Abstract
Among persons living with human immunodeficiency virus (HIV) infection, liver disease remains a major cause of morbidity and mortality. While the etiologies are varied and often overlapping in the individual patient, the underlying mechanisms, including oxidative stress, direct activation of stellate cells, HIV interaction with hepatocytes, and bacterial translocation with systemic immune activation, seem to be unifying characteristics. Early and fully suppressive HIV antiretroviral therapy is a mainstay of management either before or concurrent with treatment of etiologic cofactors, including hepatitis C virus, hepatitis B virus, and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Significant barriers to care that still exist include liver disease recognition, appropriate linkage to care, ongoing substance abuse, and psychiatric comorbidities in the HIV‐infected population. Emerging issues in these patients include acute and chronic hepatitis E, underreported hepatitis D, and a rising incidence of hepatocellular carcinoma. (Hepatology Communications 2017;1:987–1001)
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- AIDS
acquired immune deficiency syndrome
- ALD
alcoholic liver disease
- ART
antiretroviral therapy
- CAP
controlled attenuation parameter
- cART
combination antiretroviral therapy
- CD
clusters of differentiation
- CHB
chronic hepatitis B virus
- DAA
direct‐acting antiviral
- HAV
hepatitis A virus
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HDV
hepatitis D virus
- HEV
hepatitis E virus
- HIV
human immunodeficiency virus
- IDSA
Infectious Diseases Society of America
- MSM
men who have sex with men
- NAFL
nonalcoholic fatty liver
- NASH
nonalcoholic steatohepatitis
- NNRTI
non‐nucleoside reverse transcriptase inhibitor
- NP
nurse practitioner
- PCP
primary care physician
- PD
programmed cell death protein
- PI
protease inhibitor
- SVR
sustained virological response
- TAF
tenofovir alafenamide fumarate
- TDF
tenofovir disoproxil fumarate
Introduction
Although accelerated liver disease progression in people infected with human immunodeficiency virus (HIV) was first described during the 1990s, it is only in recent years that we have begun to understand the physiology, comorbidities, and biopsychosocial issues that contribute to altered rates of hepatic injury and fibrosis in these patients (Fig. 1). Improvements in treatment with regard to both regimen and timing have been associated with improved outcomes. With the availability of increasingly effective and safe antiretroviral regimens, patients with HIV now have life expectancies that approach that of non‐HIV‐infected individuals. However, many with HIV infection remain unidentified, and a large subset of those with known HIV infection remains persistently HIV viremic. Infection is associated with immune activation, development of hepatic fibrosis, and rates of hepatic decompensation that exceed decompensation rates seen in hepatitis B virus (HBV) or hepatitis C virus (HCV) monoinfection. Although increased numbers of HIV care providers have embraced provision of newer direct‐acting antiviral (DAA) HCV therapies, the linkage between hepatologists and those managing individuals infected with HIV remains limited in most places. In 2006, the National Institutes of Health and multiple industry partners supported an international meeting aimed at bridging the gap between disparate health professionals, researchers, government regulators, and the pharmaceutical industry to address key issues relevant to the development and management of liver disease in those with HIV infection. In September 2016, the Sixth Biennial HIV and Liver Disease Conference was held in Moran, Wyoming, to identify key issues in the field and to provide expert updates on progress and trends in epidemiology, treatment, and management of current and emerging liver disease in the unique HIV‐infected population. This report summarizes key information presented at the meeting and provides a synthesis of the significance of recent results and findings as it pertains to the research agenda and to care models for managing liver disease in those with HIV.
Figure 1.
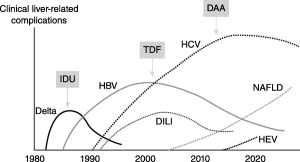
Changing epidemiology of liver disease etiologies in patients with HIV. Abbreviations: DILI, drug‐induced liver injury; IDU, intravenous drug users. (From Soriano et al., AIDS Rev 2013;15:25‐31. Reprinted with permission from Permanyer Publications.)
Pathogenesis/Immunobiology of Liver Disease in Patients Infected with HIV
Whether caused by alcohol, HIV, HCV, HBV, or other etiologies, the progression of liver fibrosis is accelerated in individuals infected with HIV.1, 2, 3, 4 Multiple mechanisms have been proposed to explain why and to ask the related question of to what extent antiretroviral therapy (ART) attenuates the risk. In a series of elegant in vitro studies, Chung and coworkers5, 6 showed that the HIV envelope stimulates greater transforming growth factor‐β1 production by Huh7.5.1 cells and together with HCV enhances collagen and tissue inhibitor of metalloproteinase‐1 production by stellate cells through the production of reactive oxygen species. In addition, by developing a coculture system, the group recently was able to demonstrate that both HCV and HIV independently activate transforming growth factor‐β1 signaling through reactive oxygen species in both cell lines, and activation of these profibrotic pathways was additive following exposure to both viruses.7 Expression of these profibrotic genes was significantly higher in the coculture model compared to either cell type in monoculture, suggesting an interaction and feedback mechanism between Huh7.5.1 and LX2 cells. Thus, it appears that HIV exacerbates a profibrogenic program in hepatocyte and hepatic stellate cell lines, and this model is at least relevant to HCV‐related liver fibrosis progression (see Fig. 2).
Figure 2.
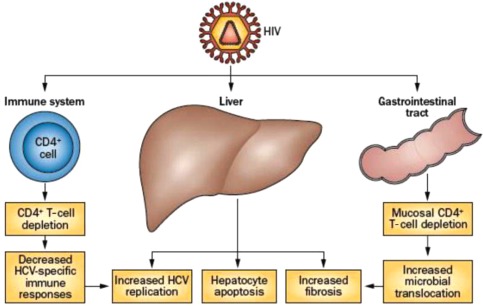
Factors in liver disease pathogenesis in HCV/HIV coinfection. HIV infection leads to impaired immune response against HCV, increased HCV replication, hepatic inflammation and apoptosis, increased microbial translocation from the gastrointestinal tract, and increased fibrosis. (From Chen et al., Nat Rev Gastroenterol Hepatol 2014;11:362‐371.)
In vivo, the situation is undoubtedly more complex because HIV may affect other cell populations; for example, HIV infects Kupffer cells.8, 9 Although HIV infection has never been reconstituted with virus derived from Kupffer cells, there is evidence the total Kupffer cell density might be affected.8 HIV also appears to reduce the killing potential of intrahepatic natural killer cells and may reduce the ability of clusters of differentiation (CD)4 lymphocytes to restrain natural killer profibrotic signaling.10, 11 The extent to which ART attenuates these mechanisms is difficult to determine; however, clinical studies reveal residual increased risk of liver fibrosis progression even among those taking ART.
HBV is a noncytopathic virus, and pathogenesis is largely immune mediated. When compared to controls, HBV‐specific T‐cell responses to HBV peptides in patients with chronic HBV (CHB) are suppressed and expression of programmed cell death protein 1 (PD‐1) is increased.12 Restoration of T‐cell responses have been noted with blockade of PD‐1 or cytotoxic T lymphocyte antigen 412 and a decrease in HBV DNA by nucleotides.13 Of great interest, HBV‐specific cell responses do not appear to clearly distinguish between patients with immune‐active, immune‐tolerant, and inactive CHB phases.12
This is not surprising as CHB is a dynamic disease, and others have shown that the phases may not be distinct virologically or immunologically.14 Mason showed varying levels of hepatitis B surface antigen even in inactive disease and that recognition of specific HBV peptides did not distinguish between phases.15 This diversity within phases was noted in a study of hepatitis B surface antigen epitope changes in patients with hepatitis B e antigen‐positive immune‐active CHB during treatment with tenofovir.16 HBV‐specific CD8 T‐cell cytokine responses to tumor necrosis factor α and interferon‐γ were weaker in HBV/HIV‐coinfected than in HBV‐monoinfected patients.17 HIV further enhances T‐cell exhaustion, and HIV coinfection is associated with increased PD‐1 and reduced CD28/CD127 expression in T cells.18 Flares during immune reconstitution were associated with increased plasma chemokine (C‐X‐C motif) ligand 10 and soluble CD30 levels.19 While T cells are functionally suppressed in chronic hepatitis B through multiple regulatory mechanisms, HBV therapy and blockade of inhibitory pathways can enhance antiviral T‐cell responses. HIV coinfection further provides immune dysregulation and suppression. Highly active ART is associated with an immune reconstitution inflammatory response in HBV/HIV or HCV/HIV coinfection. Following T‐cell receptor engagement, the activated T cell will proliferate, secrete cytokines, and CD8 T cells will become cytotoxic. However, after continued antigen stimulus, PD‐1 is expressed on the T cell and its ligand PD‐L1 on the antigen‐presenting cell. This leads to T‐cell inactivation or exhaustion. T‐cell function can be restored with antibodies to PD‐1 or PD‐L1.
In addition to liver fibrosis, which is the dominant expression of liver disease progression due to HCV and HBV, fat can accumulate in the liver and be associated with progressive inflammation and liver failure. Fatty liver disease can occur with heavy alcohol use (alcoholic liver disease; ALD) as well as without (nonalcoholic fatty liver; NAFL). Certain early generation ART caused steatosis, and its diminished use was associated with a reduction in steatosis.20 Nonetheless, even with newer generation ART there appears to be a growing risk of steatosis in individuals infected with HIV and some suggestion of greater severity. For example, one study compared 33 individuals with NAFL plus infection with HIV and 33 with NAFL but not infected with HIV.21 HIV infection was associated with more foci of lobular inflammation and more acidophil bodies, leading to the disease‐associated diagnosis of nonalcoholic steatohepatitis (NASH) in a greater proportion of persons.
One potential mechanism of promoting liver disease that is common to alcohol and HIV is increased translocation of gut microbial content.22, 23 HIV infection has also been associated with alterations in the gut microbiome.24 Thus, enhanced translocation of different microbial products might alter liver disease pathogenesis. Lipopolysaccharide has been linked to the pathogenesis of NASH and ALD by toll‐like receptor 4 stimulation, which enhances signaling through myeloid differentiation protein 88 (NASH) or TIR‐domain‐containing adapter‐inducing interferon‐β (ALD) to activate the inflammasome. HIV (and HCV) also activate the inflammasome and markedly elevate serum interleukin‐18 production.25, 26 The extent to which these processes are synergistic and the mechanisms are largely unknown; however, it is possible they explain some of the extrahepatic manifestations of liver disease (and these viral infections). For example, cardiovascular disease incidence appears to be increased with HIV and HCV infection as well as nonalcoholic fatty liver disease (NAFLD),27, 28, 29 and macrophage activation has been linked to coronary and carotid artery inflammation.30, 31 Clearly more work is needed to disentangle these associations and particularly to guide approaches to reducing the residual risk of inflammation that appears to be present in persons on long‐term ART.
Epidemiology/Natural History/Assessment of Liver Disease in HIV
VIRAL HEPATITIS A, B, C, D, E
Consideration of liver disease in the HIV‐infected patient requires identification and characterization of the multiple processes that may contribute to liver disease. Most significant are the viral hepatitides, which can cause acute, acute‐on‐chronic, and chronic liver injury with concomitant development and progression of hepatic fibrosis. All forms of viral hepatitis demonstrate increased overall prevalence in those with HIV. Several recent studies now clearly demonstrate a divergence of acquired immune deficiency syndrome (AIDS)‐related and non‐AIDS‐related causes of death among individuals infected with HIV. In recent years, persons dying of non‐AIDS‐related causes of death are more frequently HIV virologically suppressed and older. They are more likely to have cirrhosis, diabetes, and other complications associated with both aging and metabolic syndrome.32
Hepatitis A virus (HAV) is easily transmitted among men who have sex with men (MSM) and less frequently by injection drug use. Multiple outbreaks of acute HAV among MSM have been reported.33, 34, 35 Modeling has suggested that a threshold level of 70% immunity among sexually active MSM is needed to break the transmission cycle.36 This requires an active immunization program in most developed countries as rates of naturally acquired HAV have fallen due to improved sanitation and water treatment methods. Wider outbreaks in which the index case acquired HAV by sex with men has been described and represents a change in the traditional epidemiologic patterns of HAV spread prior to universal vaccination of children in many countries.37 Targeted vaccination for HAV may result in lower protective antibody titers than among those with natural infections.38
In the United States, approximately 8%‐10% of those with HIV infection have chronic HBV infection as well. Historically, HBV has been an important etiology for development of end‐stage liver disease among those with HIV infection, at least since the initiation of the modern antiretroviral era (circa 1996). Despite recommendations for universal vaccination since the late 1990s, vaccine use remains suboptimal in this at‐risk group of patients. In France, 25% of a large HIV‐infected cohort (n = 1,175) did not have serologic markers of current or past HBV infection. Among this group, 87.1% had never received vaccination for HBV.39 Even when HBV vaccination is provided, vaccine responses are suboptimal among those with concomitant HIV coinfection.40 One study suggests that the CD4/CD8 ratio can be used to predict those with better responses. Those with a ratio >0.4 were more likely to seroconvert.41 Despite availability of dual‐active therapeutic agents that suppress both HBV and HIV, coinfection is associated with an increase in liver‐related death. This was well documented by Thio and colleagues42 in the Multicenter AIDS Cohort study, where over 5,000 men were followed for more than a decade. Liver‐related mortality among HBV/HIV‐coinfected men was significantly greater compared to those with either HIV or HBV monoinfection. Similar results were reported in the Data Collection on Adverse Events of Anti‐HIV Drugs (D:A:D) study, with HBV/HIV coinfection being associated with a 3.73 times increase in the relative risk of death.43 Some have argued that these studies did not fully capture the effect of the widespread use of a tenofovir‐containing nucleoside/tide backbone. In the North American AIDS Cohort Collaboration on Research and Design study, patients were divided into three time cohorts (1996‐2000; 2001‐2005; 2006‐2010) to examine the influence of changing ART patterns on liver mortality. HBV coinfection was a key contributor, and there was little change in incidence of liver‐associated death during any of the time periods studied despite the emergence of widespread use of tenofovir during the latter time periods. Surprisingly, a high percentage (35%) of those with known HBV/HIV coinfection was not receiving HBV‐active ART.44
Historically, the natural history of HCV/HIV coinfection was associated with an accelerated rate of liver fibrosis with more rapid progression to cirrhosis.45, 46 However, more recent studies suggest that rates of fibrotic progression may be modulated by effective combination ART (cART). In the Swiss HIV Cohort study, patients with HCV who achieved sustained virological response (SVR) had much lower mortality than either treatment failures or those not treated for HCV.47 In a large U.S. Department of Veterans Affairs cohort, effective cART was associated with a decrease in hepatic decompensation, but among patients coinfected with HCV/HIV, the risk remained higher than among those not coinfected.48 A Canadian multicenter cohort was assessed to determine if the class of agent used as the anchor agent and backbone affected fibrotic progression rates. Both protease inhibitor (PI) and non‐nucleoside reverse transcriptase inhibitor (NNRTI) use was associated with increased progression as measured by aspartate aminotransferase‐to‐platelet ratio index over time. Among backbone agents, only abacavir/ lamivudine regimens were associated with an increase among HCV/HIV‐coinfected patients.49
Hepatitis D virus (HDV) occurs only in the presence of active HBV infection. There is growing evidence that HDV may be more common in areas previously recognized as low risk than in prior years. Gish and colleagues reported an 8% prevalence in California, but a significant proportion of this may have been driven by immigration from high‐risk areas.50 In Midwestern United States, a prevalence of over 3% was identified, but the investigators also noted that only a small subset of patients were screened for HDV.51 There are few seroprevalence or viral prevalence studies that focus on those with HIV infection. In recent years, most newly diagnosed HDV in Europe is among HIV‐positive immigrants from HDV‐endemic regions.52 Fibrotic progression in those with HDV/HBV/HIV coinfection appears to be faster than in non‐HIV‐infected persons.53 In Spain, liver complications and death attributable to HDV have become more prominent as treatment for HCV and better HBV control has decreased severe outcomes from those etiologies.54
Hepatitis E virus (HEV) infection continues to be an emerging issue in those with HIV infection. Hepatitis E acquisition appears to be more common in those with HIV than other population groups. It is associated with the presence of HCV, although the epidemiologic linkage in terms of risk factors remains unclear. In a longitudinal cohort of individuals infected with HIV in southern Spain, the incidence was 7.2 cases/100 patient years. Living in a rural environment was more likely to lead to HEV seroconversion.55 The authors suggest that increased exposure to wildlife, including wild boar and deer, could be a factor. A seroprevalence study in South Africa reported that genotype 3 was endemic with antibody present in 27.9% of tested individuals, but no difference between those with and without HIV infection was noted.56 Pork/bacon consumption was associated with the presence of HEV antibodies. However, other studies suggest increased prevalence in those with HIV, which has been reviewed by Debes et al.57 Chronicity is reported, but its overall frequency is poorly characterized. Kuniholm and colleagues58 used a nucleic acid testing platform to evaluate 2,919 samples, which included 2,606 women infected with HIV, for HEV RNA. HEV viremia was identified in three samples, and only one was repeatedly positive at multiple time points. This patient had CD4 counts greater than 200 cells/mm3. Chronic HEV in the setting of HIV has been associated with progression of hepatic fibrosis to cirrhosis, however, and should be considered as a cause of cryptogenic cirrhosis.59
HEPATOXICITY
It is clear that hepatotoxicity related to the use of antiretroviral agents has decreased in recent years. Integrase inhibitors appear to have little or no intrinsic hepatotoxicity. However, patients who were treated previously with certain agents may continue to experience issues related to prior exposures. Data from the D:A:D study were used to examine 319 individuals infected with HIV who died of end‐stage liver disease or developed hepatocellular carcinoma (HCC) over a median follow‐up time of 8.4 years. After adjustment for confounders, there was a significant association with severe liver outcomes among those with the greatest cumulative exposure to stavudine (Zerit), didanosine, or tenofovir disoproxil fumarate (TDF).60 These outcomes include noncirrhotic portal hypertension, which has been strongly associated with the use of didanosine.61, 62
NASH
Both HIV and cART may increase the risk for fatty liver. Evaluation of fatty liver in the Multicenter AIDS Cohort study identified 254 HIV‐uninfected and 265 HIV‐infected patients. Most of the patients infected with HIV were on cART. The overall prevalence of fatty liver, as determined by noncontrast computed tomography evaluation of the liver and spleen attenuation, was 19% in the HIV‐uninfected and only 13% in the HIV‐positive patients (P = 0.002). The homeostasis model assessment of insulin resistance, which indicates the presence of insulin resistance, was higher in men infected with HIV compared to controls (P = 0.007). Cumulative exposure to dideoxynucleotide analogs was the only key risk factor identified for fatty liver in the HIV‐positive population.63 A flaw of this study and others is the failure to distinguish liver fat from the presence of NASH. Because diagnosis of NASH requires a liver biopsy, few studies in individuals infected with HIV have evaluated NASH prevalence. Sterling et al.64 identified subjects without viral hepatitis, known alcohol abuse, or diabetes who had persistent alanine aminotransferase abnormalities for longer than 6 months. Using liver histology as the gold standard, NAFLD was identified in 65% of patients and 25% met criteria for NASH. Morse et al.65 used similar criteria for patient selection and found NAFLD in 73% with 55% having NASH.
HEPATOCELLULAR CARCINOMA
Both the incidence and prevalence of HCC appears to be increasing in patients with HIV infection.66, 67 The increase in HCC may represent a complex mix of altered natural history changing epidemiology and ascertainment bias. During the early decades of the HIV epidemic, premature mortality due to HIV‐associated opportunistic infections limited the risk of HCC development, which typically occurs over a period of years. Recent studies suggest that HCC may be more aggressive in terms of both doubling time and invasiveness in those with HIV infection.68 Finally, the diagnosis of liver cancer in persons with HIV frequently was not reported on death certificates because HIV providers were less familiar with the definitive diagnosis of this entity.
HIV Treatment
WHEN TO TREAT
Liver disease no longer affects the decision regarding when HIV is treated because ART is indicated in all individuals infected with HIV. The benefits of cART outweigh the risks in every patient group and setting that has been carefully studied. The largest and most impactful was the Strategic Timing of Antiretroviral Treatment study, which randomized HIV‐infected treatment‐naive individuals with CD4 lymphocyte counts above 500 cells/mm3 to immediate ART (n = 2,326) versus deferred ART (n = 2,359).69 The risks of both AIDS‐ and non‐AIDS‐defining events were lower in the immediate ART group. There were relatively few individuals in the study with HBV (2.8%) or HCV (3.7%) infections. However, overall fibrosis‐4 scores were less likely to rise >1.45 in those in the immediate compared to delayed ART groups, an effect that was evident 12 months after randomization and was persistent for 60 months.70 Thus, underlying liver disease caused by HCV, HBV, alcohol, or another condition is an additional reason to treat all individuals infected with HIV as soon as possible.
HIV identification and linkage to care remain problematic in the United States. New HIV cases continue to occur, and the lifetime risk of HIV infection remains exceptionally high in some racial and ethnic groups (Fig. 3). Up to 25% of those with HIV are undiagnosed.71 A recent analysis suggests that it takes approximately 3.1 months following HIV diagnosis to link to care and 8% are lost to care linkage at that stage. Those in care do not achieve HIV viral suppression for at least 1 year.72 For those in care, simplified one‐pill regimens improve adherence and outcomes.
Figure 3.
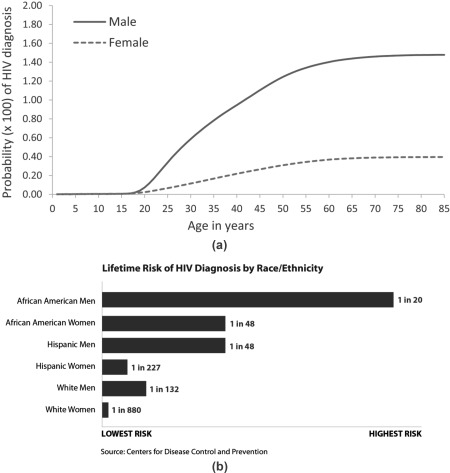
Lifetime risk of HIV diagnosis. (A) By age and sex, United States. (From Hess et al., Ann Epidemiol 2017:27:238‐243. Reprinted with permission.) (B) By race/ethnicity. (Source: Centers for Disease Control and Prevention. 2016 Conference on Retroviruses and Opportunistic Infections. https://www.cdc.gov/nchhstp/newsroom/2016/croi‐2016.html.)
WHAT TO START
Liver disease may affect how HIV is treated (see Table 1). As discussed in detail below for persons with HBV coinfection, use of cART that is active against both viruses is recommended. For individuals coinfected with HCV/HIV, it is prudent to anticipate the drug interaction that might occur when HCV treatment commences. In one study, a high proportion of patients required an alteration in their antiretroviral regimen prior to HCV treatment.73 In the rare instance in which serious drug interactions cannot be managed and the person has a CD4 lymphocyte count >500 cells/mm3, some experts would withhold ART for 12 weeks to treat HCV first. For persons with decompensated cirrhosis (Childs B or C), there are few data and some concern regarding use of currently approved PI regimens. American Association for the Study of Liver Diseases (AASLD)/Infectious Diseases Society of America (IDSA) HCV guidance suggests that until further data are available, elbasvir/grazoprevir use is not recommended in patients with decompensated cirrhosis. Other HCV PI‐based regimens are also not approved for treatment of this group (AASLD/IDSA HCV Guidance, www.hcvguidelines.org; accessed August 17, 2017).
Table 1.
CHRONIC VIRAL HEPATITIS TREATMENT GUIDANCE IN HIV‐COINFECTED PATIENTS
| Viral Coinfection | Preferred Regimens and Cautions |
|---|---|
| Hepatitis B |
• Start two HBV‐active agents simultaneously with cART. Dual activity agents preferred • If unable to use tenofovir, use entecavir in conjunction with cART |
| Hepatitis C |
• Treat using same regimen as recommended for HCV monoinfection • Short duration (8‐week) regimen use limited • Beware of drug–drug interactions • Modify cART to match DAA regimen • If cART cannot be modified, sofosbuvir and daclatasvir are compatible with most DAA regimens |
| Hepatitis D | • No effective treatment options. Consider pegylated interferon use |
| Hepatitis E | • Use ribavirin for a 12‐week course |
CONSIDERATIONS WHEN STARTING
There are emerging and, as of March 2017, unpublished data that might affect the optimal cART selection for patients with significant steatosis. One study randomized individuals who were on an efavirenz‐containing regimen to maintain (n = 18) or switch (n = 19) to raltegravir.74 Elastography with controlled attenuation parameter (CAP) was performed before and after the randomization. The median (Q1‐Q3) of the difference in CAP values between baseline and 48 weeks was –20 (range, –67, 15) dB/m for the raltegravir group and 28.5 (–18.8, 47.8) dB/m for the efavirenz group (P = 0.019). A related study examined 37 women with lipohypertrophy and well‐controlled HIV infection on NNRTI‐ or PI‐based regimens who were randomized to immediate versus delayed (24 weeks) switch to raltegravir.75 Adiponectin and chitinase‐3‐like protein 1 (also known as YKL 40) levels decreased more in women who switched to raltegravir immediately compared to those continuing NNRTI‐ or PI‐based ART. While these data are very preliminary, they underscore a possible nuance in the approach to ART among persons with hepatic steatosis.
Management of HCV/HIV
In clinical trials, individuals coinfected with HCV/HIV respond extremely well to direct‐acting anti‐HCV treatment. However, reports from real‐life settings have suggested that the validity of phase 3 results should be confirmed in real‐world settings. Saeed and coworkers76 reported that most patients in the Canadian Coinfection cohort would have been excluded from registration trials due to ART (63%‐79%) or active illicit drug use (53%‐55%). Thus, it is notable that high SVR rates are also being reported in real‐world settings. For example, Bhattacharya and coworkers77 describe the experience of 996 consecutive HCV/HIV‐coinfected U.S. veterans with genotype 1 HCV infection who were treated with ledipasvir/sofosbuvir (n = 757), ledipasvir/sofosbuvir and ribavirin (n = 138), ombitasvir/ paritaprevir/ritonavir plus dasabuvir (n = 28), or ombitasvir/ paritaprevir/ritonavir plus dasabuvir and ribavirin (n = 73). Overall SVR was 90.9%, which was lower in those with cirrhosis (85.9%) compared to those without cirrhosis (92.4%). In a French HCV/HIV cohort, an overall SVR rate of 93% was reported with no difference among those with cirrhosis.78 Similarly high real‐world rates of SVR have been described elsewhere, making drug interactions the main clinical issue with HCV treatment.
Management of HBV/HIV
Patients who are coinfected with HBV and HIV should receive ART regardless of HBV DNA and serum alanine aminotransferase levels . ART should contain tenofovir‐based therapy (TDF or tenofovir alafenamide fumarate [TAF]) as lamivudine is insufficient as monotherapy (U.S. Department of Health and Human Services guidelines https://aidsinfo.nih.gov/guidelines/html/4/adult‐and‐adolescent‐oi‐prevention‐and‐treatment‐guidelines/344/hbv). The availability of TAF has increased the accessibility of tenofovir‐based regimens to those at risk of nephrotoxicity and those with significant bone disease. The switch from TDF to TAF can be accomplished successfully with improvement in creatinine clearance and bone mineral density.79, 80 For those with creatinine clearance from 30‐59 mL/minute, TAF is preferred and has been shown to control both HIV and HBV infections.79, 80 A recent meta‐analysis of 10 randomized controlled trials comparing TDF and TAF did not show a difference in treatment efficacy, resistance, or adverse events between the two drugs. TAF showed significantly better bone mineral density and less renal toxicity but higher lipid levels. In fact, in the TAF arms there was higher use of lipid‐lowering agents.81 TAF is not recommended for those with creatinine clearance <30 mL/minute. For these patients, either dose‐adjusted TDF or entecavir can be used. If a patient coinfected with HBV/HIV requires a non‐TDF non‐TAF regimen, then entecavir should be added as lamivudine is not sufficient as the sole HBV medication due to the high rate of resistance to HBV that develops.82
With regard to vaccination, current guidelines recommend that all patients infected with HIV and susceptible to HBV should receive hepatitis B vaccination or combination hepatitis A and hepatitis B vaccination, but the response rates are lower than in HIV‐negative persons. The response rate increases with better HIV control (higher CD4 and lower HIV viral load).83 Ideally, CD4 counts should be >350 mm3, but vaccination should not be delayed because of lower CD4 counts. Antibodies to hepatitis B surface antigen should be checked 1 month after completion of the vaccine series to determine immunity. Numerous strategies have been undertaken to increase vaccine response rates, including increasing the number of doses or double‐dose vaccine with some success.84, 85, 86 Addition of adjuvants has not shown higher efficacy.86 The Swiss cohort found that tenofovir‐based cART therapy decreased incident HBV infection in susceptible individuals infected with HIV who had not developed protective antibodies to hepatitis B surface antigen after vaccination. This effect was independent of CD4 cell count and high‐risk behavior, showing that pre‐exposure prophylaxis for HBV coinfection may be of benefit in individuals infected with HIV.87
Management of HIV and HCC
There is increasing prevalence of HCC with longer lifespan of patients with HIV and viral hepatitis, alcohol, or fatty liver disease. HCV/HIV‐coinfected patients have a 23‐fold higher prevalence of HCC than HCV alone.88 It is critical to diagnose cirrhosis in patients with HIV so that screening can be performed in these high‐risk individuals. This appears even more important than screening individuals without cirrhosis, who have a lower prevalence and incidence of HCC.
Prospective studies of HCC surveillance have shown that screening every 6 months improves survival as this interval of screening has the highest ability to successfully treat the tumor. Unfortunately, less than one third of patients with cirrhosis in Europe and the United States are screened every 6 months, despite guidelines from the United States, European, and Asian‐Pacific societies.89, 90 This is also true of patients coinfected with HIV, with survival and successful therapy adversely affected by lack of appropriate screening.91 Some studies have found that HCC in patients coinfected with HIV is more likely to be infiltrative and have portal vein invasion with multifocal HCC, all factors leading to worse survival.92 Screening for early diagnosis allows patients to have access to locoregional therapy and liver transplantation. The outcome of HCC in patients with HIV is affected by the absence of surveillance, the failure of detection, delay in follow‐up, poor HIV status, and perhaps HIV immune dysregulation. It may be that HIV subjects with immune dysregulation have a different biology of HCC with different gene signatures associated with a faster doubling time. It has been suggested that poorer HIV status may be associated with worse HCC biology. These issues need further investigation. Although guidelines do not currently recommend surveillance of all HIV‐infected patients for assessment of underlying hepatic fibrosis, studies in this regard may be useful and indicated. Use of noninvasive screening strategies may allow earlier identification of patients at risk. Clearly more needs to be done to ensure appropriate screening of all patients with cirrhosis and HCV/HIV patients with liver fibrosis stage F3/4 before expanding screening of all patients with HIV and viral hepatitis.
Issues in Access to Care
While all HCV/HIV‐infected patients are candidates for therapy, there are significant barriers to access to care and treatment in the United States and worldwide. New models that focus on delivering care in HIV settings with multidisciplinary collaboration, including hepatology teams, are becoming popular as a more efficient way to improve HCV treatment uptake among patients infected with HIV. Similar to HIV care, care pathways for the patient with HCV can be developed to determine if the patient is ready for treatment, including issues that might impact adherence, such as substance use, psychiatric comorbidity, harm reduction strategies, unstable housing, and inadequate insurance. Clinics providing HIV care can also provide HCV care with prepared checklists useful for initial evaluation and prior authorizations and templates useful for notes and patient teaching. Access to pharmacy support is invaluable for DAA treatment choice, but if not available, the IDSA/AASLD guidelines website www.hcvguidelines.org or the University of Liverpool website www.hep‐druginteractions.org will highlight known drug–drug interactions and facilitate choices of antiretroviral regimens in those with HCV/HIV coinfection. Because drug–drug interactions are of major importance in treating HCV in patients with HIV, the AASLD/IDSA guidelines should be used to provide the most current up‐to‐date information on DAAs and didanosines. Providers need to be able to diagnose cirrhosis with readily available clinical algorithms (aspartate aminotransferase‐to‐platelet ratio index and fibrosis‐4) or transient elastography.93 If a patient has decompensated cirrhosis, they should be cared for by a hepatologist and evaluated for possible liver transplantation. Patients with cirrhosis require upper endoscopy to assess for varices, imaging every 6 months, and alpha fetoprotein to assess for HCC as well as regular determination of Model for End‐Stage Liver Disease and Child‐Turcotte‐Pugh scores. The ASCEND study (A Study of Cardiovascular Events iN Diabetes) evaluated the efficacy and safety of HCV treatment of 600 black patients at two urban health centers, managed by three community‐based provider types: specialist, primary care physician (PCP), or nurse practitioner (NP). They found no difference in SVR between providers, although there was a trend toward better adherence with NPs and PCPs.94 This shows that HCV therapy can be expanded and scaled up to include NPs and PCPs, thus bridging gaps in the HCV care continuum. They also found no difference in adherence and SVR between HCV/HIV‐coinfected and HCV‐monoinfected patients. Cachay et al.95 examined an HIV primary care model versus a hepatology‐based model and found increased uptake with similar outcomes following use of the HIV primary care providers.
Another group with significant barriers to care includes correctional populations.96 The United States has 25% of the world's prison population, 2.2 million prisoners, and another 4.8 million under community corrections. The prevalence of HCV varies by state and by prison or jail, with some facilities testing all inmates and other short‐term facilities not testing or linking to care.97 However, linkage to care and treatment are feasible in correctional facilities, but it is often not feasible when prisoners are released as they may lack ongoing access to care.
Alcohol, Drugs, Psychiatric Issues
In the United States and elsewhere, patients with HIV infection are more likely to have behavioral issues and psychiatric diagnoses that measurably contribute to liver injury. Behavioral issues include misuse of alcohol and the use of recreational substances that may increase risk of liver injury either directly or through increased risk of parenteral exposure to infectious agents. The prevalence of hazardous or unhealthy alcohol use among persons living with HIV infection varies by cohort study and the definitions used for alcohol misuse. The Center for AIDS Research Network of Integrated Clinical Systems study included seven sites and over 12,000 patients. Of these, 17% had Alcohol Use Disorder Identification Test for Consumption (AUDIT‐C) scores ≥4 in women and ≥5 in men.98 The Veterans Aging Cohort study included 18,145 veterans and found that 24% had an AUDIT‐C score of ≥4.99 In the women's interagency HIV study, 14% to 24% of 2,770 women met the definition for hazardous unhealthy use. Interestingly, excess alcohol use is associated with an increased risk of HIV acquisition.100 This is attributable in part to an increased risk of unprotected sex associated with alcohol misuse.101 Many studies demonstrate an association between alcohol use and level of adherence with ARTs; therefore, it is not surprising that alcohol use is also a predictor of liver disease progression in those with HIV infection.102 Despite these facts, most healthcare providers managing those with HIV do not use formal instruments to evaluate alcohol use and misuse, although most indicate they do ask patients about alcohol.103 Biomarkers, including ethylglucuronide, have been used as a tool to evaluate alcohol use.104 This modality should be studied further in individuals infected with HIV as recent studies suggest that alcohol screening questionnaires may underestimate alcohol use.105 Both counseling interventions as well as the use of pharmacologic therapies can be useful in reducing alcohol consumption and relapse following periods of abstinence.
Similar to alcohol, substance abuse is also a risk factor for the transmission of HIV and HCV infections; however, some agents used as recreational drugs specifically affect hepatic fibrosis. One of these is cocaine, which appears to increase rates of liver fibrosis. Cocaine appears to provide specific activation of profibrogenic cytokines, which increase levels of intrahepatic interferon‐γ and tumor necrosis factor α.106 Cocaine use also increases hepatic apoptosis and oxidative stress.107
Research Agenda
The last decade has seen tremendous progress in terms of our understanding and management of liver disease in the patient infected with HIV. However, many issues identified by experts in the field remain poorly understood in terms of pathophysiology or are suboptimally managed in lieu of high‐quality data derived from well‐designed clinical trials. We continue to see changing patterns of HIV risk. The risk of new HIV infections in portions of the United States is equivalent to or greater than that seen in sub‐Saharan Africa. Compounding this is a new epidemic of injection drug use that is driving a significant increase in new cases of hepatitis C infection. In one community in Scott County, Indiana, the concomitant introduction of HIV led to a severe, albeit limited, epidemic.108 Concern that other localized HIV epidemics might also appear in areas of high injection drug use highlights the need for increased epidemiologic surveillance as well as additional studies that support the theoretical rationales for disease prevention using harm reduction techniques.109 These include but are not limited to treatment as prevention of HCV and HIV and pre‐exposure prophylaxis hepatitis B as well as HIV. While needle exchange programs have been shown to be a useful adjunct in some epidemiologic settings, the unique and evolving nature of the current heroine epidemic in parts of the United States requires careful study of the effect of these interventions in that population and perhaps modification in limitation methods. Certain disease processes appear to be persistently underdiagnosed in the HIV‐infected population. These include hepatitis D, hepatitis E, and occult hepatitis B. Both education and application of implementation science methods will be needed to improve diagnostic outcomes. Recognition and understanding of NASH remains limited among HIV care providers. Although there is growing recognition of the prevalence of fatty liver disease, additional development of noninvasive biomarkers with high degrees of sensitivity and specificity for NASH in the HIV‐infected population is imperative. Immune activation, which results from gut leakage soon after acute HIV infection, remains an ongoing issue as related to liver injury even after the effective use of antiretroviral agents. Increased endotoxin entering the liver through the portal system activates a cascade of immunologic responses with outcomes that include stellate cell activation and hepatic fibrosis. There is increased interest in antifibrotic agents, which may be particularly relevant in those with HIV infection. However, research strategies that mitigate translocation in the patient infected with HIV remain limited and should be explored. While we have excellent suppressive therapies for hepatitis B that are well integrated in combination antiretroviral treatment regimens, the achievement of a functional cure for hepatitis B remains elusive. Some strategies under investigation, including the development of antibodies against checkpoint inhibitors, are promising for the treatment of both hepatitis B and HIV infections. However, at this time we remain at the proof of concept stage of development, and concerns about safety with regard to the development of autoimmune disease processes are significant in some quarters. It is clear that effective hepatitis B vaccination in HIV remains an elusive goal as well. The combination of a suboptimal vaccine response with ongoing risk behavior requires research into either new adjuvant therapies or improved primary vaccination strategies. The evolution in hepatitis C treatment has been dramatic, and the successes of direct‐acting agents have not bypassed those with HCV/HIV coinfection. However, additional research into shorter duration therapies and improved management for those with acute HCV infection is indicated. The use of alternate forms of drug administration, including long‐acting depot preparations, may help to reduce barriers to care in both resource‐limited settings and underserved populations with HIV infection. Continued research into the development of a hepatitis C vaccine is imperative. It is unlikely that we can treat our way to hepatitis C eradication without a prevention strategy. Finally, we look forward to research that includes a recalibration of biomarkers, including transient elastography, following hepatitis C cure. There is an ongoing evolution in treatment strategies for those using alcohol and injection drugs. These problems are complex and will require a multidisciplinary approach that incorporates concepts of social science, political science, and medical science. Many of these issues have been addressed in a recent publication of the National Academies of Sciences, Engineering, and Medicine that was released in March 2017 (www.nationalacademies.org/HepatitisElimination). Continued investment in the research agenda is needed to build on improvements in both the quality of life and the life expectancy of the individual infected with HIV.
Acknowledgment
Meeting Participants (speakers whose lectures contributed to the content of this meeting summary): Andrew Aronsohn, M.D., University of Chicago Medical Center; Marianna K. Baum, Ph.D., Florida International University; John T. Brooks, M.D., Centers for Disease Control and Prevention; Geetanjali Chander, M.D., M.P.H., Johns Hopkins University; Kyong‐Mi Chang, M.D., Perelman School of Medicine, University of Pennsylvania; Raymond T. Chung, M.D., Harvard Medical School, Massachusetts General Hospital; Stuart C. Gordon, M.D., Henry Ford Hospital; Camilla S. Graham, M.D., Beth Israel Deaconess Medical Center; Roy M. Gulick, M.D., M.P.H., Weill Cornell Medicine; Jag Khalsa, Ph.D., National Institutes of Health, National Institute on Drug Abuse; David Kleiner, M.D., Ph.D., National Cancer Institute; Shyam Kottilil M.D., Ph.D., Johns Hopkins University; Kristen Marks, M.D., Weill Cornell Medicine; Natasha Martin, Ph.D., University of California San Diego, School of Medicine; Marion Peters, M.D., University of California, San Francisco; Kenneth E. Sherman, M.D., Ph.D., University of Cincinnati College of Medicine; Vincent Soriano, M.D., Ph.D., La Paz University Hospital; Anne C. Spaulding, M.D., M.P.H., University of California, San Francisco; Gyongyi Szabo, M.D., Ph.D., University of Massachusetts Medical School; Phyllis Tien, M.D., University of California San Francisco, School of Medicine; Glenn Treisman, M.D., Ph.D., Johns Hopkins University School of Medicine. *HIV and Liver Disease 2016, September 15‐17, 2016.
Potential conflict of interest: Dr. Sherman received grants through his institution from AbbVie, BMS, Gilead, Innovio, Intercept, MedImmune, and Merck; he is also an advisor to Gilead, Merck, and MedImmune and is on the Data and Safety Monitoring Board for MedPace and Watermark. The other authors declare no conflict of interest.
Supported by educational grants from Gilead Sciences, Inc.; Janssen Therapeutics, Division of Janssen Products; Merck & Co.; ViiV Healthcare; Intercept Pharmaceuticals, Inc.; and Tobira Therapeutics, Inc. Funding for this conference was made possible in part by the National Institutes of Health under Award Number R13AI071925 to K.E.S. from the National Institute of Allergy and Infectious Diseases, with cofunding from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the United States Government.
Footnotes
*Correction added on 14 November 2017, after first publication on 6 November 2017: Meeting report and date added.
REFERENCES
- 1. Thio CL, Seaberg EC, Skolasky R Jr, Phair J, Visscher B, Muñoz A, et al.; Multicenter AIDS Cohort Study . HIV‐1, hepatitis B virus, and risk of liver‐related mortality in the Multicenter AIDS Cohort Study (MACS). Lancet 2002;360:1921‐1926. [DOI] [PubMed] [Google Scholar]
- 2. Kirk GD, Mehta SH, Astemborski J, Galai N, Washington J, Higgins Y, et al. HIV, age, and the severity of hepatitis C virus‐related liver disease: a cohort study. Ann Intern Med 2013;158:658‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goedert JJ, Eyster ME, Lederman MM, Mandalaki T, De Moerloose P, White GC 2 nd, et al. End‐stage liver disease in persons with hemophilia and transfusion‐associated infections. Blood 2002;100:1584‐1589. [PubMed] [Google Scholar]
- 4. Lo Re V, 3rd , Kallan MJ, Tate JP, Localio AR, Lim JK, Goetz MB, et al. Hepatic decompensation in antiretroviral‐treated patients co‐infected with HIV and hepatitis C virus compared with hepatitis C virus‐monoinfected patients: a cohort study. Ann Intern Med 2014;160:369‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin W, Weinberg EM, Tai AW, Peng LF, Brockman MA, Kim KA, et al. HIV increases HCV replication in a TGF‐beta1‐dependent manner. Gastroenterology 2008;134:803‐811. [DOI] [PubMed] [Google Scholar]
- 6. Lin W, Wu G, Li S, Weinberg EM, Kumthip K, Peng LF, et al. HIV and HCV cooperatively promote hepatic fibrogenesis via induction of reactive oxygen species and NFkappaB. J Biol Chem 2011;286:2665‐2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salloum S, Holmes JA, Jindal R, Bale SS, Brisac C, Alatrakchi N, et al. Exposure to human immunodeficiency virus/hepatitis C virus in hepatic and stellate cell lines reveals cooperative profibrotic transcriptional activation between viruses and cell types. Hepatology 2016;64:1951‐1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balagopal A, Ray SC, De Oca RM, Sutcliffe CG, Vivekanandan P, Higgins Y, et al. Kupffer cells are depleted with HIV immunodeficiency and partially recovered with antiretroviral immune reconstitution. AIDS 2009;23:2397‐2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao YZ, Dieterich D, Thomas PA, Huang YX, Mirabile M, Ho DD. Identification and quantitation of HIV‐1 in the liver of patients with AIDS. AIDS 1992;6:65‐70. [DOI] [PubMed] [Google Scholar]
- 10. Glassner A, Eisenhardt M, Kokordelis P, Krämer B, Wolter F, Nischalke HD, et al. Impaired CD4(+) T cell stimulation of NK cell anti‐fibrotic activity may contribute to accelerated liver fibrosis progression in HIV/HCV patients. J Hepatol 2013;59:427‐433. [DOI] [PubMed] [Google Scholar]
- 11. Goeser F, Glassner A, Kokordelis P, Wolter F, Lutz P, Kaczmarek DJ, et al. HIV mono‐infection is associated with an impaired anti‐hepatitis C virus activity of natural killer cells. AIDS 2016;30:355‐363. [DOI] [PubMed] [Google Scholar]
- 12. Park JJ, Wong DK, Wahed AS, Lee WM, Feld JJ, Terrault N, et al.; Hepatitis B Research Network . Hepatitis B virus‐specific and global T‐cell dysfunction in chronic hepatitis B. Gastroenterology 2016;150:684‐695.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, et al. Restored function of HBV‐specific T cells after long‐term effective therapy with nucleos(t)ide analogues. Gastroenterology 2012;143:963‐973.e9. [DOI] [PubMed] [Google Scholar]
- 14. Vanwolleghem T, Hou J, van Oord G, Andeweg AC, Osterhaus AD, Pas SD, et al. Re-evaluation of hepatitis B virus clinical phases by systems biology identifies unappreciated roles for the innate immune response and B cells. Hepatology. 2015;62(1):87‐100. [DOI] [PubMed] [Google Scholar]
- 15. Mason WS, Gill US, Litwin S, Zhou Y, Peri S, Pop O, et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology 2016;151:986‐998.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walsh R, Hammond R, Yuen L, Bayliss J, Revill P, Leary T, et al. Mapping HBsAg epitope profiles to predict HBsAg loss/seroconversion in a treatment naive cohort of genotype A chronic hepatitis B (CHB) patients receiving tenofovir disoproxil fumarate (TDF) therapy. Hepatology 2015;62:966A‐967A. [Google Scholar]
- 17. Chang JJ, Sirivichayakul S, Avihingsanon A, Thompson AJ, Revill P, Iser D, et al. Impaired quality of the hepatitis B virus (HBV)‐specific T‐cell response in human immunodeficiency virus type 1‐HBV coinfection. J Virol 2009;83:7649‐7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cho H, Kikuchi M, Li Y, Nakamoto N, Amorosa VK, Valiga ME, et al. Induction of multiple immune regulatory pathways with differential impact in HCV/HIV coinfection. Front Immunol 2014;5:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crane M, Oliver B, Matthews G, Avihingsanon A, Ubolyam S, Markovska V, et al. Immunopathogenesis of hepatic flare in HIV/hepatitis B virus (HBV)‐coinfected individuals after the initiation of HBV‐active antiretroviral therapy. J Infect Dis 2009;199:974‐981. [DOI] [PubMed] [Google Scholar]
- 20. Woreta TA, Sutcliffe CG, Mehta SH, Brown TT, Higgins Y, Thomas DL, et al. Incidence and risk factors for steatosis progression in adults coinfected with HIV and hepatitis C virus. Gastroenterology 2011;140:809‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R. Clinical, biochemical and histological differences between HIV‐associated NAFLD and primary NAFLD: a case‐control study. Aliment Pharmacol Ther 2015;41:368‐378. [DOI] [PubMed] [Google Scholar]
- 22. Szabo G. Gut‐liver axis in alcoholic liver disease. Gastroenterology 2015;148:30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balagopal A, Philp FH, Astemborski J, Block TM, Mehta A, Long R, et al. Human immunodeficiency virus‐related microbial translocation and progression of hepatitis C. Gastroenterology 2008;135:226‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vujkovic‐Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013;5:193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chattergoon MA, Latanich R, Quinn J, Winter ME, Buckheit RW 3rd, Blankson JN, et al. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal toll‐like receptors without induction of type 1 interferon. PLoS Pathog 2014;10:e1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chattergoon MA, Levine JS, Latanich R, Osburn WO, Thomas DL, Cox AL. High plasma interleukin‐18 levels mark the acute phase of hepatitis C virus infection. J Infect Dis 2011;204:1730‐1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506‐2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, et al.; R.E.V.E.A.L.‐HCV Study Group . Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community‐based long‐term prospective study. J Infect Dis 2012;206:469‐477. [DOI] [PubMed] [Google Scholar]
- 29. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non‐alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta‐analysis. J Hepatol 2016;65:589‐600. [DOI] [PubMed] [Google Scholar]
- 30. Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA 2012;308:379‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hanna DB, Lin J, Post WS, Hodis HN, Xue X, Anastos K, et al. Association of macrophage inflammation biomarkers with progression of subclinical carotid artery atherosclerosis in HIV‐infected women and men. J Infect Dis 2017; 215:1352‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cowell A, Shenoi SV, Kyriakides TC, Friedland G, Barakat LA. Trends in hospital deaths among human immunodeficiency virus‐infected patients during the antiretroviral therapy era, 1995 to 2011. J Hosp Med 2015;10:608‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Freidl GS, Sonder GJ, Bovee LP, Friesema IH, van Rijckevorsel GG, Ruijs WL, et al. Hepatitis A outbreak among men who have sex with men (MSM) predominantly linked with the EuroPride, the Netherlands, July 2016 to February 2017. Euro Surveill 2017;22:pii:30468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beebeejaun K, Degala S, Balogun K, Simms I, Woodhall SC, Heinsbroek E, et al. Outbreak of hepatitis A associated with men who have sex with men (MSM), England, July 2016 to January 2017. Euro Surveill 2017;22:pii:30454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Werber D, Michaelis K, Hausner M, Sissolak D, Wenzel J, Bitzegeio J, et al. Ongoing outbreaks of hepatitis A among men who have sex with men (MSM), Berlin, November 2016 to January 2017 ‐ linked to other German cities and European countries. Euro Surveill 2017;22:pii:30457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Regan DG, Wood JG, Benevent C, Ali H, Smith LW, Robertson PW, et al. Estimating the critical immunity threshold for preventing hepatitis A outbreaks in men who have sex with men. Epidemiol Infect 2016;144:1528‐1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martinez A, Broner S, Sala MR, Manzanares‐Laya S, Godoy P, Planas C, et al.; For The Study Of The Immune Status In Health Care TW, Hepatitis A In Catalonia FT . Changes in the epidemiology of hepatitis A outbreaks 13 years after the introduction of a mass vaccination program. Hum Vaccin Immunother 2015;11:192‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kourkounti S, Paparizos V, Leuow K, Kyriakis K, Antoniou C. Prevalence and titre of antibodies against Hepatitis A virus in HIV‐infected men having sex with men in Greece. Infez Med 2014;22:206‐212. [PubMed] [Google Scholar]
- 39. Winnock M, Bani‐Sadr F, Pambrun E, Loko MA, Lascoux‐Combe C, Garipuy D, et al. Prevalence of immunity to hepatitis viruses A and B in a large cohort of HIV/HCV‐coinfected patients, and factors associated with HAV and HBV vaccination. Vaccine 2011;29:8656‐8660. [DOI] [PubMed] [Google Scholar]
- 40. Sun HY, Sheng WH, Tsai MS, Lee KY, Chang SY, Hung CC. Hepatitis B virus coinfection in human immunodeficiency virus‐infected patients: a review. World J Gastroenterol 2014;20:14598‐14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fuster F, Vargas JI, Jensen D, Sarmiento V, Acuña P, Peirano F, et al.; Core‐HIV Study Group . CD4/CD8 ratio as a predictor of the response to HBV vaccination in HIV‐positive patients: a prospective cohort study. Vaccine 2016;34:1889‐1895. [DOI] [PubMed] [Google Scholar]
- 42. Thio CL, Seaberg EC, Skolasky R Jr, Phair J, Visscher B, Muñoz A, et al.; Multicenter AIDS Cohort Study . HIV‐1, hepatitis B virus, and risk of liver‐related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360:1921‐1926. [DOI] [PubMed] [Google Scholar]
- 43. Weber R, Sabin CA, Friis‐Moller N, Reiss P, El‐Sadr WM, Kirk O, et al. Liver‐related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632‐1641. [DOI] [PubMed] [Google Scholar]
- 44. Klein MB, Althoff KN, Jing Y, Lau B, Kitahata M, Lo Re V 3rd, et al.; North American AIDS Cohort Collaboration on Research and Design of IeDEA ; North American AIDS Cohort Collaboration on Research and Design (NA‐ACCORD) of IeDEA . Risk of end‐stage liver disease in HIV‐viral hepatitis coinfected persons in North America from the early to modern antiretroviral therapy eras. Clin Infect Dis 2016;63:1160‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 1999;30:1054‐1058. [DOI] [PubMed] [Google Scholar]
- 46. Benhamou Y, Di Martino V, Bochet M, Colombet G, Thibault V, Liou A, et al; MultivirC Group . Factors affecting liver fibrosis in human immunodeficiency virus‐and hepatitis C virus‐coinfected patients: impact of protease inhibitor therapy. Hepatology 2001;34:283‐287. [DOI] [PubMed] [Google Scholar]
- 47. Kovari H, Ledergerber B, Cavassini M, Ambrosioni J, Bregenzer A, Stöckle M, et al.; Swiss HIV Cohort Study . High hepatic and extrahepatic mortality and low treatment uptake in HCV‐coinfected persons in the Swiss HIV cohort study between 2001 and 2013. J Hepatol 2015;63:573‐580. [DOI] [PubMed] [Google Scholar]
- 48. Lo Re V 3rd, Wang L, Devine S, Baser O, Olufade T. Hepatic decompensation in patients with HIV/Hepatitis B virus (HBV)/Hepatitis C virus (HCV) triple infection versus HIV/HCV coinfection and the effect of anti‐HBV nucleos(t)ide therapy. Clin Infect Dis 2014;59:1027‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brunet L, Moodie EEM, Young J, Cox J, Hull M, Cooper C, et al. Progression of liver fibrosis and modern combination antiretroviral therapy regimens in HIV‐Hepatitis C‐coinfected persons. Clin Infect Dis 2016;62:242‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gish RG, Yi DH, Kane S, Clark M, Mangahas M, Baqai S, et al. Coinfection with hepatitis B and D: epidemiology, prevalence and disease in patients in Northern California. J Gastroenterol Hepatol. 2013;28:1521‐1525. [DOI] [PubMed] [Google Scholar]
- 51. Safaie P, Privitera I, Sherman KE. Underutilization of hepatitis D virus testing among HBV monoinfected and HBV/HIV coinfected patients [DDW Abstracts 741c]. Gastroenterology 2017;152(Suppl. 1):S162 [Google Scholar]
- 52. Ordieres C, Navascues CA, Gonzalez‐Dieguez ML, Rodríguez M, Cadahía V, Varela M, et al. Prevalence and epidemiology of hepatitis D among patients with chronic hepatitis B virus infection: a report from Northern Spain. Eur J Gastroenterol Hepatol 2017;29:277‐283. [DOI] [PubMed] [Google Scholar]
- 53. Castellares C, Barreiro P, Martin‐Carbonero L, Labarga P, Vispo ME, Casado R, et al. Liver cirrhosis in HIV‐infected patients: prevalence, aetiology and clinical outcome. J Viral Hepat 2008;15:165‐172. [DOI] [PubMed] [Google Scholar]
- 54. Fernandez‐Montero JV, Vispo E, Barreiro P, Sierra‐Enguita R, de Mendoza C, Labarga P, et al. Hepatitis delta is a major determinant of liver decompensation events and death in HIV‐infected patients. Clin Infect Dis 2014;58:1549‐1553. [DOI] [PubMed] [Google Scholar]
- 55. Rivero‐Juarez A, Cuenca‐Lopez F, Martinez‐Peinado A, Camacho A, Real LM, Frias M, et al. Rural habitat as risk factor for hepatitis E virus seroconversion in HIV‐infected patients: a prospective longitudinal study. Zoonoses Public Health 2017; doi:10.1111/zph.12347. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56. Madden RG, Wallace S, Sonderup M, Korsman S, Chivese T, Gavine B, et al. Hepatitis E virus: Western Cape, South Africa. World J Gastroenterol 2016;22:9853‐9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Debes JD, Pisano MB, Lotto M, Re V. Hepatitis E virus infection in the HIV‐positive patient. J Clin Virol 2016;80:102‐106. Corrigendum in: J Clin Virol 2016;181‐182. [DOI] [PubMed] [Google Scholar]
- 58. Kuniholm MH, Ong E, Hogema BM, Koppelman M, Anastos K, Peters MG, et al. Acute and chronic hepatitis E virus infection in human immunodeficiency virus‐infected U.S. women. Hepatology 2016;63:712‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jagjit Singh GK, Ijaz S, Rockwood N, Farnworth SP, Devitt E, Atkins M, et al. Chronic hepatitis E as a cause for cryptogenic cirrhosis in HIV. J Infect 2013;66:103‐106. [DOI] [PubMed] [Google Scholar]
- 60. Ryom L, Lundgren JD, De Wit S, Kovari H, Reiss P, Law M, et al.; D:A:D Study Group . Use of antiretroviral therapy and risk of end‐stage liver disease and hepatocellular carcinoma in HIV‐positive persons. AIDS 2016;30:1731‐1743. [DOI] [PubMed] [Google Scholar]
- 61. Schiano TD, Uriel A, Dieterich DT, Fiel MI. The development of hepatoportal sclerosis and portal hypertension due to didanosine use in HIV. Virchows Arch 2011;458:231‐235. [DOI] [PubMed] [Google Scholar]
- 62. Vispo E, Cevik M, Rockstroh JK, Barreiro P, Nelson M, Scourfield A, et al.; European Network of Clinical Trials (NEAT) . Genetic determinants of idiopathic noncirrhotic portal hypertension in HIV‐infected patients. Clin Infect Dis 2013;56:1117‐1122. [DOI] [PubMed] [Google Scholar]
- 63. Price JC, Seaberg EC, Latanich R, Budoff MJ, Kingsley LA, Palella FJ Jr, et al. Risk factors for fatty liver in the Multicenter AIDS Cohort Study. Am J Gastroenterol 2014;109:695‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sterling RK, Smith PG, Brunt EM. Hepatic steatosis in human immunodeficiency virus: a prospective study in patients without viral hepatitis, diabetes, or alcohol abuse. J Clin Gastroenterol 2013;47:182‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Morse CG, McLaughlin M, Matthews L, Proschan M, Thomas F, Gharib AM, et al. Nonalcoholic steatohepatitis and hepatic fibrosis in HIV‐1‐monoinfected adults with elevated aminotransferase levels on antiretroviral therapy. Clin Infect Dis 2015;60:1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kramer JR, Kowalkowski MA, Duan Z, Chiao EY. The effect of HIV viral control on the incidence of hepatocellular carcinoma in veterans with hepatitis C and HIV coinfection. J Acquir Immune Defic Syndr 2015;68:456‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rosenthal E, Roussillon C, Salmon‐Ceron D, Georget A, Hénard S, Huleux T, et al.; Mortalité 2010 and GERMIVIC study groups . Liver‐related deaths in HIV‐infected patients between 1995 and 2010 in France: the Mortavic 2010 study in collaboration with the Agence Nationale de Recherche sur le SIDA (ANRS) EN 20 Mortalite 2010 survey. HIV Med 2015;16:230‐239. [DOI] [PubMed] [Google Scholar]
- 68. Gelu‐Simeon M, Sobesky R, Haim‐Boukobza S, Ostos M, Teicher E, Fontaine H, et al. Do the epidemiology, physiological mechanisms and characteristics of hepatocellular carcinoma in HIV‐infected patients justify specific screening policies? AIDS 2014;28:1379‐1391. [DOI] [PubMed] [Google Scholar]
- 69. INSIGHT START Study Group , Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Matthews GV, Neuhaus J, Bhagani S, Mehta SH, Vlahakis E, Doroana M, et al.; International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) START Study Group . Baseline prevalence and predictors of liver fibrosis among HIV‐positive individuals: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med 2015;16 (Suppl. 1):129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV‐‐United States, 2011. MMWR Morb Mortal Wkly Rep 2014;63:1113‐1117. [PMC free article] [PubMed] [Google Scholar]
- 72. Gonsalves GS, Paltiel AD, Cleary PD, Gill MJ, Kitahata MM, Rebeiro PF, et al. A flow‐based model of the HIV care continuum in the United States. J Acquir Immune Defic Syndr 2017;75:548‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Macias J, Monge P, Mancebo M, Merchante N, Neukam K, Real LM, et al. High frequency of potential interactions between direct‐acting antivirals and concomitant therapy in HIV/hepatitis C virus‐coinfected patients in clinical practice. HIV Med 2017;18:445‐451. [DOI] [PubMed] [Google Scholar]
- 74. Macias J, Merino, D , Tellez F, Pulido F, Gonzalez J, Marquez M, et al. Changes in liver steatosis after switching efavirenz to raltegravir: the STERAL study. [Abstract]. In: Conference on Retroviruses and Opportunistic Infections (CROI); February 13‐16, 2017; Seattle, WA. Abstract 697.
- 75. Utay NS, Reynoso D, Somasunderam A, Nigalye M, Currier J, Lake JE. Raltegravir switch and biomarkers of liver steatosis and metabolic syndrome in women. Conference on Retroviruses and Opportunistic Infections (CROI); February 13‐16, 2017; Seattle, WA. Abstract 696.
- 76. Saeed S, Strumpf EC, Walmsley SL, Rollet‐Kurhajec K, Pick N, Martel‐Laferrière V, et al. How generalizable are the results from trials of direct antiviral agents to people coinfected with HIV/HCV in the real world? Clin Infect Dis 2016;62:919‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bhattacharya D, Belperio PS, Shahoumian TA, Loomis TP, Goetz MB, Mole LA, et al. Effectiveness of all‐oral antiviral regimens in 996 genotype 1 HIV/HCV coinfected patients treated in routine practice. Clin Infect Dis 2017;64:1711‐1720. [DOI] [PubMed] [Google Scholar]
- 78. Piroth L, Wittkop L, Lacombe K, Rosenthal E, Gilbert C, Miailhes P, et al.; ANRS CO13 HEPAVIH study group . Efficacy and safety of direct‐acting antiviral regimens in HIV/HCV‐coinfected patients ‐ French ANRS CO13 HEPAVIH cohort. J Hepatol 2017;67:23‐31. [DOI] [PubMed] [Google Scholar]
- 79. Huhn GD, Tebas P, Gallant J, Wilkin T, Cheng A, Yan M, et al. A randomized, open‐label trial to evaluate switching to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide plus darunavir in treatment‐experienced HIV‐1‐infected adults. J Acquir Immune Defic Syndr 2017;74:193‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pozniak A, Arribas JR, Gathe J, Gupta SK, Post FA, Bloch M, et al.; GS‐US‐292‐0112 Study Team . Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV‐infected patients with renal impairment: 48‐week results from a single‐arm, multicenter, open‐label phase 3 study. J Acquir Immune Defic Syndr 2016;71:530‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gotham D, Hill A, Pozniak AL. Candidates for inclusion in a universal antiretroviral regimen: tenofovir alafenamide. Curr Opin HIV AIDS 2017;12:324‐333. [DOI] [PubMed] [Google Scholar]
- 82. Benhamou Y, Bochet M, Thibault V, Di Martino V, Caumes E, Bricaire F, et al. Long‐term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus‐infected patients. Hepatology 1999;30:1302‐1306. [DOI] [PubMed] [Google Scholar]
- 83. Overton ET, Kang M, Peters MG, Umbleja T, Alston‐Smith BL, Bastow B, et al. Immune response to hepatitis B vaccine in HIV‐infected subjects using granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) as a vaccine adjuvant: ACTG study 5220. Vaccine 2010;28:5597‐5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fonseca MO, Pang LW, de Paula Cavalheiro N, Barone AA, Heloisa Lopes M. Randomized trial of recombinant hepatitis B vaccine in HIV‐infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902‐2908. [DOI] [PubMed] [Google Scholar]
- 85. Launay O, van der Vliet D, Rosenberg AR, Michel ML, Piroth L, Rey D, et al.; ANRS HB03 VIHVAC‐B Trial . Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV‐1: a randomized controlled trial. JAMA 2011;305:1432‐1440. [DOI] [PubMed] [Google Scholar]
- 86. Catherine FX, Piroth L. Hepatitis B virus vaccination in HIV‐infected people: a review. Hum Vaccin Immunother 2017;13:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shilaih M, Marzel A, Scherrer AU, Braun DL, Kovari H, Rougemont M, et al.; Swiss HIV Cohort Study a ; Swiss HIV Cohort Study . Dually active HIV/HBV antiretrovirals as protection against incident hepatitis B infections: potential for prophylaxis. J Infect Dis 2016;214:599‐606. [DOI] [PubMed] [Google Scholar]
- 88. Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology 2013;57:249‐257. [DOI] [PubMed] [Google Scholar]
- 89. Sherman M, Bruix J, Porayko M, Tran T. Screening for hepatocellular carcinoma: the rationale for the American Association for the Study of Liver Diseases recommendations. Hepatology 2012;56:793‐796. [DOI] [PubMed] [Google Scholar]
- 90. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian‐Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Merchante N, Merino E, Lopez‐Aldeguer J, Jover F, Delgado‐Fernández M, Galindo MJ, et al. Increasing incidence of hepatocellular carcinoma in HIV‐infected patients in Spain. Clin Infect Dis 2013;56:143‐150. [DOI] [PubMed] [Google Scholar]
- 92. Lewin M, Gelu‐Simeon M, Ostos M, Boufassa F, Sobesky R, Teicher E, et al. Imaging features and prognosis of hepatocellular carcinoma in patients with cirrhosis who are coinfected with human immunodeficiency virus and hepatitis C virus. Radiology 2015;277:443‐453. [DOI] [PubMed] [Google Scholar]
- 93. Castera L, Winnock M, Pambrun E, Paradis V, Perez P, Loko MA, et al. Comparison of transient elastography (FibroScan), FibroTest, APRI and two algorithms combining these non‐invasive tests for liver fibrosis staging in HIV/HCV coinfected patients: ANRS CO13 HEPAVIH and FIBROSTIC collaboration. HIV Med 2014;15:30‐39. [DOI] [PubMed] [Google Scholar]
- 94. Akoth E, Gross C, Silk R, Rosenthal E, Kattakuzhy S. A practical approach and model of care for HCV treatment with direct acting antivirals in an urban setting. J Assoc Nurses AIDS Care 2017;28:680‐684. [DOI] [PubMed] [Google Scholar]
- 95. Cachay ER, Hill L, Ballard C, Colwell B, Torriani F, Wyles D, et al. Increasing hepatitis C treatment uptake among HIV‐infected patients using an HIV primary care model. AIDS Res Ther 2013;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. He T, Li K, Roberts MS, Spaulding AC, Ayer T, Grefenstette JJ, et al. Prevention of hepatitis C by screening and treatment in U.S. prisons. Ann Intern Med 2016;164:84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schoenbachler BT, Smith BD, Sena AC, Hilton A, Bachman S, Lunda M, et al. Hepatitis C virus testing and linkage to care in North Carolina and South Carolina jails, 2012‐2014. Public Health Rep 2016;131(Suppl. 2):98‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kim HN, Crane HM, Rodriguez CV, van Rompaey S, Mayer KH, Christopoulos K, et a. The role of current and historical alcohol use in hepatic fibrosis among HIV‐infected individuals. AIDS Behav 2017;21:1878‐1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend 2016;161:95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Howe CJ, Cole SR, Ostrow DG, Mehta SH, Kirk GD. A prospective study of alcohol consumption and HIV acquisition among injection drug users. AIDS 2011;25:221‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Shuper PA, Neuman M, Kanteres F, Baliunas D, Joharchi N, Rehm J. Causal considerations on alcohol and HIV/AIDS‐‐a systematic review. Alcohol Alcohol 2010;45:159‐166. [DOI] [PubMed] [Google Scholar]
- 102. Lim JK, Tate JP, Fultz SL, Goulet JL, Conigliaro J, Bryant KJ, et al. Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV‐infected, chronic hepatitis C virus‐infected, and uninfected patients. Clin Infect Dis 2014;58:1449‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chander G, Monroe AK, Crane HM, Hutton HE, Saag MS, Cropsey K, et al. HIV primary care providers‐‐screening, knowledge, attitudes and behaviors related to alcohol interventions. Drug Alcohol Depend 2016;161:59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kissack JC, Bishop J, Roper AL. Ethylglucuronide as a biomarker for ethanol detection. Pharmacotherapy 2008;28:769‐781. [DOI] [PubMed] [Google Scholar]
- 105. Ferraguti G, Ciolli P, Carito V, Battagliese G, Mancinelli R, Ciafrè S, et al. Ethylglucuronide in the urine as a marker of alcohol consumption during pregnancy: comparison with four alcohol screening questionnaires. Toxicol Lett 2017;275:49‐56. [DOI] [PubMed] [Google Scholar]
- 106. Rios‐Olivares E, Vila LM, Reyes JC, Rodríguez JW, Colón JH, Pagán NO, et al. Impaired cytokine production and suppressed lymphocyte proliferation activity in HCV‐infected cocaine and heroin (“speedball”) users. Drug Alcohol Depend 2006;85:236‐243. [DOI] [PubMed] [Google Scholar]
- 107. Wang JF, Ren X, DeAngelis J, Min J, Zhang Y, Hampton TG, et al. Differential patterns of cocaine‐induced organ toxicity in murine heart versus liver. Exp Biol Med (Maywood) 2001;226:52‐60. [DOI] [PubMed] [Google Scholar]
- 108. Peters PJ, Pontones P, Hoover KW, Patel MR, Galang RR, Shields J, et al.; Indiana HIV Outbreak Investigation Team . HIV infection linked to injection use of oxymorphone in Indiana, 2014‐2015. N Engl J Med 2016;375:229‐239. [DOI] [PubMed] [Google Scholar]
- 109. Van Handel MM, Rose CE, Hallisey EJ, Kolling JL, Zibbell JE, Lewis B, et al. County‐level vulnerability assessment for rapid dissemination of HIV or HCV infections among persons who inject drugs, United States. J Acquir Immune Defic Syndr 2016;73:323‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]


