Abbreviations
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IFN
interferon
- IVDA
intravenous drug abuse
- PEG‐IFN
pegylated‐interferon
- RBV
ribavirin
- SVR
sustained viral response
Introduction
Hepatitis C virus (HCV) infection remains a major public health burden, with an estimated worldwide prevalence of 2.5% of the population (177.5 million infected adults); this ranges from 1.3% in the Americas to 2.9% in Africa.1 In the United States alone, an estimated 3 million to 4 million persons are chronically infected with HCV, and approximately half are unaware of their infectious status and do not receive appropriate care. The current Centers for Disease Control recommendations for HCV screening and advances in HCV treatment are anticipated to diminish the clinical burden of the disease; however, medication cost and access to care remain significant barriers.2 In order to provide a more structured approach to the dynamic landscape of HCV, adult‐centric guidelines have been established.3 These provide health care professionals with timely guidance as new therapies become available and are integrated into treatment regimens.
In children and adolescents as in adults, HCV infection is suspected to be grossly underestimated. HCV infection across the pediatric age spectrum differs from infection acquired later in life in a variety of ways, including modes of transmission, rates of spontaneous clearance or progression of fibrosis, the potential duration of chronic infection when acquired at birth, and significantly, available treatment options.4 While several reviews have recently been published regarding aspects of HCV infection in adults,3, 5, 6 our goal is to present a practical overview of the epidemiology, diagnosis, clinical features, natural history, and management of HCV infection in children and adolescents.
Epidemiology
The prevalence of HCV infection in children and adolescents has been reported to vary from 0.05%‐0.36% in the United States and Europe to 1.8%‐5.8% in certain developing countries.7 However, these reports likely underestimate the true prevalence since current ascertainment practices enable only a small fraction of children expected to be infected with HCV to be identified.8, 9
Six distinct HCV genotypes have been identified. In adults, HCV genotype 1, subtypes 1a, and 1b as well as genotype 2, subtypes 2a, 2b, and 2c represent the most common variants in Western countries. HCV genotype 3 is widely distributed in South and East Asia, with subtype 3a common among intravenous drug users from Europe; genotype 4 in North Africa and the Middle East; genotype 5 in South Africa; and genotype 6 in Asia.10 While all six genotypes have been identified in children, robust epidemiological reports on the disease burden in children and adolescents are lacking. When reported, it appears affected children often demonstrate similar regional distribution patterns that are described in adults. For example, genotypes 1‐3 dominate the HCV burden of both children and adults in the United States.11, 12, 13
Historically, HCV was considered to be a transfusion‐related disease in children and adolescents; however, with the advent of blood‐bank screening practices, no new pediatric cases of transfusion‐transmitted acute HCV infection have been detected in the United States since 1994.14 Consequently, mother to infant transmission during the perinatal period has emerged as the most common mode of acquisition of infection in children, accounting for approximately 60% of cases. Mechanisms leading to perinatal HCV transmission are currently not well understood. Likely risk factors include perinatal practices (fetal scalp monitoring and caesarean‐section delivery), extended exposure to maternal blood, high levels of HCV viremia during pregnancy, and co‐infection with human immunodeficiency virus (HIV).15, 16, 17, 18, 19 Although some studies have challenged several of these assumptions,20, 21 a recent meta‐analysis concluded that maternal HIV co‐infection is the most important determinant of the risk of perinatal transmission.15
While the rate of perinatal transmission of HCV has remained stable at 5%‐6%, from 2006 to 2012 the incidence of HCV infection among women of child‐bearing age increased 13% annually in nonurban counties and 5% annually in urban counties in the United States.22 This raises concern about an increasing number of pregnant women exposing their infants to HCV at birth. In parallel, we have noted a greater than 4‐fold increase in the number of HCV‐infected infants referred to our centers.
Unfortunately, even when maternal HCV infection is identified, most of the at‐risk children born to these women remain untested at 18 months of age,23 underscoring an important challenge. Currently, universal screening for HCV is not performed routinely in pregnant women and is reserved for those who are known to engage in “at‐risk” activities; this is highly likely to miss a significant number of infected children.24, 25 However, studies have shown that large‐scale antenatal screening programs can be feasible, effective, and affordable.26
Outside of the perinatal period, additional routes of acquisition of HCV infection in children result from engagement in high‐risk practices, such as intravenous drug abuse (IVDA), and intrafamilial transmission.
THE RECENT SURGE
IVDA is a significant and increasingly common route of HCV infection in adolescents and young adults. Mirroring the IVDA epidemic, there has been a significant increase in reported HCV infections over the last decade, with a recent study demonstrating a 364% increase in HCV infection among people 12 to 29 years of age living in the Appalachian region of the United States.22, 27, 28 These findings show that a new wave of young individuals will require HCV‐specific treatment or risk the development of progressive liver disease and its complications. Furthermore, these data suggest a potential surge in the rate of mother to infant HCV transmission with resultant increased disease burden among children.
HCV infection has been associated with other high‐risk activities, including receiving tattoos in an unregulated setting, intranasal cocaine use, and engaging in sexual practices that involve multiple partners and/or sexual activity with trauma.
HORIZONTAL TRANSMISSION
Intrafamilial transmission of HCV is a complex phenomenon with routes of transmission not established and the risk of infection uncertain.29 In the developing world, studies suggest that some HCV infections in children could not be fully accounted for by exposure to blood transfusions or unsafe injections, suggesting other modes of transmission.30, 31 Follow‐up studies have demonstrated that the robust intrafamilial component seen in HCV occurrences within families could, in part, be explained by specific genetic predisposition to persistent HCV infection.32 Future work will need to detect the exact routes of transmission in order to identify and implement preventative strategies.
Diagnosis
Blood tests available for the identification of HCV are the antibody assays for HCV (anti‐HCV) and the nucleic acid tests intended for detection and quantification of HCV RNA. In older children, the recommended testing sequence parallels adult guidelines and begins with an investigation for the presence of anti‐HCV. Current immunoassays for anti‐HCV are at least 97% sensitive and 99% specific4, 33, 34; importantly however, both false positive (patients with autoimmune disease, mononucleosis, pregnancy) and false negatives (patients with hypogammaglobulinemia or immunosuppressed patients) can occur.33 If the anti‐HCV is positive, HCV RNA quantification and genotype should be performed to confirm the infection. Knowledge of the HCV genotype is an important factor as it can determine both the specific antiviral regimen and the length of therapy.33
Challenges associated with HCV testing on a global scale include access to health care, inadequate laboratory capabilities, and a lack of data to guide country‐specific hepatitis testing and are significant barriers to the identification of affected children. Recent World Health Organization testing guidelines look to strengthen and expand current testing practices to address who and how to test for HCV infection when such obstacles exist.35
UNIQUE ASPECTS OF HCV DIAGNOSIS IN NEWBORNS/INFANTS
In general, testing for HCV infection should occur in all children suspected to be “at risk” (Table 1). However, unique to children is the sequence of testing recommended for infants born to mothers with HCV infection and who are in danger of becoming infected (perinatal transmission of HCV). Mothers infected with HCV will have circulating anti‐HCV immunoglobulin G, which crosses the placenta and can be measured in the serum of their infants. Maternal antibody can persist in the child for over a year, consequently testing for anti‐HCV in infants is not informative during this period. The American Academy of Pediatrics' recommendations are to delay measurement of anti‐HCV antibody until after 18 months of age.4 HCV RNA testing can reliably indicate perinatal transmission; however, infants should be at least 2 months old for this test to be reliable.4, 36 Retesting at 12 months should occur to confirm chronic HCV infection and to rule out the possibility of spontaneous seroconversion, defined as the absence of detectable HCV RNA on two occasions >6 months apart,37, 38 which occurs in 25%‐40% of infants infected via perinatal transmission.4, 39 In the absence of evidence suggesting active liver disease, delaying testing until 15‐18 months of age is likely to produce the clearest results in cases of suspected perinatal transmission.
Table 1.
CHILDREN AND ADOLESCENTS FOR WHOM SCREENING FOR HCV INFECTION SHOULD BE CONSIDERED
| • Children born to HCV‐infected mothers (after 18 months of age). |
| • Children and adolescents with intrafamilial exposure. |
| • Children and adolescents emigrating from a region with a high prevalence of HCV infection. |
| • Children and adolescents with signs or symptoms of hepatitis. |
| • Adolescents incarcerated in a correctional institution. |
| • Adolescents who received tattooing and body piercing in unregulated settings. |
| • Adolescents who have injected illicit drugs in the recent and remote past, including those who injected only once and do not consider themselves to be drug users. |
| • Children and adolescents with conditions associated with a high prevalence of HCV infection, including |
| ○ HIV infection |
| ○ Unexplained abnormal aminotransferase levels |
| ○ Adolescents who were recipients of transfusions or organ transplants before July 1992. |
| • Adolescents after a needle stick injury or mucosal exposure to HCV‐positive blood. |
Clinical Features
Although rare, clinically apparent acute HCV infection has been reported in children and adolescents in both isolated cases and in the setting of widespread outbreaks.40, 41 Few studies describe the features of acute HCV infection in children, although in adults, nonspecific symptoms of fatigue, jaundice, dyspepsia, and abdominal pain are often reported.42, 43, 44 At‐risk children presenting with these symptoms should be considered for testing. While perhaps more common in endemic areas, HCV‐induced fulminant hepatic failure is infrequent, with only a single case reported in the first 348 children enrolled into the Pediatric Acute Liver Failure Study Group database.41
More commonly, children and adolescents develop chronic hepatitis C infection, defined as the persistence of HCV RNA for at least 6 months.45, 46 The typical pattern of virologic, clinical, and serological events in children with perinatal infection is demonstrated in Fig. 1. Children with chronic hepatitis C infection are most often asymptomatic, although mild nonspecific symptoms can occur. In one study of children who acquired the infection in the perinatal period, approximately 10% were found to have hepatomegaly over the first 4 years of life; however, the same study confirmed the low overall prevalence of HCV‐related clinical signs and symptoms during the first 15 years of life.47 The majority of HCV‐infected children will have intermittent or persistent liver enzyme elevations. Importantly, aminotransferase levels do not correlate with histological severity.46 Accordingly, a liver biopsy is not necessary for most pediatric patients with HCV. The exceptions to this recommendation are patients in whom a concomitant or alternative diagnosis is being considered, if the biopsy may influence their treatment, or in patients with comorbid conditions where hepatotoxic medications should be avoided. Prior studies that have included liver biopsies have shown that for the majority of infected children, inflammation is mild with severe inflammation in only 3%; moderate fibrosis and cirrhosis are found in only 4% and 2%, respectively.48
Figure 1.
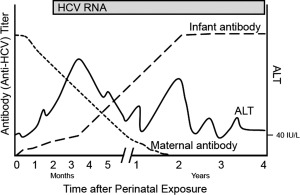
The pattern of virologic, clinical, and serological events in children following perinatal HCV infection. Following viral acquisition (time 0), there is an increase in the serum ALT level, indicating the onset of clinical hepatitis. The ALT level follows a chronic undulant pattern, reflecting ongoing infection. Maternal antibody to HCV can be detected in the serum of the newborn; however, the level begins to decline in the first weeks of life, with complete disappearance by 18‐24 months of age. Anti‐HCV produced by the infant appears shortly after infection; however, it is indistinguishable from maternal antibody. Polymerase chain reaction can indicate HCV infection within several weeks of exposure and will persist unless spontaneous viral clearance occurs. Abbreviations: ALT, alanine aminotransferase; RNA, ribonucleic acid.
Extrahepatic manifestations of chronic hepatitis C infection occur in 40%‐74% of adults during their lifetime.7 Similar manifestations, while described, are less prevalent in children with HCV infection. For example, membranoproliferative glomerulonephritis, the most common renal disease associated with HCV infection in adults,49 has been reported in only three children.50, 51, 52 Thyroid dysfunction and thyroid autoimmune disease is rare, but thyroid‐specific antibodies, subclinical hypothyroidism, and autoimmune thyroiditis have been described in children.53, 54, 55 Development of nonorgan‐specific autoantibodies, such as antinuclear antibodies, is well recognized,53, 54, 56, 57, 58 although their clinical significance is debated.54, 59 Finally, some manifestations, such as the cutaneous features of vasculitis and porphyria cutanea tarda described in adults, have not been reported in children.42, 60
Natural History of HCV Infection in Children and Adolescents
In children and adolescents, acute hepatitis due to HCV is not common and fulminant infection is rare. Chronic hepatitis C infection in children and adolescents can follow several different paths of progression with a variety of outcomes. One important outcome is the attainment of a spontaneous resolution of viremia (viral clearance).37, 38 Viral clearance is estimated to occur in approximately 20% of adults, while children have a slightly greater chance of viral clearance.61, 62, 63 In children with perinatal transmission, 25%‐40% may spontaneously undergo viral clearance, usually by age 2; this has been described as a resolution of neonatal HCV infection.4 Another 6%‐12% of those with chronic hepatitis C infection may clear the virus before adulthood.4, 39, 64, 65, 66 Spontaneous viral clearance, which is associated with biochemical remission of hepatitis, has been reported to occur more frequently in children with higher alanine aminotransferase levels in the first 2 years of life.30, 38, 66, 67, 68, 69, 70 Both host and viral factors have been associated with the attainment of spontaneous viral clearance; these include infection with genotype 3 and the interleukin 28B rs12979860 single‐nucleotide polymorphism.38, 67, 71, 72 Spontaneous viral clearance has historically been considered a permanent state and an essential “cure” of the HCV infection in the child; however, a recent case report described recurrence of viremia following seroconversion and suggests that a more nuanced approach to the care of these children may be warranted.64
In children with chronic hepatitis C infection who fail to clear the virus, liver disease is typically minor, with little evidence of progression. This pattern has been validated in multiple studies.4, 42, 46, 73, 74, 75, 76 One study included up to 35 years of follow‐up and established that HCV infection acquired in early life typically shows a slow progression and mild course and outcome in the absence of other risk factors, such as obesity.77
POTENTIAL FOR PROGRESSION
Hepatocellular damage with the development of fibrosis related to HCV infection in children can occur and tends to increase with age; however, advanced liver disease is uncommon before adulthood.77, 78, 79, 80, 81, 82 Nevertheless, disease progression can be hastened in the presence of certain risk factors. In adults, more rapid disease progression has been shown to be affected by viral load, serum aminotransferase levels, gender, ethnicity, obesity, toxins, and other environmental factors. Comorbidities, including hemolytic anemia, malignancy, immunosuppression, HIV, and hepatitis B virus (HBV) co‐infection and certain genetic factors, like single‐nucleotide polymorphisms, may also promote progression.83 Similarly, children with comorbid conditions, such as obesity, HIV, and HBV co‐infections, cancer, and anemia, are at risk for more severe disease.4, 84 In addition, high‐risk behaviors are associated with poor outcomes of disease, including alcohol use, IVDA, homelessness, and incarceration.70, 85, 86, 87, 88
Complications from chronic HCV‐related liver disease in children and adolescents, such as portal hypertension, ascites, variceal bleeding, and hepatocellular carcinoma, although uncommon, have been reported.2, 73, 89, 90, 91 Decompensated cirrhosis in children as young as 4 years of age has been described.48, 78, 91, 92 HCV infection in children has also been suggested to negatively affect both health‐related quality of life and cognitive functioning; however, these findings would need to be confirmed in larger cohorts.93, 94
Management
Given the rarity of acute HCV infection, there is a paucity of data determining when and how to initiate treatment in affected children. Therefore, this section will focus on the management of chronic HCV infection specific to children and adolescents.
The general goals of treatment in children mirror those of adults; mainly, to eradicate the virus, induce a remission of liver injury, prevent transmission, and ultimately avert and improve the outcomes relating to chronic inflammation and liver injury by decreasing or eliminating the development of fibrosis, cirrhosis, and hepatocellular carcinoma. The development of new antivirals (Table 2) has ushered in an era of well‐tolerated medications with high efficacy. Recent cure rates attained with the use of direct‐acting antivirals (DAA) in adults enables optimism. However, clinical trials in children are in their early stages (Table 3), and thus the pace of DAA approval for children and adolescents is lagging behind that set by adult studies and approvals.
Table 2.
CHARACTERISTICS OF DIRECT‐ACTING ANTIVIRAL AGENTS FOR HEPATITIS C VIRUS
| Protease Inhibitors | Polymerase Inhibitors | NS5A Inhibitors | |
|---|---|---|---|
| Viral enzyme target | NS3/4A | NS5B | NS5A |
| Mechanism(s) of action | Inhibit the NS3/4A enzyme involved in posttranslational processing and replication of HCV. |
2 classes: Nucleot(s)ide analogues target the catalytic site of NS5B and result in chain termination Nonnucleoside inhibitors allosteric inhibitors of NS5B |
Unknown. Thought to be related to viral replication and virion assembly |
| Drug | Simeprevir, Paritaprevir, Grazoprevir | Sofosbuvir, Dasabuvir | Ledipasvir, Daclatasvir, Ombitasvir, Elbasvir, Velpatasvir |
Table 3.
REGISTERED CLINICAL TRIALS OF DAAS IN CHILDREN AND ADOLESCENTS WITH HEPATITIS C INFECTION
| Clinicaltrials.gov | Drug | Phase | Status |
|---|---|---|---|
| NCT02249182 | Ledipasvir/Sofosbuvir ± Ribavirin | 2 | Recruiting |
| NCT02175758 | Sofosbuvir + Ribavirin | 2 | Recruiting |
| NCT02486406 | Ombitasvir ± Dasabuvir and ± Ribavirin | 3 | Recruiting |
| Paritaprevir ± Dasabuvir and ± Ribavirin | |||
| Ritonavir ± Dasabuvir and ± Ribavirin | |||
| NCT01701063 | Telaprevir + Peginterferon + Ribavirin | 1,2 | Terminated ‐ has results |
| NCT02985281 | Sofosbuvir + Ribavirin | 2,3 | Enrolling by invitation |
CURRENT STATUS OF HCV TREATMENT IN CHILDREN AND ADOLESCENTS
The new era of DAA therapy has enabled dramatic changes in the medical management of adults with HCV. Ever‐evolving treatment regimens are available that reliably achieve a >95% “real‐world” cure rate for all HCV genotypes.6 Efficacy has also been demonstrated in historically difficult to treat populations, such as patients with pretreatment cirrhosis, renal disease, patients previously exposed to antiviral therapy, prior null responders, those with HIV co‐infection, and patients following liver transplantation.95, 96, 97, 98, 99
Despite these advances, DAAs are not yet licensed for pediatric use. Currently, the only Food and Drug Administration‐approved options for children with chronic HCV infection are ribavirin (RBV) and pegylated‐interferon (PEG‐IFN). While sustained viral responses (SVRs) have improved over the last several decades from ∼16% with IFN monotherapy to >50% with the combination of RBV and PEG‐IFN, multiple studies have shown that SVR rates remain frustratingly low compared to adults with access to DAA regimens.6, 13, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116 Consequently, in the absence of approved DAA therapies in the United States, the chance of attaining SVR in HCV‐infected children and adolescents is little more than 50% (Table 4). Further complicating treatment decisions are the known side‐effect profiles of IFN and RBV. Adverse events, such as anemia, neutropenia, leukopenia, and thrombocytopenia, have been reported in up to 52% of those treated, leading to a discontinuation rate of 4%.103 Therapy has also been shown to negatively affect body weight, linear growth, and body composition, putting children at risk for developmental blunting.117 However, recent follow‐up studies have not observed any long‐term effects on height‐for‐age z scores that could be attributed to HCV treatment.118 Neuropsychiatric disturbances, such as mood alteration, irritability, agitation, and aggressive behavior, have been reported in up to 30% of children119; depression, anxiety, and suicidal ideation have been reported as well.13, 110
Table 4.
TREATMENT TRIALS OF HCV IN CHILDREN AND ADOLESCENTS
| Author, Year [ref] | Number Studied | Treatment Regimen | %SVR for Genotype 1/4 | %SVR for Genotype 2/3 |
|---|---|---|---|---|
| Wirth, 2002 [115] | 41 | IFN2b + ribavirin | 53 | 100 |
| Gonzalez‐Peralta, 2005 [112] | 118 | IFN2b + ribavirin | 36 | 84 |
| Wirth, 2005 [116] | 62 | Peg‐IFN2b + ribavirin | 48 | 100 |
| Jara, 2008 [107] | 30 | Peg‐IFN2b + ribavirin | 44 | 100 |
| Sokal, 2010 [113] | 65 | Peg‐IFN2a + ribavirin | 57 | 89 |
| Wirth, 2010 [110] | 107 | Peg‐IFN2b + ribavirin | 53 | 93 |
| Schwarz, 2011 [14] | 55 | Peg‐IFN2a + ribavirin | 47 | 80 |
| Wisniewska‐Ligier, 2013 [111] | 79 | Peg‐IFN2b + ribavirin | 44 | ‐ |
| Suzuki, 2016 [114] | 84 | Peg‐IFN2b + ribavirin | 72 | 100 |
Note: Table adapted from ref 117
Several studies have suggested that certain factors may predict a more favorable response to PEG‐IFN/RBV therapy, including host characteristics, such as the interleukin 28B polymorphisms, having genotype 2 or 3, and mode of transmission (iatrogenic versus vertical transmission).114, 119, 120, 121, 122 Ultimately, the current standard of care in the treatment of HCV‐infected children with IFN and RBV regimens with their well‐documented toxicities, inconvenient modes of administration (subcutaneous IFN injections), longer durations of treatment, and poor overall efficacy often leaves pediatric hepatologists searching for alternatives. More often than not, this results in the deferring of treatment in expectation of increased availability of DAAs in children.
Since there are no all‐oral DAA regimens that have been approved for children with chronic HCV infection, we conducted a phase 2, multicenter, open‐label study to evaluate the efficacy and safety of ledipasvir–sofosbuvir in 100 adolescents with chronic HCV genotype 1 infection.123 Each subject received a combination tablet of 90 mg ledipasvir and 400 mg sofosbuvir once daily for 12 weeks.123 The primary efficacy endpoint was the percentage of patients with a sustained virologic response 12 weeks posttreatment (SVR12). A majority of subjects (80%) were HCV treatment naïve, and 84% of subjects were infected through perinatal transmission. Overall, 98% of patients reached SVR12, and no patient had virologic failure. The 2 subjects who did not achieve SVR12 were lost to follow‐up either during or after treatment. No serious adverse events were reported; commonly reported events were headache, diarrhea, and fatigue. We also noted that the dose of ledipasvir−sofosbuvir currently used in adults was well tolerated in adolescents and had an appropriate pharmacokinetic profile.
MONITORING
As progression of HCV infection can occur in children and adolescents, it is appropriate to ensure consistent monitoring of these patients to ensure timely intervening measures can be taken. Sequential testing of serum aminotransferase concentrations and regular office visits to assess for evidence of disease progression or complications is suggested. In adults with chronic hepatitis C infection, recent advancements have enabled the validation of both circulating biomarker tests and vibration‐controlled transient elastography in determining the stage of fibrosis,3, 124, 125, 126, 127, 128 progression and regression of fibrosis,129, 130, 131 as well as their use in determining liver‐related complications and overall survival.132, 133 Validation of these technologies in children and adolescents is emerging; however, the low incidence of progressive hepatitis C infection in the pediatric population will require large cohorts with extended follow‐up to determine their efficacy. Ultimately, the most adept approach to fibrosis assessment in children and adolescents with chronic hepatitis C infection will likely combine biomarker assessment, physical exam findings, and vibration‐controlled transient elastography. In patients who demonstrate a more severe clinical course with the development of fibrosis, even with the current SVR outcomes with IFN/RBV, treatment may be warranted in hopes of interrupting disease progression. Furthermore, in patients with cirrhosis, hepatocellular carcinoma can occur and regular ultrasound and alpha fetoprotein surveillance should be considered.89
In addition, a “liver‐healthy lifestyle” should be discussed early in the course of HCV infection in children and adolescents, focusing on appropriate lifestyle choices, prevention of obesity, avoidance of alcohol, and proper medication management. Because of the high rate of severe hepatitis in patients with chronic liver disease from HCV infection who become co‐infected with hepatitis A or B virus, all patients should be immunized against hepatitis A and hepatitis B.
Liver transplantation for HCV‐related liver disease is rare in children and adolescents, accounting for less than 1% of cases. When transplantation is required, outcomes are generally good. In an analysis of the United Network for Organ Sharing database assessing outcomes in children who received a liver transplant for complications related to HCV, 1‐year and 3‐year graft survival rates were reported to be 89.7% and 76.2%, respectively, and 1‐year and 3‐year patient survival rates were 97.5% and 89.4%, respectively. Retransplantation, mainly due to disease recurrence, was reported as 10%.134
Future Directions
Despite the success of DAA agents in adults and the preliminary data that suggest similar safety and efficacy will be demonstrated in children, there remain additional avenues of pursuit in the march toward eradication of HCV worldwide. In addition to expanding pharmaceutical options and determining optimal antiviral regimens, strategies aimed at improving diagnosis, identifying patients who would benefit from treatment, and eliminating perinatal transmission from mother to child are needed to optimize the overall management of HCV in children. Finally, and in parallel, is the continued march toward the development of a broadly directed HCV vaccine that would target both humoral and cellular immune responses and assist with worldwide viral eradication.
The majority of children and adolescents with HCV remain undiagnosed; therefore enhanced efforts focused on case identification are needed so that patients can be appropriately managed. While universal screening for adults of a certain age is recommended,135 no such guidelines exist for children. One large study investigating the prevalence of HCV in urban children suggested that screening is not warranted.136 However, this study was undertaken prior to the explosive IVDA epidemic and the dramatic increase in HCV infection among young persons.22, 27, 28 Increased patient identification must be followed with a linkage to care that enables patients to receive evaluation and treatment by an experienced health care provider.137
Antenatal screening programs also represent an opportunity to possibly prevent the passage of viral infection from mother to child. Current antenatal screening programs for HCV are not standard of care, and cost effectiveness is debated.26, 138, 139 However, strategies aimed at preventing perinatal transmission will be impactful. Recent data have demonstrated that reductions in perinatal transmission of HCV can be achieved in select populations. Mothers with HCV–HIV co‐infection who were treated with combined anti‐retroviral therapy at the time of delivery demonstrated a lower rate of HCV transmission to their newborns than what has historically been described.140 Additional advances seen in the prevention of mother to child transmission of hepatitis B infection during pregnancy141 suggests that future efficacy and safety data will be informative as it relates to newer anti‐HCV therapies during pregnancy and the prevention of perinatal transmission of the HCV.
VACCINE UPDATE
Improved treatment regimens, greater disease awareness, improved access to care, and lower cost will undoubtedly decrease the global disease burden of HCV infection. However, no virus to date has been globally eradicated without the development of a prophylactic vaccine. A preventative vaccine is needed to stop HCV transmission to uninfected individuals and to those who are cured with DAA but remain at risk for re‐exposure and persistence of infection.5 Major obstacles to HCV vaccine development are the diversity of the virus, the ability of the virus to evade the immune response in infected individuals with high rates of mutation, and development of “quasispecies,” which are distinct but closely related HCV variants that can be present in a single individual.5, 137, 142 Additional immune‐evading strategies that have been identified include antibody avoidance, cytotoxic T lymphocyte escape, and a failure to initiate an appropriate T cell response during the beginning of infection, among others.143, 144, 145, 146, 147, 148 Despite these challenges, several investigations have demonstrated success in preclinical animal studies showing induction of both humoral and cellular immunity against HCV.149, 150 Promising preliminary results have been demonstrated in trials conducted in humans based in part on these findings.151 As a result, a phase II human clinical trial (https://clinicaltrials.gov/ct2/show/NCT01296451) is underway. Ultimately, the path to a successful preventative vaccine requires comprehensive evaluation of all aspects of protective immunity, innovative application of state‐of‐the‐art vaccine technology, and properly designed clinical trials that can affirm definitive endpoints of safety and efficacy.
Author names in bold designate shared co‐first authorship.
Dr. Balistreri is an investigator for Gilead regarding clinical trials in the treatment of hepatitis C virus.
REFERENCES
- 1. Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up‐date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol 2016;22:7824‐7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.[No authors listed]. Hepatitis C: global ambition, national realities. Lancet 2016;387:1970. [DOI] [PubMed] [Google Scholar]
- 3. AASLD/IDSA HCV Guidance Panel . Hepatitis C guidance: AASLD‐IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015;62:932‐954. [DOI] [PubMed] [Google Scholar]
- 4. Mack CL, Gonzalez‐Peralta RP, Gupta N, Leung D, Narkewicz MR, Roberts EA, et al. NASPGHAN practice guidelines: Diagnosis and management of hepatitis C infection in infants, children, and adolescents. J Pediatr Gastroenterol Nutr 2012;54:838‐855. [DOI] [PubMed] [Google Scholar]
- 5. Moradpour D, Grakoui A, Manns MP. Future landscape of hepatitis C research ‐ basic, translational and clinical perspectives. J Hepatol 2016;65:S143‐155. [DOI] [PubMed] [Google Scholar]
- 6. Rehermann B. HCV in 2015: advances in hepatitis C research and treatment. Nat Rev Gastroenterol Hepatol 2016;13:70‐72. [DOI] [PubMed] [Google Scholar]
- 7. El‐Shabrawi MH, Kamal NM. Burden of pediatric hepatitis C. World J Gastroenterol 2013;19:7880‐7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delgado‐Borrego A, Smith L, Jonas MM, Hall CA, Negre B, Jordan SH, et al. Expected and actual case ascertainment and treatment rates for children infected with hepatitis C in Florida and the United States: epidemiologic evidence from statewide and nationwide surveys. J Pediatr 2012;161:915‐921. [DOI] [PubMed] [Google Scholar]
- 9. Raynes‐Greenow C, Polis S, Elliott E, Hardikar W, Kesson A, Kaldor J, et al. Childhood hepatitis C virus infection: an Australian national surveillance study of incident cases over five years. J Paediatr Child Health 2015;51:1115‐1120. [DOI] [PubMed] [Google Scholar]
- 10. Kuiken C, Simmonds P. Nomenclature and numbering of the hepatitis C virus. Methods Mol Biol 2009;510:33‐53. [DOI] [PubMed] [Google Scholar]
- 11. Liakina V, Hamid S, Tanaka J, Olafsson S, Sharara AI, Alavian SM, et al. Historical epidemiology of hepatitis C virus (HCV) in select countries ‐ volume 3. J Viral Hepat 2015;22 Suppl 4:4‐20. [DOI] [PubMed] [Google Scholar]
- 12. Lowe NJ, Rizk D, Grimes P, Billips M, Pincus S. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker‐skinned patients. Clin Ther 1998;20:945‐959. [DOI] [PubMed] [Google Scholar]
- 13. Schwarz KB, Gonzalez‐Peralta RP, Murray KF, Molleston JP, Haber BA, Jonas MM, et al. The combination of ribavirin and peginterferon is superior to peginterferon and placebo for children and adolescents with chronic hepatitis C. Gastroenterology 2011;140:450‐458e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention . Viral Hepatitis Statistics and Surveillance. Atlanta, GA; Centers for Disease Control and Prevention, 2009. http://www.cdc.gov/hepatitis/Statistics/. Accessed July 22, 2013. [Google Scholar]
- 15. Benova L, Mohamoud YA, Calvert C, Abu‐Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta‐analysis. Clin Infect Dis 2014;59:765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dal Molin G, D'Agaro P, Ansaldi F, Ciana G, Fertz C, Alberico S, et al. Mother‐to‐infant transmission of hepatitis C virus: rate of infection and assessment of viral load and IgM anti‐HCV as risk factors. J Med Virol 2002;67:137‐142. [DOI] [PubMed] [Google Scholar]
- 17. European Paediatric Hepatitis C Virus Network . A significant sex‐‐but not elective cesarean section‐‐effect on mother‐to‐child transmission of hepatitis C virus infection. J Infect Dis 2005;192:1872‐1879. [DOI] [PubMed] [Google Scholar]
- 18. Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis 2005;192:1880‐1889. [DOI] [PubMed] [Google Scholar]
- 19. Wen JW, Haber BA. Maternal‐fetal transmission of hepatitis C infection: what is so special about babies? J Pediatr Gastroenterol Nutr 2014;58:278‐282. [DOI] [PubMed] [Google Scholar]
- 20. Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology 2000;31:751‐755. [DOI] [PubMed] [Google Scholar]
- 21. Resti M, Azzari C, Mannelli F, Moriondo M, Novembre E, de Martino M, et al. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV‐1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ 1998;317:437‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suryaprasad AG, White JZ, Xu F, Eichler BA, Hamilton J, Patel A, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006‐2012. Clin Infect Dis 2014;59:1411‐1419. [DOI] [PubMed] [Google Scholar]
- 23. Kuncio DE, Newbern EC, Johnson CC, Viner KM. Failure to test and identify perinatally infected children born to hepatitis C Virus‐infected women. Clin Infect Dis 2016;62:980‐985. [DOI] [PubMed] [Google Scholar]
- 24. American College of Obstetricians and Gynecologists . ACOG Practice Bulletin No. 86: viral hepatitis in pregnancy. Obstet Gynecol 2007;110:941‐956. [DOI] [PubMed] [Google Scholar]
- 25. Kanninen TT, Dieterich D, Asciutti S. HCV vertical transmission in pregnancy: new horizons in the era of DAAs. Hepatology 2015;62:1656‐1658. [DOI] [PubMed] [Google Scholar]
- 26. Selvapatt N, Ward T, Bailey H, Bennett H, Thorne C, See LM, et al. Is antenatal screening for hepatitis C virus cost‐effective? A decade's experience at a London centre. J Hepatol 2015;63:797‐804. [DOI] [PubMed] [Google Scholar]
- 27. Centers for Disease Control and Prevention . Hepatitis C virus infection among adolescents and young adults:Massachusetts, 2002‐2009. MMWR Morb Mortal Wkly Rep 2011;60:537‐541. [PubMed] [Google Scholar]
- 28. Zibbell JE, Iqbal K, Patel RC, Suryaprasad A, Sanders KJ, Moore‐Moravian L, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years ‐ Kentucky, Tennessee, Virginia, and West Virginia, 2006‐2012. MMWR Morb Mortal Wkly Rep 2015;64:453‐458. [PMC free article] [PubMed] [Google Scholar]
- 29. Indolfi G, Nesi A, Resti M. Intrafamilial transmission of hepatitis C virus. J Med Virol 2013;85:608‐614. [DOI] [PubMed] [Google Scholar]
- 30. Arafa N, El Hoseiny M, Rekacewicz C, Bakr I, El‐Kafrawy S, El Daly M, et al. Changing pattern of hepatitis C virus spread in rural areas of Egypt. J Hepatol 2005;43:418‐424. [DOI] [PubMed] [Google Scholar]
- 31. Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005;5:558‐567. [DOI] [PubMed] [Google Scholar]
- 32. Plancoulaine S, Mohamed MK, Arafa N, Bakr I, Rekacewicz C, Tregouet DA, et al. Dissection of familial correlations in hepatitis C virus (HCV) seroprevalence suggests intrafamilial viral transmission and genetic predisposition to infection. Gut 2008;57:1268‐1274. [DOI] [PubMed] [Google Scholar]
- 33. Ciotti M, D'Agostini C, Marrone A. Advances in the diagnosis and monitoring of hepatitis C virus infection. Gastroenterology Res 2013;6:161‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Leuw P, Sarrazin C, Zeuzem S. How to use virological tools for the optimal management of chronic hepatitis C. Liver Int 2011;31 Suppl 1:3‐12. [DOI] [PubMed] [Google Scholar]
- 35. Easterbrook PJ, WHO Guidelines Development Group . Who to test and how to test for chronic hepatitis C infection ‐ 2016 WHO testing guidance for low‐ and middle‐income countries. J Hepatol 2016;65:S46‐66. [DOI] [PubMed] [Google Scholar]
- 36. Polywka S, Pembrey L, Tovo PA, Newell ML. Accuracy of HCV‐RNA PCR tests for diagnosis or exclusion of vertically acquired HCV infection. J Med Virol 2006;78:305‐310. [DOI] [PubMed] [Google Scholar]
- 37. Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet 2008;372:321‐332. [DOI] [PubMed] [Google Scholar]
- 38. Resti M, Bortolotti F, Vajro P, Maggiore G, Committee of Hepatology of the Italian Society of Pediatric Gastroenterology and Hepatology . Guidelines for the screening and follow‐up of infants born to anti‐HCV positive mothers. Dig Liver Dis 2003;35:453‐457. [DOI] [PubMed] [Google Scholar]
- 39. Sheiko MA, Golden‐Mason L, Giugliano S, Hurtado CW, Mack CL, Narkewicz MR, et al. CD4+ and CD8+ T cell activation in children with hepatitis C. J Pediatr 2016;170:142‐148 e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jonas MM, Baron MJ, Bresee JS, Schneider LC. Clinical and virologic features of hepatitis C virus infection associated with intravenous immunoglobulin. Pediatrics 1996;98:211‐215. [PubMed] [Google Scholar]
- 41. Squires RH, Jr. , Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr 2006;148:652‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. El‐Guindi MA. Hepatitis C viral infection in children: updated review. Pediatr Gastroenterol Hepatol Nutr 2016;19:83‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Santantonio T, Sinisi E, Guastadisegni A, Casalino C, Mazzola M, Gentile A, et al. Natural course of acute hepatitis C: a long‐term prospective study. Dig Liver Dis 2003;35:104‐113. [DOI] [PubMed] [Google Scholar]
- 44. Santantonio T, Wiegand J, Gerlach JT. Acute hepatitis C: current status and remaining challenges. J Hepatol 2008;49:625‐633. [DOI] [PubMed] [Google Scholar]
- 45. Davison SM, Mieli‐Vergani G, Sira J, Kelly DA. Perinatal hepatitis C virus infection: diagnosis and management. Arch Dis Child 2006;91:781‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mohan N, Gonzalez‐Peralta RP, Fujisawa T, Chang MH, Heller S, Jara P, et al. Chronic hepatitis C virus infection in children. J Pediatr Gastroenterol Nutr 2010;50:123‐131. [DOI] [PubMed] [Google Scholar]
- 47. European Paediatric Hepatitis C Virus Network . Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis 2005;41:45‐51. [DOI] [PubMed] [Google Scholar]
- 48. Goodman ZD, Makhlouf HR, Liu L, Balistreri W, Gonzalez‐Peralta RP, Haber B, et al. Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds‐C Trial. Hepatology 2008;47:836‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zignego AL, Ferri C, Pileri SA, Caini P, Bianchi FB, Italian Association of the Study of Liver Commission on Extrahepatic Manifestations of HCV infection . Extrahepatic manifestations of hepatitis C virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis 2007;39:2‐17. [DOI] [PubMed] [Google Scholar]
- 50. Matsumoto S, Nakajima S, Nakamura K, Etani Y, Hirai H, Shimizu N, et al. Interferon treatment on glomerulonephritis associated with hepatitis C virus. Pediatr Nephrol 2000;15:271‐273. [DOI] [PubMed] [Google Scholar]
- 51. Romas E, Power DA, Machet D, Powell H, d'Apice AJ. Membranous glomerulonephritis associated with hepatitis C virus infection in an adolescent. Pathology 1994;26:399‐402. [DOI] [PubMed] [Google Scholar]
- 52. Sugiura T, Yamada T, Kimpara Y, Fujita N, Goto K, Koyama N. Effects of pegylated interferon alpha‐2a on hepatitis‐C‐virus‐associated glomerulonephritis. Pediatr Nephrol 2009;24:199‐202. [DOI] [PubMed] [Google Scholar]
- 53. Gehring S, Kullmer U, Koeppelmann S, Gerner P, Wintermeyer P, Wirth S. Prevalence of autoantibodies and the risk of autoimmune thyroid disease in children with chronic hepatitis C virus infection treated with interferon‐alpha. World J Gastroenterol 2006;12:5787‐5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Indolfi G, Bartolini E, Olivito B, Azzari C, Resti M. Autoimmunity and extrahepatic manifestations in treatment‐naive children with chronic hepatitis C virus infection. Clin Dev Immunol 2012;2012:785627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Indolfi G, Stagi S, Bartolini E, Salti R, de Martino M, Azzari C, et al. Thyroid function and anti‐thyroid autoantibodies in untreated children with vertically acquired chronic hepatitis C virus infection. Clin Endocrinol (Oxf) 2008;68:117‐121. [DOI] [PubMed] [Google Scholar]
- 56. Bortolotti F, Vajro P, Balli F, Giacchino R, Crivellaro C, Barbera C, et al. Non‐organ specific autoantibodies in children with chronic hepatitis C. J Hepatol 1996;25:614‐620. [DOI] [PubMed] [Google Scholar]
- 57. Gregorio GV, Pensati P, Iorio R, Vegnente A, Mieli‐Vergani G, Vergani D. Autoantibody prevalence in children with liver disease due to chronic hepatitis C virus (HCV) infection. Clin Exp Immunol 1998;112:471‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muratori P, Muratori L, Verucchi G, Attard L, Bianchi FB, Lenzi M. Non‐organ‐specific autoantibodies in children with chronic hepatitis C: clinical significance and impact on interferon treatment. Clin Infect Dis 2003;37:1320‐1326. [DOI] [PubMed] [Google Scholar]
- 59. Bogdanos DP, Mieli‐Vergani G, Vergani D. Non‐organ‐specific autoantibodies in hepatitis C virus infection: do they matter? Clin Infect Dis 2005;40:508‐510. [DOI] [PubMed] [Google Scholar]
- 60. Akhter A, Said A. Cutaneous manifestations of viral hepatitis. Curr Infect Dis Rep 2015;17:452. [DOI] [PubMed] [Google Scholar]
- 61. Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000;284:450‐456. [DOI] [PubMed] [Google Scholar]
- 62. Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long‐term follow‐up after acute hepatitis C infection. Hepatology 1999;29:908‐914. [DOI] [PubMed] [Google Scholar]
- 63. Wang CC, Krantz E, Klarquist J, Krows M, McBride L, Scott EP, et al. Acute hepatitis C in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J Infect Dis 2007;196:1474‐1482. [DOI] [PubMed] [Google Scholar]
- 64. Indolfi G, Mangone G, Bartolini E, Moriondo M, Azzari C, Resti M. Hepatitis C viraemia after apparent spontaneous clearance in a vertically infected child. Lancet 2016;387:1967‐1968. [DOI] [PubMed] [Google Scholar]
- 65. Iorio R, Giannattasio A, Sepe A, Terracciano LM, Vecchione R, Vegnente A. Chronic hepatitis C in childhood: an 18‐year experience. Clin Infect Dis 2005;41:1431‐1437. [DOI] [PubMed] [Google Scholar]
- 66. Yeung LT, To T, King SM, Roberts EA. Spontaneous clearance of childhood hepatitis C virus infection. J Viral Hepat 2007;14:797‐805. [DOI] [PubMed] [Google Scholar]
- 67. Garazzino S, Calitri C, Versace A, Alfarano A, Scolfaro C, Bertaina C, et al. Natural history of vertically acquired HCV infection and associated autoimmune phenomena. Eur J Pediatr 2014;173:1025‐1031. [DOI] [PubMed] [Google Scholar]
- 68. Tovo PA, Calitri C, Scolfaro C, Gabiano C, Garazzino S. Vertically acquired hepatitis C virus infection: correlates of transmission and disease progression. World J Gastroenterol 2016;22:1382‐1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Farmand S, Wirth S, Loffler H, Woltering T, Kenzel S, Lainka E, et al. Spontaneous clearance of hepatitis C virus in vertically infected children. Eur J Pediatr 2012;171:253‐258. [DOI] [PubMed] [Google Scholar]
- 70. Serra MA, Escudero A, Rodriguez F, del Olmo JA, Rodrigo JM. Effect of hepatitis C virus infection and abstinence from alcohol on survival in patients with alcoholic cirrhosis. J Clin Gastroenterol 2003;36:170‐174. [DOI] [PubMed] [Google Scholar]
- 71. Indolfi G, Mangone G, Bartolini E, Nebbia G, Calvo PL, Moriondo M, et al. Comparative analysis of rs12979860 SNP of the IFNL3 gene in children with hepatitis C and ethnic matched controls using 1000 Genomes Project data. PLoS One 2014;9:e85899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Indolfi G, Mangone G, Calvo PL, Bartolini E, Regoli M, Serranti D, et al. Interleukin 28B rs12979860 single‐nucleotide polymorphism predicts spontaneous clearance of hepatitis C virus in children. J Pediatr Gastroenterol Nutr 2014;58:666‐668. [DOI] [PubMed] [Google Scholar]
- 73. Bortolotti F, Verucchi G, Camma C, Cabibbo G, Zancan L, Indolfi G, et al. Long‐term course of chronic hepatitis C in children: from viral clearance to end‐stage liver disease. Gastroenterology 2008;134:1900‐1907. [DOI] [PubMed] [Google Scholar]
- 74. Jonas MM. Children with hepatitis C. Hepatology 2002;36:S173‐178. [DOI] [PubMed] [Google Scholar]
- 75. Lee CK, Jonas MM. Hepatitis C: Issues in Children. Gastroenterol Clin North Am 2015;44:901‐909. [DOI] [PubMed] [Google Scholar]
- 76. Zein NN. Hepatitis C in children: recent advances. Curr Opin Pediatr 2007;19:570‐574. [DOI] [PubMed] [Google Scholar]
- 77. Casiraghi MA, De Paschale M, Romano L, Biffi R, Assi A, Binelli G, et al. Long‐term outcome (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology 2004;39:90‐96. [DOI] [PubMed] [Google Scholar]
- 78. Birnbaum AH, Shneider BL, Moy L. Hepatitis C in children. N Engl J Med 2000;342:290‐291. [DOI] [PubMed] [Google Scholar]
- 79. Guido M, Bortolotti F, Leandro G, Jara P, Hierro L, Larrauri J, et al. Fibrosis in chronic hepatitis C acquired in infancy: is it only a matter of time? Am J Gastroenterol 2003;98:660‐663. [DOI] [PubMed] [Google Scholar]
- 80. Matsuoka S, Tatara K, Hayabuchi Y, Taguchi Y, Mori K, Honda H, et al. Serologic, virologic, and histologic characteristics of chronic phase hepatitis C virus disease in children infected by transfusion. Pediatrics 1994;94:919‐922. [PubMed] [Google Scholar]
- 81. Minola E, Prati D, Suter F, Maggiolo F, Caprioli F, Sonzogni A, et al. Age at infection affects the long‐term outcome of transfusion‐associated chronic hepatitis C. Blood 2002;99:4588‐4591. [DOI] [PubMed] [Google Scholar]
- 82. Stallings‐Smith S, Krull KR, Brinkman TM, Hudson MM, Ojha RP. Long‐term follow‐up for incident cirrhosis among pediatric cancer survivors with hepatitis C virus infection. J Clin Virol 2015;71:18‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Maasoumy B, Wiegand SB, Jaroszewicz J, Bremer B, Lehmann P, Deterding K, et al. Hepatitis B core‐related antigen (HBcrAg) levels in the natural history of hepatitis B virus infection in a large European cohort predominantly infected with genotypes A and D. Clin Microbiol Infect 2015;21:606 e601‐610. [DOI] [PubMed] [Google Scholar]
- 84. Cesaro S, Bortolotti F, Petris MG, Brugiolo A, Guido M, Carli M. An updated follow‐up of chronic hepatitis C after three decades of observation in pediatric patients cured of malignancy. Pediatr Blood Cancer 2010;55:108‐112. [DOI] [PubMed] [Google Scholar]
- 85. Beech BM, Myers L, Beech DJ. Hepatitis B and C infections among homeless adolescents. Fam Community Health 2002;25:28‐36. [DOI] [PubMed] [Google Scholar]
- 86. Murray KF, Richardson LP, Morishima C, Owens JW, Gretch DR. Prevalence of hepatitis C virus infection and risk factors in an incarcerated juvenile population: a pilot study. Pediatrics 2003;111:153‐157. [DOI] [PubMed] [Google Scholar]
- 87. Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis 2009;200:1216‐1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wise M, Finelli L, Sorvillo F. Prognostic factors associated with hepatitis C disease: a case‐control study utilizing U.S. multiple‐cause‐of‐death data. Public Health Rep 2010;125:414‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gonzalez‐Peralta RP, Langham MR, Jr. , Andres JM, Mohan P, Colombani PM, Alford MK, et al. Hepatocellular carcinoma in 2 young adolescents with chronic hepatitis C. J Pediatr Gastroenterol Nutr 2009;48:630‐635. [DOI] [PubMed] [Google Scholar]
- 90. Jara P, Resti M, Hierro L, Giacchino R, Barbera C, Zancan L, et al. Chronic hepatitis C virus infection in childhood: clinical patterns and evolution in 224 white children. Clin Infect Dis 2003;36:275‐280. [DOI] [PubMed] [Google Scholar]
- 91. Rumbo C, Fawaz RL, Emre SH, Suchy FJ, Kerkar N, Morotti RA, et al. Hepatitis C in children: a quaternary referral center perspective. J Pediatr Gastroenterol Nutr 2006;43:209‐216. [DOI] [PubMed] [Google Scholar]
- 92. Badizadegan K, Jonas MM, Ott MJ, Nelson SP, Perez‐Atayde AR. Histopathology of the liver in children with chronic hepatitis C viral infection. Hepatology 1998;28:1416‐1423. [DOI] [PubMed] [Google Scholar]
- 93. Nydegger A, Srivastava A, Wake M, Smith AL, Hardikar W. Health‐related quality of life in children with hepatitis C acquired in the first year of life. J Gastroenterol Hepatol 2008;23:226‐230. [DOI] [PubMed] [Google Scholar]
- 94. Rodrigue JR, Balistreri W, Haber B, Jonas MM, Mohan P, Molleston JP, et al. Impact of hepatitis C virus infection on children and their caregivers: quality of life, cognitive, and emotional outcomes. J Pediatr Gastroenterol Nutr 2009;48:341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R, Jr. , et al. An interferon‐free antiviral regimen for HCV after liver transplantation. N Engl J Med 2014;371:2375‐2382. [DOI] [PubMed] [Google Scholar]
- 96. Lawitz E, Gane E, Pearlman B, Tam E, Ghesquiere W, Guyader D, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK‐5172) and elbasvir (MK‐8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C‐WORTHY): a randomised, open‐label phase 2 trial. Lancet 2015;385:1075‐1086. [DOI] [PubMed] [Google Scholar]
- 97. Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT‐450/r‐ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014;370:1973‐1982. [DOI] [PubMed] [Google Scholar]
- 98. Sulkowski M, Hezode C, Gerstoft J, Vierling JM, Mallolas J, Pol S, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK‐5172) and elbasvir (MK‐8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono‐infection and HIV/hepatitis C virus co‐infection (C‐WORTHY): a randomised, open‐label phase 2 trial. Lancet 2015;385:1087‐1097. [DOI] [PubMed] [Google Scholar]
- 99. Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourliere M, et al. Retreatment of HCV with ABT‐450/r‐ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014;370:1604‐1614. [DOI] [PubMed] [Google Scholar]
- 100. Abdel‐Hady M, Bansal S, Davison SM, Brown M, Tizzard SA, Mulla S, et al. Treatment of chronic viral hepatitis C in children and adolescents: UK experience. Arch Dis Child 2014;99:505‐510. [DOI] [PubMed] [Google Scholar]
- 101. Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370:1889‐1898. [DOI] [PubMed] [Google Scholar]
- 102. Curry MP, O'Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med 2015;373:2618‐2628. [DOI] [PubMed] [Google Scholar]
- 103. Druyts E, Thorlund K, Wu P, Kanters S, Yaya S, Cooper CL, et al. Efficacy and safety of pegylated interferon alfa‐2a or alfa‐2b plus ribavirin for the treatment of chronic hepatitis C in children and adolescents: a systematic review and meta‐analysis. Clin Infect Dis 2013;56:961‐967. [DOI] [PubMed] [Google Scholar]
- 104. Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015;373:2599‐2607. [DOI] [PubMed] [Google Scholar]
- 105. Foster GR, Afdhal N, Roberts SK, Brau N, Gane EJ, Pianko S, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3infection. N Engl J Med 2015;373:2608‐2617. [DOI] [PubMed] [Google Scholar]
- 106. Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 2013;368:34‐44. [DOI] [PubMed] [Google Scholar]
- 107. Jara P, Hierro L, de la Vega A, Diaz C, Camarena C, Frauca E, et al. Efficacy and safety of peginterferon‐alpha2b and ribavirin combination therapy in children with chronic hepatitis C infection. Pediatr Infect Dis J 2008;27:142‐148. [DOI] [PubMed] [Google Scholar]
- 108. Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed‐dose combination with and without ribavirin in treatment‐naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open‐label, randomised, phase 2 trial. Lancet 2014;383:515‐523. [DOI] [PubMed] [Google Scholar]
- 109. Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology 2014;146:1176‐1192. [DOI] [PubMed] [Google Scholar]
- 110. Wirth S, Ribes‐Koninckx C, Calzado MA, Bortolotti F, Zancan L, Jara P, et al. High sustained virologic response rates in children with chronic hepatitis C receiving peginterferon alfa‐2b plus ribavirin. J Hepatol 2010;52:501‐507. [DOI] [PubMed] [Google Scholar]
- 111. Wisniewska‐Ligier M, Pawlowska M, Pilarczyk M, Halota W, Wozniakowska‐Gesicka T. Efficacy of pegylated interferon alpha‐2b and ribavirin in chronic hepatitis C virus (genotypes 1 and 4) infection. J Pediatr Gastroenterol Nutr 2013;57:694‐699. [DOI] [PubMed] [Google Scholar]
- 112. Gonzalez‐Peralta RP, Kelly DA, Haber B, Molleston J, Murray KF, Jonas MM, et al. Interferon alfa‐2b in combination with ribavirin for the treatment of chronic hepatitis C in children: efficacy, safety, and pharmacokinetics. Hepatology 2005;42:1010‐1018. [DOI] [PubMed] [Google Scholar]
- 113. Sokal EM, Bourgois A, Stephenne X, Silveira T, Porta G, Gardovska D, et al. Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection in children and adolescents. J Hepatol 2010;52:827‐831. [DOI] [PubMed] [Google Scholar]
- 114. Suzuki M, Tajiri H, Tanaka Y, Takano T, Miyoshi Y, Murakami J, et al. Peginterferon therapy in children with chronic hepatitis C: a nationwide, multicenter study in Japan, 2004‐2013. J Pediatr Gastroenterol Nutr 2016;63:88‐93. [DOI] [PubMed] [Google Scholar]
- 115. Wirth S, Lang T, Gehring S, Gerner P. Recombinant alfa‐interferon plus ribavirin therapy in children and adolescents with chronic hepatitis C. Hepatology 2002;36:1280‐1284. [DOI] [PubMed] [Google Scholar]
- 116. Wirth S, Pieper‐Boustani H, Lang T, Ballauff A, Kullmer U, Gerner P, et al. Peginterferon alfa‐2b plus ribavirin treatment in children and adolescents with chronic hepatitis C. Hepatology 2005;41:1013‐1018. [DOI] [PubMed] [Google Scholar]
- 117. Jonas MM, Balistreri W, Gonzalez‐Peralta RP, Haber B, Lobritto S, Mohan P, et al. Pegylated interferon for chronic hepatitis C in children affects growth and body composition: results from the pediatric study of hepatitis C (PEDS‐C) trial. Hepatology 2012;56:523‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jonas MM, Schwarz KB, Gonzalez‐Peralta R, Lobritto S, Molleston JP, Murray KF, et al. Long‐term growth outcomes in children treated for chronic hepatitis C. J Pediatr 2014;165:1252‐1254. [DOI] [PubMed] [Google Scholar]
- 119. Granot E, Sokal EM. Hepatitis C virus in children: deferring treatment in expectation of direct‐acting antiviral agents. Isr Med Assoc J 2015;17:707‐711. [PubMed] [Google Scholar]
- 120. Schwarz KB, Molleston JP, Jonas MM, Wen J, Murray KF, Rosenthal P, et al. Durability of response in children treated with pegylated interferon alfa [corrected] 2a +/‐ ribavirin for chronic hepatitis C. J Pediatr Gastroenterol Nutr 2016;62:93‐96. [DOI] [PubMed] [Google Scholar]
- 121. Zhong YW, Zhang HF, Shi YM, Li YL, Chu F, Xu ZQ, et al. IL28B SNP rs12979860 is the critical predictor for sustained viral response in Chinese children aged 1 to 6 years with chronic hepatitis C. Int J Biol Sci 2016;12:1357‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zhu SS, Zeng QL, Dong Y, Xu ZQ, Wang LM, Chen DW, et al. Interferon‐alpha plus ribavirin yields 98% sustained virologic response in children aged 1‐5 years with iatrogenic chronic hepatitis C. Hepatol Int 2015;9:578‐585. [DOI] [PubMed] [Google Scholar]
- 123. Balistreri WF, Murray KF, Rosenthal P, Bansal S, Chuan‐Hao L, Kersey K, et al. The safety and effectiveness of ledipasvir‐sofosbuvir in adolescents 12 to 17 years old with hepatitis C virus genotype 1 infection. Hepatology 2016; doi: 10.1002/hep.28995. [DOI] [PubMed] [Google Scholar]
- 124. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Ann Intern Med 2013;159:372. [DOI] [PubMed] [Google Scholar]
- 125. European Association for Study of Liver . EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2014;60:392‐420. [DOI] [PubMed] [Google Scholar]
- 126. Poynard T, Morra R, Halfon P, Castera L, Ratziu V, Imbert‐Bismut F, et al. Meta‐analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Steadman R, Myers RP, Leggett L, Lorenzetti D, Noseworthy T, Rose S, et al. A health technology assessment of transient elastography in adult liver disease. Can J Gastroenterol 2013;27:149‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta‐analysis of diagnostic accuracy. J Hepatol 2011;54:650‐659. [DOI] [PubMed] [Google Scholar]
- 129. Poynard T, Moussalli J, Munteanu M, Thabut D, Lebray P, Rudler M, et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol 2013;59:675‐683. [DOI] [PubMed] [Google Scholar]
- 130. Poynard T, Munteanu M, Deckmyn O, Ngo Y, Drane F, Castille JM, et al. Validation of liver fibrosis biomarker (FibroTest) for assessing liver fibrosis progression: proof of concept and first application in a large population. J Hepatol 2012;57:541‐548. [DOI] [PubMed] [Google Scholar]
- 131. Poynard T, Ngo Y, Munteanu M, Thabut D, Massard J, Moussalli J, et al. Biomarkers of liver injury for hepatitis clinical trials: a meta‐analysis of longitudinal studies. Antivir Ther 2010;15:617‐631. [DOI] [PubMed] [Google Scholar]
- 132. Poynard T, Vergniol J, Ngo Y, Foucher J, Munteanu M, Merrouche W, et al. Staging chronic hepatitis C in seven categories using fibrosis biomarker (FibroTest) and transient elastography (FibroScan). J Hepatol 2014;60:706‐714. [DOI] [PubMed] [Google Scholar]
- 133. Vergniol J, Foucher J, Terrebonne E, Bernard PH, le Bail B, Merrouche W, et al. Noninvasive tests for fibrosis and liver stiffness predict 5‐year outcomes of patients with chronic hepatitis C. Gastroenterology 2011;140:1970‐1979, 1979 e1971‐1973. [DOI] [PubMed] [Google Scholar]
- 134. Gupta M, Bahirwani R, Levine MH, Malik S, Goldberg D, Reddy KR, et al. Outcomes in pediatric hepatitis C transplant recipients: analysis of the UNOS database. Pediatr Transplant 2015;19:153‐163. [DOI] [PubMed] [Google Scholar]
- 135. Smith BD, Morgan RL, Beckett GA, Falck‐Ytter Y, Holtzman D, Teo CG, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945‐1965. MMWR Recomm Rep 2012;61:1‐32. [PubMed] [Google Scholar]
- 136. El‐Kamary SS, Serwint JR, Joffe A, Santosham M, Duggan AK. Prevalence of hepatitis C virus infection in urban children. J Pediatr 2003;143:54‐59. [DOI] [PubMed] [Google Scholar]
- 137. Trucchi C, Orsi A, Alicino C, Sticchi L, Icardi G, Ansaldi F. State of the art, unresolved issues, and future research directions in the fight against hepatitis C virus: perspectives for screening, diagnostics of resistances, and immunization. J Immunol Res 2016;2016:1412840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Plunkett BA, Grobman WA. Routine hepatitis C virus screening in pregnancy: a cost‐effectiveness analysis. Am J Obstet Gynecol 2005;192:1153‐1161. [DOI] [PubMed] [Google Scholar]
- 139. Urbanus AT, van Keep M, Matser AA, Rozenbaum MH, Weegink CJ, van den Hoek A, et al. Is adding HCV screening to the antenatal national screening program in Amsterdam, the Netherlands, cost‐effective? PLoS One 2013;8:e70319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Snijdewind IJ, Smit C, Schutten M, Nellen FJ, Kroon FP, Reiss P, et al. Low mother‐to‐child‐transmission rate of hepatitis C virus in cART treated HIV‐1 infected mothers. J Clin Virol 2015;68:11‐15. [DOI] [PubMed] [Google Scholar]
- 141. Brown RS, Jr. , McMahon BJ, Lok AS, Wong JB, Ahmed AT, Mouchli MA, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta‐analysis. Hepatology 2016;63:319‐333. [DOI] [PubMed] [Google Scholar]
- 142. Pierce BG, Keck ZY, Lau P, Fauvelle C, Gowthaman R, Baumert TF, et al. Global mapping of antibody recognition of the hepatitis C virus E2 glycoprotein: implications for vaccine design. Proc Natl Acad Sci U S A 2016; pii: 201614942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Bowen DG, Walker CM. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J Exp Med 2005;201:1709‐1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Larrubia JR, Lokhande MU, Garcia‐Garzon S, Miquel J, Gonzalez‐Praetorius A, Parra‐Cid T, et al. Persistent hepatitis C virus (HCV) infection impairs HCV‐specific cytotoxic T cell reactivity through Mcl‐1/Bim imbalance due to CD127 down‐regulation. J Viral Hepat 2013;20:85‐94. [DOI] [PubMed] [Google Scholar]
- 145. Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, et al. Dysfunction and functional restoration of HCV‐specific CD8 responses in chronic hepatitis C virus infection. Hepatology 2007;45:588‐601. [DOI] [PubMed] [Google Scholar]
- 146. Rehermann B. Chronic infections with hepatotropic viruses: mechanisms of impairment of cellular immune responses. Semin Liver Dis 2007;27:152‐160. [DOI] [PubMed] [Google Scholar]
- 147. Timpe JM, Stamataki Z, Jennings A, Hu K, Farquhar MJ, Harris HJ, et al. Hepatitis C virus cell‐cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology 2008;47:17‐24. [DOI] [PubMed] [Google Scholar]
- 148. von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P, et al. Hepatitis C virus continuously escapes from neutralizing antibody and T‐cell responses during chronic infection in vivo. Gastroenterology 2007;132:667‐678. [DOI] [PubMed] [Google Scholar]
- 149. Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, et al. A T‐cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med 2006;12:190‐197. [DOI] [PubMed] [Google Scholar]
- 150. Yokokawa H, Higashino A, Suzuki S, Moriyama M, Nakamura N, Suzuki T, et al. Induction of humoural and cellular immunity by immunisation with HCV particle vaccine in a non‐human primate model. Gut 2016; doi: 10.1136/gutjnl-0000-0000. [DOI] [PubMed] [Google Scholar]
- 151. Swadling L, Capone S, Antrobus RD, Brown A, Richardson R, Newell EW, et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV‐specific T cell memory. Sci Transl Med 2014;6:261ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]


