Abstract
Despite guideline recommendations, access to hepatitis C virus (HCV) treatment is frequently restricted, with some payers approving therapy for only those with advanced disease or cirrhosis. However, delaying potentially curative treatment until the development of advanced liver disease may have costly consequences in terms of both hepatic complications and extrahepatic manifestations (EHMs) of HCV. Using a large claims database from the United States, we measured the risks and medical costs of 20 EHMs and investigated the role of treatment in different stages of liver fibrosis for mitigating the clinical and economic burden of these EHMs. After adjusting for potential confounders, including comorbid liver disease, patients with HCV had a significantly higher risk for any EHM (adjusted odds ratio, 2.23; P < 0.05) and higher EHM‐related annual medical costs (adjusted medical cost difference, $6,458; P < 0.05) compared to matched patients without HCV. HCV treatment can offset the higher medical costs in patients with HCV by saving ∼$25,000 in all‐cause medical costs per patient per year, with a large proportion attributable to savings in EHM‐related medical costs (adjusted cost difference $12,773, P < 0.05). Finally, additional EHM‐related medical costs could be saved by initiating HCV therapy in early stage fibrosis as opposed to late‐stage fibrosis (adjusted medical cost difference, $10,409; P < 0.05). Conclusion: The clinical and economic burden of EHMs is substantial and can be reduced through viral eradication, especially if treatment is initiated early and not delayed until fibrosis advances. Considering that the wholesale acquisition cost of a 12‐week course of therapy ranges from $55,000 to $147,000, the results of the current study suggest the cost of these treatments could be offset within 3 to 6 years by savings in all‐cause medical costs. (Hepatology Communications 2017;1:439–452)
Abbreviations
- APRI
aspartate aminotransferase to platelet ratio index
- CCI
Charlson Comorbidity Index
- CVD
cardiovascular disease
- DAA
direct‐acting antiviral
- EHM
extrahepatic manifestation
- HCV
hepatitis C virus
- ICD‐9
International Classification of Diseases, Ninth Edition
- ICD‐10
International Classification of Diseases, Tenth Revision
- Q1
first quarter (of a year)
- SD
standard deviation
Introduction
Hepatitis C virus (HCV) infection is one of the most common blood‐borne virus infections, affecting approximately 2.7 million to 3.9 million people in the United Sates.1 HCV becomes chronic in up to 85% of infected individuals,2 placing patients at risk of developing severe hepatic complications, such as cirrhosis, hepatocellular carcinoma, and liver failure.2, 3 Besides hepatic complications, HCV infection has also been associated with numerous extrahepatic manifestations (EHMs) that are secondary to HCV‐related inflammatory responses and/or autoimmune reactions.4, 5 Previously established or hypothesized EHMs for patients with HCV include mixed cryoglobulinemia, non‐Hodgkin lymphoma, type 2 diabetes mellitus, depression, renal insufficiency, cognitive impairments, head and neck cancer, cardiovascular disease (CVD), and atherosclerosis.6, 7, 8, 9, 10, 11, 12 Although up to 74% of patients with HCV have been reported to experience at least one EHM,7 no single study has assessed the risk of a comprehensive list of EHMs.6
Several studies assessing the all‐cause economic burden of HCV have found these costs to be substantial13, 14, 15, 16, 17; however, none of these studies have separated the economic burden attributable to EHMs in their estimates. To the best of our knowledge, the only evidence on the economic burden of HCV‐related EHMs is from an economic model study in the United States. That study estimated the total direct medical costs associated with nine EHMs (including kidney disease, diabetes, and B‐cell lymphoma) at approximately $1.5 billion, with total per patient per year costs associated with specific EHMs ranging from $127 for lichen planus to $5,589 for stroke (CVD, $4,066; type 2 diabetes, $2,903; depression, $2,201; chronic kidney disease excluding end‐stage liver disease, $189).18
The treatment landscape for HCV is rapidly evolving, with several direct‐acting antiviral agents (DAAs) being effective in achieving high levels of sustained virologic response.19 However, the impact of treatment on the economic burden associated with HCV‐related EHMs remains unclear, and whether such impact would differ should patients be treated earlier versus later in their fibrosis stages has not been assessed.
This study aims to provide a more comprehensive understanding of the risks and medical costs of EHMs among patients with HCV in the United States as well as the role of treatment in different stages of liver fibrosis for mitigating these clinical and economic burdens.
Patients and Methods
DATA SOURCES
We used data from the Optum Claims Data‐Clinformatics Data Mart, a closed system of de‐identified health claims data that includes over 15 million lives annually and contains patients’ medical, prescription drug, laboratory, and eligibility information since 2007. The geographically diverse data come from a large national U.S. health insurer and was collected for all patients diagnosed with chronic HCV from the first quarter (Q1)/2009‐Q1/2016 and a random sample of 500,000 patients from the general population in the same period. Data are certified as de‐identified and comply with the Health Insurance Portability and Accountability Act requirements.
STUDY DESIGN AND STUDY COHORTS
Risks of EHMs in HCV and Associated Medical Costs
A retrospective cross‐sectional design was used to assess risks for EHMs in HCV patients, and a retrospective cohort design was used to compare the medical costs between patients with and without HCV (Supporting Fig. S1). Adult patients with at least two HCV diagnoses (HCV cohort; n = 17,054) were matched 1:5 on age, sex, region, and availability of laboratory data to HCV‐free adults from the random sample of the general population (no‐HCV cohort; n = 85,270; Fig. 1; Supporting Fig. S1). HCV was identified based on International Classification of Disease, Ninth Edition (ICD‐9) diagnosis codes 070.44 and 070.54 and ICD‐10 diagnosis code B18.2. All patients were required to have continuous health plan eligibility for at least 6 months before and 12 months after an index date selected randomly from all dates with an HCV diagnosis (HCV cohort; to ensure the inclusion of prevalent cases of HCV) or all dates of observation (no‐HCV cohort). EHMs were assessed in the two cohorts from 6 months before to 12 months after the index date. Annualized medical costs were assessed from the index date to the end of patient follow‐up, defined across the study as the end of health care insurance coverage or the end of data availability (March 31, 2016), whichever occurred first. Patient baseline characteristics were assessed in the 6 months prior to the index date.
Figure 1.
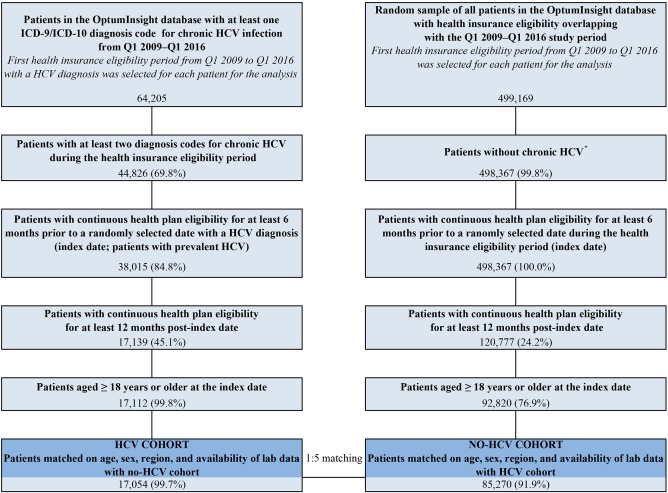
Sample selection showing matched HCV versus no‐HCV cohorts. *Patients without any HCV diagnosis from Q1 2009 to Q1 2015 or patients who had a single HCV diagnosis with more than six months of follow‐up after this diagnosis (if the initiation HCV diagnosis was not followed within six months by another diagnosis it is likely that the initial diagnosis was linked to a test / exam for HCV and not to a confirmed HCV diagnosis).
Impact of Treatment on EHM‐Associated Medical Costs
A retrospective cohort design was used to compare the medical costs between treated and untreated time in patients newly diagnosed with HCV (Supporting Fig. S2). Patients were considered to have newly diagnosed HCV if their first HCV diagnosis in the data was after 6 or more months of continuous health plan eligibility (i.e., washout period). For this analysis, the date of the first HCV diagnosis was defined as the index date, and medical costs were assessed from the index date to the end of patient follow‐up. Patients who initiated HCV treatment (e.g., interferon, ribavirin, DAAs) between the index date and the end of follow‐up were included in the treated cohort (n = 6,827; 11,473 person‐years posttreatment), while the remaining patients were included in the untreated cohort (n = 21,358; 44,715 person‐years postdiagnosis; Fig. 2; Supporting Fig. S2). Medical costs incurred by the treated patients between the index date and treatment initiation (i.e., during the period when they were untreated; 8,523 person‐years) were added to the calculation of the medical costs in the untreated patients. Patients were required to have at least 6 months of continuous health care insurance coverage from 6 months prior to the index date to 1 month after the index date (untreated cohort) or to 1 month after the date of HCV treatment initiation (treated cohort). Patient baseline characteristics were assessed in the 6 months prior to the index date (untreated time) and the 6 months prior to the HCV treatment initiation (treated time).
Figure 2.
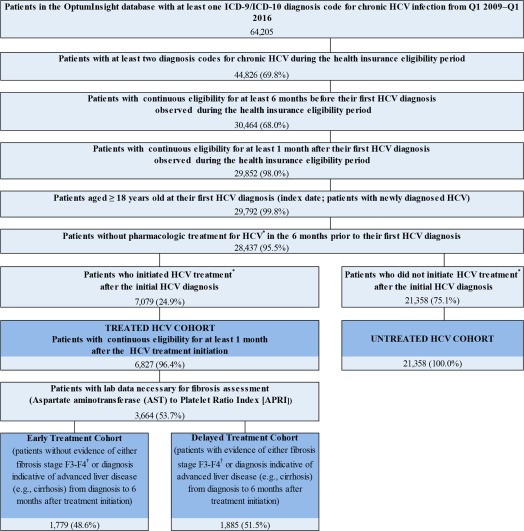
Sample selection showing treated versus untreated cohorts and early versus delayed‐treatment cohorts. *HCV treatment was identified using the prescription drug claims portion of the database based on National Drug Codes (NDC) specific to currently available HCV treatments and, where applicable, on Healthcare Common Procedure Coding System (HCPCS) procedure codes from the medical claims portion of the database. †Patients with an APRI score > 1.0 were considered to have fibrosis F3‐F4 by METAVIR criteria (Lin et al. Hepatology 2011).
Impact of Treatment in Earlier Versus Later Fibrosis Stages on EHM‐Associated Medical Costs
A retrospective cohort design was used to compare the medical costs between patients newly diagnosed with HCV who initiated treatment in early METAVIR fibrosis stages F0‐F2 (early treatment) versus late fibrosis stages F3‐F4 (delayed treatment; Supporting Fig. S3). The analysis was conducted among the subset of patients in the treated cohort who had laboratory measurements needed to ascertain fibrosis stage (i.e., aspartate aminotransferase and platelet counts, which were used to measure the aspartate aminotransferase to platelet ratio index [APRI])20, 21 at the time of treatment initiation (index date). Patients with an APRI score ≥120 and/or an ICD‐9/10 diagnosis for a condition suggestive of advanced liver disease (i.e., cirrhosis of the liver, esophageal varices, spontaneous bacterial peritonitis, hepatic encephalopathy, portal hypertension, hepatorenal syndrome, ascites) from the initial HCV diagnosis up to 6 months after the index date were considered to be in late‐stage fibrosis at the time of treatment initiation (i.e., METAVIR fibrosis stage F3‐F4) and were included in the delayed‐treatment cohort (n = 1,885). Patients with an APRI <1 and with no diagnosis for advanced liver disease over the same period were considered to be in early stage fibrosis at the time of treatment initiation (i.e., METAVIR fibrosis stage F0‐F2) and were included in the early treatment cohort (n = 1,779; Fig. 2; Supporting Fig. S3). Medical costs were assessed from the index date to the end of patient follow‐up. Patient baseline characteristics were assessed in the 6 months prior to the index date.
STUDY OUTCOMES AND STATISTICAL ANALYSES
Risks of EHMs in HCV
Twenty EHMs were investigated in this study, including well‐documented HCV‐related EHMs (e.g., CVD, metabolic conditions, kidney disease) as well as EHMs hypothesized to be associated with HCV given the inflammatory processes that can be triggered by the HCV infection5 (e.g., psoriasis, fibromyalgia, celiac disease, irritable bowel syndrome, and gastroesophageal reflux disease). Patients living with the selected EHMs were identified in the HCV and no‐HCV cohorts based on EHM‐specific ICD‐9/10 diagnosis codes (prevalent cases of EHM). The risks of these EHMs were compared between the HCV and the matched no‐HCV cohorts using odds ratios (ORs) and 95% confidence intervals (CIs) estimated from conditional logistic regression models accounting for matching one model for each EHM outcome. ORs are presented both unadjusted and adjusted for the conditions included in the Charlson Comorbidity Index (CCI), with the exception of conditions related to the study cohort definition (i.e., HCV infection) and conditions related to the outcome of the regression model (e.g., malignancy was not included in the model for malignancy risk).
Medical Costs
Medical costs investigated in this study included EHM‐related medical costs, HCV and/or hepatic complications‐related medical costs, and all‐cause medical costs. We did not directly estimate the cost of HCV treatment because drugs used at the beginning of the study period (e.g., interferon, ribavirin) had lower costs than the DAA treatments that are currently available to the patients. For discussion purposes, the study relied on the wholesale acquisition cost of a 12‐week course of treatment with the newer DAAs; this cost ranges from $55,000 to $147,000.22 EHM and HCV/hepatic complications‐related medical costs were estimated from claims with ICD‐9/10 diagnosis codes identifying the conditions of interest. Hepatic complications included cirrhosis of the liver, esophageal varices, spontaneous bacterial peritonitis, hepatic encephalopathy (liver failure), portal hypertension, ascites, splenomegaly, hepatorenal syndrome, hepatocellular carcinoma, porphyria cutanea tarda, and liver transplantation. The cost of a claim associated with diagnosis codes for both hepatic complication(s) and one or more EHMs (i.e., overlapping claims) was attributed to both hepatic and EHM‐related costs.
Medical costs were calculated as average charged amounts and adjusted to Q1 2016 U.S. dollars. Costs were weighted for each patient based on the length of follow‐up, such that patients with longer follow‐up were given more weight in the analyses, and were reported on an annual basis (per patient per year). Medical costs were compared between the study cohorts (HCV versus no‐HCV, treated versus untreated, early versus delayed treatment) using mean cost differences estimated from unadjusted and adjusted two‐part regression models.
All regression models for costs were adjusted for comorbidities known to be associated with high costs (i.e., other CVD, organ transplant, epilepsy, and obesity) and for conditions included in the CCI, with the exception of conditions related to the study cohort definition and conditions related to the outcome of the regression model. Cost models for treated versus untreated cohorts and early versus delayed‐treatment cohorts were also adjusted for the matching variables used for the HCV and no‐HCV cohorts. In addition, the fibrosis stage was included as an adjustment variable in the model for treated versus untreated cohorts as a covariate with three categories: fibrosis stage F3‐F4, fibrosis stage F0‐F2, and unknown fibrosis status.
Other Statistical Analyses
Patient characteristics were described using means ± SD, medians, and proportions. For the comparison of patient characteristics between the matched HCV and no‐HCV cohorts, P values were calculated from univariate regression models that account for matching (generalized linear regressions for continuous variables and conditional logistic regressions for categorical variables). Comparisons of patient characteristics between treated and untreated patients were based on Wilcoxon and chi‐square tests.
Results
DESCRIPTION OF STUDY COHORTS
Patient characteristics are described in Table 1 for all cohorts. Matching variables had the same distribution in the HCV and no‐HCV cohorts (mean age, 53 years; 63.0% males). Despite statistically significant differences (due to large samples), there were no clinically important differences between cohorts other than a few noteworthy exceptions. First, the overall disease burden, measured by the CCI, was higher in the HCV versus the no‐HCV cohort (mean 1.6 versus 0.4), in the untreated versus the treated cohort (mean 1.1 versus 0.9), and the delayed‐treatment versus the early treatment cohort (mean 2.3 versus 1.6). Second, untreated patients were less likely than treated patients to have laboratory data (60.8% versus 76.6%) and to have an index date after year 2011 (46.9% versus 58.9%). Third, there were fewer male patients in the early treatment cohort compared to the delayed‐treatment cohort (60.6% versus 70.1%; P < 0.05 for all; Table 1). Length of the postindex follow‐up was between 1.6 and 2.5 years for all cohorts.
Table 1.
COMPARISON OF PATIENT CHARACTERISTICS BETWEEN STUDY COHORTS
| Patients With Prevalent HCV and Matched HCV‐Free Controls | Patients With Newly Diagnosed HCV | Treated Patients With Newly Diagnosed HCV and Laboratory Data | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No‐HCV Cohort | HCV Cohort | Treated Cohort | Untreated Cohort | Early Treatment Cohort | Delayed‐Treatment Cohort | ||||
| Characteristics | n = 85,270 | n = 17,054 | n = 6,827 | n = 21,358 | n = 1,779 | n = 1,885 | |||
| Agec (years), mean ± SD [median] | 53.0 ± 11.7 [55.0] | 52.9 ± 9.8 [55.0] | b | 52.2 ± 9.4 [54.0] | 52.5 ± 10.1 [54.0] | a | 52.0 ± 10.5 [54.0] | 55.4 ± 8.3 [57.0] | a |
| Males, n (%) | 53,715 (63.0%) | 10,743 (63.0%) | b | 4,503 (66.0%) | 13,566 (63.5%) | a | 1,078 (60.6%) | 1,321 (70.1%) | a |
| Regiond, n (%) | |||||||||
| Northeast | 8,020 (9.4%) | 1,604 (9.4%) | b | 602 (8.8%) | 1,947 (9.1%) | 137 (7.7%) | 130 (6.9%) | ||
| Midwest | 14,680 (17.2%) | 2,936 (17.2%) | b | 1,260 (18.5%) | 3,635 (17.0%) | a | 164 (9.2%) | 151 (8.0%) | |
| South | 47,310 (55.5%) | 9,462 (55.5%) | b | 3,855 (56.5%) | 12,044 (56.4%) | 1,191 (66.9%) | 1,332 (70.7%) | a | |
| West | 15,260 (17.9%) | 3,052 (17.9%) | b | 1,105 (16.2%) | 3,719 (17.4%) | a | 286 (16.1%) | 272 (14.4%) | |
| Unknown | 5 (0.1%) | 13 (0.1%) | 1 (0.1%) | 0 (0.0%) | |||||
| Lab data available, n (%) | 54,705 (64.2%) | 10,941 (64.2%) | b | 5,230 (76.6%) | 12,981 (60.8%) | a | 531 (100.0%) | 3,133 (100.0%) | ‐ |
| Type of health plan, n (%) | |||||||||
| Point of service | 63,473 (74.4%) | 12,395 (72.7%) | a | 5,104 (74.8%) | 15,019 (70.3%) | a | 1,331 (74.8%) | 1,349 (71.6%) | a |
| Othere | 21,797 (25.6%) | 4,659 (27.3%) | a | 1,723 (25.2%) | 6,339 (29.7%) | a | 448 (25.2%) | 536 (28.4%) | a |
| Calendar yearc ≥ 2011, n (%) | 60,041 (70.4%) | 11,496 (67.4%) | a | 4,022 (58.9%) | 10,015 (46.9%) | a | 1,469 (82.6%) | 1,527 (81.0%) | |
| Charlson Comorbidity Indexf mean ± SD [median] | 0.4 ± 1.0 [0.0] | 1.6 ± 1.8 [1.0] | a | 0.9 ± 1.5 [0.0] | 1.1 ± 1.8 [0.0] | a | 1.6 ± 1.3 [1.0] | 2.3 ± 1.9 [1.0] | a |
Differences between cohorts statistically significant at α = 0.05.
P‐value estimation for difference between cohorts not applicable due to matching.
Measured at the index date of each sample. The HCV cohort index date was selected randomly from all days with an HCV diagnosis; the No‐HCV cohort index date was selected randomly from all days more than 6 months after the beginning of the eligibility period; the treated versus untreated cohorts index date was defined as the day of first HCV diagnosis; the early versus delayed‐treatment cohort index date was defined as the day of treatment initiation.
Database provides patient geographic location provided by state. Defining by region using U.S. census definitions. United States Census Bureau. Census regions and divisions of the United States. http://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf. Accessed June 22, 2016.
Includes Exclusive Provider Organization (EPO), Health Maintenance Organization (HMO), Preferred Provider Organization (PPO), Indemnity and other plans.
Measured in the 6 months prior to the index date.
RISKS OF EHMS IN HCV AND ASSOCIATED MEDICAL COSTS
Compared with the patients in the matched no‐HCV cohort, patients in the HCV cohort exhibited significantly greater risks, both for any EHM (70.2% versus 52.2%; adjusted OR, 2.23; P < 0.05; Table 2) and for 17 of the 20 EHMs analyzed. EHMs that affected > 5% of the patients with HCV and had more than 2‐fold higher risk in the HCV compared to the no‐HCV cohort included kidney disease (9.4% versus 3.5%; adjusted OR, 2.58) and depression (16.9% versus 7.9%; adjusted OR, 2.26).
Table 2.
CLINICAL BURDEN OF HCV SHOWING THE EHM RISK IN THE MATCHED HCV VERSUS NO‐HCV COHORTS
| Frequency of Specific EHMs n (%) | Odds Ratio HCV Versus No‐HCV Cohorts (95% CI) | |||
|---|---|---|---|---|
| No‐HCV Cohort | HCV Cohort | Before Adjustment | After Adjustment | |
| Specific EHMs | n = 85,270 | n = 17,054 | for Comorbidities | for Comorbidities |
| Cardiovascular disease | 12,020 (14.1%) | 3,853 (22.6%) | 1.83 (1.76, 1.91)a | 1.64 (1.57, 1.71)a |
| Metabolic conditionsb | 13,844 (16.2%) | 3,495 (20.5%) | 1.35 (1.29, 1.41)a | 1.26 (1.20, 1.31)a |
| Type 2 diabetes mellitus | 13,271 (15.6%) | 3,415 (20.0%) | 1.38 (1.32, 1.44)a | 1.28 (1.23, 1.34)a |
| Insulin resistance | 896 (1.1%) | 146 (0.9%) | 0.81 (0.68, 0.97)a | 0.80 (0.67, 0.96)a |
| Kidney diseaseb | 2,960 (3.5%) | 1,608 (9.4%) | 2.98 (2.79, 3.18)a | 2.58 (2.40, 2.77)a |
| Nephritis/Nephrotic syndrome /Nephrosis | 1,493 (1.8%) | 1,062 (6.2%) | 3.79 (3.49, 4.11)a | 3.22 (2.94, 3.54)a |
| Chronic kidney disease | 2,303 (2.7%) | 1,200 (7.0%) | 2.80 (2.60, 3.01)a | 2.39 (2.20, 2.59)a |
| Auto‐immune conditionsb | 2,218 (2.6%) | 758 (4.4%) | 1.75 (1.60, 1.90)a | 1.70 (1.56, 1.86)a |
| Celiac disease | 133 (0.2%) | 66 (0.4%) | 2.49 (1.85, 3.34)a | 2.39 (1.75, 3.25)a |
| Inflammatory bowel disease | 766 (0.9%) | 296 (1.7%) | 1.95 (1.70, 2.23)a | 1.81 (1.57, 2.08)a |
| Psoriasis | 1,344 (1.6%) | 408 (2.4%) | 1.53 (1.37, 1.71)a | 1.56 (1.39, 1.75)a |
| Malignancyb | 2,152 (2.5%) | 533 (3.1%) | 1.25 (1.14, 1.38)a | 1.21 (1.10, 1.34)a |
| Non‐Hodgkin's lymphoma | 424 (0.5%) | 196 (1.1%) | 2.33 (1.97, 2.77)a | 2.23 (1.87, 2.66)a |
| Prostate cancer | 1,234 (1.4%) | 199 (1.2%) | 0.80 (0.69, 0.93)a | 0.79 (0.67, 0.92)a |
| Head and neck cancers | 236 (0.3%) | 95 (0.6%) | 2.02 (1.59, 2.56)a | 2.09 (1.62, 2.69)a |
| Thyroid cancer | 278 (0.3%) | 36 (0.2%) | 0.65 (0.46, 0.92)a | 0.65 (0.46, 0.93)a |
| Esophageal cancer | 35 (0.0%) | 27 (0.2%) | 3.86 (2.33, 6.37)a | 4.48 (2.58, 7.75)a |
| Neuromuscular manifestationsb | 19,020 (22.3%) | 5,659 (33.2%) | 1.76 (1.70, 1.83)a | 1.68 (1.62, 1.74)a |
| Chronic fatigue syndrome/Fatigue | 15,708 (18.4%) | 4,842 (28.4%) | 1.79 (1.72, 1.86)a | 1.70 (1.63, 1.77)a |
| Fibromyalgia | 4,935 (5.8%) | 1,440 (8.4%) | 1.51 (1.42, 1.61)a | 1.47 (1.38, 1.56)a |
| Parkinson's disease | 166 (0.2%) | 43 (0.3%) | 1.30 (0.93, 1.82) | 1.19 (0.83, 1.70) |
| Otherb | 18,190 (21.3%) | 6,216 (36.4%) | 2.15 (2.08, 2.23)a | 2.03 (1.95, 2.10)a |
| Irritable bowel syndrome | 1,291 (1.5%) | 343 (2.0%) | 1.34 (1.19, 1.51)a | 1.31 (1.16, 1.48)a |
| Cognitive impairment | 387 (0.5%) | 161 (0.9%) | 2.11 (1.75, 2.54)a | 1.91 (1.55, 2.37)a |
| Depression | 6,773 (7.9%) | 2,885 (16.9%) | 2.40 (2.29, 2.52)a | 2.26 (2.15, 2.38)a |
| Gastroesophageal reflux disease | 12,284 (14.4%) | 4,034 (23.7%) | 1.86 (1.79, 1.94)a | 1.75 (1.68, 1.83)a |
| Any of the above listed EHMs, n (%) | 44,510 (52.2%) | 11,971 (70.2%) | 2.29 (2.21, 2.38)a | 2.23 (2.15, 2.31)a |
P < 0.05.
Any of the specific EHMs listed in the category.
The annualized medical costs were significantly higher for patients in the HCV cohort compared to patients in the no‐HCV cohort for all cost components analyzed. The mean annual all‐cause medical cost was $43,891 in the HCV cohort versus $17,989 in the no‐HCV cohort, corresponding to an unadjusted mean difference of $25,901 and an adjusted mean difference of $13,933 (P < 0.05 for both; Table 3). The divergence between the two cost‐difference estimates was largely driven by the adjustment for liver diseases. Because liver disease is more prevalent in patients with HCV than those without HCV (29% versus 0%), there are more excess costs due to liver disease in the HCV than the no‐HCV cohort. As a result, the excess costs associated with HCV are greater in the unadjusted analyses than in the adjusted analyses.
Table 3.
ECONOMIC BURDEN OF HCV SHOWING CHARGED MEDICAL COSTS IN THE MATCHED HCV VERSUS NO‐HCV COHORTS
| Weighted Mean Medical Costs per Patient per Year (2016 US$) | Weighted Two‐Part Regression | |||
|---|---|---|---|---|
| No‐HCV | HCV | |||
| Cohort [A] | Cohort [B] | Unadjusted Cost Difference | Adjusted Cost Difference | |
| Cost category | n = 85,270 | n = 17,054 | (95% CI) [B]‐[A] | (95% CI) [B]‐[A] |
| Total all‐cause medical costs | 17,989 ± 171 | 43,891 ± 398 | 25,901 (24,932; 26,732)a | 13,933 (13,248; 14,620)a |
| EHM‐related cost, any EHM (Table 1) | 6,550 ± 117 | 17,416 ± 277 | 10,866 (10,246; 11,488)a | 6,458 (5,930; 7,055)a |
| Associated with diagnoses for EHMs but not HCV/liver disease (nonoverlapping claims) | 6,450 ± 114 | 13,432 ± 238 | 6,982 (6,507; 7,526)a | 5,104 (4,589; 5,712)a |
| Associated with diagnoses for both EHMs and HCV/liver disease (overlapping claims) | 101 ± 13 | 3,984 ± 121 | 3,884 (3,586; 4,162)a | 3,703 (3,453; 3,940)a |
| EHM‐related cost, selected specific EHMs | ||||
| Cardiovascular diseaseb | 2,362 ± 57 | 4,387 ± 111 | 2,025 (1,786; 2,228)a | 1,062 (873; 1,270)a |
| Metabolic conditions | 1,222 ± 26 | 2,511 ± 47 | 1,289 (1,201; 1,379)a | 527 (428; 634)a |
| Kidney diseaseb | 1,878 ± 92 | 9,317 ± 240 | 7,439 (6,953; 7,982)a | 4,024 (3,531; 4,548)a |
| Auto‐immune conditionsb | 222 ± 15 | 299 ± 15 | 77 (44; 114)a | 32 (1; 69) |
| Malignancyb | 605 ± 33 | 861 ± 42 | 256 (172; 339)a | 123 (27; 209)a |
| Neuromuscular manifestationsb | 315 ± 9 | 629 ± 38 | 314 (231; 419)a | 200 (170; 241)a |
| Depression | 248 ± 13 | 610 ± 19 | 361 (326; 402)a | 318 (274; 369)a |
| GERD | 737 ± 16 | 1,142 ± 22 | 405 (357; 453)a | 318 (269; 367)a |
| HCV and/or hepatic complications‐related cost | 240 ± 21 | 11,650 ± 206 | 11,409 (10,938; 11,801)a | 9,366 (9,001; 9,672)a |
| Associated with diagnoses for HCV/liver disease but not EHMs (nonoverlapping claims) | 140 ± 12 | 7,665 ± 136 | 7,525 (7,252; 7,777)a | 6,343 (6,111; 6,559)a |
| Associated with diagnoses for both EHMs and HCV/liver disease (overlapping claims) | 101 ± 13 | 3,984 ± 121 | 3,884 (3,586; 4,162)a | 3,703 (3,453; 3,940)a |
P < 0.05.
Any of the specific EHMs listed in the category.
Abbreviation: GERD, gastroesophageal reflux disease.
The mean annual EHM‐related costs in the HCV and no‐HCV cohorts were $17,416 and $6,550, respectively (Table 3). In both cohorts, the majority of the EHM‐related costs (77% and 98% of the EHM‐related costs, respectively) were related to nonoverlapping claims (i.e., claims associated with diagnosis codes for EHMs, without any diagnosis for liver disease). After adjustment for potential confounders, including comorbid liver disease, EHM‐related costs remained significantly higher for the HCV than the no‐HCV cohort, both overall (unadjusted and adjusted cost differences, $10,866 and $6,458, respectively; P < 0.05 for both) and in the subset of nonoverlapping claims (unadjusted and adjusted cost differences, $6,982 and $5,104, respectively; P < 0.05 for both; Table 3). Similar to the all‐cause cost, the divergence between the unadjusted and adjusted cost‐difference estimates was largely driven by the adjustment for liver diseases, which were more prevalent in patients with HCV than those without HCV (29% versus 0%). With an adjusted mean difference of $4,024 per patient per year (P < 0.05; Table 3), kidney disease was the main driver of the higher EHM‐related costs.
HCV and/or hepatic complications‐related costs were also higher in the HCV compared to the no‐HCV cohort, both overall and in the subset of nonoverlapping claims (adjusted cost differences, $9,366 and $6,343, respectively; P < 0.05 for both).
IMPACT OF TREATMENT ON EHM‐ASSOCIATED MEDICAL COSTS
Treatment significantly mitigates the economic burden from HCV/hepatic complications and EHMs. Compared with the treated cohort, the untreated cohort incurred higher average annual total all‐cause medical costs per patient ($54,240 versus $39,659), corresponding to an unadjusted mean difference of $14,581 and an adjusted mean difference of $24,834 (P < 0.05 for both; Table 4). The divergence between the two cost‐difference estimates was largely driven by the adjustment for liver diseases; because liver disease is more prevalent in treated than untreated patients (48% versus 22%), there are more excess costs due to liver disease in the treated than untreated cohort. As a result, the cost savings associated with treatment (i.e., the cost difference between untreated and treated patients) are lower in unadjusted analyses than in adjusted analyses.
Table 4.
ECONOMIC BURDEN BY TREATMENT STATUS SHOWING CHARGED MEDICAL COSTS IN TREATED VERSUS UNTREATED PERSON‐TIME
| Weighted Mean Medical Costs per Patient per Year (2016 US$) | Weighted Two‐Part Regression | |||
|---|---|---|---|---|
| Treated Person‐Time | Untreated Person‐Time | |||
| [A] | [B] | |||
| Cost Category | n = 11,473 person‐years (6,827 treated patients) | n = 53,238 person‐years (6,164 treated & 21,358 untreated patients) | Unadjusted Cost Difference (95% CI) [B]‐[A] | Adjusted Cost Difference (95% CI) [B]‐[A] |
| Total all‐cause medical costs | 39,659 ± 571 | 54,240 ± 1,018 | 14,581 (11,416; 18,035)a | 24,834 (21,220; 29,007)a |
| EHM‐related cost, any EHM (Table 1) | 13,933 ± 428 | 21,916 ± 711 | 7,983 (5,685; 10,554)a | 12,773 (10,092; 16,660)a |
| Associated with diagnoses for EHMs, but not HCV/liver disease (nonoverlapping claims) | 9,552 ± 307 | 15,943 ± 560 | 6,391 (4,571; 8,051)a | 8,238 (6,069; 11,011)a |
| Associated with diagnoses for both EHMs and HCV/liver disease (overlapping claims) | 4,381 ± 276 | 5,973 ± 335 | 1,592 (125; 3,064)a | 1,500 (236; 2,909)a |
| EHM‐related cost, selected specific EHMs | ||||
| Cardiovascular diseaseb | 3,506 ± 136 | 5,308 ± 259 | 1,801 (1,045; 2,722)a | 2,124 (1,176; 3,467)a |
| Metabolic conditions | 1,857 ± 51 | 2,888 ± 100 | 1,031 (699; 1,406)a | 1,497 (1,077; 1,946)a |
| Kidney diseaseb | 6,849 ± 395 | 11,841 ± 517 | 4,992 (2,776; 7,153)a | 7,707 (4,955; 10,803)a |
| Auto‐immune conditionsb | 142 ± 13 | 394 ± 46 | 252 (143; 361)a | 354 (205; 548)a |
| Malignancyb | 943 ± 85 | 1,291 ± 138 | 348 (−96; 709) | 717 (219; 1,221)a |
| Neuromuscular manifestationsb | 541 ± 14 | 617 ± 47 | 77 (−60; 267) | 43 (−61; 143) |
| Depression | 559 ± 26 | 696 ± 34 | 137 (−11; 278) | 158 (10; 326)a |
| GERD | 1,008 ± 34 | 1,761 ± 386 | 753 (346; 1,361)a | 880 (430; 1,520)a |
| HCV and/or hepatic complications‐related cost | 14,086 ± 379 | 16,871 ± 504 | 2,785 (898; 4,793)a | 3,790 (1,950; 5,916)a |
| Associated with diagnoses for HCV/liver disease but not EHMs (nonoverlapping claims) | 9,705 ± 227 | 10,897 ± 318 | 1,193 (67; 2,195) | 1,929 (846; 2,959)a |
| Associated with diagnoses for both EHMs and HCV/liver disease (overlapping claims) | 4,381 ± 276 | 5,973 ± 335 | 1,592 (125; 3,064)a | 1,500 (236; 2,909)a |
P < 0.05.
Any of the specific EHMs listed in the category.
Abbreviation: GERD, gastroesophageal reflux disease.
The mean annual EHM‐related costs in the untreated and treated cohorts were $21,916 and $13,933, respectively (Table 4). In both cohorts, the majority of the EHM‐related costs (73% and 69% of the EHM‐related costs, respectively) were related to nonoverlapping claims. After adjustment for potential confounders, including comorbid liver disease, EHM‐related costs remained significantly higher for the untreated than the treated cohort, both overall (unadjusted and adjusted cost differences, $7,983 and $12,773; P < 0.05 for both) and in the subset of nonoverlapping claims (unadjusted and adjusted cost differences, $6,391 and $8,238, respectively; P < 0.05 for both; Table 4). Similar to all‐cause cost, the divergence between the unadjusted and adjusted cost‐difference estimates was largely driven by the adjustment for liver diseases, which were more prevalent in treated than untreated patients (48% versus 22%). With an adjusted mean difference of $7,707 per patient per year (P < 0.05; Table 4), kidney disease was the main driver of the higher EHM‐related costs.
HCV and/or hepatic complications‐related costs were also higher in the untreated compared to the treated cohort, both overall and in the subset of nonoverlapping claims (adjusted cost differences, $3,790 and $1,929, respectively; P < 0.05 for both).
IMPACT OF TREATMENT IN EARLIER VERSUS LATER FIBROSIS STAGES ON EHM ASSOCIATED MEDICAL COSTS
Treatment initiated in early fibrosis stages significantly mitigates the economic burden from HCV/hepatic complications and EHM. Compared with the early treatment cohort (fibrosis stages F0‐F2), the delayed‐treatment cohort (fibrosis stages F3‐F4) incurred higher average annual total all‐cause medical costs ($52,782 versus $26,582), corresponding to a statistically significant adjusted mean difference of $21,078 (P < 0.05; Table 5).
Table 5.
ECONOMIC BURDEN BY LIVER DISEASE STAGE TREATMENT SHOWING CHARGED MEDICAL COSTS IN EARLY (FIBROSIS F0‐F2) VERSUS DELAYED‐ (FIBROSIS F3‐F4) TREATMENT COHORTS
| Weighted Mean Medical Costs per Patient per Year (2016 US$) | Weighted Two‐Part Regression | |||
|---|---|---|---|---|
| Early Treatment (Fibrosis F0‐F2) | Delayed Treatment (Fibrosis F3‐F4) | |||
| Cohort [A] | Cohort [B] | Unadjusted Cost Difference | Adjusted Cost Difference | |
| Cost category | n = 1,779 | n = 1,885 | (95% CI) [B]‐[A] | (95% CI) [B]‐[A] |
| Total all‐cause medical costs | 26,582 ± 345 | 52,782 ± 794 | 26,200 (18,964; 33,604)a | 21,078 (14,782; 27,929)a |
| EHM‐related cost, any EHM (Table 1) | 8,423 ± 289 | 20,522 ± 605 | 12,100 (6,749; 18,689)a | 10,409 (5,215; 15,299)a |
| Associated with diagnoses for EHMs but not HCV/liver disease (nonoverlapping claims) | 7,773 ± 287 | 11,955 ± 373 | 4,183 (39; 8,725)a | 3,722 (–688; 7,200) |
| Associated with diagnoses for both EHMs and HCV/liver disease (overlapping claims) | 650 ± 22 | 8,567 ± 463 | 7,917 (4,688; 12,529)a | 6,080 (4,154; 9,311)a |
| EHM–related cost, selected specific EHMs | ||||
| Cardiovascular diseaseb | 2,593 ± 100 | 4,072 ± 169 | 1,478 (–337; 3,376) | 560 (–3,227; 3,490) |
| Metabolic conditions | 1,064 ± 30 | 2,325 ± 58 | 1,262 (698; 1,947)a | 880 (336; 1,606)a |
| Kidney diseaseb | 2,669 ± 262 | 12,276 ± 572 | 9,607 (4,467; 16,007)a | 10,896 (6,057; 44,719)a |
| Auto–immune conditionsb | 132 ± 18 | 153 ± 11 | 21 (–105; 160) | –65 (–437; 97) |
| Malignancyb | 811 ± 50 | 1,112 ± 103 | 300 (–732; 1,433) | 1,601 (–2,885; 208,404) |
| Neuromuscular manifestationsb | 522 ± 10 | 568 ± 11 | 47 (–112; 191) | 81 (–61; 216) |
| Depression | 404 ± 15 | 827 ± 41 | 423 (–10; 841) | 392 (33; 1,067)a |
| GERD | 1,230 ± 43 | 988 ± 36 | –241 (–704; 199) | –365 (–1,001; 217) |
| HCV and/or hepatic complications–related costs | 4,652 ± 72 | 24,080 ± 605 | 19,429 (14,800; 24,798)a | 16,343 (12,662; 20,400)a |
| Associated with diagnoses for HCV/liver disease but not EHMs (nonoverlapping claims) | 4,002 ± 68 | 15,513 ± 348 | 11,511 (8,828; 14,286)a | 10,294 (8,072; 12,721)a |
| Associated with diagnoses for both EHMs and HCV/liver disease (overlapping claims) | 650 ± 22 | 8,567 ± 463 | 7,917 (4,688; 12,529)a | 6,080 (4,154; 9,311)a |
P < 0.05.
Any of the specific EHMs listed in the category.
Abbreviation: GERD, gastroesophageal reflux disease.
The mean annual EHM‐related costs in the delayed‐treatment and early treatment cohorts were $20,522 and $8,423, respectively (Table 5). In both cohorts, the majority of the EHM‐related costs (58% and 92%, respectively) were related to nonoverlapping claims. After adjustment for potential confounders, including comorbid liver disease, EHM‐related costs remained higher for the untreated than the treated cohort. Cost‐difference estimates reached statistical significance in overall analyses (unadjusted and adjusted cost differences, $12,100 and $10,409, respectively; P < 0.05 for both) and in the unadjusted analysis of nonoverlapping claims but not in the adjusted analysis of nonoverlapping claims (unadjusted and adjusted cost differences, $4,183 and $3,722, respectively; P < 0.05 and P = 0.13, respectively; Table 5). With an adjusted mean difference of $10,896 per patient per year (P < 0.05; Table 5), kidney disease was the main driver of the higher EHM‐related costs.
HCV and/or hepatic complications‐related costs were also higher in the untreated compared to the treated cohort, both overall and in the subset of nonoverlapping claims (adjusted cost differences, $16,343 and $10,294, respectively; P < 0.05 for both).
Discussion
This large U.S. claims data study found that patients with HCV have higher EHM risks (adjusted OR for any EHM, 2.23) and all‐cause and EHM‐related medical costs (adjusted annual cost differences, $13,933 and $6,458, respectively) than patients without HCV. Furthermore, HCV treatment can reduce the higher medical costs in patients with HCV by saving ∼$25,000 in all‐cause medical costs per patient per year, with a large proportion attributable to savings in EHM‐related medical costs (adjusted cost difference compared to patients without HCV, $12,773). Considering that the wholesale acquisition cost of a 12‐week course of treatment with DAA ranges from $55,000 to $147,000,22 results of this study suggest the cost of a curative DAA treatment could be offset within 3 to 6 years by savings in all‐cause medical costs, which are mostly accounted for by chronic EHM and hepatic conditions. Importantly, initiating HCV therapy in early rather than late‐stage fibrosis is associated with a cost savings of ∼$21,000 annually in all‐cause medical costs, including ∼$10,000 EHM‐related costs.
A strength of the current study is the inclusion of a comprehensive set of HCV‐related EHMs, including well‐documented EHMs (e.g., CVD, kidney disease) as well as several EHMs that have drawn little attention in the published literature (e.g., psoriasis, fibromyalgia, celiac disease, irritable bowel syndrome, and gastroesophageal reflux disease). To the best of our knowledge, this is the first study that used real‐world data to directly compare medical costs, and in particular EHM‐related medical costs, between patients with and without HCV, treated and untreated, and patients who initiated treatment in early versus late stages of fibrosis. We found that EHMs pose a high clinical and economic burden on patients with HCV and that treatment, in particular treatment initiated at early fibrosis stages, could result in medical cost savings by allowing patients to avoid or delay the onset of clinically risky and economically costly EHMs. These results may be particularly relevant to inform therapeutic and policy decisions.
Given that kidney disease was the most costly EHM identified in the current study, substantial savings in medical costs could be achieved by initiating treatment early in patients with HCV and with or at risk of developing kidney disease. In light of this evidence, the kidney toxicity profile of different DAAs may be considered for selecting therapy in this particular population. Even though some EHMs investigated in this study were associated with a smaller excess in medical costs, their contribution to the overall costs should not be underestimated given the high number of HCV‐infected individuals in the United States and the compounding effect of these conditions over time. It should also be noted that there is a substantial clinical burden of EHMs from the patient's perspective irrespective of the economic burden accrued to the health care system.
Results of the current study are consistent with the literature with respect to EHM‐related risks and costs. A 2016 meta‐analysis by Younossi et al.18 also found that HCV is an independent risk factor for developing nine EHMs investigated, including chronic kidney disease, end‐stage renal disease, CVD, stroke, lymphoma, and depression. While some differences were observed between the study by Younossi et al. and the current study in the proportion of patients with specific EHMs, these are likely due to methodological differences between the two studies. Because Younossi et al. combined clinical trial and observational studies from multiple countries, their estimates for the proportion of patients with specific EHMs may be driven by disease prevalence variations across countries, differences in criteria used to define the EHMs across the studies included in the meta‐analysis, and differences in the underlying populations considered.
Similar to findings from the current study, Younossi et al.18 found that EHMs are associated with a substantial economic burden in patients with HCV. When compared to the current study, EHM‐specific medical costs were similar for some EHMs (e.g., CVD, $4,066$ in Younossi et al. versus $4,387 in the current study) but different for other EHMs (e.g., depression, $2,201 versus $610). The most likely explanation for these observed differences is related to the methodology and input sources used to estimate medical costs. Younussi et al. used an economic model based on Medicare costs and assumptions for the outpatient and inpatient services utilization, whereas charged amounts from the actual patients’ medical claims were used in the current study. Despite these differences, both studies highlight the substantial impact that EHMs have on the overall economic burden of HCV. Furthermore, both studies point to kidney disease as one of the most costly HCV‐related EHMs.
Other previous U.S. studies that have considered medical costs associated with HCV focused on either all‐cause or HCV/hepatic complications‐related costs and, similar to the current study, showed that the excess medical costs of patients with HCV are substantial. Davis et al.14 found an excess all‐cause cost of $15,510 for patients with HCV in the first year postdiagnosis using 2002‐2006 claims data from commercial, Medicare, and Medicaid payers. McCombs et al.16 found an excess all‐cause cost of $23,406 in the first year postdiagnosis using 2003‐2008 claims data from commercial payers; Khoury et al.23 estimated the costs of hepatic complications ranging from $585 to $1,110 per year for patients with compensated cirrhosis to $201,110 per year for patients with liver transplantation. Despite differences in study periods, data sources, and methodologies employed, estimates from these previous studies appear to be consistent, although generally higher, compared with those from the current study for all‐cause and HCV/hepatic complications‐related medical costs (adjusted cost difference, $13,933 and $9,366 per patient per year, respectively). Finally, while no other previous study has directly compared EHM‐related medical costs between patients who initiated treatment in early versus late fibrosis stages, previous economic models did suggest higher medical costs for hepatic complications among patients in later fibrosis stages compared to those in earlier fibrosis stages.15, 24
Findings from the present study should be interpreted in light of its limitations. First, despite sample matching and covariate adjustment in the analyses, residual confounding may persist. In particular, claims data do not include information on behavioral factors, such as injectable drug use, which may be more common in the HCV cohort. However, many injectable drug users are uninsured or have government‐supported health insurance25 and may not be captured in this cohort of patients with commercial insurance. Second, due to the chronic nature of HCV, there may be a lag between HCV infection and diagnosis. If some of the patients included in the no‐HCV cohort were HCV infected but not yet diagnosed, this would underestimate the OR of EHMs, leading to conservative estimates of the risk of EHM among patients with HCV. Third, medical costs were analyzed as charged amounts because paid amounts were not available. While the medical charges may overestimate the paid amounts, this probably affects the study cohorts equally and should not change the conclusion of higher medical costs among patients with HCV. However, a longer time may be needed to offset the cost of treatment than that estimated in the current study. Fourth, in this data set, one medical claim can be associated with several diagnoses, with no indicator for the primary diagnosis. Thus, the same medical cost may be attributed to more than one EHM or to both EHM and HCV/hepatic complications, resulting in costs overlapping and being counted twice when calculating hepatic and EHM‐specific costs. However, statistical adjustment separates effects at the patient level, and the stratification by nonoverlapping/overlapping claims provides more transparency on how EHM‐related costs are distinct from the costs related to liver disease. Furthermore, the results are robust with regards to indicating lower costs for treated patients compared to untreated patients for all cost categories investigated, and all‐cause cost is unaffected by this limitation. Fifth, fibrosis stage was only measured for patients followed by providers that contributed laboratory data, and these patients may not be representative of the full sample. Similarly, our sample of commercially insured patients may not be representative of the broader HCV population. Sixth, fibrosis stage measured by APRI may be less accurate than the gold standard of liver biopsy. Seventh, the HCV cohort only included patients with at least two diagnoses of chronic HCV. The practice of requesting two or more diagnoses is common in claims‐based studies.17, 26, 27, 28, 29, 30, 31 Given that an HCV diagnosis is sometimes recorded when HCV tests are ordered, this criterion was applied to ensure that the HCV cohort did not include HCV‐free patients. While patients with no physician follow‐up after their first HCV diagnosis were excluded from the study, individuals who incorrectly received an HCV diagnosis that was subsequently not confirmed were successfully excluded. Lastly, claims data might be subject to coding errors or omissions, potentially resulting in patient misclassification.
This study found that HCV is associated with high EHM risks and EHM‐related medical costs. However, it is possible to significantly reduce this clinical and economic burden through viral eradication, especially if treatment is initiated early and not delayed until fibrosis advances.
Author names in bold designate shared co‐first authorship.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep4.1049/suppinfo.
Supplemental Information
Acknowledgment
Medical writing assistance was provided by Dr. Cinzia Metallo, an employee of Analysis Group, Inc. Raluca Ionescu‐Ittu, Willy Wynant, and Hela Romdhani, employees of Analysis Group, Inc., contributed to data analysis and drafting the manuscript.
Potential conflict of interest: Nancy Reau is an employee of Rush University Medical Center and is a consultant for AbbVie Inc. She is also a consultant for Gilead Sciences, Inc., Merck and Co, Inc., and Bristol Myers Squibb Company, and her institution has received research support from AbbVie Inc. and Gilead Sciences, Inc. Eric Wu is an employee of Analysis Group, Inc., which received consultancy fees from AbbVie for conducting research analysis. Francis Vekeman was an employee of Analysis Group, Inc. at the time this analysis was conducted. Yanjun Bao and Yuri Sanchez Gonzalez are employees of AbbVie Inc. and may own AbbVie stock or stock options. Analysis Group, Inc. received payment from AbbVie for providing medical writing assistance.
Design, analysis, and financial support provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the study.
REFERENCES
- 1. Centers for Disease Control and Prevention . Hepatitis C FAQs for health professionals. http://www.cdc.gov/hepatitis/hcv/hcvfaq.htm#section1. Accessed September 2016.
- 2. Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci 2006;3:47‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002;36(5 Suppl 1):S35‐46. [DOI] [PubMed] [Google Scholar]
- 4. Medina J, Garcia‐Buey L, Moreno‐Otero R. Hepatitis C virus‐related extra‐hepatic disease‐‐aetiopathogenesis and management. Aliment Pharmacol Ther 2004;20:129‐141. [DOI] [PubMed] [Google Scholar]
- 5. Zampino R, Marrone A, Restivo L, Guerrera B, Sellitto A, Rinaldi L, et al. Chronic HCV infection and inflammation: clinical impact on hepatic and extra‐hepatic manifestations. World J Hepatol 2013;5:528‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cacoub P, Gragnani L, Comarmond C, Zignego AL. Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver Dis 2014;46 Suppl 5:S165‐S173. [DOI] [PubMed] [Google Scholar]
- 7. Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, et al. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment virus C. Arthritis Rheum 1999;42:2204‐2212. [DOI] [PubMed] [Google Scholar]
- 8. Cacoub P, Comarmond C, Domont F, Savey L, Desbois AC, Saadoun D. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther Adv Infect Dis 2016;3:3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahale P, Sturgis EM, Tweardy DJ, Ariza‐Heredia EJ, Torres HA. Association between hepatitis C virus and head and neck cancers. J Natl Cancer Inst 2016;108:pii djw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Voulgaris T, Sevastianos VA. Atherosclerosis as extrahepatic manifestation of chronic infection with hepatitis C virus. Hepat Research Treat 2016;2016:7629318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gill K, Ghazinian H, Manch R, Gish R. Hepatitis C virus as a systemic disease: reaching beyond the liver. Hepatol Int 2016;10:415‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moorman AC, Tong X, Spradling PR, Rupp LB, Gordon SC, Lu M, et al. Prevalence of renal impairment and associated conditions among HCV‐infected persons in the Chronic Hepatitis Cohort Study (CHeCS). Dig Dis Sci 2016;61:2087‐2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leigh JP, Bowlus CL, Leistikow BN, Schenker M. Costs of hepatitis C. Arch Intern Med 2001;161:2231‐2237. [DOI] [PubMed] [Google Scholar]
- 14. Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol 2011;45:e17‐24. [DOI] [PubMed] [Google Scholar]
- 15. Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology 2013;57:2164‐2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCombs JS, Yuan Y, Shin J, Saab S. Economic burden associated with patients diagnosed with hepatitis C. Clin Ther 2011;33:1268‐1280. [DOI] [PubMed] [Google Scholar]
- 17. McAdam‐Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All‐cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J Manag Care Pharm 2011;17:531‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta‐analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150:1599‐1608. [DOI] [PubMed] [Google Scholar]
- 19. Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. Jama. 2014;312:631‐640. [DOI] [PubMed] [Google Scholar]
- 20. Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase‐to‐platelet ratio index for the staging of hepatitis C‐related fibrosis: an updated meta‐analysis. Hepatology 2011;53:726‐736. [DOI] [PubMed] [Google Scholar]
- 21. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518‐526. [DOI] [PubMed] [Google Scholar]
- 22. Rosenthal ES, Graham CS. Price and affordability of direct‐acting antiviral regimens for hepatitis C virus in the United States. Infect Agent Cancer 2016;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. El Khoury AC, Klimack WK, Wallace C, Razavi H. Economic burden of hepatitis C‐associated diseases in the United States. J Viral Hepat 2012;19:153‐160. [DOI] [PubMed] [Google Scholar]
- 24. Najafzadeh M, Andersson K, Shrank WH, et al. Cost‐effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med 2015;162:407‐419. [DOI] [PubMed] [Google Scholar]
- 25. Heimer R, Barbour R, Palacios WR, Nichols LG, Grau LE. Associations between injection risk and community disadvantage among suburban injection drug users in southwestern Connecticut, USA. AIDS Behav. 2014;18:452‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blumentals WA, Arreglado A, Napalkov P, Toovey S. Rheumatoid arthritis and the incidence of influenza and influenza‐related complications: a retrospective cohort study. BMC Musculoskelet Disord 2012;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coleman KJ, Lutsky MA, Yau V, Qian Y, Pomichowski ME, Crawford PM, et al. Validation of autism spectrum disorder diagnoses in large healthcare systems with electronic medical records. J Autism Dev Disord 2015;45:1989‐1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cramer JA, Wang ZJ, Chang E, Copher R, Cherepanov D, Broder MS. Health‐care costs and utilization related to long‐ or short‐acting antiepileptic monotherapy use. Epilepsy Behav 2015;44:40‐46. [DOI] [PubMed] [Google Scholar]
- 29. Day S, Acquah K, Mruthyunjaya P, Grossman DS, Lee PP, Sloan FA. Ocular complications after anti‐vascular endothelial growth factor therapy in Medicare patients with age‐related macular degeneration. Am J Ophthalmol. 2011;152:266‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine (Phila Pa 1976) 2012;37:E668‐677. [DOI] [PubMed] [Google Scholar]
- 31. Shi L, Ye X, Lu M, Wu EQ, Sharma H, Thomason D, et al. Glycemic and cholesterol control versus single‐goal control in US veterans with newly diagnosed type 2 diabetes: a retrospective observational study. Diabetes Ther. 2015;6:339‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep4.1049/suppinfo.
Supplemental Information


