than in HuCCT‐1 (a moderately differentiated ICC cell). In addition, high‐level secretion of periostin into culture media was observed in HuH28 but not in HuCCT‐1. To identify the biological significance of periostin in EMT, gene silencing of periostin by small interfering RNA was performed in HuH28 cells. Periostin knockdown in HuH28 cells significantly down‐regulated mesenchymal markers and up‐regulated epithelial markers, suggesting the reversal of EMT, namely mesenchymal‐epithelial transition. Along with these changes, cell proliferation was significantly suppressed by 52%. In addition, cell migration and invasion were significantly suppressed by 62% and 61%, respectively, with reduced gene expression of matrix metalloproteinase 2. Interestingly, chemosensitivity to gemcitabine was also significantly improved by periostin depletion. Conclusion: Periostin plays an important role in the regulation of malignant potential through EMT and is suggested to be a novel target for the treatment of ICC. (Hepatology Communications 2017;1:1099–1109)
Abbreviations
- αSMA
alpha smooth muscle actin
- BrdU
bromodeoxyuridine
- CK19
cytokeratin 19
- ELISA
enzyme‐linked immunosorbent assay
- EMT
epithelial‐mesenchymal transition
- FBS
fetal bovine serum
- Gem
gemcitabine
- HSC
hepatic stellate cell
- ICC
intrahepatic cholangiocarcinoma
- MMP
matrix metalloproteinase
- PCR
polymerase chain reaction
- siRNA
small interfering RNA
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary malignancy in the liver and has a poor prognosis because of the late onset of symptoms, high recurrence rate after surgical resection, and limited effective nonsurgical therapies.1 Although epidemiologic studies have revealed risk factors for ICC, including primary sclerosing cholangitis, liver fluke infection, and chemical carcinogens, such as nitrosamines, the precise etiology and pathogenesis remain incompletely understood.2, 3, 4 ICC incidence and mortality continue to increase worldwide, particularly in Western countries; therefore, the pathophysiologic mechanisms that regulate tumor progression and metastasis of this cancer need to be explored.
Periostin is a nonstructural matricellular protein originally isolated from a mouse osteoblast cell line.5, 6 In addition to its ability to interact with other extracellular matrix components, periostin regulates cell adhesion, migration, and proliferation and alters cell phenotypes through binding to cell‐surface receptor integrins.7 Analyses of periostin−/− mice revealed that periostin is required for tissue development and repair.8, 9, 10 Periostin is also involved in the pathogenesis of proliferative vitreoretinopathy and allergic inflammation.11, 12
Periostin is implicated in the tumorigenesis and progression of various cancers, including breast, lung, colon, pancreatic, and ovarian cancers.13 In cancer cells, the binding of periostin to integrins activates phosphoinositide 3‐kinase/protein kinase B‐mediated and focal adhesion kinase‐mediated signaling pathways, which lead to increased cell survival, angiogenesis, invasion, metastasis, and epithelial‐mesenchymal transition (EMT).14 In ICC, the serum level of periostin is significantly elevated and thus may be a novel serodiagnostic marker for this disease.15 In addition, high periostin expression, as assessed by immunostaining, was reported as an independent risk factor for the poor prognosis of ICC patients after hepatectomy.16 Thus, periostin is a potentially important modulator of ICC progression and metastasis.
EMT is a reversible and dynamic process in which epithelial cells acquire the structural and functional characteristics of mesenchymal cells.17 EMT is an early event of metastasis that is required for tumor cell migration and invasion from the primary site. EMT consists of the repression of genes related to an epithelial phenotype, including E‐cadherin and β‐catenin, concomitantly with the activation of genes related to a mesenchymal phenotype, including N‐cadherin, vimentin, fibronectin, and matrix metalloproteinase (MMPs). The acquisition of a mesenchymal‐like phenotype endows the tumor cells with invasive properties; therefore, EMT is an important factor that regulates the malignant potential of cancer cells.
Hepatic stellate cells (HSCs) are a major source of extracellular matrix during the development of hepatic fibrosis. When the liver is injured, quiescent HSCs transdifferentiate into myofibroblasts and thus acquire the ability to proliferate, contract, and migrate with a switch from E‐ to N‐cadherin expression as well as the increased expression of mesenchymal markers, including α smooth muscle actin (αSMA), vimentin, and fibronectin.18, 19 This phenotype change raises the interesting prospect that HSCs undergo EMT. We previously reported that the gene silencing of periostin led to the deactivation of HSCs and down‐regulation of profibrotic markers, including αSMA. These observations raise intriguing hypotheses that depletion of periostin in ICC may reverse EMT.
In the current study, we investigated the expression of periostin in human ICC tissues and tumor cell lines. To examine its biological significance in EMT and malignant cell behavior, we used small interfering RNA (siRNA) to knockdown periostin in an ICC cell line that overexpresses this protein.
Materials and Methods
SURGICAL RESECTED TISSUES
ICC tissues were obtained from patients who underwent surgery in the Department of Gastroenterological and Transplant Surgery at Hiroshima University Hospital. The tissues collected at the time of surgery were kept in RNAlater (Thermo Fisher Scientific, Waltham, MA) until analysis. A total of 11 ICC tissues were subjected to quantitative real‐time polymerase chain reaction (PCR). All procedures using clinical patients' samples were approved by the Ethical Committee of Hiroshima University Hospital.
CELL LINES AND CELL CULTURE
Human undifferentiated ICC cells (HuH28), moderately differentiated ICC cells (HuCCT1), hepatocellular carcinoma cells (HepG2), and hepatocellular cells (HuH7) cell lines were obtained from the Japanese Collection of Research Bioresources Cell Bank. Immortalized human cholangiocyte (MMNK1) cells were a kind gift from the National Institute for Environmental Studies in Ibaraki, Japan.20 The cells were cultured in Dulbecco's modified eagle's medium (Wako Pure Chemical Industries Ltd., Osaka, Japan) containing 10% fetal bovine serum (FBS) (Invitrogen, Grand Island, NY) and incubated in 95% air and 5% CO2 at 37°C.
QUANTITATIVE REAL‐TIME PCR
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Quantitative real‐time PCR was performed as described.8 The specific primer sequences are listed in Supporting Table S1.
WESTERN BLOT ANALYSIS
Cells were lysed with radioimmunoprecipitation assay buffer and centrifuged at 10,000g for 5 minutes. Five micrograms of protein from the supernatant was subjected to western blot analysis with primary antibodies against periostin (Shino‐Test Corp., Tokyo, Japan), E‐cadherin (ab76055; Abcam, Cambridge, United Kingdom), N‐cadherin (ab76011; Abcam), vimentin (ab92547; Abcam), and glyceraldehyde‐3 phosphate dehydrogenase (G9545; Sigma‐Aldrich, St Louis, MO).
GENE SILENCING WITH siRNA
siRNAs specific to human periostin, αv integrin (Invitrogen), and a nonsilencing negative control (Sigma‐Aldrich Japan, Hokkaido, Japan) were transfected using Lipofectamine RNAiMAX (Invitrogen) as described.8 The siRNA sequences used in this study were as follows: human periostin, 5′‐ rGrArCrA rArCrArArAUrGrGUrGUrArAUUTT‐3′ and 5′‐rArAUUrArCrArCrCrAUUUrGUUrGUrCTT‐3′; human αv integrin, 5′‐rCUUUrArCUrGrCUrGrAUr ArGUrGrCUTT‐3′ and 5′‐rArGrCrArCUrAUrCrAr GrCrArGUrArArArGTT‐3′.
BROMODEOXYURIDINE CELL PROLIFERATION ASSAY
Cell proliferation was analyzed using a bromodeoxyuridine (BrdU)‐based colorimetric immunoassay. After 3 days of siRNA transfection, cells were seeded at a density of 1 × 104 cells/well in 96‐well plates and incubated for 24 hours. Following an additional 24 hours of serum starvation, cells were analyzed with a BrdU Cell Proliferation Assay kit (Millipore, Schwalbach, Germany) according to the manufacturer's instructions. Absorbance values of the samples were determined using a spectrophotometric plate reader at 450 nm.
INVASION ASSAY
Cell invasion was determined using a CytoSelect 24‐well Cell Invasion Assay kit with basement membrane‐coated inserts (Cell Biolabs, San Diego, CA). After 6 days of siRNA transfection, HuH28 cells were serum starved for 24 hours. Next, 3 × 105 cells per insert were cultured in the upper chamber containing a serum‐free medium. Medium containing 10% FBS was applied to the lower chamber as a chemoattractant. After 48 hours incubation at 37°C in 5% CO2, cells remaining above the insert were gently scraped off with a cotton swab. The invaded cells that had adhered to the lower surface of the filter were fixed with 1% formalin and stained with crystal violet for visualization. The cell lysate was quantified with a spectrometer at 560 nm.
TRANSWELL MIGRATION ASSAY
After 6 days of siRNA transfection, a total of 5 × 104 cells were incubated in FBS‐free Dulbecco's modified eagle's medium for 24 hours and added to the top of a transwell membrane in the upper chamber (8.0 μm pore; Corning, NY). Following a 48‐hour incubation period, migrated cells were visualized and quantified.
EVALUATION OF CHEMOSENSITIVITY TO GEMCITABINE
HuH28 cells were transfected with siRNA targeting periostin or a control siRNA 7 days prior to gemcitabine (Gem) treatment. Cells were plated in 96‐well plates at a density of 5 × 103 cells/well and then treated with Gem hydrochloride (Wako) at different final concentrations diluted with phosphate‐buffered saline (1 × 10–5 to 1 × 10–2 M). Cell viability was assayed 72 hours after Gem treatment using Cell Counting Kit‐8 (Dojindo Laboratories, Kumamoto, Japan).
CELL APOPTOSIS ASSAY
Cell apoptosis was evaluated using the APOSTRAND enzyme‐linked immunosorbent assay (ELISA) apoptosis detection kit (Enzo Life Sciences, Farmingdale, NY) according to the manufacturer's instructions.
ELISA
The concentrations of periostin in the culture media were measured with a human periostin ELISA kit (Shino‐Test) according to the manufacturer's instructions.
STATISTICAL ANALYSIS
Data were analyzed with a two‐way analysis of variance or two‐sided unpaired or paired Student t tests. Results were expressed as mean ± SEM. Values were considered significant when P < 0.05.
Results
Periostin overexpression correlated with the levels of mesenchymal markers in ICC. Gene expression was analyzed in a total of 11 ICC tissues and adjacent nontumor liver tissue. Periostin expression was significantly higher in tumor tissue compared with nontumor tissue; however, the expression level varied in each ICC case (Fig. 1A). The expression level of periostin closely correlated with the expression of mesenchymal markers N‐cadherin (R = 0.767; P = 0.0058), vimentin (R = 0.9613; P = 2.4E‐6), and fibronectin (R = 0.8023; P = 0.0029) (Fig. 1B). In addition, the expression level of E‐cadherin, an epithelial marker, tended to be negatively associated with periostin expression. These results suggest a potential link between periostin expression and EMT regulation in ICC.
Figure 1.
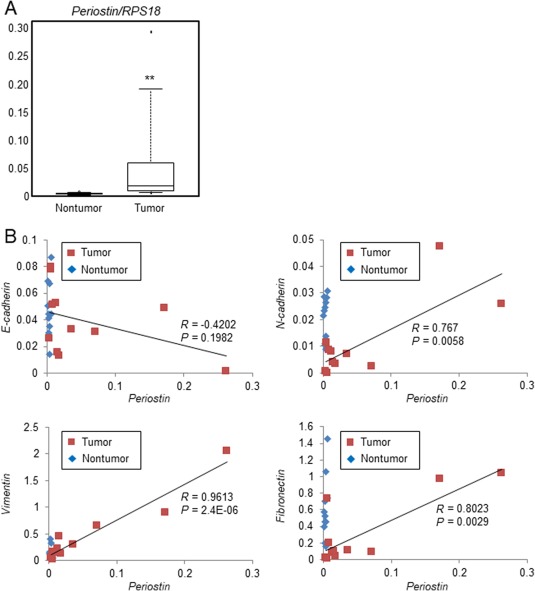
Expression levels of periostin and mesenchymal markers in ICC tissues. Surgical ICC tissues (n = 11) were subjected to real‐time PCR for the quantification of periostin and EMT‐related markers. (A) The gene expression of periostin was compared in ICC tissues and adjacent nontumor tissues. Data are shown as box plots that indicate the minimum, first quartile, median, third quartile, and maximum. Statistical comparisons were completed using a two‐sided paired Student t test; **P < 0.01. (B) Associations between the levels of periostin (x axis) and each EMT‐related marker (y axis) are shown in scatter plots. Abbreviation: RPS18, ribosomal protein S18.
PERIOSTIN EXPRESSION WAS ASSOCIATED WITH MESENCHYMAL MARKERS IN ICC CELL LINES
A wide range of periostin expression levels was observed in clinical ICC samples; therefore, two distinct ICC cell lines, HuH28 (an undifferentiated ICC cell line) and HuCCT1 (a moderately differentiated ICC cell line), were used to compare periostin expression levels. There was abundant periostin expression in HuH28 cells, whereas HuCCT1 cells and MMNK1 cells, an immortalized cholangiocyte cell line, showed faint periostin expression (Fig. 2A). Accordingly, periostin levels secreted into the culture media were extremely high in HuH28 cells only. These data suggest that periostin expression depends on the cellular differentiation of ICC.
Figure 2.
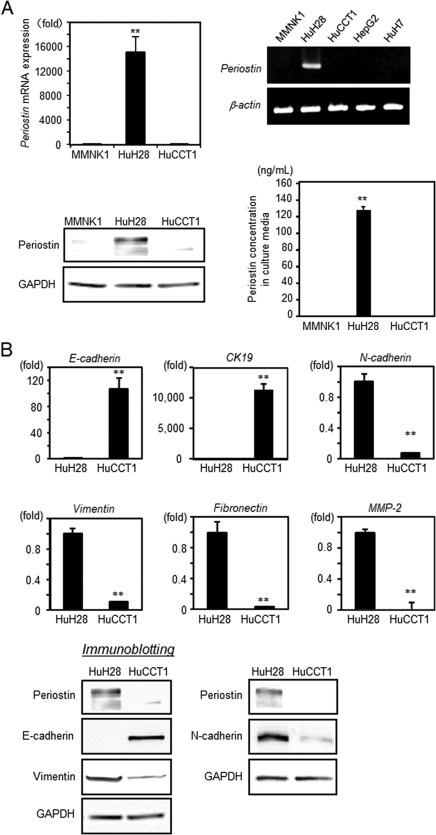
Expression levels of periostin and mesenchymal markers in ICC cell lines. (A) Periostin expression in MMNK1, HuH28, and HuCCT1 cells was investigated using real‐time PCR (n = 6) (upper panels) and immunoblotting (lower left panel). Periostin concentrations in culture media after 72 hours were measured using ELISA (n = 6) (lower right panel). Data were analyzed with a two‐way analysis of variance and expressed as the mean ± SEM. (B) The expression of EMT‐related markers in HuH28 and HuCCT1 cells was assessed using real‐time PCR (n = 6) and immunoblotting. Statistical comparisons were performed with a two‐sided unpaired Student t test. Data are expressed as the mean ± SEM; **P < 0.01. Abbreviations: GAPDH, glyceraldehyde‐3 phosphate dehydrogenase; mRNA, messenger RNA.
As demonstrated in human clinical samples (Fig. 1B), the expression of periostin was associated with the expression of mesenchymal markers. We hypothesized that HuH28 cells, which express abundant periostin, exhibited a mesenchymal phenotype. Real‐time PCR revealed a markedly high expression of mesenchymal markers, including N‐cadherin, vimentin, fibronectin, and MMP2, in HuH28 cells compared to HuCCT1 and MMNK1. In contrast, epithelial markers, including E‐cadherin and cytokeratin (CK)19, were significantly higher in HuCCT1 cells (Fig. 2B, upper panels). Our immunoblot analysis confirmed that the expression level of periostin correlated with the overexpression of mesenchymal markers and down‐regulation of epithelial markers (Fig. 2B, lower panels).
GENE SILENCING OF PERIOSTIN REVERSES EMT IN HuH28 CELLS
To explore the functional role of periostin in EMT, we silenced the gene expression of periostin in HuH28 cells. Periostin expression was sufficiently suppressed at both the messenger RNA and protein level after 1 day of siRNA transfection, and these levels remained low for 7 days (Fig. 3A,C). The amount of periostin secreted into the culture media was also significantly reduced in HuH28 cells transfected with siRNA targeting periostin. Interestingly, the expression of mesenchymal markers, including N‐cadherin, vimentin, and fibronectin, were significantly reduced concomitantly with an increase in the expression of the epithelial markers E‐cadherin and CK19 (Fig. 3B,C). These findings indicate that suppression of periostin reverts ICC cells to an epithelial‐like state; therefore, periostin is a critical regulator of EMT.
Figure 3.
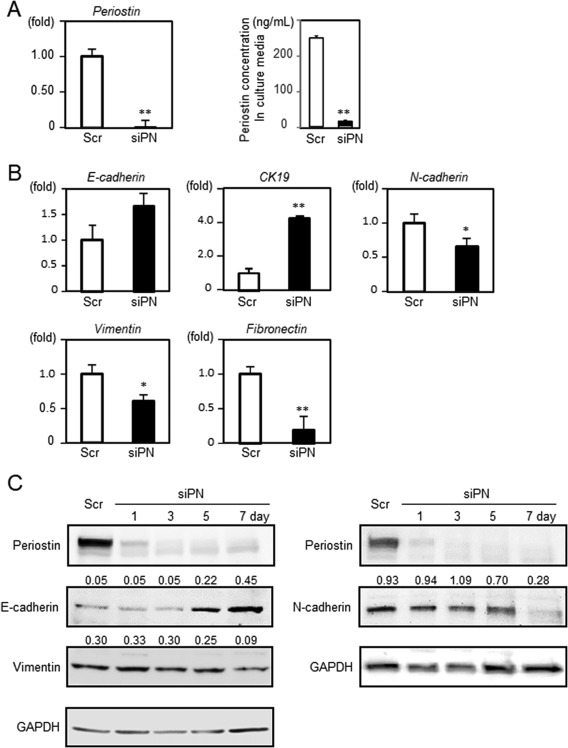
Reversal of EMT induced by periostin knockdown in HuH28 cells. HuH28 cells were transfected with siRNA targeting human periostin or a nontargeting scrambled control. (A) Knockdown of periostin was confirmed using real‐time PCR (n = 6). The periostin concentration in culture media after 7 days of transfection was quantified using ELISA (n = 3). (B) Gene expression of epithelial (E‐cadherin and CK19) and mesenchymal (N‐cadherin, vimentin, and fibronectin) markers was analyzed using real‐time PCR after 7 days of siRNA transfection. (C) Protein expression of periostin and EMT‐related markers was determined using immunoblotting. Data are expressed as the mean ± SEM; *P < 0.05, **P < 0.01. Abbreviations: GAPDH, glyceraldehyde‐3 phosphate dehydrogenase; Scr, scrambled control; siPN, siRNA targeting human periostin.
DEPLETION OF PERIOSTIN REDUCES THE MALIGNANT POTENTIAL OF ICC CELLS
EMT promotes tumor invasion and metastasis as well as chemoresistance. Accordingly, we investigated the influence of periostin depletion on cellular phenotype with a focus on malignant potential. Periostin knockdown significantly reduced cell proliferation by 52%, as assessed by a BrdU assay, and significantly down‐regulated the cell proliferation markers Ki67 and proliferative cell nuclear antigen (Fig. 4A). Unexpectedly, periostin knockdown modestly but significantly reduced baseline cell apoptosis with down‐regulated messenger RNA expression of Bax, an apoptosis‐related gene (Supporting Fig. S1). The ability of invasion and migration is important for the metastasis of tumor cells. Thus, we performed an in vitro chamber assay. The transwell migration assay demonstrated that periostin depletion in HuH28 cells significantly reduced cell migration by 62% (Fig. 4B). An invasion assay also demonstrated a significant decrease in the number of HuH28 cells invading through the basement membrane‐coated insert by 61% after periostin depletion (Fig. 4C). This finding was partly explained by the down‐regulation of MMPs in periostin‐depleted HuH28 cells. In addition, despite reduced baseline cell apoptosis, chemosensitivity to Gem was significantly increased when periostin was silenced with siRNA (Fig. 4D). Collectively, these observations suggest that periostin promotes malignant potential by regulating cell proliferation, migration/invasion, and chemoresistance in ICC.
Figure 4.
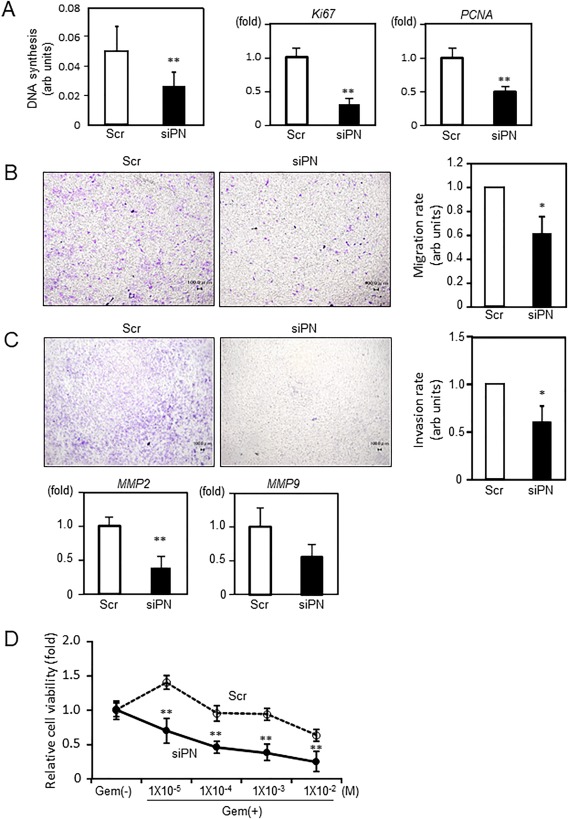
Regulation of malignant potential by periostin in ICC cells. HuH28 cells were transfected with siRNA targeting human periostin or a nontargeting scrambled control. (A) Cell proliferation was evaluated using a BrdU assay after 7 days of transfection (n = 11‐12) (left panel) and real‐time PCR for Ki67 and PCNA expression (middle and right panels). (B) HuH28 cells transfected with siRNA (siPN or Scr) were seeded into the upper chamber in serum‐free conditions, and cells migrating toward the lower chamber filled with media containing 10% FBS were visualized (left panels) and quantified (right panel). (C) Transfected cells were seeded into the basement membrane‐coated upper chamber in serum‐free conditions. Medium containing 10% FBS was applied to the lower chamber. The invaded cells were visualized (left panels) and quantified (right panel). The expression of MMP2 and MMP9 was quantified using real‐time PCR (n = 6). For the quantification of migration and invasion, four independent experiments were performed with each control value set at 1.0 for comparison. (D) Chemosensitivity to Gem was compared after 7 days of siRNA transfection. Relative cell viabilities were assessed 72 hours after Gem treatment using Cell Counting Kit‐8 (n = 3). Data are expressed as the mean ± SEM; *P < 0.05, **P < 0.01. Abbreviations: arb, arbitrary; PCNA, proliferative cell nuclear antigen; Scr, scrambled control; siPN, siRNA targeting human periostin.
Discussion
We determined that periostin is a key molecule for the regulation of EMT in ICC. Our analysis of clinical resected samples and ICC cell lines demonstrated that the expression level of periostin was closely associated with that of mesenchymal markers. In addition, the silencing of periostin in ICC cells decreased mesenchymal markers in conjunction with an increase in epithelial markers. Accompanied with this reversal of EMT, ICC cell proliferation, migration, and invasion were significantly reduced and the sensitivity to Gem was markedly improved, suggesting that periostin regulates malignant potential through induction of EMT in ICC.
EMT is a reversible process by which epithelial cells lose cell polarity and cell–cell adhesion and gain the structural and functional characteristics of a mesenchymal phenotype.17 EMT was recently found to play a crucial role in cancer cell metastasis and the development of resistance to chemotherapy.21 Thus, the regulation of EMT in tumor cells may be a novel strategy for the treatment of cancer.
Accumulating evidence suggests that periostin is a critical modulator of EMT in different types of cancer. A previous study demonstrated that the ectopic expression of periostin in 293T cells, which are tumorigenic but not metastatic, induced EMT and promoted cell invasion and metastasis in vivo.22 A similar observation was reported in a prostate cancer cell line that was manipulated using a lentiviral vector to overexpress periostin.23 In contrast, the overexpression of periostin reversed EMT and suppressed cell invasiveness in bladder cancer cells, suggesting that the functional role of periostin in cancer cells is cell‐type dependent.24 Our data revealed that the expression of periostin positively correlated with the expression of mesenchymal markers in ICC tissues and cell lines. Furthermore, the down‐regulation of intrinsic periostin in ICC cells reversed EMT. These results suggest that periostin promotes EMT in ICC. The prognostic significance of EMT‐related markers has been increasingly reported in ICC.25, 26, 27 In addition, a previous in vitro study using ICC cells demonstrated that exogenous periostin stimulation promoted proliferation and invasion through the integrin α5/phosphoinositide 3‐kinase/protein kinase B pathway.16, 28
One of our interesting findings was that the reversal of EMT gradually progressed after periostin depletion, as shown by the protein levels of EMT‐related markers (Fig. 3C). The periostin concentration in culture media was markedly reduced following depletion of endogenous periostin; thus, the autocrine loop likely plays an important role in the reversal of EMT. Periostin has been reported to interact with integrin αvβ3, αvβ5, α5β1, and α6β4 and modulate cancer cell behavior.14, 28 Consistently, the expression of integrin αv and α5 was significantly higher in HuH28 compared to MMNK‐1 or HuCCT‐1 (Supporting Fig. S2). In addition, knockdown of αv integrin reduced periostin secretion almost by half with modest down‐regulation of mesenchymal markers, suggesting αv integrin as one of the functional receptors for the autocrine loop (Supporting Fig. S2). However, further analysis of the downstream signaling pathway is required to confirm this mechanism.
Accumulating reports demonstrate that periostin is overexpressed in ICC tissues. Most reports showed that periostin protein was detected in αSMA‐positive cells within the desmoplastic connective tissue stroma, whereas the cancer cells themselves showed no immunoreactivity, thus suggesting the major source of periostin is myofibroblastic cancer‐associated fibroblasts in ICC.15, 16, 29 In contrast, the report by Reiner et al.30 demonstrated strong periostin expression in tumor cells and stromal cells. These conflicting data are clearly explained by our findings that HuH28 cells, an undifferentiated ICC cell line, expressed abundant periostin whereas HuCCT‐1 cells, a moderately differentiated ICC cell line, showed negligible periostin expression. This suggests that periostin is secreted by ICC itself depending on ICC cell type. Consistently, the expression level of periostin in resected tumor specimens differed for each case.
In conclusion, periostin plays a pivotal role in the regulation of malignant potential, including cell proliferation, migration/invasion, and chemoresistance in ICC through the induction of EMT, presumably through a positive feedback loop. Although further studies in animal models should be conducted to better understand the effect of periostin depletion on tumor progression and metastasis, our data suggest that periostin is a potential target for the treatment of ICC.
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1114/full.
Supporting Information
Supporting Information
Supporting Information
Acknowledgment
We thank Mika Nakashima and Sayaka Yonezawa for technical assistance. Experiments were performed, in part, at the Analysis Center of Life Science, Hiroshima University.
Potential conflict of interest: Dr. Izuhara received a research grant from Chugai Pharmaceutical Co. Ltd. and an unrestricted grant from Shino‐Test Corp. Ltd. and serves as a consultant to Chugai Pharmaceutical Co. Ltd. The other authors have nothing to report.
Supported by Grant‐in‐Aids from the Ministry of Health, Labor, and Welfare and the Ministry of Education, Culture, Sports, Science, and Technology of Japan to S.T. and partly supported by the Program of the network‐type joint Usage/Research Center for Radiation Disaster Medical Science of Hiroshima University, Nagasaki University, and Fukushima Medical University.
Presented in part at the 118th Annual Meeting of American Gastroenterological Association.
REFERENCES
- 1. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353‐1357. [DOI] [PubMed] [Google Scholar]
- 2. Shaib Y, El‐Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115‐125. [DOI] [PubMed] [Google Scholar]
- 3. Watanapa P, Watanapa WB. Liver fluke‐associated cholangiocarcinoma. Br J Surg 2002;89:962‐970. [DOI] [PubMed] [Google Scholar]
- 4. Hardell L, Bengtsson NO, Jonsson U, Eriksson S, Larsson LG. Aetiological aspects on primary liver cancer with special regard to alcohol, organic solvents and acute intermittent porphyria‐‐an epidemiological investigation. Br J Cancer 1984;50:389‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast‐specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J 1993;294:271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 1999;14:1239‐1249. [DOI] [PubMed] [Google Scholar]
- 7. Liu AY, Zheng H, Ouyang G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol 2014;37:150‐156. [DOI] [PubMed] [Google Scholar]
- 8. Sugiyama A, Kanno K, Nishimichi N, Ohta S, Ono J, Conway SJ, et al. Periostin promotes hepatic fibrosis in mice by modulating hepatic stellate cell activation via αv integrin interaction. J Gastroenterol 2016;51:1161‐1174. [DOI] [PubMed] [Google Scholar]
- 9. Lie‐Venema H, Eralp I, Markwald RR, van den Akker NM, Wijffels MC, Kolditz DP, et al. Periostin expression by epicardium‐derived cells is involved in the development of the atrioventricular valves and fibrous heart skeleton. Differentiation 2008;76:809‐819. [DOI] [PubMed] [Google Scholar]
- 10. Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 2007;13:962‐969. [DOI] [PubMed] [Google Scholar]
- 11. Ishikawa K, Yoshida S, Nakao S, Nakama T, Kita T, Asato R, et al. Periostin promotes the generation of fibrous membranes in proliferative vitreoretinopathy. FASEB J 2014;28:131‐142. [DOI] [PubMed] [Google Scholar]
- 12. Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest 2012;122:2590‐2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci 2009;66:2219‐2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morra L, Moch H. Periostin expression and epithelial‐mesenchymal transition in cancer: a review and an update. Virchows Arch 2011;459:465‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujimoto K, Kawaguchi T, Nakashima O, Ono J, Ohta S, Kawaguchi A, et al. Periostin, a matrix protein, has potential as a novel serodiagnostic marker for cholangiocarcinoma. Oncol Rep 2011;25:1211‐1216. [DOI] [PubMed] [Google Scholar]
- 16. Utispan K, Thuwajit P, Abiko Y, Charngkaew K, Paupairoj A, Chau‐in S, et al. Gene expression profiling of cholangiocarcinoma‐derived fibroblast reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Mol Cancer 2010;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell 2016;166:21‐45. [DOI] [PubMed] [Google Scholar]
- 18. Lim YS, Lee HC, Lee HS. Switch of cadherin expression from E‐ to N‐type during the activation of rat hepatic stellate cells. Histochem Cell Biol 2007;127:149‐160. [DOI] [PubMed] [Google Scholar]
- 19. Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T, et al. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation 2004;77:446‐451. [DOI] [PubMed] [Google Scholar]
- 21. Du B, Shim JS. Targeting epithelial‐mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules 2016;21.pii: E965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yan W, Shao R. Transduction of a mesenchyme‐specific gene periostin into 293T cells induces cell invasive activity through epithelial‐mesenchymal transformation. J Biol Chem 2006;281:19700‐19708. [DOI] [PubMed] [Google Scholar]
- 23. Hu Q, Tong S, Zhao X, Ding W, Gou Y, Xu K, et al. Periostin mediates TGF‐β‐induced epithelial mesenchymal transition in prostate cancer cells. Cell Physiol Biochem 2015;36:799‐809. [DOI] [PubMed] [Google Scholar]
- 24. Kim CJ, Sakamoto K, Tambe Y, Inoue H. Opposite regulation of epithelial‐to‐mesenchymal transition and cell invasiveness by periostin between prostate and bladder cancer cells. Int J Oncol 2011;38:1759‐1766. [DOI] [PubMed] [Google Scholar]
- 25. Yao X, Wang X, Wang Z, Dai L, Zhang G, Yan Q, et al. Clinicopathological and prognostic significance of epithelial mesenchymal transition‐related protein expression in intrahepatic cholangiocarcinoma. Onco Targets Ther 2012;5:255‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang XY, Zhang C, Cai JB, Shi GM, Ke AW, Dong ZR, et al. Comprehensive multiple molecular profile of epithelial mesenchymal transition in intrahepatic cholangiocarcinoma patients. PLoS One 2014;9:e96860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhai B, Yan HX, Liu SQ, Chen L, Wu MC, Wang HY. Reduced expression of P120 catenin in cholangiocarcinoma correlated with tumor clinicopathologic parameters. World J Gastroenterol 2008;14:3739‐3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Utispan K, Sonongbua J, Thuwajit P, Chau‐In S, Pairojkul C, Wongkham S, et al. Periostin activates integrin α5β1 through a PI3K/AKT‐dependent pathway in invasion of cholangiocarcinoma. Int J Oncol 2012;41:1110‐1118. [DOI] [PubMed] [Google Scholar]
- 29. Darby IA, Vuillier‐Devillers K, Pinault E, Sarrazy V, Lepreux S, Balabaud C, et al. Proteomic analysis of differentially expressed proteins in peripheral cholangiocarcinoma. Cancer Microenviron 2010;4:73‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riener MO, Fritzsche FR, Soll C, Pestalozzi BC, Probst‐Hensch N, Clavien PA, et al. Expression of the extracellular matrix protein periostin in liver tumours and bile duct carcinomas. Histopathology 2010;56:600‐606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1114/full.
Supporting Information
Supporting Information
Supporting Information


