Abstract
Nonalcoholic steatohepatitis (NASH) is the progressive form of nonalcoholic fatty liver disease (NAFLD). The minimal pathologic criteria for NASH include hepatic steatosis, ballooning degeneration, and lobular inflammation. The resolution of NASH, which relies on the loss of ballooning degeneration, is subject to sampling and observer variability in pathologic interpretation. Ballooning is associated with advanced hepatic fibrosis in cross‐sectional studies but is not a predictor of mortality in NAFLD. Fibrosis staging, while still subject to some sampling variability, has less observer variability and is a robust predictor of liver‐related mortality in NAFLD. In this study, we hypothesize that, regardless of the diagnosis of NASH, the presence of steatofibrosis (steatosis accompanied by fibrosis) regardless of other pathologic features can also be a robust predictor of mortality in NAFLD. We used our previously reported cohort of patients with NAFLD with liver biopsies and long‐term mortality follow‐up. Cox proportional hazard models were used to determine the predictors of overall and liver‐related mortality. Of 209 enrolled NAFLD subjects, 97 can be classified as having steatofibrosis. During follow‐up (median 150 months), 64 (30.6%) patients died, with 18 (8.6%) from liver‐related causes. Adjusted for age, both diagnostic categories of NASH and steatofibrosis were significantly and similarly associated with liver‐related mortality (adjusted hazard ratio [aHR], 9.9; 95% confidence interval (CI), 1.3‐74.9; P = 0.027; aHR, 6.7; 95% CI, 1.5‐29.8; P = 0.013, respectively). However, only steatofibrosis showed independent association with overall mortality (aHR, 1.76; 95% CI, 1.02‐3.05; P = 0.043). Conclusion: Steatofibrosis and NASH are similarly associated with liver‐related mortality, but only steatofibrosis is associated with overall mortality in patients with NAFLD. Given the inherent observer variability in ballooning degeneration, a key diagnostic component of NASH, we suggest that steatofibrosis should be considered a viable diagnostic classification for NAFLD subjects at risk or adverse outcomes and provides a simpler endpoint for clinical trials of therapeutic agents. (Hepatology Communications 2017;1:421–428)
Abbreviations
- aHR
adjusted hazard ratio
- CI
confidence interval
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
Introduction
Nonalcoholic fatty liver disease (NAFLD) is thought to affect approximately 24% of the world population, with nonalcoholic steatohepatitis (NASH) being its predominantly progressive subtype.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 In 2013, NAFLD became the second indication for liver transplantation and is among the top causes of hepatocellular carcinoma in the United States.13, 14, 15 Besides its clinical burden, NAFLD has an important negative impact on health‐related quality of life and other patient‐reported outcomes.16 In addition, the economic burden of NAFLD and NASH to society is tremendous and increasing.17 Despite clear evidence supporting its clinical, economic, and patient‐reported outcomes burden, there are important controversies about the rate of progression and spontaneous regression of NASH as well as the most appropriate endpoints for clinical trials or the natural history of NASH.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
To better understand the initial subclassification of NAFLD (NASH versus non‐NASH), it is important to understand the context in which NASH became an important subtype of NAFLD. After the introduction of the term NASH in 1980, there was little clarity regarding its clinical importance, its natural history, and the histologic criteria required for its diagnosis.11 In fact, most clinicians in the early 1990s did not believe that NASH was a progressive or a common form of chronic liver disease. In an attempt to shed some light about the natural history of NASH, it became clear that NASH must be considered a part of the spectrum of NAFLD.2, 20 In this context, the majority of subjects with NAFLD did not have NASH and did not develop progressive liver disease.2, 3, 4, 5, 6, 7 On the other hand, NAFLD subjects who met a strict histologic criterion for NASH seemed to have a higher rate of adverse outcomes.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Over the years, accumulating evidence has supported the notion that NASH is a subtype of NAFLD with a potential for progressive liver disease.1, 2, 3, 4, 5, 6, 7, 8, 9 Although the non‐NASH subtype of NAFLD was historically considered nonprogressive, recent data suggest otherwise.10 In this context, it is plausible that a small number of patients with non‐NASH NAFLD can progress to NASH and follow a progressive course. It is also possible that the initial diagnosis of non‐NASH NAFLD in those subjects may have been misclassified due to sampling and interobserver variabilities in the initial liver biopsy specimen. According to the current American Association for the Study of Liver Diseases guidelines,4 the minimal histologic requirements for diagnosing NASH include steatosis, lobular inflammation, and ballooning of hepatocytes. Although these criteria provide an overall categorization of a subtype of NAFLD that can be at risk for progressive liver disease, it also has created substantial uncertainty2, 3, 5, 20 that is primarily related to the reliance on hepatocyte ballooning for diagnosing NASH and its disappearance as the endpoint of the “resolution of NASH” for clinical trials.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 It therefore became important to assess the predictive ability of the different histologic features in NAFLD and the reliability of these features in establishing the diagnosis of NASH.18
In the context of predicting long‐term outcomes, a number of studies have documented that the stage of hepatic fibrosis was the only histologic feature that predicted liver‐related mortality among patients with NAFLD.18, 19, 32, 33 Although other pathologic features of NASH were found to be associated with the presence of advanced fibrosis in cross‐sectional assessments, they did not add any predictive power when tested in survival models on top of fibrosis. Furthermore, in addition to the inability of hepatocyte ballooning to predict long‐term mortality outcomes in NAFLD, hepatocyte ballooning suffers from significant interobserver variability. In one study, the interobserver agreements were low (kappa scores for injury, 0.35 and lobular inflammation, 0.33) in contrast to significantly better interobserver agreement for steatosis and fibrosis (kappa scores for fibrosis, 0.61 and steatosis, 0.64).20 In another study with a predetermined pathologic protocol for NASH, the interobserver variability between expert hepatopathologists confirmed a weaker agreement for ballooning and inflammation as opposed to interobserver agreement related to fibrosis and steatosis (kappa scores for fibrosis, 0.84; steatosis, 0.79; ballooning, 0.56; lobular inflammation, 0.45).21
These findings may be partly due to the difficulty in identifying ballooning degeneration of hepatocytes in liver biopsy slides leading to underdiagnosis of NASH in the initial biopsy or subsequent follow‐up biopsies. Despite this, resolution of NASH, which can be defined as the disappearance of liver cell ballooning injury/inflammation in NAFLD, has been proposed as a primary endpoint for clinical trials of NASH.34 However, to anchor drug development for NASH on a relatively unreliable pathologic feature, which is also not a good surrogate for the hard outcome of mortality, can lead to a great deal of uncertainty about the true long‐term efficacy endpoint of these trials. Adding to these complexities in the field of NAFLD is the rate of spontaneous regression of NASH in sequential liver biopsies.22 In this context, a question remains whether this regression reflects the true dynamic nature of the natural history of NASH or it relates to a flaw in the diagnostic criteria for NASH influenced by the weak agreement between two liver biopsy specimens identifying hepatocyte ballooning of hepatocytes.
These issues have increasingly become important as we develop new treatments for NASH. In this context, a number of clinical trials have been designed, all requiring histologic documentation of NASH as entry criteria and the resolution of NASH as one of the primary endpoints.24, 25, 26 We believe this approach has led to significant difficulty in recruiting “at risk” patients with NAFLD for clinical trials and has placed an undue burden to document the efficacy of drug development in the field of NASH and NAFLD. In anticipation for more clinical trials involving patients with NAFLD and NASH in the near future, it is important to encourage investigators and regulatory bodies to prioritize the most important outcomes in patients with NAFLD and to determine endpoints that would be the best surrogates of mortality. It is also important to recognize that outside of the clinical trial setting, this inherent interobserver variability associated with the diagnosis of NASH can create more problems with the generalizability of data because practicing clinical pathologists' readings of these liver biopsies may have weaker interobserver agreement than those assessed by the expert study pathologists. Therefore, to simplify the diagnostic and therapeutic conundrum of NAFLD, it seems logical to focus on the most relevant and reliable surrogate marker of the hard outcomes of NASH, which seems to be hepatic fibrosis. Although subclassification of NAFLD to NASH and non‐NASH has served the purpose of providing an overall risk assessment for NAFLD, it is time to clarify that patients with NAFLD and fibrosis are at the highest risk and in the most urgent need of attention. Additionally, fibrosis is significantly more robust than ballooning and should become the primary endpoint for clinical trials of NASH. Thus, we hypothesize that “steatofibrosis” (fibrosis accompanying steatosis among patients with NAFLD) could be a more relevant and robust diagnostic classification than NASH.
In this study, we used our NAFLD database to assess the long‐term outcomes of a well‐defined cohort of patients with NAFLD who were histologically categorized as NASH and compared those outcomes with subjects meeting the criteria for nonalcoholic steatofibrosis.
Methods
In this study, we used a historic cohort of patients with biopsy‐proven NAFLD and mortality follow‐up of at least 5 years. The previously reported cohort with inclusion criteria into the NAFLD database have been published.18, 19, 20 Briefly, patients were included if they were at least 18 years of age, had an available liver biopsy slide and mortality follow‐up, and did not have other causes of chronic liver disease, such as excessive alcohol consumption, viral hepatitis, autoimmune, or drug‐induced hepatitis. Patients who died in follow‐up had a recorded cause of death; patients with liver‐related causes of death were specifically identified as described.18, 19, 20
The biopsy slides were reviewed jointly by two hepatopathologists as described.18, 19, 20 Each liver biopsy was categorized into NASH (minimum criteria: steatosis with hepatocellular ballooning, lobular inflammation, with the optional features of Mallory‐Denk bodies or fibrosis) and non‐NASH NAFLD. In the scoring protocol, pericellular/perisinusoidal fibrosis and portal fibrosis were graded into four categories on a scale of 0 to 3: (0) none, (1) mild or few, (2) moderate, or (3) marked or many. Using these pathologic features, we defined steatofibrosis as steatosis (>5%) in the presence of moderate or marked hepatic fibrosis (stage >1) regardless of other features.
STATISTICAL ANALYSIS
Demographic and clinical parameters of patients with and without steatofibrosis were compared using nonparametric tests. Survival curves of these patients were compared using a log‐rank test. The same test was then used to compare survival curves of patients with and without NASH. Cox proportional hazard models were built using steatofibrosis and NASH as interchangeable or combined predictors of liver‐related mortality and overall mortality with adjustment for significant confounders (demographics, comorbidities; only confounders with P < 0.05 were kept in the models). Goodness of fit was assessed using a partial likelihood ratio test and the Harrell c‐index. All calculations were run in SAS 9.3 (SAS Institute, Cary, NC). The study was approved by the Inova Institutional Review Board.
Results
Of the 209 patients with NAFLD with long‐term follow‐up and liver biopsy slides, 97 patients met the histologic criteria for steatofibrosis while 112 NAFLD subjects did not have steatofibrosis. With the same NAFLD cohort, 131 patients fulfilled the criteria for NASH and 78 had non‐NASH NAFLD. Demographics, medical history, and pathologic features of enrolled patients are summarized in Table 1 according to their steatofibrosis status. As shown, patients with steatofibrosis were significantly older, more were female, and body mass index was lower but type 2 diabetes was more prevalent (all P < 0.05) (Table 1).
Table 1.
DEMOGRAPHICS AND BASIC CLINICAL PRESENTATION OF PATIENTS WITH AND WITHOUT STEATOFIBROSIS
| Steatofibrosis | No Steatofibrosis | P a | All | |
|---|---|---|---|---|
| N | 97 | 112 | 209 | |
| Died in follow‐up | 44 (45.4%) | 20 (17.9%) | <0.0001 | 64 (30.6%) |
| Died of liver‐related causes | 16 (16.5%) | 2 (1.8%) | 0.0002 | 18 (8.6%) |
| Died of cardiovascular causes | 15 (15.5%) | 7 (6.3%) | 0.0322 | 22 (10.6%) |
| Duration of follow‐up, months (median[IQR]) | 150 (114‐180) | 138 (68‐216) | 0.74 | 150 (69‐186) |
| Age at biopsy, years (mean ± SD) | 53.8 ± 13.8 | 44.4 ± 14.2 | <0.0001 | 48.7 ± 14.7 |
| Male sex | 29 (29.9%) | 50 (44.6%) | 0.0283 | 79 (37.8%) |
| Caucasian race | 68 (79.1%) | 74 (71.8%) | 0.25 | 142 (75.1%) |
| Body mass index, kg/m2 (mean ± SD) | 32.7 ± 7.5 | 37.8 ± 11.5 | 0.0163 | 36.1 ± 10.6 |
| Obesity (BMI >30) | 40 (41.2%) | 62 (55.4%) | 0.0417 | 102 (48.8%) |
| Type 2 diabetes | 29 (29.9%) | 14 (12.5%) | 0.0019 | 43 (20.6%) |
| Hyperlipidemia | 17 (18.1%) | 30 (28.3%) | 0.09 | 47 (23.5%) |
| Pathologic features: | ||||
| NASH | 86 (88.7%) | 45 (40.2%) | <0.0001 | 131 (62.7%) |
| Fat | ||||
| <5% | 13 (13.4%) | 32 (28.6%) | 45 (21.5%) | |
| 6%‐33% | 51 (52.6%) | 45 (40.2%) | 96 (45.9%) | |
| 34%‐66% | 29 (29.9%) | 25 (22.3%) | 54 (25.8%) | |
| 67%‐100% | 4 (4.1%) | 10 (8.9%) | 0.0317 | 14 (6.7%) |
| Lobular inflammation | ||||
| 0 (none) | 4 (4.1%) | 16 (14.3%) | 20 (9.6%) | |
| 1 (mild) | 30 (30.9%) | 67 (59.8%) | 97 (46.4%) | |
| 2 (moderate) | 46 (47.4%) | 25 (22.3%) | 71 (34.0%) | |
| 3 (severe) | 17 (17.5%) | 4 (3.6%) | <0.0001 | 21 (10.0%) |
| Portal inflammation | ||||
| 0 (none) | 5 (5.2%) | 38 (33.9%) | 43 (20.6%) | |
| 1 (mild) | 29 (29.9%) | 65 (58.0%) | 94 (45.0%) | |
| 2 (moderate) | 58 (59.8%) | 9 (8.0%) | 67 (32.1%) | |
| 3 (severe) | 5 (5.2%) | 0 (0.0%) | <0.0001 | 5 (2.4%) |
| Hepatocellular ballooning | ||||
| 0 (none) | 18 (18.6%) | 81 (72.3%) | 99 (47.4%) | |
| 1 (rare) | 21 (21.7%) | 22 (19.6%) | 43 (20.6%) | |
| 2 (frequent) | 43 (44.3%) | 8 (7.1%) | 51 (24.4%) | |
| 3 (severe/numerous) | 15 (15.5%) | 1 (0.9%) | <0.00001 | 16 (7.7%) |
| Mallory‐Denk bodies | ||||
| 0 (none) | 24 (24.7%) | 93 (83.0%) | 117 (56.0%) | |
| 1 (rare) | 31 (32.0%) | 15 (13.4%) | 46 (22.0%) | |
| 2 (frequent) | 31 (32.0%) | 4 (3.6%) | 35 (16.7%) | |
| 3 (severe/numerous) | 11 (11.3%) | 0 (0.0%) | <0.0001 | 11 (5.3%) |
| Pericellular/perisinusoidal fibrosis | ||||
| 0 (none) | 14 (14.4%) | 77 (68.7%) | 91 (43.5%) | |
| 1 (mild) | 16 (16.5%) | 35 (31.3%) | 51 (24.4%) | |
| 2 (moderate) | 51 (52.6%) | 0 (0.0%) | 51 (24.4%) | |
| 3 (severe) | 16 (16.5%) | 0 (0.0%) | <0.0001 | 16 (7.7%) |
| Portal fibrosis | ||||
| 0 (none) | 5 (5.2%) | 55 (49.1%) | 60 (28.7%) | |
| 1 (mild) | 6 (6.2%) | 57 (50.9%) | 63 (30.1%) | |
| 2 (moderate) | 62 (63.9%) | 0 (0.0%) | 62 (29.7%) | |
| 3 (severe) | 24 (24.7%) | 0 (0.0%) | <0.0001 | 24 (11.5%) |
Chi‐square or Mann‐Whitney nonparametric test.
Abbreviations: BMI, body mass index; IQR, interquartile range.
In follow‐up (median 150 months; interquartile range, 69‐186 months), 64 (30.6%) patients died, with 18 (8.6%) patients dying from liver‐related causes. Liver‐related mortality curves by patients' steatofibrosis status are shown in Fig. 1A. The log‐rank test indicated a significant association of liver‐related mortality with steatofibrosis (hazard ratio [HR], 8.7; 95% confidence interval [CI], 2.0‐37.9; P = 0.0040). A similar association of liver‐related mortality was documented for NASH (HR, 10.4; 95% CI, 1.4‐78.7; P = 0.0229) (Fig. 1B).
Figure 1.
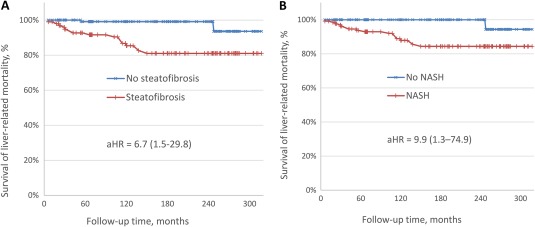
Liver‐related mortality by (A) steatofibrosis and (B) NASH status. The 95% CI is shown in parentheses.
In multiple Cox proportional hazard models, after inclusion of age, sex, obesity, and diabetes status of patients in addition to diagnostic categories of steatofibrosis and NASH, only age at the time of diagnosis demonstrated a statistically significant association with liver‐related mortality (other P values > 0.05). Thus, in proportional hazard models adjusted for age (aHR), both NASH and steatofibrosis were significantly and similarly associated with liver‐related mortality (aHR, 9.9; 95% CI, 1.3‐74.9; P = 0.027 for NASH; aHR, 6.7; 95% CI, 1.5‐29.8; P = 0.013 for steatofibrosis). Both models also had nearly identical fit statistics (likelihood ratio, 15.5; P = 0.0004 for NASH; likelihood ratio, 15.2; P = 0.0005 for steatofibrosis) and Harrell c‐indices (0.56; 95% CI, 0.31‐0.80 for NASH; 0.58; 95% CI, 0.33‐0.82 for steatofibrosis).
Additionally, for the purpose of the sensitivity analysis, we also studied other thresholds for the definition of steatofibrosis. Steatofibrosis defined as the pericellular or portal fibrosis of a stage greater than 0 was not found to be associated with liver‐related mortality (P = 0.99). The threshold of 1 was the one chosen in this study with the results cited above. Finally, severe steatofibrosis defined as the pericellular or portal fibrosis of a stage greater than 2 (bridging fibrosis or cirrhosis) was more strongly associated with liver‐related mortality (aHR, 17.5; 95% CI, 5.6‐54.3; P < 0.0001) but also resulted in twice as many false negatives because 4 of the 18 subjects who eventually died of liver‐related causes had fibrosis of stage 1 or 2. We also studied patients who had both NASH and steatofibrosis; there were 86 such patients of whom 16 died of liver‐related causes (age‐adjusted association with liver‐related mortality: aHR, 9.1; 95% CI, 2.1‐40.3; P = 0.0036; likelihood ratio, 19.5). This definition had the same false‐negative rate as steatofibrosis alone while returning 16% fewer false positives; however, this came at the cost of a substantially more complicated algorithm that included the diagnosis of NASH with all its flaws discussed above.
Because liver‐related mortality accounted for only 28% of all deaths in the cohort, we also studied the association of the diagnostic categories of steatofibrosis and NASH with overall mortality. In contrast to liver‐related mortality, which was similarly associated with both steatofibrosis and NASH, overall mortality in subjects with NAFLD was only associated with steatofibrosis (aHR, 1.76; 95% CI, 1.02‐3.05; P = 0.043) but not with NASH (P = 0.28) (Fig. 2). Notably, the most prevalent cause of death in the study cohort was related to cardiovascular diseases (Table 1). However, consistent with a prior report,3 it was not found to be associated with steatofibrosis or NASH after adjustment for age, sex, and components of metabolic syndrome (both P > 0.10).
Figure 2.
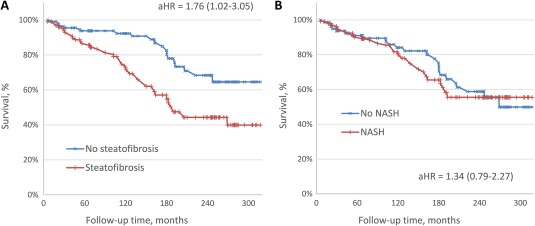
Overall mortality by (A) steatofibrosis and (B) NASH status. The 95% CI is shown in parentheses.
Discussion
This was a study of patients with NAFLD with a baseline histopathologic spectrum and long‐term follow‐up data. Although the study confirms that the subgroup of patients with NAFLD who meet the histologic criteria of NASH are at increased risk for liver‐related mortality, we document an almost identical association with liver‐related mortality for patients who meet the histologic diagnosis of steatofibrosis. In addition, our data show that NASH is not independently predictive of overall mortality while steatofibrosis is an independent predictor of not only liver‐related mortality but also overall mortality. Thus, we suggest that steatofibrosis rather than steatohepatitis (NASH) should be used as a more meaningful, more reliably assessed, and more clinically relevant category of patients with NAFLD who are at the highest risk for liver‐specific and all‐cause mortality.
These data should also be considered in the context of reported suboptimal interobserver agreement of hepatocyte injury (ballooning degeneration) and inflammation as well as the superior interobserver agreement for histologic fibrosis.11, 12, 21 In the context of reliability, the strong interobserver concordance for fibrosis further contributes to the superiority of steatofibrosis as a diagnostic category for NAFLD, making fibrosis the single most reliable endpoint for clinical trials of NASH. Furthermore, the most promising noninvasive tests for diagnosing the progressive form of NAFLD have focused on indirect measures of fibrosis or its surrogates (e.g., the NAFLD fibrosis score, ELF score, elastography).32 In this context, these noninvasive tests should be more useful in assessing steatofibrosis rather than NASH. They may document improvement of fibrosis but certainly are not going to be able to document resolution of NASH.
These data have important clinical relevance as well as important implications for the regulatory bodies that oversee drug development for NAFLD patients with the most urgent need for treatment. Although subclassification of NAFLD into NASH and non‐NASH have important epidemiologic relevance to allow separation of benign NAFLD from a potentially progressive subtype of NAFLD, we suggest that the clinical trials for NAFLD should be designed for those patients who are at the highest risk for mortality, i.e., patients with steatofibrosis regardless of whether they meet the histologic criteria for NASH. We also encourage regulatory bodies and pharmaceutical companies to abandon their insistence on requiring the resolution of NASH as a primary endpoint of intervention for clinical trials in NAFLD. It is important to remember that resolution of NASH has never been shown to improve survival of patients with NAFLD, while resolution of fibrosis has been associated with a decrease of mortality in patients with other chronic liver diseases.27, 28, 29, 30, 31 It is also important to recognize that the interobserver agreements of the aggregate pathologic diagnostic criteria for NASH are also very weak (kappa scores were as low as 0.18).18 Furthermore, it is important to note that pathologic scores, such as the nonalcoholic fatty liver disease activity score, have never been shown to predict liver‐related mortality.18, 19 In this context, we believe that focusing on patients with steatofibrosis and relying on the improvement of fibrosis as a primary endpoint will be a superior approach to design clinical trials for patients with NAFLD. This simple, straightforward, and reliable endpoint, for clinical trial as well as the best surrogate of liver‐related mortality and all‐cause mortality, should replace the cumbersome and unreliable primary endpoint of the resolution of NASH in future clinical trials and clinical management of patients with NAFLD.
In summary, these data confirm that NASH is associated with liver‐related mortality but not overall mortality. Evaluation of the histologic components of NASH is hampered by interobserver variability, which makes them less reliable. On the other hand, the definition of steatofibrosis allows for a simpler and more reliable categorization of patients with NAFLD that is predictive of both liver‐related and overall mortality. This approach would provide a more meaningful and reliable outcome for drug development and for designing noninvasive tests for the patients with NAFLD who have the most urgent needs. Further studies are needed to better define the contribution of each stage of steatofibrosis to the risk of developing adverse outcomes and liver‐related mortality.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413‐1493. [DOI] [PubMed] [Google Scholar]
- 3. Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, et al. Predictors of all‐cause mortality and liver‐related mortality in patients with non‐alcoholic fatty liver disease (NAFLD). Dig Dis Sci 2013;58:3017‐3023. [DOI] [PubMed] [Google Scholar]
- 4. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005‐2023. [DOI] [PubMed] [Google Scholar]
- 5. Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, et al. Long‐term follow‐up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol 2009;7:234‐238. [DOI] [PubMed] [Google Scholar]
- 6. Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 7. Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005;42:132‐138. [DOI] [PubMed] [Google Scholar]
- 8. Fassio E, Alvarez E, Domínguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology 2004;40:820‐826. [DOI] [PubMed] [Google Scholar]
- 9. Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, et al. Disease progression of non‐alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 2010;59:969‐974. [DOI] [PubMed] [Google Scholar]
- 10. McPherson S, Hardy T, Henderson E, Burt A, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing‐steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148‐1155. [DOI] [PubMed] [Google Scholar]
- 11. Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980;55:434‐438. [PubMed] [Google Scholar]
- 12. Kleiner DE, Bedossa P. Liver histology and clinical trials for nonalcoholic steatohepatitis‐perspectives from 2 pathologists. Gastroenterology 2015;149:1305‐1308. [DOI] [PubMed] [Google Scholar]
- 13. Wong R, Aguilar M, Cheung R, Perumpail R, Harrison S, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 14. Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62:1723‐1730. [DOI] [PubMed] [Google Scholar]
- 15. Stepanova M, Wai H, Saab S, Mishra A, Venkatesan C, Younossi ZM. The portrait of an adult liver transplant recipient in the United States from 1987 to 2013. JAMA Intern Med 2014;174:1407‐1409. [DOI] [PubMed] [Google Scholar]
- 16. Sayiner M, Stepanova M, Pham H, Noor B, Walters M, Younossi ZM. Assessment of health utilities and quality of life in patients with non‐alcoholic fatty liver disease. BMJ Open Gastroenterol 2016;3:e000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577‐1586. [DOI] [PubMed] [Google Scholar]
- 18. Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver‐related mortality. Hepatology 2011;53:1874‐1882. [DOI] [PubMed] [Google Scholar]
- 19. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Younossi ZM, Gramlich T, Liu YC, Matteoni C, Petrelli M, Goldblum J, et al. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod Pathol 1998;11:560‐565. [PubMed] [Google Scholar]
- 21. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 22. Loomba R, Chalasani N. The hierarchical model of NAFLD: prognostic significance of histologic features in NASH. Gastroenterology 2015;149:278‐281. [DOI] [PubMed] [Google Scholar]
- 23. Gramlich T, Kleiner DE, McCullough AJ, Matteoni CA, Boparai N, Younossi ZM. Pathologic features associated with fibrosis in nonalcoholic fatty liver disease. Hum Pathol 2004;35:196‐199. [DOI] [PubMed] [Google Scholar]
- 24. He L, Liu X, Wang L, Yang Z. Thiazolidinediones for nonalcoholic steatohepatitis: a meta‐analysis of randomized clinical trials. Medicine (Baltimore). 2016;95:e4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non‐invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol 2016;65:1006‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perazzo H, Dufour JF. The therapeutic landscape of non‐alcoholic steatohepatitis. Liver Int. 2017;37:634‐647. [DOI] [PubMed] [Google Scholar]
- 27. Goossens N, Hoshida Y, Song WM, Jung M, Morel P, Nakagawa S, et al. Nonalcoholic steatohepatitis is associated with increased mortality in obese patients undergoing bariatric surgery. Clin Gastroenterol Hepatol 2016;14:1619‐1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hagström H, Nasr P, Ekstedt M, Kechagias S, Stål P, Bedossa P, et al. SAF score and mortality in NAFLD after up to 41 years of follow‐up. Scand J Gastroenterol 2017;52:87‐91. [DOI] [PubMed] [Google Scholar]
- 29. Alkhouri N, McCullough AJ. Noninvasive diagnosis of NASH and liver fibrosis within the spectrum of NAFLD. Gastroenterol Hepatol (N Y) 2012;8:661‐668. [PMC free article] [PubMed] [Google Scholar]
- 30. Angulo P. Long‐Term Mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology 2010;51:373‐375. Erratum in: Hepatology 2010;51:1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol 2012;56:1171‐1180. [DOI] [PubMed] [Google Scholar]
- 32. Sebastiani G, Alshaalan R, Wong P, Rubino M, Salman A, Metrakos P, et al. Prognostic value of non‐invasive fibrosis and steatosis tools, hepatic venous pressure gradient (HVPG) and histology in nonalcoholic steatohepatitis. PLoS One 2015;10:e0128774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, Choi PC, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology 2017;65:54‐64. [DOI] [PubMed] [Google Scholar]
- 34. Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011;54:344‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]


