Abstract
Hepatocellular carcinoma (HCC) is the main cause of mortality in patients with chronic viral hepatitis (CVH). We determined the impact of surveillance and treatments on long‐term outcomes in patients with CVH who developed HCC. Between 1984 and 2014, 333 patients with HCC and with hepatitis B or hepatitis C virus infection were evaluated. An adjusted lead time bias interval was added to patients with HCC who presented with HCC (no surveillance), and their survival was compared to patients whose HCC was detected by surveillance. After HCC treatments, survival rates within and beyond 3 years of follow‐up were compared. In 175 (53%) patients, HCC was detected through surveillance using alpha‐fetoprotein and abdominal ultrasound examinations. Compared to 158 (47%) patients with HCC who had no surveillance, more patients with HCC detected by surveillance received surgical and locoregional treatments (P < 0.0001 to P < 0.001), and their 1‐, 3‐, and 5‐year overall and disease‐free survival rates were significantly higher (P < 0.001 for both). During the first 3 years of follow‐up, patients with HCC receiving liver transplantation had similar survival rates as those with liver resection or radiofrequency ablation (RFA); however, due to HCC recurrence, survival in resection and RFA patients became significantly less when followed beyond 3 years (P = 0.001 to P = 0.04). Factors associated with mortality included tumors beyond University of California at San Francisco criteria (hazard ratio [HR] 2.02; P < 0.0001), Child‐Pugh class B and C (HR, 1.58‐2.26; P = 0.043 to P = 0.015, respectively), alpha‐fetoprotein per log ng/mL increase (HR, 1.30; P < 0.0001), previous antiviral therapy in hepatitis B virus patients (HR, 0.62; P = 0.032), and treatments other than liver transplantation (HR, 2.38‐6.45; P < 0.0001 to P < 0.003).
Conclusion. Patients with HCC detected by surveillance had prolonged survival. Due to HCC recurrence, survival rates after liver resection and RFA were lower when followed beyond 3 years after treatments. (Hepatology Communications 2017;1:595–608)
Abbreviations
- AFP
alpha‐fetoprotein
- CI
confidence interval
- CT
computerize tomography
- CVH
chronic viral hepatitis
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- LT
liver transplantation
- MRI
magnetic resonance imaging
- RFA
radiofrequency ablation
- TACE
transarterial chemoembolization
- UCSF
University of California at San Francisco
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and causes up to 600,000 deaths each year.1, 2 It is now the second‐leading cause of cancer‐related mortality in the world.2 More than 80% of HCC cases occur in Sub‐Saharan Africa or Eastern Asia and over 50% are from China alone.3 Hepatitis B virus (HBV) and hepatitis C virus (HCV) are associated with at least 50% and 33% of HCC cases globally.4, 5 The remaining HCC cases are from other causes, such as chronic alcohol intake and nonalcoholic steatohepatitis. HCC incidence rates are increasing in many countries, including the United States where the incidence more than tripled from 1975 through 2007.6 Furthermore, molecular evolutionary models based on the prevalence of HCV have predicted that HCC incidence rates will continue to rise over the next several decades.7 In the United States, the etiology of HCC differs among racial groups. HBV is the main etiologic agent in Asian Americans with HCC, while HCV is the leading cause of HCC in whites and blacks.8 HBV and HCV are associated with 10%‐15% and 55%‐60% of HCC cases in the United States, respectively.9, 10 Meta‐analyses have reported that the risk of developing HCC is 15‐20 times greater in hepatitis B surface antigen (HBsAg)‐positive individuals compared to HBsAg‐negative individuals.10 The relative risks for developing HCC in the presence of chronic HBV infection range from 5 to 49 in case‐control studies and from 7 to 49 in cohort studies.11, 12 Similarly, epidemiological evidence suggests a strong association between chronic HCV infection and HCC.13 One prospective cohort study found that the rate of developing HCC for a patient with HCV‐related cirrhosis was 2%‐6% per year.14 Nearly all cases of HCV‐related HCC develop in the presence of cirrhosis; however, 10%‐30% of HBV‐related HCC cases can arise without underlying cirrhosis.15
The prognosis of HCC depends on tumor size, the severity of underlying liver disease, and the presence of macrovascular invasion and extrahepatic metastasis. When HCC is detected at an early stage, patients may achieve 5‐year survival rates as high as 70% with liver transplantation or liver resection.16 However, patients with advanced HCC are only eligible for palliative treatments. Unfortunately, patients with HCC are often asymptomatic until the most advanced stages, making early detection difficult. Patients who have HCC detected after the onset of symptoms have an extremely poor prognosis, with an overall 3‐year survival rate of 0%‐10%.17
The goal of HCC surveillance is to detect tumors at an early stage when potentially curative treatments may be offered. In a randomized controlled trial of 18,816 individuals with HBV infection in Shanghai, China, surveillance with biannual ultrasounds and alpha‐fetoprotein (AFP) tests detected subclinical and small stage 1 tumors in 60% of screened patients compared to 0% in the control group.18 Although the surveillance group had only 58% compliance with screening, the 1‐year, 3‐year, and 5‐year survival rates were significantly higher and the HCC mortality rate decreased by 37%. In addition, a recent meta‐analysis of 47 cohort and case‐control studies found that HCC surveillance improved early stage detection rates, curative treatment rates, and overall survival in patients with cirrhosis.19 However, other reports have discussed methodological limitations of these studies and questioned whether routine screening for HCC leads to a survival advantage over clinical diagnosis. A systematic review of 22 studies concluded that there is very low‐strength evidence from which to draw conclusions about the effects of HCC screening on mortality in high‐risk patients with chronic liver disease.20 Among the 22 studies that fulfilled the authors' inclusion criteria, only five adjusted for lead time bias in their assessment of survival. Indeed, few studies on HCC surveillance in the United States have adjusted for lead time bias that incorporated patients with chronic HBV and HCV infection. The purpose of this study is to evaluate the impact of surveillance and treatments on long‐term survival in 333 patients with HCC who were evaluated in a community‐based clinic.
Patients and Methods
PATIENT POPULATION
From 1984 to 2014, 357 patients with HCC were evaluated at the Liver Center in Pasadena, CA. Patients who had no specific measurement of tumor size at diagnosis (22 individuals) or who were diagnosed with HCC within 6 months of the final date of patient entry (November 19, 2014; 2 individuals) were excluded from this study. The remaining 333 patients are reported herein. In 175 patients, HCC was detected through surveillance using serum AFP testing and abdominal ultrasound examinations. Most patients in the surveillance group were screened every 6 or 12 months; however, for this report, we expanded the maximal surveillance interval to 36 months because some patients did not return for follow‐up visits until later time periods. Thus, the surveillance group was subdivided into patients who had HCC screening within 6 months (56 patients), 7‐12 months (61 patients), 13‐24 months (43 patients), and 25‐36 months (13 patients) of the date of HCC diagnosis. In the latter two groups, the delay in surveillance was due to the time lapse between clinic visits. The remaining 32 of 175 patients in the surveillance group had HCC surveillance by the referring physician prior to their first visit to our clinic. The no surveillance group comprised 158 patients who did not have ultrasound or AFP tests prior to HCC diagnosis. Of these, 116 presented to their physicians prior to referral to our Liver Center; in 50 patients, HCC was found incidentally, and 66 others presented with symptoms, including abdominal pain, anorexia, and jaundice, which prompted further imaging studies. Because it has been our policy to perform abdominal ultrasound examination and AFT testing on every new patient with HBV or HCV, the other 42 patients in the no surveillance group were diagnosed with HCC during their first visit to our clinic. A summary of the patients with HCC among the surveillance and no surveillance groups is shown in Supporting Fig. 1.
BASELINE LABORATORY TESTS
All patients with HCC had baseline laboratory tests that measured platelet counts and levels of serum albumin, total bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine transaminase, and AFP. The patients with cirrhosis were stratified according to Child‐Pugh class A, B, or C. Sera from patients whose HCC was diagnosed prior to 1991 were retrospectively tested for anti‐HCV.
TUMOR CHARACTERISTICS AND HCC DIAGNOSIS
The number, location, and size of HCC lesions were determined by either computed tomography (CT) scan or magnetic resonance imaging (MRI), and patients were classified by the Milan criteria (single lesion ≤ 5 cm, maximum of three lesions with none >3 cm) and by the University of California at San Francisco (UCSF) criteria (single lesion ≤ 6.5 cm, maximum of three lesions with none >4.5 cm, or a total tumor burden of ≤8 cm). Large infiltrating lesions without definite borders and tumors ≥10 cm in total diameter were categorized as diffuse HCC because patients with either presentation have similarly poor prognoses. The presence of macrovascular invasion of HCC was determined by abdominal CT scan or MRI. In addition, patients were evaluated for lung or bone metastasis using chest CT scans and bone scans, respectively.
HCC TREATMENTS
In this study, 225 patients received treatments while 108 were offered supportive care. Patients with HCC were referred to academic centers for surgical and/or locoregional therapies, which included liver transplantation (LT), liver resection, radiofrequency ablation (RFA), percutaneous ethanol injection, or transcatheter arterial chemoembolization (TACE). If patients with HCC received more than one treatment, the patient was assigned to a category based on the most definitive treatment; LT was considered to be the most definitive treatment, followed by liver resection, and then by RFA, TACE, and percutaneous ethanol injection. Patients with HCC who did not receive the above treatments were treated with chemotherapy or supportive care.
POSTTREATMENT OUTCOMES
Patients with HCC who received treatments developed recurrent HCC, had residual HCC, or were cancer free at the last date of follow‐up. Patients in the “recurrence” group were free of HCC for at least 3‐6 months after initial treatment according to follow‐up imaging studies (MRI or CT scan) and correlative AFP tests. These patients then developed new intrahepatic lesions or extrahepatic metastases. Patients in the “residual” group had enhancing lesions at the site of initial treatment within the first 6 months of initial treatment. Posttreatment lesions were not considered viable unless early arterial enhancement and venous washout were observed on MRI or CT. Patients in the “cancer‐free group” remained free of HCC at the last date of follow‐up or until death occurred from other causes. Dates of diagnosis, initial treatment, last follow‐up visit, and death were recorded for calculation of overall survival. If a patient developed recurrent HCC after definitive treatments, the date of recurrence was also recorded for the calculation of disease‐free survival.
STATISTICAL ANALYSIS
Univariate Analysis
Continuous data were summarized with median or means and discrete data with percentages (proportions). The P value for comparing the medians or means was calculated using the Kruskal‐Wallis test, and the P values for comparing proportions were determined by Fisher's exact test. Overall and disease‐free survival intervals were evaluated from the date of treatments to the time of recurrence, date of last visit, or date of death. Survival curves were estimated using Kaplan‐Meier analysis and comparing the log rank test. A P value < 0.05 was considered statistically significant.
Multivariate Analysis
Baseline factors that were considered to be significantly associated with survival by univariate analysis were entered into a Cox proportional hazards regression model to test for significant effects while simultaneously adjusting for multiple factors. The 11 candidate variables were age, sex, symptoms at diagnosis, diabetes, HCC surveillance, Milan criteria, UCSF criteria, Child‐Pugh class (A, B, or C), AFP, previous antiviral therapy in patients with HBV or HCV, and HCC treatments. All candidate variables were initially entered into a model, and the final model was chosen backwards in a stepwise manner. Statistical analysis was carried out using SAS version 9.1 (SAS Inc., Cary, NC).
We evaluated the relationship between treatment modalities before and after adjusting for selected covariates. We determined that the differences in the rates of death across treatments were not the same over the entire follow‐up time. Therefore, the Cox models for mortality allowed the hazard ratio (HR) estimates for the effect of treatment to vary over time (time × treatment interaction). In particular, we defined an early follow‐up interval (≤36 months) and a late follow‐up interval (36 months) and estimated the HRs for each of these intervals separately. We chose 36 months as the cutoff for distinguishing early versus late follow‐up as this is about the point in time where the survival curves appear to start crossing over. The final models were selected using the backwards procedure for variable selection with a liberal P < 0.15 as the retention criterion. We used the date of the most definitive treatment as time “zero” in the analysis.
We attempted to minimize lead time bias, which is defined as the apparent improvement in survival due to tumor detection at an earlier stage by surveillance.21 To calculate lead time, we used the formula developed by Schwartz, 22
where T is the lead time interval in days, DT is the median tumor volume doubling time, d1 and d0 are median tumor diameters of our no surveillance patients and the median tumor diameters of our patients whose HCC was detected by surveillance, respectively. To calculate the rate of tumor growth and its corresponding DT, we used data obtained from 166 of our 333 patients with HCC who had a second imaging study prior to HCC treatment. In these 166 of 175 patients with HCC detected by surveillance, we had the date of the last tumor‐free imaging ultrasound, the date of the first imaging study confirming appearance of HCC (by CT scan or MRI) and its size, and the date of the second imaging (by CT scan or MRI) and its size. For survival analyses, we added the adjusted estimated lead time bias interval to the survival time of patients in the no surveillance group.
Results
BASELINE CHARACTERISTICS
From 1984 to 2014, 333 patients with HCC were evaluated in our clinic. Of these, 170 (51.1%) were HBsAg‐positive, 159 (47.7%) were anti‐HCV‐positive, and four (1.2%) were co‐infected with both viruses (Table 1). The mean age at HCC presentation was 61.7 ± 12.2 years, 235 (70.6%) were male, 234 (70%) were Asian, and 249 (74%) were born outside the United States. The baseline laboratory tests at HCC diagnosis showed that the AFP levels were elevated (>10 ng/mL) in 71% of patients; the median AFP value in this subset of patients was 191.1 ng/mL. Cirrhosis was present in 257 (77.2%) patients; 183 (71.2%) were Child‐Pugh class A, 59 (22.9%) class B, and 15 (5.8%) class C. At presentation, 190 (57.1%) had tumors within Milan criteria, 223 (67%) were within UCSF criteria, and 66 (19.8%) presented with diffuse tumors. In those with evaluable imaging data, 33 of 267 (9.9%) presented with macrovascular invasion, 22 of 229 (6.6%) had evidence for lung metastasis, and 21 of 230 (6.3%) had evidence for bone metastasis. Overall median survival rates were higher among patients with AFP <10 ng/mL (P = 0.001), within Milan and UCSF (P < 0.001 for both), and those without cirrhosis or in Child‐Pugh class A (Fig. 1A‐D; P < 0.001 for all).
Table 1.
BASELINE CHARACTERISTICS OF 333 PATIENTS WITH HEPATOCELLULAR CARCINOMA
| Characteristic | Number (%) or Mean ± SD Unless Noted Otherwise |
|---|---|
| Age at diagnosis (years) | 61.7 ± 12.2 |
| Gender | |
| Female | 98 (29.4) |
| Male | 235 (70.6) |
| Ethnicity | |
| African American | 7 (2.1) |
| Asian | 234 (70.3) |
| Hispanic | 31 (9.3) |
| White | 61 (18.3) |
| Virology | |
| HBV | 170 (51.1) |
| HCV | 159 (47.7) |
| HBV+HCV | 4 (1.2) |
| Birthplace | |
| United States | 84 (25) |
| Non‐United States | 249 (74) |
| Albumin (g/dL) | 3.7 ± 0.7 |
| Total bilirubin (mg/dL) | 1.6 ± 2.8 |
| Alkaline phosphate (U/L) | 149.4 ± 149.8 |
| AST (U/L) | 102.3 ± 108.8 |
| ALT (U/L) | 76.2 ± 56.5 |
| Platelet (×103 mm3) | 155.1 ± 99.8 |
| AFP (ng/mL, %) | |
| No ≤ 10 | 96 (28.8) |
| No > 10 | 237 (71.2) |
| AFP median (in those > 10 ng/mL) | 191.1 |
| First quartile | 42.5 |
| Second quartile | 1,064.3 |
| Minimum | 10.4 |
| Maximum | 1,800,000 |
| Diabetes | |
| No | 265 (79.6) |
| Yes | 50 (15) |
| Missing | 18 (5.4) |
| Family history of HCC | |
| No | 263 (80) |
| Yes | 51 (15.3) |
| Missing | 19 (5.7) |
| Cirrhosis | |
| No | 71 (21.3) |
| Yes | 257 (77.2) |
| Missing | 5 (1.5) |
| Child‐Pugh score (no. 257, %) | |
| A | 183 (71.2) |
| B | 59 (22.9) |
| C | 15 (5.8) |
| Milan | |
| Beyond | 143 (42.9) |
| Within | 190 (57.1) |
| UCSF | |
| Beyond | 110 (33) |
| Within | 223 (67) |
| Diffuse* | |
| No | 267 (80.2) |
| Yes | 66 (19.8) |
| Macrovascular invasion | |
| No | 267 (80.2) |
| Yes | 33 (9.9) |
| Missing | 33 (9.9) |
| Metastasis to lunga | |
| No | 229 (68.7) |
| Yes | 22 (6.6) |
| Missing | 82 (24.6) |
| Metastasis to bonea | |
| No | 230 (69) |
| Yes | 21 (6.4) |
| Missing | 82 (24.6) |
Diffuse tumors: multifocal or single tumor >10 cm diameter.
2 patients presented with both bone and lung metastasis
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HBV, hepatitis B virus DNA; HCV, hepatitis C virus RNA.
Figure 1.
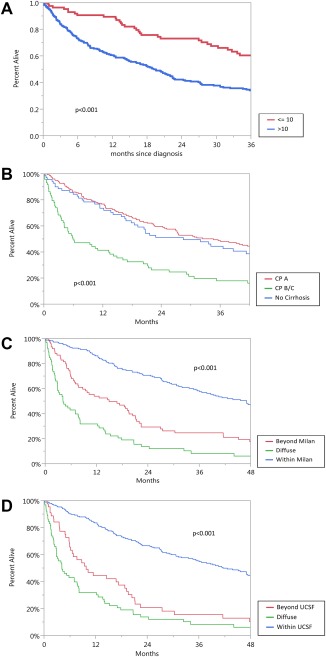
Overall survival of 333 patients with HCC by (A) AFP level (ng/mL); (B) Child‐Pugh class; (C) Milan criteria; (D) UCSF criteria.
SURVEILLANCE
In 175 (53%) patients, HCC was detected through surveillance using AFP and abdominal ultrasound examinations. The no surveillance group comprised 158 (47%) patients who had neither of the above tests prior to HCC diagnosis. As shown in Table 2, patients with HCC detected by surveillance were older in age (63.5 versus 59.8 years; P = 0.006), and their values of alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, platelets, and AFP were significantly lower than in the no surveillance patients (P < 0.0001 to P = 0.0015). Patients who had surveillance were more likely to have received antiviral therapy prior to diagnosis of HCC compared to the no surveillance group (HBV subset, 47.5% versus 15.6%, P < 0.0001; HCV subset, 23.4% versus 7.7%, P = 0.01). After adjusting for prior antiviral treatment, surveillance patients had significantly lower rates of mortality in both the HBV (HR = 0.44; P < 0.0001) and HCV (HR = 0.57; P = 0.007) subsets. In addition, the surveillance group had more patients in Child‐Pugh class A and within Milan and UCSF criteria, fewer patients with diffuse tumors, and fewer with macrovascular invasion and extrahepatic metastasis (P ≤ 0.001 for all). More patients with HCC detected by surveillance had solitary tumors (P < 0.0001), and the median size of their tumors was 3 cm compared to 5 cm in the no surveillance group (P < 0.001). More patients in the surveillance group received LT, liver resection, RFA, and TACE than patients in the no surveillance group (Table 3; P < 0.001).
Table 2.
A COMPARISON OF 333 PATIENTS WITH HEPATOCELLULAR CARCINOMA: SURVEILLANCE VERSUS NO SURVEILLANCE
| Surveillance | |||
|---|---|---|---|
| No | Yes | P value | |
| Number of patients | 158 | 175 | |
| Age (years)a | 59.8 ± 13.2 | 63.5 ± 11.1 | 0.0065 |
| Virology (number, %) | 0.049 | ||
| HBV | 90 (57) | 80 (45.7) | |
| HCV | 65 (41.1) | 94 (53.7) | |
| Both | 3 (1.9) | 1 (0.60) | |
| Albumin (g/dL)a | 3.6 ± 0.7 | 3.8 ± 0.7 | 0.27 |
| Total bilirubin (mg/dL)a | 1.9 ± 3.3 | 1.4 ± 2.2 | 0.25 |
| Alkaline phosphate (U/L)a | 183.3 ± 203.6 | 118.9 ± 61.3 | <0.0001 |
| AST (U/L)a | 123.7 ± 109.7 | 83 ± 104.7 | <0.0001 |
| ALT (U/L)a | 85.9 ± 62.4 | 67.6 ± 49.3 | 0.0065 |
| Platelets ( × 103 mm3)a | 187.6 ± 119.3 | 126.6 ± 70.5 | <0.0001 |
| AFP (ng/mL, %) | 0.0015 | ||
| Number < 10 | 150 | 174 | |
| Number > 10 | |||
| AFP in those > 10 (ng/mL) | 4,9279.9 ± 221,286.6 | 2,645.9 ± 11,480.7 | |
| Cirrhosis (number, %) | 0.24 | ||
| No | 37 (23.4) | 34 (19.4) | |
| Yes | 117 (74.1) | 140 (80.0) | |
| Missing | 4 (2.5) | 1 (0.60) | |
| Hepatitis B | <0.0001 | ||
| No treatment (number, %) | 69 (76.7) | 42 (52.5) | |
| Treatment (number, %) | 14 (15.6) | 38 (47.5) | |
| Unknown | 7 (7.8) | ‐ | |
| Hepatitis C | 0.01 | ||
| No treatment (number, %) | 56 (86.2) | 68 (72.3) | |
| Treatment (number, %) | 5 (7.7) | 22 (23.4) | |
| Unknown | 4 (6.2) | 4 (4.3) | |
| Child‐Pugh score (number, %) A | 0.0001 | ||
| B | 99 (62.7) | 145 (82.9) | |
| C | 50 (31.6) | 22 (12.6) | |
| Missing | 7 (4.4) | 8 (4.60) | |
| 2 (1.3) | 0 | ||
| Milan (number, %) | <0.0001 | ||
| Beyond | 113 (71.5) | 30 (17.1) | |
| Within | 45 (28.5) | 145 (82.9) | |
| UCSF (number, %) | <0.0001 | ||
| Beyond | 94 (59.5) | 16 (9.10) | |
| Within | 64 (40.5) | 159 (90.9) | |
| Diffuse (number, %) No | <0.0001 | ||
| Yes | 100 (63.3) | 167 (95.4) | |
| 58 (36.7) | 8 (4.60) | ||
| Macrovascular invasion (number, %) | <0.0001 | ||
| No | 103 (65.2) | 164 (93.7) | |
| Yes | 27 (17.1) | 6 (3.40) | |
| Missing | 28 (17.7) | 5 (2.90) | |
| Metastasis to lung (number, %) | |||
| No | 87 (55.0) | 142 (81.1) | |
| Yes | 18 (11.4) | 4 (2.30) | |
| Missing | 53 (33.5) | 29 (16.6) | <0.0001 |
| Metastasis to bone (number, %) | <0.0001 | ||
| No | 87 (55.1) | 143 (81.7) | |
| Yes | 18 (11.4) | 3 (1.7) | |
| Missing | 53 (33.5) | 29 (16.6) | |
| Median size of largest tumor (cm) | 5 | 3 | <0.001 |
| Number of tumors (number, %) | <0.0001 | ||
| 1 | 82 (51.9) | 132 (75.4) | |
| ≥2 | 76 (48.1) | 43 (24.6) | |
Mean ± SD
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HBV, hepatitis B virus DNA; HCV, hepatitis C virus RNA.
Table 3.
TREATMENTS BY SURVEILLANCE VERSUS NO SURVEILLANCE
| Surveillance | |||
|---|---|---|---|
| No | Yes | P Value | |
| HCC treatment | <0.0001 | ||
| No | 77 (48.7) | 31 (17.7) | |
| Yes | 81 (51.3) | 144 (82.3) | |
| Treatment modality | <0.0001 | ||
| OLT | 9 (5.70) | 38 (21.7) | |
| Resection | 21 (13.3) | 31 (17.7) | |
| RFA | 12 (7.60) | 37 (21.1) | |
| TACE | 25 (15.8) | 26 (14.9) | |
| PEI | 4 (2.50) | 4 (2.30) | |
| Chemotherapy | 10 (6.30) | 8 (4.60) | |
| Supportive | 77 (45.7) | 31 (17.7) | |
| Local regional | 41 (25.9) | 67 (38.3) | |
| Treatment modality | <0.0001 | ||
| OLT only | 0 (0) | 7 (4.00) | |
| OLT plus other | 9 (5.70) | 31 (17.7) | |
| Resection only | 11 (7.00) | 17 (9.70) | |
| Resection plus other | 10 (6.30) | 14 (8.00) | |
| RFA | 7 (4.40) | 27 (15.4) | |
| RFA plus other | 5 (3.20) | 10 (5.70) | |
| TACE | 24 (15.2) | 22 (12.6) | |
| TACE plus other | 1 (0.60) | 4 (2.30) | |
| PEI only | 4 (2.50) | 4 (2.30) | |
| Chemotherapy only | 10 (6.30) | 8 (4.60) | |
| Supportive only | 77 (48.7) | 31 (17.7) | |
| Overall survived (%, SE) | <0.001 | ||
| 1 year | 53.9 (4.0) | 81.1 (3.0) | |
| 3 years | 25.6 (3.7) | 53.3 (4.0) | |
| 5 years | 14.2 (3.2) | 37.5 (4.2) | |
| Median (months) | 14.5 | 40.5 | |
| Disease‐free survival (%, SE) | <0.0001 | ||
| 1 year | 43.8 (4.0) | 72.8 (3.4) | |
| 3 years | 13.5 (2.9) | 37.9 (3.9) | |
| 5 years | 7.4 (2.3) | 22.4 (3.6) | |
| Median (months) | 11 | 26.9 | |
Abbreviations: OLT, orthotopic liver transplantation; PEI, percutaneous ethanol injection.
In 166 of 175 patients whose HCC was detected by surveillance, two consecutive imaging studies by CT scan or MRI showing tumor size growth prior to initiation of HCC treatments were available for analyses. Based on these data, the average tumor DT was calculated to be 4.3 months (129 days), which corresponds to an average tumor growth rate of 16% per month (Fig. 2). For some of these patients, the date of the calculated time of the start of tumor growth was before the time of the last tumor‐free abdominal ultrasound examination. There was no difference in the tumor DT between the patients with HCC and HBV or those with HCC and HCV.
Figure 2.
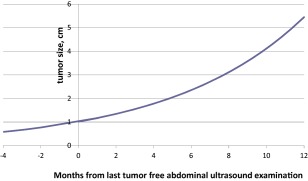
Projected tumor growth in 166 patients whose HCC was detected by surveillance (based on an average growth rate of 16% per month).
For survival analyses, we added the adjusted estimated lead time bias interval of 3.4 months to the survival time of patients who presented with HCC (no surveillance). The overall median survival was significantly higher in surveillance compared to no surveillance patients (40.5 months versus 14.5 months; P < 0.0001; Fig. 3). Furthermore, the 1‐, 3‐, and 5‐year overall and disease‐free survival rates were significantly higher in the surveillance group compared to the no surveillance group (81.1%, 53.3%, and 37.5% versus 53.9%, 25.6%, and 14.2%, respectively, and 72.8%, 37.9%, and 22.4% versus 43.8%, 13.5%, and 7.4%, respectively; P < 0.001 for both; Table 3). In addition, we further analyzed the impact on surveillance versus no surveillance in the subset of 257 patients with HCC with cirrhosis. The overall survival at 1, 3, and 5 years remained significant in patients with cirrhosis who have surveillance at intervals of 6 months, 7‐12 months, and at greater than 12 months compared to the no surveillance patients (P < 0.001 for all three comparisons).
Figure 3.
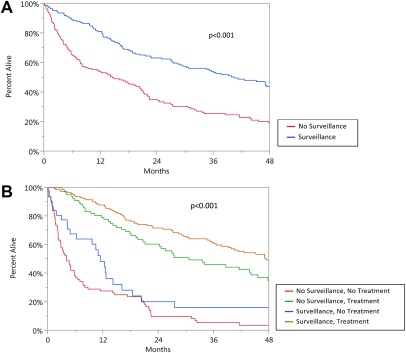
Overall patient survival by (A) surveillance and (B) surveillance and treatments.
To illustrate the importance of surveillance in combination with treatments, we compared groups with and without surveillance and those with and without treatments (Fig. 3B). Those with the longest survival rates were patients with HCC who had both surveillance and treatments but also in those who had no surveillance but presented with tumors that were still eligible for treatments (P < 0.0001 for both). However, patients who had surveillance but received no treatments had similarly poor survival rates as patients with HCC who had no surveillance and no treatments.
A comparison of tumor burden by time interval of surveillance is shown in Table 4A. Patients who had surveillance within a 6‐month period had the smallest size tumors (mean 2.66 ± 1.5 cm), while patients who had surveillance between 25 and 36 months prior to HCC diagnosis had the largest tumors (mean, 4.3 ± 2 cm; P = 0.0037). In addition, patients who had 6‐ and 12‐ month interval surveillance were more likely to have one tumor compared to those in the 13‐24‐ or 25‐36‐month interval surveillance groups (P = 0.0126; Table 4B).
Table 4.
TUMOR BURDEN BY TIME INTERVAL OF SURVEILLANCE IN 175 PATIENTS WITH HCC. (A) TUMOR SIZE (CM) BY TIME INTERVAL OF SURVEILLANCE. (B) NUMBER OF TUMORS BY TIME INTERVAL OF SURVEILLANCE
| A | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Min. | Q1 | Median | Mean | SD | Q3 | Max | P Value | |
| Time | 0.0037 | ||||||||
| 0‐6 months | 56 | 0.8 | 1.7 | 2.15 | 2.66 | 1.5 | 3.5 | 10.0 | |
| 7‐12 months | 61 | 0.9 | 2.2 | 3.00 | 3.15 | 1.6 | 3.7 | 8.2 | |
| 13‐24 months | 43 | 0.8 | 2.0 | 3.40 | 3.96 | 3.2 | 4.9 | 20.0 | |
| 25‐36 months | 13 | 1.8 | 3.0 | 3.30 | 4.30 | 2.0 | 5.6 | 8.5 | |
| B | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0‐6 Months | 7‐12 Months | 13‐24 Months | 25‐36 Months | ||||||
| No. | % | No. | % | No. | % | No. | % | P Value | |
| One tumor | 48 | 84.2 | 48 | 78.7 | 27 | 61.4 | 9 | 69.2 | 0.0126 |
| >One tumor | 9 | 15.8 | 13 | 21.3 | 17 | 38.6 | 4 | 30.8 | |
TREATMENTS
A comparison of patient mortality by treatment modality is shown in Table 5. For 39 patients, other treatments preceded the most definitive treatment. Eight of 39 patients who had residual or recurrent disease after initial treatments received either RFA or liver resection as their most definitive treatment; however, these sample sizes were inadequate to evaluate the effect of prior recurrence or residual HCC on mortality. For 50 patients who received LT as their most definitive treatment, 16 had prior recurrence and 15 had residual HCC following initial treatments. Compared to LT patients without prior recurrence or residual disease, there was no apparent difference on overall mortality in those with prior residual (P = 0.285) or prior recurrence (P = 0.079) following LT during the first 3 years (early follow‐up period). During this time period, the survival in all three categories of LT patients was similar compared to those with liver resection and RFA, while patients receiving TACE had significantly lower survival compared to other treatments (P = 0.0001 to P = 0.001, Fig. 4). However, when followed beyond 3 years (late follow‐up period), patients treated with liver resection and RFA showed significantly lower survival rates compared to patients who received LT (P < 0.001 to P < 0.043). During this late follow‐up period, there was no difference in survival in patients who were treated with liver resection, RFA, or TACE.
Table 5.
COMPARISON OF EARLY AND LATE MORTALITY IN 139 PATIENTS WITH HCC RECEIVING HCC TREATMENTS
| Treatment Modality | No. Patients | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|---|
| A. Early follow‐up (<3 years) | ||||
| OLT/no prior recurrence or residual | 19 | 1.00 | Reference | Reference |
| OLT/prior recurrence | 16 | 2.53 | 0.90‐7.13 | 0.0790 |
| OLT/prior residual | 15 | 1.83 | 0.61‐5.52 | 0.2850 |
| Resection | 52 | 1.82 | 0.69‐4.83 | 0.2280 |
| RFA | 44 | 1.96 | 0.68‐5.63 | 0.2100 |
| TACE | 53 | 7.90 | 3.43‐18.22 | 0.0000 |
| PEI | 8 | 7.90 | 3.43‐18.22 | 0.0000 |
| Chemotherapy | 18 | 28.38 | 10.38‐77.57 | 0.0000 |
| Resection vs. OLT/prior recurrence | 0.72 | 0.29‐1.82 | 0.4890 | |
| Resection vs. OLT/prior residual | 1.00 | 0.37‐2.71 | 0.9950 | |
| RFA vs. OLT/prior recurrence | 0.78 | 0.29‐2.10 | 0.6170 | |
| RFA vs. OLT/prior residual | 1.07 | 0.37‐3.13 | 0.8970 | |
| TACE vs. OLT/prior recurrence | 3.13 | 1.42‐6.90 | 0.0050 | |
| TACE vs. OLT/prior residual | 4.31 | 1.79‐10.44 | 0.0010 | |
| RFA vs. Resection | 1.08 | 0.45‐2.56 | 0.8670 | |
| TACE vs. Resection | 4.34 | 2.30‐8.17 | 0.0000 | |
| TACE vs. RFA | 4.03 | 1.90‐8.52 | 0.0000 | |
| B. Late follow‐up (≥3 years) | ||||
| OLT/no prior recurrence or residual | 19 | 1.00 | Reference | Reference |
| OLT/prior recurrence | 16 | 2.53 | 0.90‐7.13 | 0.0790 |
| OLT/prior residual | 15 | 1.83 | 0.61‐5.52 | 0.2850 |
| Resection | 52 | 6.56 | 2.52‐17.05 | 0.0000 |
| RFA | 44 | 6.46 | 2.21‐18.83 | 0.0010 |
| TACE | 53 | 7.90 | 3.43‐18.22 | 0.0000 |
| PEI | 8 | 7.90 | 3.43‐18.22 | 0.0000 |
| Chemotherapy | 18 | 28.38 | 10.38‐77.57 | 0.0000 |
| Resection vs. OLT/prior recurrence | 2.60 | 1.03‐6.55 | 0.0430 | |
| Resection vs. OLT/prior residual | 3.59 | 1.28‐10.07 | 0.0150 | |
| RFA vs. OLT/prior recurrence | 2.55 | 0.91‐7.16 | 0.0750 | |
| RFA vs. OLT/prior residual | 3.53 | 1.14‐10.91 | 0.0280 | |
| TACE vs. OLT/prior recurrence | 3.13 | 1.42‐6.90 | 0.0050 | |
| TACE vs. OLT/prior residual | 4.32 | 1.79‐10.44 | 0.0010 | |
| RFA vs. Resection | 0.98 | 0.42‐2.28 | 0.9710 | |
| TACE vs. Resection | 1.20 | 0.56‐2.60 | 0.6340 | |
| TACE vs. RFA | 1.22 | 0.49‐3.03 | 0.6620 | |
Abbreviations: OLT, orthotopic liver transplantation; PEI, percutaneous ethanol injection.
Figure 4.
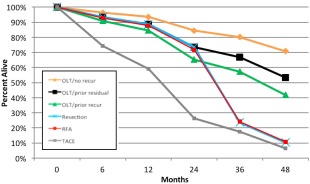
Overall survival in patients with HCC receiving therapies after adjusting for lead time bias intervals divided into early follow‐up period (<3 years) and late follow‐up period (>3 years). Abbreviation: OLT, orthotopic liver transplantation.
The HCC residual and recurrence rates after definitive treatments for HCC are shown in Table 6. Residual rates in patients treated with TACE were significantly higher than other treatments (P = 0.001 for all comparisons). The recurrence rates were lowest in patients receiving LT compared to liver resection and RFA‐treated patients (P = 0.0001 for both comparisons).
Table 6.
RESIDUAL AND RECURRENCE OF HCC AFTER DEFINITIVE TREATMENTS
| A Residual or Recurrent HCC Rates After Treatments | |||||
|---|---|---|---|---|---|
| Treatment Modality | No. Patients | Residual* HCC | Recurrence† at 1 Year | Recurrence at 3 Years | Recurrence at 5 Years |
| Liver transplantation | 50 | 10% | 0% | 6.4% | 8.6% |
| Liver resection | 52 | 19.2% | 9.9% | 31.7% | 47.9% |
| Radiofrequency ablation | 44 | 15.9% | 7.4% | 27.7% | 37.4% |
| Transarterial chemoembolization | 53 | 56.6% | 14.0% | 16.3% | 18.6% |
| *Residual: Enhancing lesions at the site of initial treatment within the first 6 months. | |||||
| †Recurrence: Free of HCC for 3‐6 months after initial treatment and then developed new intrahepatic lesions or extrahepatic metastases. | |||||
| B Comparison of Residual and Recurrence HCC Rates After Treatment | ||
|---|---|---|
| P Value for Residual HCC | P Value for Recurrent HCC | |
| LT vs. Resection | 0.19 | 0.0001 |
| LT vs. RFA | 0.39 | 0.0001 |
| LT vs. TACE | 0.0001 | 0.0863 |
| Resection vs. RFA | 0.67 | 0.9913 |
| Resection vs. TACE | 0.0001 | 0.0134 |
| RFA vs. TACE | 0.0001 | 0.0111 |
Factors significantly associated with mortality included sex (HR, 1.33; 95% CI, 0.99‐1.80; P = 0.058), Child‐Pugh class B (HR, 1.58; 95% CI, 1.02‐2.46; P = 0.043) and Child‐Pugh class C (HR, 2.26; 95% CI, 1.18‐4.33; P = 0.015), AFP per log ng/mL increase (HR, 1.30; 95% CI, 1.18‐1.44; P < 0.0001), tumors beyond UCSF criteria (HR, 2.03; 95% CI, 1.47‐2.78; P < 0.0001), previous antiviral therapy in HBV patients (HR, 0.63; 95% CI, 0.40‐0.96; P = 0.032), and receiving treatments other than LT (HR, 2.38‐6.45; 95% CI, 1.35‐3.82 to 4.32‐17.85; P < 0.0001 to P < 0.003) (Table 7).
Table 7.
PREDICTORS OF OVERALL DISEASE‐FREE SURVIVAL IN PATIENTS WITH HCC
| Predictor | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Sex | |||
| Female | 1.00 | Reference | Reference |
| Male | 1.33 | 0.99‐1.80 | 0.058 |
| Cirrhosis | |||
| No | 1.00 | Reference | Reference |
| Yes, Child‐Pugh | |||
| Class A | 1.10 | 0.76‐1.58 | 0.611 |
| Class B | 1.58 | 1.02‐2.46 | 0.043 |
| Class C | 2.26 | 1.18‐4.33 | 0.015 |
| AFP per log/mL increase | 1.30 | 1.18‐1.44 | <0.0001 |
| UCSF | |||
| Within | 1.00 | Reference | Reference |
| Beyond | 2.02 | 1.47‐2.78 | <0.0001 |
| Antiviral therapy | |||
| HBV not treated | 1.00 | Reference | Reference |
| HBV treated | 0.62 | 0.40‐0.96 | 0.032 |
| HCV not treated | 1.00 | Reference | Reference |
| HCV treated | 1.43 | 0.87‐2.34 | 0.161 |
| Treatments | |||
| OLT | 1.00 | Reference | Reference |
| Resection | 2.38 | 1.35‐4.21 | 0.003 |
| RFA | 3.38 | 1.88‐6.08 | <0.0001 |
| PEI | 5.94 | 1.98‐17.85 | 0.002 |
| TACE | 3.80 | 2.22‐6.52 | <0.0001 |
| Chemotherapy | 6.34 | 3.02‐13.08 | <0.0001 |
| Supportive | 6.45 | 3.82‐10.89 | <0.0001 |
Abbreviations: OLT, orthotopic liver transplantation; PEI, percutaneous ethanol injection.
Discussion
In order to improve survival, surveillance for HCC must be routinely performed while caring for patients with CVH. In our study, the surveillance group had more patients within Milan and UCSF criteria and had fewer patients with diffuse tumors, macrovascular invasion, and extrahepatic metastasis. Other studies have similarly reported that HCC surveillance was associated with early tumor detection and improvement of overall survival. A recent meta‐analysis of 47 studies showed that the pooled rate of early stage HCC among patients undergoing surveillance was 70.9% compared to only 29.9% among those who presented with symptoms or were incidentally diagnosed.19 These observations support American Association for the Study of Liver Diseases and European Association for the Study of the Liver guidelines for HCC screening in patients with chronic HBV, HCV, and cirrhosis of any etiology.5, 23 Yet, studies have reported very low rates of HCC surveillance in patients with HBV and HCV despite screening guidelines.24, 25, 26, 27 In addition, some authors questioned whether routine screening for HCC actually offered a survival advantage over clinical diagnosis. While several retrospective cohort studies suggest that HCC surveillance was associated with improved survival, lead time bias may have led to an overestimation of survival in HCC cases detected by surveillance.20 In three of the reports, the survival advantage of surveillance disappeared with assumed tumor DTs of 90 days or more. However, a large cohort study from Italy comparing surveillance to patients presenting with symptomatic HCC showed a survival benefit even after adjusting for a lead time interval of 6.5 months for surveillance patients.28 In the current study, our average tumor DT was 129 days and the lead time interval was estimated to be 3.4 months (102 days). After adjustment for lead time bias, the overall survival in our patients with HCC detected by surveillance was significantly longer, confirming the importance of routine surveillance in patients with CVH. Thus, detection of early stage HCC by surveillance is the initial step for improving patient survival.
AFP and ultrasound examination are routinely used for HCC surveillance. However, it has been previously shown that the sensitivity of ultrasound for detecting lesions less than 2 cm is 46% compared to 65% for CT scan and 72% for MRI.29 The low sensitivity of ultrasound may account for the last “negative” ultrasound examinations in our patients who had 6‐month surveillance intervals. As mentioned, CT scan or MRI would be more accurate in detecting HCC lesions less than 2 cm, but the use of the latter two imaging modalities may not be cost effective as screening tools.
After HCC diagnoses, patients must be referred to centers with a multidisciplinary tumor board and where definitive treatment options are available. In our cohort of patients with HCC, 30% and 32% were able to receive surgical and locoregional therapies, respectively. In patients within Milan and UCSF criteria, LT remains the most definitive treatment, and long‐term overall survival rates and recurrence‐free survival rates of 75% and 83%, respectively, may be achieved.30, 31, 32, 33 In patients with sufficient hepatic reserve and absence of macrovascular invasion and metastasis, liver resection of HCC may achieve 5‐year overall survival rates and recurrence‐free survival rates of 75% and 50%, respectively.34, 35 RFA has been shown to be highly effective as a standalone therapy with overall survival and tumor control rates of 40%‐50% at 5 years.36, 37 Two randomized controlled trials using TACE in unresectable HCC showed 2‐year survival rates of 31% in predominately patients with HBV and 63% in patients with HCV, respectively.38, 39
During the first 3 years of follow‐up, there were no significant differences in survival in our patients who received LT, liver resection, or RFA. However, beyond 3 years follow‐up, there was a significant drop in survival in patients who had liver resection or RFA, and their survival rates at that point were similar to the patients who had TACE. One of the reasons for the decrease in survival is the high rates of HCC recurrence during the course of follow‐up. In our study, 7.4%, 27.7%, and 37.4% of patients who were treated with RFA developed recurrent HCC at 1, 2, and 3 years, respectively. Another study of 303 patients with single HCC tumors found overall recurrence rates of 19.6%, 61.8%, and 78.3% at 1, 3, and 5 years following RFA.40 Tumor recurrence after surgical resection also is common. Poon et al.34 reported HCC recurrence rates of 24%, 48%, 60%, and 76% at 1, 3, 5, and 10 years following resection. In our study, 9.9%, 31.7%, and 47.9% of our patients who were treated with liver resection developed recurrent HCC after 1, 2, and 3 years, respectively. Thus, due to the high rate of HCC recurrence after treatments, we recommend continued surveillance with either CT or MRI examinations along with AFP tests every 3 months following surgical and locoregional therapies. Few guidelines exist, but the National Comprehensive Cancer Network currently recommends surveillance after HCC resection with imaging every 3‐6 months for 2 years, then annually thereafter.41 The latest American Association for the Study of Liver Diseases and European Association for the Study of the Liver guidelines on the management of HCC do not address surveillance after treatments for HCC. Further studies on surveillance and subsequent treatments following surgical and locoregional therapies and their effect on survival remain to be elucidated.
The time course of this observational study on patients with HCC ranged over a number of years beginning with a period of 1 no surveillance and no treatments to 2 surveillance and few treatment options and finally to 3 surveillance and available “curative” treatments (Supporting Table 1). Thus, we were able to observe the gradual improvement in survival in our patients with HCC. More patients in era 1 were in the no surveillance group compared to the surveillance group (36.7% versus 10.9%; P < 0.001), and the increasing use of RFA was the main intervention. The use of RFA increased over the 3 eras, while the percentage of patients who received LT, resection, and TACE remained relatively unchanged. Also, both surveillance and definitive treatments are necessary to prolong survival (Fig. 2B). A randomized control trial in Qidong, China, on screening for liver cancer detected early stage tumors, but there was no reduction in mortality because the authors concluded that the therapies for patients with HCC found on screening were ineffective.42 Another report from Hong Kong that screened over 1,000 HBV carriers using AFP and ultrasound detected HCC with mean tumor sizes of less than 5 cm.43 However, this latter study also was not able to demonstrate any clinical benefit after early detection because therapies for HCC were considered to be inadequate. These observations were similar to our initial surveillance report on 602 patients with CVH.44 The accessibility to surgical and locoregional treatments is the major factor that prolongs survival, so patients with HCC detected by surveillance must be referred to centers with LT, liver resection, and up to date interventional treatments.
Finally, antiviral therapy in patients with CVH has been shown to decrease occurrence of HCC and to reduce mortality.45, 46 In the report herein, patients with HBV and HCV who had surveillance were more likely to receive antiviral therapy but nevertheless progressed to HCC. In our multivariate analysis, prior antiviral therapy significantly improved survival in patients with HBV, but no improvement was noted in patients with HCV and HCC. In addition, HCC has been reported to develop in patients with HCV after clearance of viremia, especially in patients treated with direct‐acting antiviral agents.47 Thus, the effects of prior antiviral treatments on survival in patients with established HCC are unknown and require further study. However, surveillance and HCC treatments remain the mainstay for improved survival.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep4.1047/suppinfo.
Supporting Information Figure 1.
Supporting Information Table 1.
Acknowledgment
Jeffrey Gornbein, Ph.D., and Daniella Markovic, M.S., assisted in part in the statistical analysis of this study. Lori Tong, R.N., M.S.N., participated in the care of these patients.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Ferenci P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M, et al; World Gastroenterology Organisation Guidelines and Publications Committee. World Gastroenterology Organisation Guideline . Hepatocellular carcinoma (HCC): a global perspective. J Gastrointestin Liver Dis 2010;19:311‐317. [PubMed] [Google Scholar]
- 2. Wallace MC, Preen D, Jeffrey GP, Adams LA. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol 2015;9:765‐769. [DOI] [PubMed] [Google Scholar]
- 3. El‐Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557‐2576. [DOI] [PubMed] [Google Scholar]
- 4. Parkin DM. The global health burden of infection‐associated cancers in the year 2002. Int J Cancer 2006;118:3030‐3044. [DOI] [PubMed] [Google Scholar]
- 5. European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer . EASL‐EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908‐943. [DOI] [PubMed] [Google Scholar]
- 6. Davila JA, El‐Serag HB. The rising incidence of hepatocellular carcinoma in the United States: an update. Gastroenterol 2012;142(Suppl 1):S914. [Google Scholar]
- 7. Mizokami M, Tanaka Y. Molecular evolutionary analysis predicts the incidence of hepatocellular carcinoma in the United States and Japan. Cancer Chemother Pharmacol 2004;54(Suppl 1):S83‐86. [DOI] [PubMed] [Google Scholar]
- 8. Di Bisceglie AM, Lyra AC, Schwartz M, Reddy RK, Martin P, Gores G, et al; Liver Cancer Network . Hepatitis C‐related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol 2003;98:2060‐2063. [DOI] [PubMed] [Google Scholar]
- 9. Mittal S, El Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;47(Suppl):S2‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El‐Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen VT, Law MG, Dore GJ. Hepatitis B‐related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat 2009;16:453‐463. [DOI] [PubMed] [Google Scholar]
- 12. Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus‐induced hepatocellular carcinoma. Pathol Biol (Paris) 2010;58:273‐277. [DOI] [PubMed] [Google Scholar]
- 13. Goodgame B, Shaheen NJ, Galanko J, El‐Serag HB. The risk of end stage liver disease and hepatocellular carcinoma among persons infected with hepatitis C virus: publication bias? Am J Gastroenterol 2003;98:2535‐2542. [DOI] [PubMed] [Google Scholar]
- 14. Sangiovanni A, Del Ninno E, De Fazio C, De Fazio C, Ronchi G, Romeo R, et al. Increased survival of cirrhotic patients with hepatocellular carcinoma detected during surveillance. Gastroenterology 2004;126:1005‐1014. [DOI] [PubMed] [Google Scholar]
- 15. Yang JD, Kim WR, Coelho R, Mettler TA, Benson JT, Sanderson SO, et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol 2011;9:64‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Padhya KT, Marrero JA, Singal AG. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol 2013;29:285‐292. [DOI] [PubMed] [Google Scholar]
- 17. Sala M, Forner A, Varela M, and Bruix J. Prognostic prediction in patients with hepatocellular carcinoma. Semin Liver Dis 2005;25:171‐180. [DOI] [PubMed] [Google Scholar]
- 18. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417‐422. [DOI] [PubMed] [Google Scholar]
- 19. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta‐analysis. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kansagara D, Papak J, Pasha AS, O'Neil M, Freeman M, Relevo R, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med 2014;161:261‐269. [DOI] [PubMed] [Google Scholar]
- 21. Adams PC, Arthur MJ, Boyer TD, DeLeve LD, Di Bisceglie AM, Hall M, et al. Screening in liver disease: report of an AASLD clinical workshop. Hepatology 2004;39:1204‐1212. [DOI] [PubMed] [Google Scholar]
- 22. Schwartz M. A biomathematical approach to clinical tumor growth. Cancer 1961;14:1272‐1294. [DOI] [PubMed] [Google Scholar]
- 23. Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus‐infected veterans in the United States. Ann Intern Med 2011;154:85‐93. [DOI] [PubMed] [Google Scholar]
- 25. Davila JA, Weston A, Smalley W, El‐Serag HB. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol 2007;41:777‐782. [DOI] [PubMed] [Google Scholar]
- 26. Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El‐Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology 2010;52:132‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leykum LK, El‐Serag HB, Cornell J, Papadopoulos KP. Screening for hepatocellular carcinoma among veterans with hepatitis C on disease stage, treatment received, and survival. Clin Gastroenterol Hepatol 2007;5:508‐512. [DOI] [PubMed] [Google Scholar]
- 28. Cucchetti A, Trevisani F, Pecorelli A, Erroi V, Farinati F, Ciccarese F, et al; Italian Liver Cancer Group . Estimation of lead‐time bias and its impact on the outcome of surveillance for the early diagnosis of hepatocellular carcinoma. J Hepatol 2014;61:333‐341. [DOI] [PubMed] [Google Scholar]
- 29. Yu NC, Chaudhari V, Raman SS, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:161‐167. [DOI] [PubMed] [Google Scholar]
- 30. Mazzaferro V., Regalia E., Doci R., et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693‐699. [DOI] [PubMed] [Google Scholar]
- 31. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394‐1403. [DOI] [PubMed] [Google Scholar]
- 32. Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF‐expanded criteria based on preoperative imaging. Am J Transplant 2007;7:2587‐2596. [DOI] [PubMed] [Google Scholar]
- 33. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35‐43. [DOI] [PubMed] [Google Scholar]
- 34. Poon RT, Fan ST, Lo CM, Liu CL, Wong J, et al. Long‐term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function; implications for a strategy of salvage transplantation. Ann Surg 2002;235:373‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duffy JP, Hiatt JR, Busuttil RW. Surgical resection of hepatocellular carcinoma. Cancer J 2008;14:100‐110. [DOI] [PubMed] [Google Scholar]
- 36. Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10‐year outcome and prognostic factors. Am J Gastroenterol 2012;107:569‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten‐year outcomes of percutaneous radiofrequency ablation as first‐line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol 2013;58:89‐97. [DOI] [PubMed] [Google Scholar]
- 38. Lo CM, Ngan H, Tso W, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164‐1171. [DOI] [PubMed] [Google Scholar]
- 39. Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trial. Lancet 2002;359:1734‐1739. [DOI] [PubMed] [Google Scholar]
- 40. Tateishi R, Shiina S, Akahane M, Sato J, Kondo Y, Masuzaki R, et al. Frequency, risk factors and survival associated with an intrasubsegmental recurrence after radiofrequency ablation for hepatocellular carcinoma. PLoS ONE 2013;8:e59040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benson AB, Abrams TA, Ben‐Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 2009;7:350‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, et al. Screening for liver cancer: results of a randomized controlled trial in Qidong, China. J Med Screen 2003;10:204‐209. [DOI] [PubMed] [Google Scholar]
- 43. Mok TS, Yeo W, Yu S, Lai P, Chan HL, Chan AT, et al. An intensive surveillance program detected a high incidence of hepatocellular carcinoma among hepatitis B virus carriers with abnormal alpha‐fetoprotein levels or abdominal ultrasonography results. J Clin Oncol 2005;23:8041‐8047. [DOI] [PubMed] [Google Scholar]
- 44. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol 2001;16:553‐559. [DOI] [PubMed] [Google Scholar]
- 45. Liaw, YF , Sung, JY , Chow, WC , Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521‐1531. [DOI] [PubMed] [Google Scholar]
- 46. Kimer N, Dahl EK, Gluud LL, Krag A. Antiviral therapy for prevention of hepatocellular carcinoma in chronic hepatitis C: systematic review and meta‐analysis of randomised controlled trials. BMJ Open 2012;2:e001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reig M, Marino Z, Perello C, Inarrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV‐related HCC undergoing interferon‐free therapy. J Hepatol 2016;65:719‐726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep4.1047/suppinfo.
Supporting Information Figure 1.
Supporting Information Table 1.


