Abstract
Sofosbuvir (SOF) is a nonstructural 5B polymerase inhibitor with activity in all hepatitis C virus (HCV) genotypes and is the backbone of many anti‐HCV drug regimens. SOF is converted into inactive metabolites that undergo renal excretion. Patients with an estimated glomerular filtration rate (eGFR) < 30 mL/minute/1.73 m2 may experience increased drug exposure and thus potential toxicities along with decreased efficacy due to dose reduction or drug discontinuation. This is a single‐center study evaluating safety and effectiveness of SOF‐based regimens in patients with severe renal dysfunction, defined as eGFR <30 mL/minute/1.73 m2, including those receiving concurrent hemodialysis. Data were collected from patients with HCV and severe renal dysfunction who started full‐dose (400 mg) SOF‐based antiviral therapy ± ribavirin between April 2014 and February 2016. Medical records were reviewed for demographics, medical history, laboratory, radiologic imaging, echocardiography, transplant status, and liver pathologic findings. Twenty‐nine patients were identified; 12 had cirrhosis and 4 of those had decompensated cirrhosis. Fourteen patients had undergone transplantation of liver and/or kidney and were on calcineurin inhibitors, with 42% requiring dose increases or decreases while on therapy. All patients attained viral suppression on treatment, and 97% had a sustained viral response at 12 weeks posttreatment. There were no early treatment discontinuations. One death occurred posttreatment from a non‐ST elevation myocardial infarction in a patient with a history of coronary artery disease and ischemic cardiomyopathy. Conclusion: SOF‐based regimens appear safe in a broad range of patients with severe renal dysfunction, including those with decompensated cirrhosis and liver transplant. To confirm these retrospective findings, prospective studies that include SOF and SOF metabolite measurements coupled with prospective serial monitoring of electrocardiograms and echocardiograms are needed. (Hepatology Communications 2017;1:248‐255)
Abbreviations
- CHF
congestive heart failure
- CNI
calcineurin inhibitor
- CTP
Child‐Turcotte‐Pugh
- ECG
electrocardiogram
- eGFR
estimated glomerular filtration rate
- HCV
hepatitis C virus
- LVEF
left ventricular ejection fraction
- MPGN
membranoproliferative glomerulonephritis
- RBV
ribavirin
- SOF
sofosbuvir
- SVR
sustained viral response
Introduction
Chronic hepatitis C virus (HCV) infection is a leading cause of chronic liver disease and hepatocellular carcinoma and is affecting approximately 3% of the world's population. The prevalence of HCV infection is higher in patients with significant renal disease, particularly in patients receiving hemodialysis. Importantly, HCV liver‐related morbidity and mortality appears to be higher in patients with end‐stage renal disease than in the general population. In the United States, the prevalence of HCV in patients receiving hemodialysis ranges from 5% to 13% depending on the region evaluated.1 End‐stage renal disease is associated with an increased risk for all‐cause and liver‐related mortality compared to the general population.2, 3
Sofosbuvir (SOF) is a nonstructural 5B polymerase inhibitor with activity in all HCV genotypes and is the backbone of many anti‐HCV drug regimens. SOF is initially converted into a pharmacologically active form in the liver and subsequently into an inactive metabolite that undergoes renal excretion. This poses a particular challenge for using SOF in HCV patients with significant renal disease. When compared to those with normal renal function, SOF exposure is 450% higher in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/minute/1.73 m2.4 As a result, the use of SOF is not recommended in patients receiving hemodialysis or with an eGFR <30 mL/minute/1.73 m2.
Safety and efficacy of SOF‐containing HCV regimens have been formally evaluated in over 10,000 patients in registration studies5, 6 and real‐world cohorts.7, 8, 9 Overall, the use of SOF appears safe with few significant toxicities reported. However, potential cardiotoxicity has been reported in patients taking SOF along with amiodarone.10 Additionally, cardiotoxicity has been reported for another NS5b inhibitor (BMS‐986094), and this prevented further investigation beyond phase 2 trials.11 Given the increased exposure to SOF in severe renal impairment, it is unclear if this could lead to increased cardiovascular toxicity. The purpose of this study is to report the safety and efficacy of our center's experience using full‐dose SOF‐based regimens in patients with an eGFR <30 mL/minute/1.73 m2.
Patients and Methods
Patients with chronic HCV and an eGFR <30 mL/minute/1.73 m2 with or without hemodialysis treated with full‐dose (400 mg) SOF‐based antiviral therapy with or without ribavirin (RBV) were retrospectively identified from a database of patients with HCV who were treated at two affiliated ambulatory liver clinics, including a university transplant center and a county hospital clinic, between April 2014 and February 2016. The study was approved by the University of Washington Institutional Review Board. Medical records were reviewed for demographics, medical history, laboratory, radiologic imaging, echocardiography, transplant status, and liver pathologic findings. Renal function was measured pretreatment and posttreatment. The eGFR was calculated using the Modification of Diet in Renal Disease study equation.12, 13 The pretreatment eGFR was based on the serum creatinine measurement before initiation of HCV therapy, and the posttreatment eGFR was based on the serum creatinine measurement obtained at least 12 weeks after completion of therapy. Improvement in renal function is defined as an eGFR >30 mL/minute/1.73 m2 posttreatment. Fibrosis stage was determined by liver biopsy or noninvasive testing. The most reliable fibrosis test available was reported based on the following hierarchy: liver biopsy > transient elastography > fibrosure testing > aspartate aminotransferase to platelet ratio index. Patients with a history of hepatic encephalopathy, ascites, or variceal bleeding were classified as fibrosis stage 4 and subclassified based on the Child‐Turcotte‐Pugh (CTP) score. The pretreatment viral load was defined as the most recent hepatitis C RNA quantitation obtained before initiating HCV therapy; sustained viral response (SVR) was determined by posttreatment hepatitis C RNA quantitation, which was obtained at least 12 weeks after completion of therapy. The medical record was reviewed for the presence of cardiac disease prior to, during, or following HCV treatment. Cardiac disease was categorized as congestive heart failure (CHF), valvular heart disease, myocardial infarction, or arrhythmia. Echocardiograms and electrocardiograms (ECGs) performed within 5 years of treatment initiation were reviewed. For purposes of this study, CHF was defined as a baseline left ventricular ejection fraction (LVEF) of < 55% in patients without cirrhosis or <60% in patients with cirrhosis. Standard ECG parameters from available pretreatment and posttreatment ECGs were tabulated, which included the PR interval, QRS duration, and QT/QTc interval. A prolonged QT was defined as a QTc (Bazett's formula) of > 440 milliseconds in men or >460 milliseconds in women. The cause of kidney disease was determined by a biopsy report or by documentation from the treating renal provider. Adverse events were identified by chart documentation throughout treatment. Any event that occurred on treatment was collected and reported regardless of the need for intervention.
Results
We identified 29 patients with an eGFR <30 mL/minute/1.73 m2 who were treated with full‐dose SOF‐based regimens between April 2014 and February 2016. Baseline characteristics are presented in Table 1, which shows that the identified cohort was clinically heterogeneous. Three patients had CTP class C cirrhosis, 2 had CTP class B cirrhosis, and 8 had CTP A cirrhosis. Twelve patients had undergone liver transplantation, 3 had undergone kidney transplantation, and 19 had echocardiograms for review. The etiologies of renal disease included diabetes mellitus, hypertension, glomerulonephritis, calcineurin toxicity, hepatorenal syndrome, membranoproliferative glomerulonephritis (MPGN), poststreptococcal glomerulonephritis, thrombotic microangiopathy, renal agenesis, and chronic active tubulointerstitial nephritis.
Table 1.
Patient Demographics
| Characteristic | Results |
|---|---|
| Genotypea | |
| 1 | 1 |
| 1a | 14 |
| 1b | 6 |
| 2 | 2 |
| 3 | 5 |
| 6 | 1 |
| Fibrosis stage | |
| F0‐2 | 16 |
| F3 | 0 |
| CTP A | 8 |
| CTP B | 2 |
| CTP C | 3 |
| Organ transplant | |
| None | 14 |
| Liver | 12 |
| Kidney | 3 |
| Etiology for CKD | |
| Hypertension | 4 |
| Diabetes mellitus | 7 |
| Calcineurin toxicity | 2 |
| Membranoproliferative glomerulonephritis | 5 |
| Hepatorenal syndrome | 7 |
| Other | 4 |
| Treatment prescribed SOF/RBV × 24 wk | 2 |
| SOF/LDV × 24 wk | 11 |
| SOF/LDV × 12 wk | 7 |
| SOF/LDV/RBV × 8 wk | 1 |
| SOF/LDV/RBV × 24 wk | 1 |
| SOF/DCV × 24 wk | 5 |
| SOF/DCV × 12 wk | 1 |
| SOF/DCV/RBV × 24 wk | 1 |
1 patient was GT1 with undefined subtype.
Abbreviations: CKD, chronic kidney disease; DCV, daclatasvir; LDV, ledipasvir.
TREATMENT SAFETY AND EFFICACY
All 29 patients completed a full course of either 8, 12, or 24 weeks of treatment with a 400‐mg SOF‐based regimen. A reduced SOF dose or dosing interval was not used in any of the patients receiving dialysis. Forty‐three percent (12/28) of patients were previous treatment nonresponders. End of treatment response was 100% (29/29), and an SVR at least 12 weeks after completing therapy was achieved by 97% (28/29) of patients. One patient did not have a 12‐week posttreatment viral load due to death 2 weeks after the completion of therapy.
Only 4 patients were receiving regimens containing RBV, and all these patients developed worsening anemia. Starting doses of RBV were variable: 200 mg every other day (1 patient), 200 mg daily (2), and 1,000 mg daily (1). Hemoglobin levels were monitored every 1‐2 weeks in these patients, and anemia occurred around week 3 in 3 patients. In 2 patients, the anemia resolved with dose reduction and erythropoietin‐stimulating agents; 1 patient required dose termination. The patient on every other day dosing was able to be increased to daily dosing at treatment week 4, and this was maintained through the duration of therapy.
The mean eGFR and creatinine pretreatment in those patients not receiving dialysis was 22.2 mL/minute/1.73 m2 and 3.1 mg/dL, respectively. The mean eGFR and creatinine posttreatment was 20 mL/minute/1.73 m2 and 3.3 mg/dL, respectively, showing no significant change in renal function (P = 1.0). Two patients with an eGFR <30 mL/minute/1.73 m2 from MPGN and both with cirrhosis but not on dialysis had improvement in their posttreatment eGFR to >60 mL/minute/1.73 m2 and 31 mL/minute/1.73 m2. In addition, 2 patients who had undergone liver transplantation, were on hemodialysis, and were with and without cirrhosis had a marked improvement in posttreatment eGFR to 38 mL/minute/1.73 m2 and >60 mL/minute/1.73 m2, respectively, and were able to discontinue hemodialysis (Fig. 1).
Figure 1.
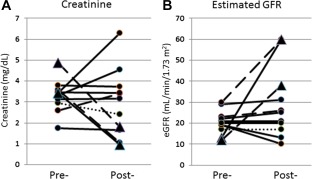
Results following treatment with a 400‐mg SOF‐based regimen. (A) Pretreatment and posttreatment creatinine levels in patients with baseline eGFR <30 mL/minute/1.73 m2 (•) and 2 patients receiving dialysis who improved (▴). There was no statistically significant change in creatinine levels or eGFR after treatment. (B) Pretreatment and posttreatment eGFR in patients with baseline eGFR <30 mL/minute/1.73 m2 (•) and 2 patients receiving dialysis who improved ((▴). Two patients with MPGN had improvement in their posttreatment estimated creatinine clearance to >60 mL/minute and 31 mL/minute. There was no statistically significant change in creatinine or eGFR after treatment in patients without cirrhosis (n = 7, solid lines), compensated cirrhosis (n = 1, dotted lines), or decompensated cirrhosis (n = 3, dashed lines). Two patients who were liver transplant recipients with renal failure from hepatorenal syndrome and who were receiving hemodialysis had marked improvement in their posttreatment eGFR to 38 mL/minute/1.73 m2 and >60 mL/minute/1.73 m2. Both were able to discontinue hemodialysis.
There were 8 patients with compensated cirrhosis. All but one of these patients were receiving dialysis prior to starting HCV therapy. There was no statistically significant change in renal function before or after treatment in this group.
There were 4 patients with decompensated cirrhosis: 3 were CTP classification C, 1 was CTP B. One of the CTP C patients had also undergone liver transplantation. There was no statistically significant change in renal function before or after treatment in this group.
There were 11 patients who had undergone liver transplantation; 2 also had cirrhosis (1 patient was CTP A, 1 CTP C). The mean eGFR and creatinine pretreatment was 11.72 mL/minute/1.73 m2 and 5.76 mg/dL, respectively, and the mean eGFR and creatinine posttreatment was 15.75 mL/minute/1.73 m2 and 7.2 mg/dL, respectively, with no statistically significant change in renal function (P = 0.65). However, the patient who had CTP C cirrhosis had posttreatment improvement in the eGFR to 38 mL/minute/1.73 m2 and was able to discontinue hemodialysis.
There were 3 patients who had undergone kidney transplantation; 2 of these patients also had CTP A cirrhosis. The mean eGFR and creatinine pretreatment was 12.67 mL/minute/1.73 m2 and 5.41 mg/dL, respectively, and the mean eGFR and creatinine posttreatment was 14.33 mL/minute/1.73 m2 and 6.17 mg/dL, respectively, with no statistically significant change in renal function (P = 0.84).
Immunosuppression levels and doses were assessed pretreatment and posttreatment in patients who had an organ transplant. All patients were receiving a calcineurin inhibitor (CNI)‐based immunosuppression; 1 patient was receiving cyclosporine, and the remainder were receiving tacrolimus. No significant differences in CNI levels were observed posttreatment compared to levels checked prior to initiating therapy. However, 42% of patients had their CNI doses reduced (3/14) or increased (3/14) while receiving HCV therapy.
Pretreatment ECGs were available for 86% (24/28) of the cohort, while only 25% (7/28) had posttreatment ECGs available. The majority of the cohort had unremarkable pretreatment ECGs read as normal (12/24), left ventricular hypertrophy, or possible left ventricular hypertrophy (4/24). Findings identified on abnormal pretreatment ECGs included prolonged QT (5/24), evidence of prior myocardial infarction (2/24), atrial fibrillation (1/24), and permanent pacemaker (1/24). The prolonged QT was universally mild, with no patients having a QT or QTc >500 milliseconds. The presence of a prolonged QT was not associated with an increased risk for adverse events. No new or worsening of baseline ECG findings were identified on the available posttreatment ECGs.
Pretreatment echocardiograms were available for 71% (20/28) of the cohort. The LVEF ranged from 43% to 72%. There were 3 patients with right heart disease, 5 patients with left heart disease, and 4 patients met study criteria for a diagnosis of CHF prior to initiating treatment. There was one cardiac‐related event in a patient on hemodialysis with cirrhosis, CHF with a pretreatment LVEF of 55% with a mildly dilated left ventricle, and with coronary artery disease with a pacemaker. The patient had a non‐ST elevation myocardial infarction and died 2 weeks after completing a 12‐week course of treatment. An echocardiogram done at the time of treatment completion showed an LVEF of 37% and a mildly dilated left ventricle with wall motion abnormalities.
Discussion
The use of SOF for HCV treatment in the setting of significant renal dysfunction continues to be a challenge due to the concern for toxicity from reduced SOF metabolite clearance. This retrospective study builds on a number of smaller studies suggesting that SOF‐based regimens can be used safely and effectively in patients with severe renal dysfunction, including those receiving hemodialysis, those with decompensated cirrhosis, and those who have undergone liver and/or kidney transplant.14, 15
All patients in this study achieved viral suppression after treatment, with 97% achieving SVR. This study included traditionally difficult to treat patients, with 43% being previously treated nonresponders and 52% being either transplant recipients or having decompensated cirrhosis. The SVR rate in this study compares favorably to those reported in other studies, which vary widely from 67% to 100%16, 17, 18, 19, 20, 21, 22, 23 (Table 2). Several aspects of these other studies may have contributed to reduced SVR rates, including the use of reduced‐dose SOF18, 19, 20 and the use of regimens with less potential to achieve SVR, such as SOF/RBV and SOF/pegylated interferon/RBV.16, 17
Table 2.
Summary of Studies Evaluating the Safety and Efficacy of Sofosbuvir‐Based Regimens in Patients with Severe Renal Dysfunction
| Author | Number with CrCl < 30 mL/min | Number on Dialysis | Regimen (%) | Cohort (%) | Safety and Comments | SVR Rate |
|---|---|---|---|---|---|---|
| Saxena et al. 16 | 13 | 5 |
SOF/PEG/RBV (5) SOF/RBV (19) SOF/SMV (48) SOF/SMV/RBV (15) |
GT1a/b (77) Liver transplant (50) Kidney transplant (8) Cirrhosis (64) Decompensated cirrhosis (73) |
‐Increased anemia in those receiving RBV ‐Worsening of RF in those with CrCl <45 ‐GFR ≤ 30 and GFR 31‐45 had similar safety outcomes ‐Total of 72 patients enrolled in study, only 18 had eGFR < 30 mL/min. |
81% |
| Hundemer et al. 17 | 4 | 2 |
SOF/SMV (50) SOF/RBV (33) SOF/PEG/RBV (16) |
Liver transplant (17) Cirrhosis (50) |
‐Increased anemia in those receiving RBV (3) ‐Worsening renal function (1) |
67% |
| Bhamidimarri et al. 18 | 3 | 12 | Half dose SOF/SMV (100) |
GT1a/b (100) Cirrhosis (60) Decompensated cirrhosis (7) |
‐No serious adverse events | 87% |
| Perumpail et al. 19 | 1 | 1 | Half dose SOF/SMV (100) | Liver transplant (100) | ‐No serious adverse events | 100% |
| Desnoyer et al. 20 | 0 | 12 | SOF daily (58) vs. SOF TIW (41) + SMV, DCV, or LDV |
GT1a/b (92) Cirrhosis (83) |
‐No serious adverse events ‐SOF‐007 plasma not linked with adverse events |
82% |
| Nazaro et al. 21 | 2 | 15 | SOF/SMV (100) |
GT1a/b (100) Cirrhosis (47) |
‐Worsening anemia requiring blood transfusion (1) | 100% |
| Singh et al. 22 | 0 | 8 |
SOF/SMV (50) SOF/LDV (50) |
GT1a/1b (75) Cirrhosis (37) |
‐Uncontrolled nausea and vomiting requiring hospitalization | 100% |
| Dumortier et al.23 | 9 | 35 |
SOF/RBV (14) SOF/RBV/PEG (4) SOF/DCV ± RBV (60) SOF/SMV (22) |
GT1a/1b (56) Cirrhosis (54) Liver transplant (22) Kidney transplant (34) Decompensated cirrhosis (4) |
‐2 deaths; liver failure at 12 weeks of treatment, and unknown reasons 2 months after completing therapy ‐Anemia requiring blood transfusion (3) |
86% |
| Cox‐North et al. (this study) | 9 | 20 |
SOF/LDV (65) SOF/LDV/RBV (6) SOF/DCV (20) SOF/RBV (6) |
GT1a/b (69) Liver transplant (38) Kidney transplant (10) Cirrhosis (45) Decompensated cirrhosis (17) |
‐One death due to myocardial infarction | 97% |
Abbreviations: PEG, pegylated interferon; SMV, simeprevir; TIW, three times a week.
All patients completed either 8, 12, or 24 weeks of treatment, and reported adverse events were rare. Anemia developed in 4 patients treated with RBV and improved with either dose reduction or discontinuation of RBV. There was one cardiac‐related event that occurred at roughly 12 weeks of therapy in a patient with cirrhosis and pre‐existing CHF, coronary artery disease on hemodialysis, a baseline LVEF of 55%, and marked worsening of LVEF to 36% at the end of treatment in a setting of non‐ST elevation myocardial infarction. For the majority of patients, including those with decompensated cirrhosis and previous liver or kidney transplants, kidney function remained unchanged before and after hepatitis C treatment with an SOF‐based regimen. There were 3 patients that had a 1‐fold to 2‐fold increase in creatinine from baseline at the end of treatment (Fig. 1). This was associated with a six‐ to eight‐point drop in eGFR but no significant change in serum electrolytes, reported urine output, or albumin. It is possible that these changes could be related to increased exposure to SOF metabolites, highlighting the need for prospective pharmacokinetic studies evaluating different SOF doses and dosing regimens in those with severe renal dysfunction. There were 4 patients with marked improvement in underlying renal function after completing treatment, with 2 patients being able to discontinue hemodialysis.
It is worth noting that worsening renal function has been reported in subjects receiving SOF‐based regimens who have moderate renal impairment16 or have undergone kidney transplant.24 Because no control was available for these studies, it is difficult to attribute the worsening renal function to SOF exposure versus natural progression of disease. Furthermore, other studies involving kidney transplant recipients did not report worsening renal function with SOF‐based regimens in this population.25, 26
Other studies that have evaluated the safety and efficacy of SOF‐based regimens in patients with severe renal impairment are summarized in Table 2. At the time of this publication and to the best of our knowledge, outcomes have been reported for a total of 150 patients with severe renal dysfunction treated with SOF‐based regimens. The most common adverse event reported in other studies was anemia in association with RBV use.
Mildly prolonged QTc was noted in 21% of the patients prior to starting SOF‐based therapy; however, there was not a sufficient amount of posttreatment ECG data available for meaningful assessment of cardiotoxicity in this study. Given the increased exposure to SOF metabolites in severe renal impairment and the potential risk of cardiotoxicity, there is a need for prospective studies assessing QTc over time in patients with severe renal dysfunction being treated with SOF‐based therapies.
There were several limitations to our study. The retrospective design and small sample size did not allow us to control for other variables, such as treatment regimen, liver fibrosis stage, genotype, transplant status, degree of cirrhosis compensation, or medical comorbidities. There was one serious adverse cardiac event in our study, but we were unable to draw any conclusions about the safety of SOF regimens in those with underlying cardiac disease due to these limitations.
The clinical observations and experiences from this study demonstrate that full‐dose SOF‐based regimens can be tolerated and effective in patients with significant renal disease with and without cirrhosis and/or having undergone liver or kidney transplantation. Our data are particularly compelling for treatment of posttransplant HCV recurrence.14 Renal insufficiency is common in recipients of liver transplantation and complicates the decision to treat. Although there are HCV regimens in evaluation27 and approved28 for use in patients with an eGFR <30 mL/minute/1.73 m2, SOF‐based regimens are often preferred in patients who have undergone liver transplantation due to high efficacy and less potential for drug–drug interactions.15 The study findings are also of particular interest in those with CTP class B or C cirrhosis as only SOF‐based treatment regimens are currently approved by the U.S. Food and Drug Administration in this population. Finally, the results also suggest that in a select patient population, such as patients with HCV‐induced MPGN, eradicating hepatitis C may significantly improve underlying renal function and prevent or reduce the need for dialysis. Prospective studies are needed to confirm our findings, which suggest that SOF‐based regimes are safe and effective in patients with renal dysfunction.
Author names in bold designate shared co‐first authorship.
Potential conflict of interest: Dr. Cox‐North is on the speaker bureau for Merck and is on the NP HBV Advisory board for Gilead. Dr. Landis is an investigator for Gilead and Abbvie regarding treatment trials for hepatitis C.
The authors did not receive financial support to conduct this study.
REFERENCES
- 1. Bianco A, Bova F, Nobile CG, Pileggi C, Pavia M. Healthcare workers and prevention of hepatitis C virus transmission: exploring knowledge, attitudes and evidence‐based practices in hemodialysis units in Italy. BMC Infect Dis 2013;13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fabrizi F, Lunghi G, Ganeshan SV, Martin P, Messa P. Hepatitis C virus infection and the dialysis patient. Semin Dial 2007;20:416‐422. [DOI] [PubMed] [Google Scholar]
- 3. Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta‐analysis of observational studies. J Viral Hepat 2007;14:697‐703. [DOI] [PubMed] [Google Scholar]
- 4. Sofosbuvir [package insert] . Foster City, CA: Gilead Sciences; 2015. http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/sovaldi/sovaldi_pi. Accessed September 2016.
- 5. Alqahtani SA, Afdhal N, Zeuzem S, Gordon SC, Mangia A, Kwo P, et al. Safety and tolerability of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic hepatitis C virus genotype 1 infection: analysis of phase III ION trials. Hepatology 2015;62:25‐30. [DOI] [PubMed] [Google Scholar]
- 6. Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370:1879‐1888. [DOI] [PubMed] [Google Scholar]
- 7. Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real‐world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment‐naive genotype 1 hepatitis C‐infected patients. Hepatology 2016;64:405‐414. [DOI] [PubMed] [Google Scholar]
- 8. Sulkowski MS, Vargas HE, Di Bisceglie AM, Kuo A, Reddy KR, Lim JK, et al. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real‐world patients with HCV genotype 1 infection. Gastroenterology 2016;150:419‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology 2016;151:457‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klein R, Struble K, Morin S. Food and Drug Administration. FDA hepatitis update—important safety information: Harvoni and Sovaldi; March 21, 2015. https://content.govdelivery.com/accounts/USFDA/bulletins/f97c71. Accessed September 2016.
- 11. Ahmad T, Yin P, Saffitz J, Pockros PJ, Lalezari J, Shiffman M, et al. Cardiac dysfunction associated with a nucleotide polymerase inhibitor for treatment of hepatitis C. Hepatology 2015;62:409‐416. [DOI] [PubMed] [Google Scholar]
- 12. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247‐254. [DOI] [PubMed] [Google Scholar]
- 14. Berenguer M, Palau A, Aguilera V, Rayon JM, Juan FS, Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant 2008; 8:679–687. [DOI] [PubMed] [Google Scholar]
- 15. Burgess S, Partovi N, Yoshida EM, Erb SR, Azalgara VM, Hussaini T. Drug interactions with direct‐acting antivirals for hepatitis C: implications for HIV and transplant patients. Ann Pharmacother 2015; 49:674–687. [DOI] [PubMed] [Google Scholar]
- 16. Saxena V, Koraishy FM, Sise ME, Lim JK, Schmidt M, Chung RT, et al.; HCV‐TARGET . Safety and efficacy of sofosbuvir‐containing regimens in hepatitis C‐infected patients with impaired renal function. Liver Int 2016;36:807‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hundemer GL, Sise ME, Wisocky J, Ufere N, Friedman LS, Corey KE, et al. Use of sofosbuvir‐based direct‐acting antiviral therapy for hepatitis C viral infection in patients with severe renal insufficiency. Infect Dis (Lond) 2015;47:924‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhamidimarri KR, Czul F, Peyton A, Levy C, Hernandez M, Jeffers L, et al. Safety, efficacy and tolerability of half‐dose sofosbuvir plus simeprevir in treatment of Hepatitis C in patients with end stage renal disease. J Hepatol 2015;63:763‐765. [DOI] [PubMed] [Google Scholar]
- 19. Perumpail RB, Wong RJ, Ha LD, Pham EA, Wang U, Luong H, et al. Sofosbuvir and simeprevir combination therapy in the setting of liver transplantation and hemodialysis. Transpl Infect Dis 2015;17:275‐278. [DOI] [PubMed] [Google Scholar]
- 20. Desnoyer A, Pospai D, Lê MP, Gervais A, Heurgué‐Berlot A, Laradi A, et al. Pharmacokinetics, safety and efficacy of a full dose sofosbuvir‐based regimen given daily in hemodialysis patients with chronic hepatitis C. J Hepatol 2016;65:40‐47. [DOI] [PubMed] [Google Scholar]
- 21. Nazario HE, Ndungu M, Modi AA. Sofosbuvir and simeprevir in hepatitis C genotype 1‐patients with end‐stage renal disease on haemodialysis or GFR <30 ml/min. Liver Int 2016;36:798‐801. [DOI] [PubMed] [Google Scholar]
- 22. Singh T, Guirguis J, Anthony S, Rivas J, Hanouneh I, Alkhouri N. Sofosbuvir‐based treatment is safe and effective in patients with chronic hepatitis C infection and end stage renal disease: a case series. Liver Int 2016;36:802–806. [DOI] [PubMed] [Google Scholar]
- 23. Dumortier J, Bailly F, Pageaux GP, Vallet‐Pichard A, Radenne S, Habersetzer F, et al. Sofosbuvir‐based antiviral therapy in hepatitis C virus patients with severe renal failure. Nephrol Dial Transplant 2016; doi: 10.1093/ndt/gfw348. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24. Colombo M, Aghemo A, Liu H, Zhang J, Dvory‐Sobol H, Hyland R, et al. Treatment with ledipasvir‐sofosbuvir for 12 or 24 weeks in kidney transplant recipients with chronic hepatitis C virus genotype 1 or 4 infection: a randomized trial. Ann Intern Med 2017;166:109‐117. [DOI] [PubMed] [Google Scholar]
- 25. Fernández I, Muñoz‐Gómez R, Pascasio JM, Baliellas C, Polanco N, Esforzado N, et al. Efficacy and tolerability of interferon‐free antiviral therapy in kidney transplant recipients with chronic hepatitis C. J Hepatol 2017;66:718‐723. [DOI] [PubMed] [Google Scholar]
- 26. Lin MV, Sise ME, Pavlakis M, Amundsen BM, Chute D, Rutherford AE, et al. Efficacy and safety of direct acting antivirals in kidney transplant recipients with chronic hepatitis C virus infection. PLoS One 2016;11:e0158431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pugatch D, Lei Y, Kosloski MP, Mensa FJ, Gane E. EXPEDITION‐IV: safety and efficacy of GLE/PIB in adults with renal impairment and chronic hepatitis C virus genotype 1‐6 infection [Abstract LB‐11]. AASLD Liver Meeting. November 11‐15, 2016. Boston, MA.
- 28. Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H Jr, et al. Grazoprevir plus elbasvir in treatment‐naive and treatment‐experienced patients with hepatitis C virus genotype 1 infection and stage 4‐5 chronic kidney disease (the C‐SURFER study): a combination phase 3 study. Lancet 2015;386:1537‐1545. [DOI] [PubMed] [Google Scholar]


