Abstract
We forecast the health and budgetary impact of hepatitis C (HCV) treatment on the Medicare program based on currently observed rates of treatment among Medicare and non‐Medicare patients and identify the impact of higher rates of treatment among non‐Medicare populations. We developed a computer microsimulation model to conduct an epidemiologic forecast, a budgetary impact analysis, and a cost‐effectiveness analysis of the treatment of HCV based on three scenarios: 1) no treatment, 2) continuation of current‐treatment rates, and 3) treatment rates among non‐Medicare patients increased to match that of Medicare patients. The simulated population is based on National Health and Nutrition Examination Survey data. HCV progression rates and costs were calculated in Surveillance, Epidemiology, and End Results Program Medicare 5% claims data from the Chronic Hepatitis Cohort Study and published literature. We estimate that 13.6% of patients with HCV in the United States are enrolled in Medicare, but 75% will enter Medicare in the next 20 years. Medicare patients were over 5 times as likely to be treated in 2014‐2015 as other patients. Medicare paid over $9 billion in treatment costs in both 2015 and 2016 and will total $28.4 billion from 2017‐2026. Increasing treatment rates among non‐Medicare patients would lead to 234,000 more patients being treated, reduce HCV mortality by 19%, and decrease Medicare costs by $18.6 billion from 2017‐2026. We find that treatment remains cost‐effective under most assumptions, costing $31,718 per quality adjusted life year gained. Conclusion: Medicare treats a disproportionately large share of HCV patients. Continued low rates of treatment among non‐Medicare HCV patients will result in both reduced and deferred treatment, shifting future treatment costs to Medicare while increasing overall medical management costs, morbidity, and mortality. (Hepatology Communications 2017;1:99‐109)
Abbreviations
- CC
compensated cirrhosis
- CHeCs
Chronic Hepatitis Cohort Study
- DAA
direct acting antiviral
- DC
decompensated cirrhosis
- ESLD
end‐stage liver disease
- HCV
hepatitis C virus
- ICER
incremental cost effectiveness ratio
- NHANES
National Health and Nutrition Examination Survey
- QALY
quality adjusted life year
- SEER
Surveillance, Epidemiology, and End Results Program
- SVR
sustained viral response
- WAC
wholesale acquisition cost
Introduction
Recent analyses have shown that without medical intervention, the large cohort of Americans infected with viral hepatitis C (HCV) will soon lead to rapid increases in incident cirrhosis and end‐stage liver disease (ESLD), including liver cancer (hepatocellular carcinoma [HCC]), liver failure, transplant, and death.1 Recently released direct acting antiviral (DAA) therapies have the potential to avert this looming epidemic, but treatment comes at a very high cost. Barriers to care as a result of the high cost of treatment have generated tremendous controversy in the areas of public discourse and have led some groups to advocate for drastic measures to expand purchasing or curtail pricing of DAA therapies.2 However, despite these high costs, multiple studies have demonstrated favorable cost‐effectiveness of these treatments.3, 4, 5 As prices continue to decrease, HCV treatment will become more cost‐effective.
Hepatitis C exhibits a unique epidemiology; high incidence rates through the 1960s and 1970s were followed by reductions in incidence in the 1980s and 1990s due to both protective behaviors in response to HIV and the initiation of blood supply screening for HCV in 1992. This has led to a prevalent population concentrated in the cohort born from 1945 to 1965. Due to the long natural history of HCV progression, many of these patients are just now reaching more advanced stages of disease. The confluence of three factors in particular will drive a large but temporary wave in demand for treatment: 1) many chronically infected HCV‐positive (HCV+) patients are progressing to more severe levels of fibrosis and ESLD, 2) expanded recommendations for one‐time testing of baby‐boomers issued in 2013 will continue to increase the number of diagnosed patients, and 3) the release of new highly effective DAA therapies with few side effects beginning in 2014 is increasing the number of patients eligible and wanting to undergo treatment.6 Thus, insurers are now faced with a high demand for treatment by patients in a large but largely finite population of HCV patients. Perhaps the primary issue of hepatitis C treatment now is not whether it is cost‐effective but how payers, particularly public payers with fixed budgets, can finance a large, albeit temporary, spike in costs due to HCV treatment.
The response of many payers thus far has been 2‐fold: negotiated price discounts and treatment restrictions.7, 8 Media reports and anecdotal evidence suggest that a number of payers have negotiated discounts for DAA drugs, particularly as additional drugs have entered the market. Politico reported that New York's Medicaid office secured a price of approximately $28,000 per prescription, a steep discount from the $94,500 wholesale acquisition cost (WAC) price.9 While it is likely that much of the market is mitigating some portion of costs through negotiated discounts, there is evidence that the primary vehicle for cost containment may be treatment restrictions set by payers. Publicly reported payer mix data show that by mid‐2015, 45% of all DAA‐treated patients were in Medicare. However, it was recently estimated that fewer than 12% of HCV+ patients are in Medicare.10 From these estimates, it would appear that Medicare patients are 5 times as likely to be treated than patients without Medicare coverage.
The probability of HCV treatment is dependent on a number of factors within the treatment cascade, including prior knowledge of HCV infection, testing rates, and access to care as well as any potential restrictions on payment for HCV drugs by various insurers. Treatment disparities among different pools of insured populations may have important long‐term health, economic, and budgetary implications for the nation and particularly for the Medicare program as well as its current and future beneficiaries.
In this analysis, we consider the potential long‐term impact of continued restricted levels of treatment among non‐Medicare patients and estimate the possible impact of increased treatment rates among non‐Medicare populations. Several forecasting analyses have considered the future epidemiology and medical treatment of HCV. However, none have specifically considered the Medicare program, included actual observed rates of treatment for HCV by insurer, or used empirically calculated rates of clinical progression and costs from the Medicare program.
Methods
We developed a computer microsimulation model to estimate the current and future HCV epidemic and simulate natural history and progression, insurance coverage, treatment, and outcomes of HCV. Briefly, the model is based on a microsimulation approach whereby a population of simulated individuals is created to represent the current prevalent HCV+ population of the United States by age, sex, stage of infection, and insurance status (Medicare or non‐Medicare). Simulated patients are tracked over time as they progress through HCV stages and face an annual probability of treatment based on currently observed treatment rates among Medicare and non‐Medicare HCV+ patients. Patients incur annual costs of medical management of HCV. Unsuccessfully treated patients may be retreated once. Costs of testing are imputed based on rates of treatment because testing rates by payer are not known. All costs are expressed in 2016 dollars. The model is programmed in AnyLogic (XJtek Inc, St. Petersburg, Russia). Major input parameters are summarized in Table 1.
Table 1.
MAJOR PARAMETERS
| Parameter | Value | 95% Interval | Distribution | Source |
|---|---|---|---|---|
| Treatment Management Costs | 831.63 | 342–1029 | Lognormal | 1 |
| Annual Medical Costs, Post Treatment | ||||
| Year 1 | 224.88 | 123–370 | Lognormal | 1 |
| Years 2–5 | 112.44 | 62–185 | Lognormal | 1 |
| Years 6+ | 56.22 | 27–81 | Lognormal | 1 |
| Genotype 1 | ||||
| Probability of SVR | 0.972 | 0.964‐0.978 | Beta | Appendix 6 |
| Cost of treatment | $66,686 | NA | NA | Appendix 7 |
| Utility decrement | 0 | 0 | NA | Assumption |
| Genotype 2 | ||||
| Probability of SVR | 0.993 | 0.98‐1.0 | Beta | Appendix 6 |
| Cost of treatment | $74,760 | NA | NA | Appendix 7 |
| Utility decrement | 0 | NA | NA | Assumption |
| Genotype 3: | ||||
| Probability of SVR | 0.848 | 0.927‐0.966 | Beta | Appendix 6 |
| Cost of treatment | $80,472 | NA | NA | Appendix 7 |
| Utility decrement | 0 | NA | NA | Assumption |
| Annual Stage Costs, Medicare Patients | ||||
| Chronic infection or cirrhosis | $2,708 | ‐$918–$6,376 | Lognormal | Medicare claims |
| Decompensated cirrhosis | $34,976 | $27,616–$42,422 | Lognormal | Medicare claims |
| HCC | $35,011 | $25,760–$43,895 | Lognormal | Medicare claims |
| Transplant, per year | $22,638 | $11,319–$33,957 | Lognormal | Medicare claims |
| Annual Stage Costs, Non‐Medicare Patients | ||||
| Chronic infection* | $5,275 | SE: $177 | Normal | 16 |
| Cirrhosis | $6,746 | SE:$672 | Normal | 16 |
| Decompensated cirrhosis | $13,589 | SE:$941 | Normal | 16 |
| HCC | $35,940 | SE:$10,505 | Normal | 16 |
| Transplant | $102,530 | SE: $30,406 | Normal | 16 |
| Annual Stage QALYs | ||||
| Chronic infection | 0.86 | NA | NA | 17, 18, 20, 21, 22, 23 |
| Cirrhosis | 0.81 | NA | ||
| Decompensated cirrhosis | 0.7 | NA | ||
| HCC | 0.67 | NA | ||
| Transplant | 0.78 | NA | ||
| Dead | 0 | NA | ||
Annual stage costs for chronic infection assumed to be zero for uninsured patients.
Abbreviations: NA, not applicable; SE, standard error, in lieu of 95% confidence interval.
ANALYSES AND SENSITIVITY ANALYSIS
We present results of the simulation in three distinct analyses: 1) an epidemiologic forecast, 2) a cost‐effectiveness analysis, and 3) a budgetary impact analysis. The epidemiologic forecast reports the lifetime incidence of HCV and treatment outcomes among the current HCV+ population. The cost‐effectiveness analysis reports the incremental cost‐effectiveness ratio (ICER), calculated as the incremental medical costs per quality adjusted life year (QALY) gained. The cost‐effectiveness analysis is reported from the medical system perspective, including payments by all insurers and patients. Future costs and QALYs are discounted to the year 2016 by 3% annually. The budgetary impact analysis reports the incremental impact on Medicare costs (total Medicare costs include direct Medicare reimbursement, reimbursement by Medicare supplemental payers or part D carriers, and any beneficiary copayments) in 2014, 2015, 2016, and over the next 10 years (2016‐2025). Future costs reported in the budgetary analysis are not discounted.
Sensitivity analyses are included to provide information on the impact of parameter uncertainty and assumptions. Univariate sensitivity analysis scenarios include alternative rates of treatment (including continuation of current‐treatment volume rather than treatment rates, disease progression (including progression rates calculated in Medicare claims and other published studies), and alternative treatment costs (including full WAC price and higher future discount scenarios). All parameters are sampled from their prior distributions for each of the 1,000 iterations of the model, with each iteration including 10,000 simulated patients. The mean and 95% credible interval are based on the 2.5, mean, and 97.5 percentiles of the iterations.
TREATMENT SCENARIOS
We conduct the simulation based on three alternative scenarios of treatment: 1) no treatment, 2) current‐treatment rates, and 3) a matched‐treatment rate scenario. The no‐treatment scenario includes zero treatment and serves as a comparator. The current‐treatment rate scenario represents a continuation of currently observed treatment rates among Medicare and non‐Medicare populations. The matched‐treatment rate scenario is meant to provide a hypothetical scenario in which treatment among non‐Medicare patients increases to match those observed among Medicare patients beginning in 2017.
POPULATION
The model creates a synthetic population of agents representing the age and insurance distribution of the prevalent HCV‐infected U.S. population, estimated using the 1999‐2012 National Health and Nutrition Examination Survey (NHANES), as shown in Supporting Information Appendix 1. We simulate future annual rates of Medicare entry by age, race, and sex among HCV patients based on two routes of entry, reaching age 65 or entering before age 65 due to disability coverage, based on an annual transition probability as shown in Supporting Information Appendix 2.
HCV STAGES AND TRANSITION RATES
The model includes the major stages of HCV, including chronic infection, compensated cirrhosis (CC), and ESLD stages, including decompensated cirrhosis (DC), HCC, liver transplant, and death. Among chronic infection, patients are allocated to four levels of fibrosis progression (F0, F1, F2, and F3). Initial HCV stages are assigned to patients based on the baseline distribution as reported in the Chronic Hepatitis Cohort Study (CHeCs).5 For patients enrolled in Medicare at model initiation, cirrhosis and ESLD stages are assigned based on stage allocations by age group as calculated in the 2009 data from the Surveillance, Epidemiology, and End Results (SEER) program's SEER‐Medicare data and linked Medicare 5% data.
Progression rates are also based on those observed in the CHeCS study for the baseline scenario.2 Initial stage allocation of the population is shown in Supporting Information Appendix 3. Sensitivity analyses include a scenario in which simulated patients in Medicare progress based on clinical progression rates calculated from SEER/Medicare 5% data for the years 2002‐2009, representing 6,173,194 life years.11 To our knowledge, this is the first time clinical progression of HCV has been measured in Medicare data; this analysis shows faster rates of progression than previously reported (Supporting Information Appendix 4).
TREATMENT
Treatment in the model consists of the administration of current‐generation DAA therapies. Treatment is initiated in the model directly by applying an annual rate of treatment to all patients per each insurance pool. The model does not simulate prior awareness or testing due to limitations in the availability of these data by Medicare and non‐Medicare insurance status. A full description and rationale for this approach are included in Supporting Information Appendix 8.
We calculate separate treatment rates for Medicare and non‐Medicare patients based on the number of patients treated in 2014 and 2015 by insurance type relative to the HCV+ population size for the corresponding insurance pool estimated in the NHANES data. In the baseline scenario, the 2015 treatment rates by payer are continued from 2016 onward. In the matched‐treatment rate scenario, non‐Medicare patients are assigned the treatment rates calculated for Medicare patients starting in 2017. Sensitivity analyses include alternative treatment scenarios, including lower treatment rates based on testing rate assumptions and limits on the number of potentially treatable patients.
Based on 2014 and 2015 earnings statements for three primary DAA drugs, sofosbuvir (Sovaldi; Gilead Sciences, Inc., CA), ledipasvir/sofosbuvir (Harvoni; Gilead Sciences), and ombitasvir, paritaprevir, and ritonavir tablets copackaged with dasabuvir tablets (VIEKIRA PAK; AbbVie Inc., IL), we estimated that 150,000 patients were treated with DAA in 2014 and 250,000 in 2015. We identified 2014 payer mix estimates for sofosbuvir in financial news services, which indicated that 45% of sales were within the Medicare program, 45% were covered through private insurance, 7% by Medicaid, and only 3% by all other payers and the uninsured.12 We requested confirmation of these estimates and updated payer mix data for DAA drugs from Gilead Sciences, who declined to provide full updated payer mix but did confirm that the Medicare share had remained at 45% through 2015.
While 45% of treated patients are in Medicare, we estimate that 13.8% of HCV+ patients were likely to be in Medicare as of 2014 based on NHANES data. This implies that in 2014, Medicare patients were nearly five times as likely to be treated as patients without Medicare coverage. Using the model, we calibrated treatment rates such that the model replicated the 150,000 and 250,000 total number of patients treated in 2014 and 2015, respectively, while maintaining the payer mix. Because we could not identify data on treatment rates by stage of HCV, we assumed diagnosed patients in all treatable stages of HCV (F0, F1, F2, F3, and CC) have the same probability of treatment. Additional detail on the calibrated treatment rates is provided in Supporting Information Appendix 5.
The efficacy of treatment is based on clinical trial data, with successful treatment defined as a sustained viral response (SVR) at 12 weeks after the end of therapy.13, 14 Calculation of SVR rates is shown in Supporting Information Appendix 6.
COSTS AND QALYs
The analysis takes a medical system perspective; modeled costs include medical costs paid by insurers and their enrollees. Thus, Medicare costs include all medical costs of Medicare beneficiaries, Medicare reimbursement, and Medicare Part D carriers. Non‐Medicare costs include all medical costs regardless of payer among patients without Medicare coverage. The model includes three types of medical costs: medical management of diagnosed HCV, the costs of HCV treatment, and costs for treatment management and follow‐up. For Medicare patients, we assign annual medical management costs based on the calculation of overall medical costs attributable to HCV by stage using a two‐part log‐link “tpm” module in STATA on 2009 SEER/5% Medicare claims data, representing 407,786 Medicare beneficiaries.3, 15 Medical management costs for non‐Medicare‐insured patients are based on published estimates of attributable costs calculated for private payers.16 By assumption, we apply these costs for uninsured patients with cirrhosis or ESLD but do not apply medical management costs for uninsured patients with chronic infection.
Treatment costs include costs for DAA therapies and medical management of treatment. Drug selection is based on genotype. Many payers on the market have now negotiated discounts for DAA drugs, and we identified discounts ranging from 23.1% for a state Medicaid program to 46% for the Veterans Administration, although this discount was recently increased to over 80% in exchange for the Veteran Administration's decision to drop coverage restrictions. The New York State Department of Health reported an average discount of 18% in 2014 and 2015, representing sales of ledipasvir/sofosbuvir and ombitasvir, paritaprevir, and ritonavir tablets copackaged with dasabuvir tablets. We assume a discount rate of 20% off WAC prices for all drugs except sofosbuvir/velpatasvir (Epclusa; Gilead Sciences) for which anecdotal evidence suggests there are limited or no discounts available. In addition, genotype 1 patients may be indicated for a reduced 8‐week course of treatment with ledipasvir/sofosbuvir; we assume 50% of patients would benefit from this reduced treatment course. We calculated an expected treatment cost for genotypes 1/4, 2, and 3 based on market share for all drugs in August 2016 (Supporting Information Appendix 7). In sensitivity analyses, we include alternative treatment cost scenarios, including full WAC price and a 50% universal discount.
We estimated that 70%, 20%, and 10% of patients had genotypes 1, 2, and 3, respectively, based on our analysis of biopsy results from several hospital and clinic‐based electronic medical record systems. For all treatments, we included an estimated cost of treatment management and follow‐up totaling $831.63 for the initial workup, and subsequent years accruing costs of $224.88 (posttreatment year 1), $112.44 per year (years 2‐5), and $56.22 per year for posttreatment years 6 and later.3
The model tracks patient well‐being based on QALYs. QALYs are derived from utility loss estimates from five studies based on HCV states F0‐1, F2‐3, CC, DC, HCC, postliver transplant, and post‐SVR.17, 18, 19, 20, 21, 22 QALYs are calculated by multiplying annual stage utility losses by expected background utility based on age for each year of life.23
Results
CURRENT HCV+ POPULATION
Based on pooled 1999‐2012 NHANES data, we estimate the prevalence of patients with HCV antibody+ to be 3,701,117 in 2012. In NHANES data, 76.92% of respondents with HCV antibody+ who were given an RNA test were RNA positive, indicating chronic infection. After controlling for RNA confirmation and general population mortality, the chronically infected population would be expected to total 2,809,698 in 2014. Of this total, 382,111 were in Medicare or would be expected to have entered Medicare between their respective response year and 2014, while 2,427,587 HCV patients are expected to not have Medicare insurance. This result is slightly higher than that of a previous analysis, which estimated 350,000 HCV patients in Medicare by 2014.10 Medicare patients currently constitute only 13.6% of the HCV+ population, but nearly three quarters of the HCV+ population are aged 45‐64 and are anticipated to enter Medicare in the next 20 years. The current distribution of the HCV+ population by Medicare insurance status is shown in Supporting Information Appendix 1.
EPIDEMIOLOGIC FORECAST
Table 2 shows the predicted lifetime incidence of HCV stage under the three scenarios: no treatment, treatment based on current‐treatment rates by insurer, and treatment with reduced treatment restrictions whereby non‐Medicare insurers match Medicare treatment rates beginning in 2016. With no treatment for HCV, our simulation finds that 60% of patients with HCV+ would reach cirrhosis or an ESLD stage before death.
Table 2.
LIFETIME INCIDENCE OF HCV OUTCOMES (95% CREDIBLE INTERVAL)
| No Treatment | Current‐Treatment Rate | Medicare‐Matched Treatment Rate | |
|---|---|---|---|
| SVR | 0 | 1,853,760 | 2,047,966 |
| (0‐0) | (1,827,800‐1,880,559) | (2,016,078‐2,075,383) | |
| Cirrhosis | 1,772,596 | 1,012,135 | 851,235 |
| (1,750,294‐1,796,254) | (987,314‐1,039,602) | (824,070‐878,192) | |
| DC | 1,228,462 | 600,969 | 495,732 |
| (1,201,090‐1,256,644) | (577,674‐620,423) | (472,992‐516,998) | |
| HCC | 423,554 | 161,361 | 120,188 |
| (400,087‐441,755) | (148,900‐172,958) | (109,417‐133,341) | |
| Transplant | 158,009 | 103,889 | 93,574 |
| (147,509‐167,191) | (92,720‐115,640) | (84,010‐103,706) | |
| Death | 1,514,458 | 751,049 | 611,801 |
| (1,489,126‐1,537,656) | (726,560‐775,772) | (584,136‐640,421) |
Treatment reduces progression to ESLD and death substantially. Under the baseline scenario of current‐treatment rates carried forward, we estimate that 68% or 1.9 million of the HCV+ population of 2.8 million would be treated in their lifetimes. Of these, we expect 1.85 million patients to achieve an SVR. Continued treatment at current rates would reduce cirrhosis incidence by 43% and ESLD incidence would decrease by 53%. HCV‐associated death would decrease by 50%, but still over 750,000 patients would be expected to die in an HCV‐associated state.
The matched‐treatment rate scenario in which treatment rates among non‐Medicare patients increase to match that of Medicare patients results in the treatment of an additional 234,000 patients. In this scenario, patients would also be treated earlier; 500,000 more would be treated by 2025. This scenario leads to larger decreases in cirrhosis (19% versus current treatment, 54% reduction versus no treatment), ESLD (21% versus current treatment, 63% versus no treatment), and death with active HCV (22% versus current treatment, 61% versus no treatment).
BUDGETARY IMPACT
The budgetary impact analysis shows forecasted undiscounted medical costs incurred by Medicare under the three treatment scenarios in Table 3. With no treatment, we estimate that the HCV management costs borne by Medicare would have totaled $3.15 billion in 2014 and would have increased to an average $4.7 billion per year from 2017‐2026, totaling $44.8 billion during this time period. Without any treatment, non‐Medicare HCV management costs would have totaled $14.7 billion in 2014 but would decrease over time as patients transition to Medicare, averaging $7.0 billion a year from 2017‐2026.
Table 3.
BUDGETARY IMPACT FORECAST, $BILLIONS
| No Treatment | 2014 | 2015 | 2016 | 2017 | 2017‐2026 |
|---|---|---|---|---|---|
| Total Medicare costs | $3.15 | $3.21 | $3.30 | $3.42 | $46.84 |
| Treatment | $0.00 | $0.00 | $0.00 | $0.00 | $0.00 |
| Other medical | $3.15 | $3.21 | $3.30 | $3.42 | $46.84 |
| Total non‐Medicare costs | $14.68 | $11.91 | $10.79 | $9.89 | $70.13 |
| Treatment | $0.00 | $0.00 | $0.00 | $0.00 | $0.00 |
| Other medical | $14.68 | $11.91 | $10.79 | $9.89 | $70.13 |
| Total costs, all payers | $17.84 | $15.11 | $14.09 | $13.31 | $116.97 |
| Current Treatment Rate | 2014 | 2015 | 2016 | 2017 | 2017‐2026 |
|---|---|---|---|---|---|
| Total Medicare costs | $7.93 | $12.29 | $12.27 | $8.16 | $70.24 |
| Treatment | $4.80 | $9.13 | $9.12 | $4.95 | $28.41 |
| Other medical | $3.13 | $3.15 | $3.16 | $3.21 | $41.83 |
| Total non‐Medicare costs | $20.42 | $21.54 | $20.30 | $18.06 | $109.39 |
| Treatment | $5.72 | $9.73 | $9.71 | $8.47 | $44.92 |
| Other medical | $14.70 | $11.81 | $10.59 | $9.59 | $64.46 |
| Total costs, all payers | $28.35 | $33.82 | $32.57 | $26.22 | $179.63 |
| Medicare‐Matched Treatment Rate | 2014 | 2015 | 2016 | 2017 | 2017‐2026 |
|---|---|---|---|---|---|
| Total Medicare costs | $7.94 | $12.23 | $12.29 | $8.21 | $51.63 |
| Treatment | $4.82 | $9.10 | $9.16 | $5.03 | $10.22 |
| Other medical | $3.12 | $3.13 | $3.13 | $3.18 | $41.41 |
| Total non‐Medicare costs | $20.33 | $21.49 | $20.27 | $69.09 | $152.43 |
| Treatment | $5.68 | $9.68 | $9.68 | $59.71 | $93.49 |
| Other medical | $14.65 | $11.81 | $10.59 | $9.37 | $58.94 |
| Total costs, all payers | $28.26 | $33.78 | $32.54 | $77.25 | $204.06 |
In the scenario with current 2014 and 2015 treatment rates, we estimate that Medicare spent a total of $7.9 billion on HCV treatment and management in 2014 and $12.3 billion in 2015. This includes treatment costs of $4.8 billion in 2014 and $9.1 billion in 2015. These estimates of treatment costs closely align with subsequently reported Medicare HCV drug spending of $4.7 billion in 2014 and $9.1 billion in 2015.24 Carrying the 2015 treatment rates forward, we predict that treatment costs for Medicare would have continued to increase until mid‐2016, at which time the majority of existing Medicare patients will have been treated and subsequent treatment volume begins to decrease as treatment is driven largely by the volume of new Medicare enrollment. Treatment costs are then expected to quickly drop to $5.0 billion in 2017 and will then continue to decrease slowly over time, averaging $2.9 billion from 2017‐2026. Non‐Medicare spending was more than 2.5 times higher in 2014 ($20.4 billion) and 1.8 times higher in 2015 ($21.5 billion). This difference will decrease over time; after 2024, Medicare will pay the majority of HCV medical costs.
Under the matched‐treatment rate scenario, the number of patients treated outside the Medicare program would increase markedly in 2017, resulting in a cost spike to $69 billion. However, treatment costs would dramatically fall as the high rates of treatment applied in this scenario would quickly reduce the pool of eligible remaining patients. Over 2017‐2026, non‐Medicare costs would average $15.2 billion per year, an increase of 39% relative to the scenario of current‐treatment rates carried forward. The matched‐treatment scenario, however, would reduce Medicare spending by $18.6 billion over 2017‐2026, a 26% reduction relative to the current‐treatment rate scenario.
COST‐EFFECTIVENESS ANALYSIS
We report the cost‐effectiveness of treatment relative to no‐treatment and higher non‐Medicare treatment rates relative to current‐treatment rates in terms of the ICER of discounted costs of the intervention divided by the incremental gains in discounted QALYs, as shown in Table 4. While the undiscounted budgetary impact analysis found a potential for cost neutrality or even potential savings over a lifetime perspective, net costs always increase in the cost‐effectiveness analysis due to the effect of the 3% discount rate, which is used in the cost‐effectiveness analysis but not in the budgetary impact analysis per consensus guidelines.23 This is because both treatment scenarios increase treatment costs in early years, while cost offsets from reduced medical management costs mostly accrue in later years where discounting has a larger impact.
Table 4.
COST‐EFFECTIVENESS ANALYSIS RESULTS (95% CREDIBLE INTERVALS)
| Medical Costs | Treatment | Total Costs | QALYs | ICER vs | ||
|---|---|---|---|---|---|---|
| No Txt | Current Txt | |||||
| No treatment | $84,174 | $0 | $84,174 | 8.99 | ||
| ($83,314‐$85,165) | $0‐$0 | ($83,314‐$85,165) | (8.90‐9.10) | |||
| Current treatment | $80,463 | $49,298 | $129,761 | 10.43 | $31,718 | |
| ($79,581‐$81,570) | ($48,126‐$50,579) | ($127,707‐$132,149) | (10.32‐10.52) | ($29,526‐$35,039) | ||
| Medicare‐matched treatment rate | $79,833 | $54,750 | $134,584 | 10.92 | $26,146 | $9,828 |
| ($78,807‐$80,626) | ($53,772‐$55,707) | ($132,579‐$136,333) | (10.78‐11.01) | ($24,653‐$28,232) | ($7,851‐$13,351) | |
Relative to no treatment, treatment at current rates is expected to cost $49,298 per patient with HCV+ but will reduce medical costs by $3,711 and increase QALYs by 1.44. A $45,586 net increase in total costs divided by an increase in QALYs of 1.44 results in an ICER of $31,718. Relative to current treatment, the matched‐treatment rate scenario would increase expected treatment costs by $5,453 while increasing medical cost offsets by $630 for a net increase in costs of $4,823 per patient with HCV. Dividing this cost by the 0.49 QALYs gained results in an ICER of $9,828, making this scenario highly cost‐effective. The matched‐treatment scenario is more cost‐effective than the current‐treatment scenario versus no treatment. This is largely because while the matched‐treatment scenario increases the number of patients treated, it has an even larger effect of achieving earlier treatment, thus avoiding years of medical management costs associated with HCV progression for patients awaiting treatment.
UNIVARIATE SENSITIVITY ANALYSIS
In addition to the probabilistic sensitivity analysis included in the primary results that captures the impact of parameter uncertainty, we included five alternative scenarios to test the impact of major assumptions. Figure 1 shows the Medicare budgetary impact of each scenario in 1 year, the annual average over the next 10 years, and the 30‐year annual average. Lowest costs occur with no treatment. Lower treatment costs, the matched‐treatment rate scenario, and an assumption of a 29% reduction in post‐SVR medical management costs all reduce near‐and long‐term costs. A reduction in treatment rate would reduce near‐term costs but would break even over the next 10 years and lead to a $0.3 billion increase in costs over 30 years. Highest costs occur under an assumption that HCV progression matches the higher rates calculated in Medicare claims data.
Figure 1.
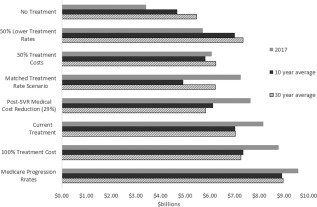
Annualized Medicare budgetary costs over 1 year, 10 year, and 30 year horizons, primary and sensitivity analysis scenarios.
Figure 2 shows the range of ICER values associated with each scenario relative to the baseline result of $31,718 per QALY versus no treatment. Results are most sensitive to the discount rate. A 50% decrease in treatment costs and Medicare‐observed progression rates both improve the ICER to under $18,000 per QALY, while a 50% increase in treatment costs increases the ICER to $35,700. Unlike the budgetary impact analysis, the cost‐effectiveness results are relatively insensitive to the treatment rate.
Figure 2.
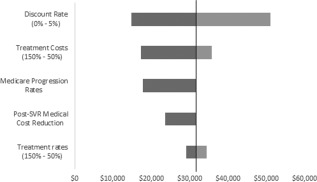
Cost‐effectiveness results of sensitivity analysis scenarios.
Discussion
This analysis is intended to show the potential long‐term outcomes from the HCV epidemic under current HCV treatment rates. We identified the current HCV prevalence by insurance type using nationally representative examination data and identified the rate of treatment for patients with and without Medicare. We empirically estimated medical costs and clinical stage prevalence using Medicare claims data and used these and published data to model the clinical progression, costs, and outcomes of HCV patients.
At an ICER of $31,718 per QALY gained, we find cost‐effectiveness is similar to earlier analyses.3, 4, 5 Although these studies focused on the cost‐effectiveness of population testing, the ICER of testing interventions that include treatment is similar to treatment alone because testing costs are negligible compared to treatment costs. Assuming a $50 testing cost and a 1% true‐positive rate, our model shows that the ICER would increase by only $2,754 to $34,472. Comparatively, Rein et al.3 found sofosbuvir and ribavirin therapy achieved an ICER of $47,304 per QALY gained; Leidner et al.5 found an ICER of $37,000 per QALY; while McEwan et al.4 identified an ICER of $28,602. These earlier studies did not report budgetary impact forecasts. However, they do assume a reduction or elimination in medical management costs post SVR. In our baseline analyses, we do not consider such a reduction. In sensitivity analyses, we find that allowing for post‐SVR cost reduction would result in lower cost projections while also improving the ICER to $23,607 per QALY.
LIMITATIONS
This study is subject to a number of limitations and constraints. The primary limitation is the fundamental unpredictability of the future; thus, this forecast does not attempt to predict the future but rather attempts to predict what would happen in the future based on a continuation of the status quo in terms of treatment rates. We do not consider potential drivers of demand for treatment such as HCV awareness, testing rates, treatment caps, or potential changes to the medical system.
Given the rapid and unpredictable changes facing HCV medical treatment in the United States, this simplified approach removes uncertainty but also limits the scope of the analysis and thus limits how results should be interpreted. The cost projections for Medicare and non‐Medicare must be considered in the context of potential changes to the underlying rates of treatment and other parameters due to any factors within these insured populations. For example, treatment rates may increase in response to reductions in drug prices, or conversely treatment rates may decrease if Medicaid enrollment decreases. We include simplified scenarios representing possible drivers of changes in treatment rates or costs in the sensitivity analysis but do not attempt to capture such outcomes in the primary results. A full rationale for the strategy of directly modeling constant treatment rates is included in Supporting Information Appendix 8.
A number of other limitations exist that could potentially bias the forecast results toward less conservative budgetary forecast or cost‐effectiveness results. The HCV+ population was estimated in NHANES data, and we do not consider successful treatment within this population prior to 2014. However, we do control for general mortality and NHANES excludes institutionalized persons, both of which bias the population estimate downward. We also do not prioritize treatment toward persons in advanced stages, although in testing we found this was unlikely to significantly change results.
This analysis is also subject to limitations related to the accuracy and availability of data to frame the HCV epidemic and current‐treatment levels. All parameters are measured with uncertainty, which we attempt to capture in the 95% credible intervals.
INTERPRETATION AND IMPLICATIONS
Rates of treatment among patients without Medicare coverage were dramatically lower than those with Medicare coverage in 2014‐2015. While some of this disparity in treatment rates could potentially be attributable to infection awareness and testing rates if these are higher among Medicare beneficiaries, little evidence exists to support this as a significant cause. Another explanation is that this disparity is driven by treatment restrictions imposed by some payers due to budgetary restrictions. For example, a North Carolina state Medicaid office report estimates that only 2.5% of its known HCV+ Medicaid beneficiaries received treatment, but illustrating the problem, this nonetheless constituted the second largest expenditure for the program.25 Unlike other payers, Medicare does not appear to be imposing restrictions and thus may be covering a disproportionate share of treatment costs.26
As the 1945‐1965 birth cohort ages fully into Medicare over the next 15 years, patients who are deferred from treatment by their current insurers may not undergo treatment until they enter Medicare, likely after they have progressed to a more severe and costly stage of disease. Thus, when these patients enter Medicare not only are costs deferred from pent‐up demand for treatment but disease management costs per patient will be greater. The predicted consequences of this deferment among the cohort of 60‐year‐old patients with HCV+ in 2014 are shown in Fig. 3. Relatively few patients are treated until the large majority of these patients enter Medicare at age 65 in year 2019, at which time the majority of patients are quickly treated over the next several years.
Figure 3.
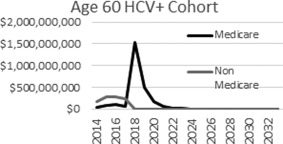
Deferment of treatment costs to Medicare among current 60‐year old HCV+ patients.
While HCV treatment costs have exploded in the past 2 years, we nonetheless predict that medical management costs from HCV may cost more than double that of treatment over the remaining lives of the current HCV‐infected population and would be even higher without treatment. If treatment rates among patients without Medicare were to increase to those observed among Medicare beneficiaries beginning in 2017, total costs from 2017‐2026 would increase by $24.4 billion, but Medicare costs would decrease by $18.6 billion, and 150,000 cases of ESLD would be avoided over the full lifetime of current patients.
Based on current levels of treatment, it is apparent that Medicare will bear a disproportionately high share of the costs of HCV treatment as well as higher future medical management costs attributable to delayed treatment.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep4.1031/suppinfo.
Supporting Information
Potential conflict of interest: Nothing to report.
This work was underwritten by an unrestricted grant from Gilead Sciences Inc.
REFERENCES
- 1. Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre‐cirrhotic chronic hepatitis C in the United States. Dig Liver Dis 2011;43:66‐72. [DOI] [PubMed] [Google Scholar]
- 2. Trooskin SB, Reynolds H, Kostman JR. Access to costly new hepatitis C drugs: medicine, money, and advocacy. Clin Infect Dis 2015;61:1825‐1830. [DOI] [PubMed] [Google Scholar]
- 3. Rein D, Wittenborn J, Smith B, Liffmann D, Ward JW. The cost‐effectiveness, health benefits, and financial costs of new antiviral treatments for hepatitis C virus. Clin Infect Dis 2015;61:157‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McEwan P, Ward T, Yuan Y, Kim R, L'Italien G. The impact of timing and prioritisation on the cost‐effectiveness of birth‐cohort testing and treatment for hepatitis C virus in the United States. Hepatology 2013;58:54‐64. [DOI] [PubMed] [Google Scholar]
- 5. Leidner AJ, Chesson HW, Xu F, Ward JW, Spradling PR, Holmberg SD. Cost‐effectiveness of hepatitis C treatment for patients in early stages of liver disease. Hepatology 2015;61:1860‐1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith BD, Morgan R, Beckett G, Falck Y, Teo C, Jewett A, et al. Recommendations for the identification of hepatitis C virus (HCV) chronic infection among persons born from 1945 through 1965. Centers for Disease Control and Prevention, 2012.
- 7. Edlin BR. Access to treatment for hepatitis C virus infection: time to put patients first. Lancet Infect Dis 2016;16:e196‐201. [DOI] [PubMed] [Google Scholar]
- 8. Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015. Aug 4;163:215‐223. [DOI] [PubMed] [Google Scholar]
- 9. Goldberg D. Hepatitis C drugs top Medicaid spending, highlighting larger concerns. Politico. 2016. March 8, 2016. http://www.politico.com/states/new-york/albany/story/2016/03/hepatitis-c-drugs-top-medicaid-spending-highlighting-larger-concerns-000000. Accessed March 15, 2017. [Google Scholar]
- 10. Neuman T, Hoadley J, Cubanski J. The cost of a cure: Medicare's role in treating hepatitis C. Health Affairs Blog June 5, 2014. http://healthaffairs.org/blog/2014/06/05/the-cost-of-a-cure-medicares-role-in-treating-hepatitis-c/. Accessed March 15, 2017. [Google Scholar]
- 11. SEER Program . Surveillance, Epidemiology, and End Results (SEER) Program, September 2013. www.seer.cancer.gov. Accessed March 15, 2017.
- 12. Goldman Sachs Comments on Gilead Sciences, April 23, 2014. https://www.streetinsider.com/Analyst+Comments/Goldman+Sachs+Comments+on+Gilead+Sciences+%28GILD%29+Q1/9401869.html. Accessed March 15, 2017.
- 13. Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370:1879‐1888. [DOI] [PubMed] [Google Scholar]
- 14. Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370:1889‐1898. [DOI] [PubMed] [Google Scholar]
- 15. Rein D, Borton J, Liffmann D, Wittenborn J. The burden of hepatitis C to the United States Medicare system in 2009: descriptive and economic characteristics. Hepatology 2016;63:1135‐1144. [DOI] [PubMed] [Google Scholar]
- 16. Baran RW, Samp JC, Walker DR, Smeeding JE, Young JW, Kleinman NL, et al. Costs and absence of HCV‐infected employees by disease stage. J Med Econ 2015;18:691‐703. [DOI] [PubMed] [Google Scholar]
- 17. Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW, et al. The cost‐effectiveness of birth‐cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med 2012;156:263‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health‐state utilities and quality of life in hepatitis C patients. Am J Gastroenterol 2003;98:630‐638. [DOI] [PubMed] [Google Scholar]
- 19. Grieve R, Roberts J, Wright M, Sweeting M, DeAngelis D, Rosenberg W, et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut 2006;55:1332‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sherman KE, Sherman SN, Chenier T, Tsevat J. Health values of patients with chronic hepatitis C infection. Arch Intern Med 2004;164:2377‐2382. [DOI] [PubMed] [Google Scholar]
- 21. Siebert U, Sroczynski G. Effectiveness and cost‐effectiveness of initial combination therapy with interferon/peginterferon plus ribavirin in patients with chronic hepatitis C in Germany: a health technology assessment commissioned by the German Federal Ministry of Health and Social Security. Int J Technol Assess Health Care 2005;21:55‐65. [DOI] [PubMed] [Google Scholar]
- 22. Thein HH, Krahn M, Kaldor JM, Dore GJ. Estimation of utilities for chronic hepatitis C from SF‐36 scores. Am J Gastroenterol 2005;100:643‐651. [DOI] [PubMed] [Google Scholar]
- 23. Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost‐utility analyses: using national measures to create condition‐specific values. Med Care 1998;36:778‐792. [DOI] [PubMed] [Google Scholar]
- 24. Hoadley J, Neuman T, Cubanski J. The cost of a cure: revisiting Medicare Part D and hepatitis C drugs. Health Affairs Blog. November 3, 2016. http://healthaffairs.org/blog/2016/11/03/the-cost-of-a-cure-revisiting-medicare-part-d-and-hepatitis-c-drugs/. Accessed March 15, 2017. [Google Scholar]
- 25. deBruyn J. Sovaldi is expensive, but this drug cost N.C. Medicaid even more. Triangle Business Journal December 15, 2015; Health Care Section. http://www.bizjournals.com/triangle/news/2015/12/15/nc-medicaid-expensive-drug-spending.html. Accessed March 15, 2017. [Google Scholar]
- 26. Younossi ZM, Bacon BR, Dieterich DT, Flamm SL, Kowdley K, Milligan S, et al. Disparate access to treatment regimens in chronic hepatitis C patients: data from the TRIO network. J Viral Hepat 2016;23:447‐454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep4.1031/suppinfo.
Supporting Information


