Abstract
In patients with nonalcoholic fatty liver disease (NAFLD), prognosis and outcome, especially non‐liver‐related mortality, remain incompletely elucidated. We clarified the mortality from all causes in patients with NAFLD. A total of 4,073 patients with NAFLD diagnosed by ultrasonography were enrolled. We investigated the causes of death and analyzed the mortality from non‐liver‐related diseases according to the degrees of steatosis and fibrosis using the competing risk method. We used the NAFLD fibrosis score (NFS) to assess fibrosis severity and the ultrasonography fatty liver score to evaluate steatosis severity. The numbers of patients with NFS indicating low, intermediate, and high probabilities of advanced fibrosis were 2,451 (60.2%), 1,462 (35.9%), and 160 (3.9%), respectively. Of the 4,073 patients, 179 died during follow‐up, but only nine deaths were due to liver‐related diseases. Of the remaining 170 patients who died due to non‐liver‐related diseases, 83 (48.8%), 42 (24.7%), and 45 (26.5%) patients died due to malignancies, cerebrovascular and cardiovascular diseases, and benign diseases (excluding cerebrovascular and cardiovascular diseases), respectively. Multivariate analysis showed that the intermediate and high NFS groups were independently associated with each disease category: hazard ratio (HR) 2.163 (95% confidence interval [CI], 1.354‐3.457) and HR 4.814 (95% CI, 2.323‐9.977) for malignancies; HR 2.265 (95% CI, 1.141‐4.497) and HR 8.482 (95% CI, 3.558‐20.220) for cerebrovascular and cardiovascular diseases; and HR 3.216 (95% CI, 1.641‐6.303) and HR 5.558 (95% CI, 1.923‐16.070) for benign diseases, respectively. Conversely, the status of steatosis was not associated with risk of mortality in multivariate analysis. Conclusion: Progression of liver fibrosis severity was associated with mortality from various non‐liver‐related causes in patients with NAFLD. (Hepatology Communications 2017;1:928–945)
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CI
confidence interval
- FIB‐4
fibrosis‐4
- HR
hazard ratio
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NFS
nonalcoholic fatty liver disease fibrosis score
- US
ultrasonography
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease and its prevalence is increasing worldwide.1, 2 In Japan, approximately 14% of screened patients reportedly have NAFLD.3 NAFLD is histologically classified into either nonalcoholic fatty liver (NAFL) or nonalcoholic steatohepatitis (NASH). The histologic findings of NAFL demonstrate hepatic steatosis without evidence of hepatocellular injury (e.g., ballooning of hepatocytes), and NAFL usually follows a benign clinical course. Conversely, the histologic findings of NASH are characterized by the presence of hepatic steatosis and inflammation with distinctive hepatocyte injury (e.g., ballooning degeneration), and NASH is considered to be a potentially health‐threatening disease that may progress to cirrhosis in 10%‐15% of patients.4
Some patients with NAFLD, including NAFL, however, may develop cirrhosis if liver transplantation is not performed and may subsequently die from complications of portal hypertension, liver failure, or liver cancer.5, 6, 7, 8 NAFLD has been reported to be a common cause of liver transplantation and, in particular, the second most common cause of liver transplantation in large medical centers.9 When compared with the general population of the same age and sex, patients with NAFLD were found to have a significantly higher mortality rate.10 However, the long‐term prognosis is not the same across the spectrum of the disease. Patients with NAFLD with minimal features of liver injury may follow a relatively benign clinical course, with overall mortality similar to the general population of similar age and sex,11 whereas patients with NAFLD with advanced liver fibrosis appear to have lower long‐term survival.4, 12 NAFLD typically develops as a result of various metabolic disorders, such as obesity, diabetes, and dyslipidemia, but it is not yet clear how prognosis and outcome, especially non‐liver‐related mortality, are related to the degrees of steatosis and fibrosis in patients with NAFLD.
Although consensus is lacking regarding optimal surrogate indicators of NAFLD and liver fibrosis in NAFLD in large‐scale, population‐based, epidemiological studies, a number of noninvasive tools have been developed. For the diagnosis of steatosis, abdominal ultrasonography (US) has been shown to have a sufficient degree of diagnostic accuracy.13 For the diagnosis of liver fibrosis in NAFLD, various noninvasive tools have been developed; these include serum markers and mechanical measures of liver stiffness, both of which have been correlated with hepatic fibrosis. Of these, the NAFLD fibrosis score (NFS) has proved to be the most accurate in comparison studies and has been validated to identify or exclude advanced fibrosis in patients with a diagnosis of NAFLD.14
In the present study, we compared mortality due to liver‐related and non‐liver‐related diseases in patients with NAFLD. In addition, we investigated the mortality from various categories of non‐liver‐related diseases based on the degree of steatosis and fibrosis in the same cohort. We used the competing risk method for analysis of mortality from different diseases.
Patients and Methods
PATIENTS
The study protocol complied with the Helsinki Declaration and was approved by the institutional review board of Ogaki Municipal Hospital. All patients provided written informed consent for the use of their clinical data.
Ogaki Municipal Hospital is the only general hospital located in a region of 400,000 inhabitants; it employs approximately 200 specialists, including more than 15 gastroenterologists. Therefore, a large number of patients with various diseases, including NAFLD, visit the hospital as outpatients. In addition, the number of individuals who drop out of the cohort due to relocation is small in this region. There is also close contact between family medicine clinics, community hospitals, and our hospital, including the sharing of patient mortality data (for patients who did not die in our hospital).
Between January 2006 and December 2015, 13,368 consecutive patients were diagnosed with fatty liver by US at Ogaki Municipal Hospital. Of these, 4,664 met the following inclusion criteria: 1) diagnostic criteria fulfilled for NAFLD, 2) height and weight available, 3) at least two visits to the hospital, 4) follow‐up duration of greater than 1 year, and 5) no evidence of malignancies for at least 1 year from the start of the follow‐up period. We then excluded patients who met the following criteria: 1) score of 0 on the US fatty liver scoring system (the majority had local fatty liver) (n = 467), 2) insufficient evaluation of the US fatty liver scoring system due to poor image quality (n = 50), and 3) incomplete clinical data (n = 74). Consequently, 4,073 patients were enrolled in the study (Fig. 1).
Figure 1.
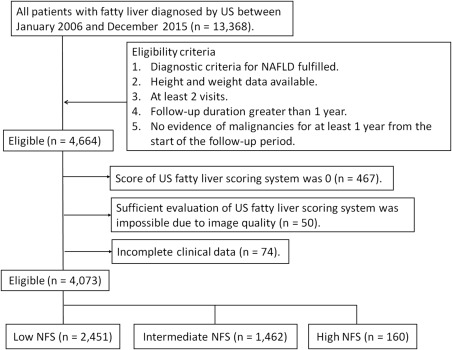
Flowchart of the patient selection process.
The date of the US examination was defined as the start of follow‐up. The end of follow‐up was defined as the date of the final visit for patients who had not died and as the date of death for patients who died during follow‐up.
DIAGNOSTIC CRITERIA OF NAFLD
NAFLD was diagnosed based on the latest guidelines established by the American Association for the Study of Liver Diseases,15 as follows: 1) fatty change of the liver was observed by imaging; 2) no marked alcohol drinking habit was present (ethanol intake of < 210 g per week for men and <140 g per week for women); 3) no presence of other factors inducing fatty change of the liver; and 4) no chronic liver disease with clear etiology, such as viruses (hepatitis C virus and hepatitis B virus), primary biliary cholangitis, or autoimmune hepatitis.
CLINICAL AND LABORATORY DATA
Patient age, sex, height, weight, resting recumbent blood pressure, presence or absence of smoking, and amount of drinking were recorded. Blood counts and blood biochemistry tests were conducted using standard methods in a fasting state within 3 months of US examination.
Patients were assigned a diagnosis of diabetes mellitus if they had documented use of oral hypoglycemic medication, a random glucose level >200 mg/dL, or a fasting plasma glucose level >126 mg/dL.16
US FATTY LIVER SCORING SYSTEM
In the present study, patients underwent US examination because of clinical symptoms or during medical check‐ups (in the community or workplace) or assessments by physicians. Subsequently, the archived US images were reviewed to assess fatty liver based on the previously reported US fatty liver scoring system (0‐6 points)17 (Table 1). The US images were evaluated by two experienced physicians, a hepatologist (T.K.) and a radiologist (Y.S.), who specialize in hepatic imaging. Both were blinded to all clinical data.
Table 1.
Fatty Liver Scoring System Using US
| US Findings | Score |
|---|---|
| Bright liver and hepatorenal echo contrast | Score of A |
| Bright liver and hepatorenal echo contrast were negative. | 0 |
| Bright liver was positive or hepatorenal echo contrast was positive. | 1 |
| Liver was mildly bright, and hepatorenal echo contrast was positive. | 2 |
| Liver was very bright, and hepatorenal echo contrast was positive. | 3 |
| Deep Attenuation | Score of B |
| Deep attenuation was negative. | 0 |
| Diaphragm was obscure but could be distinguished. | 1 |
| Diaphragm could not be distinguished. | 2 |
| Vessel Blurring | Score of C |
| Vessel blurring was negative. | 0 |
| The borders of intrahepatic vessels were unclear and the lumens of intrahepatic vessels were narrowed. | 1 |
| Add scores of A, B, and C if score of A is more than 1. | |
| Total score is 0 if score of A is 0. |
LIVER FIBROSIS MARKER
In the present study, NFS was used to assess the severity of fibrosis and was calculated based on the following previously published formula: −1.675 + 0.037 × age (years) + 0.094 × body mass index (kg/m2) + 1.13 × impaired fasting glycemia or diabetes (yes = 1, no = 0) + 0.99 × aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio – 0.013 × platelet count ( × 109/L) – 0.66 × albumin (g/dL).14 Two cutoff points were selected to categorize subjects with NAFLD into three groups, including those with low probability (NFS < −1.455), intermediate probability (NFS −1.455 to 0.676), and high probability of advanced fibrosis (NFS > 0.676).
In addition, we used the fibrosis‐4 (FIB‐4) index as another liver fibrosis marker. This index was calculated as AST (IU/L) × age (years)/platelet count ( × 109/L) × ALT (IU/L)1/2 and has been reported to be useful in diagnosing liver fibrosis in patients with NAFLD. Published cutoff values were used to define a 1.30 probability of advanced fibrosis.18
CAUSES OF DEATH
Causes of death were categorized by specialists at our hospital or by family physicians (if patients died outside our hospital) using International Statistical Classification of Diseases and Related Health Problems 10 codes.19 All determinations of causes of death were performed retrospectively by collecting and analyzing data from patient medical records.
STATISTICAL ANALYSIS
Continuous variables were expressed as medians (interquartile range). The Kruskal‐Wallis test was used for continuous variables, and the chi‐square test with Fisher's exact test was used for categorical variables.
Actuarial analysis of cumulative mortality from each categorized disease (liver‐related disease, including primary liver cancer; malignancies, excluding primary liver cancer; cerebral and cardiovascular disease; and benign diseases, excluding liver‐related, cerebrovascular, and cardiovascular diseases) was performed using the cumulative incidence with the competing risks method; differences were tested using the Gray test with Holm correction. For multivariate analysis, Fine and Gray proportional hazards models with the backward elimination method20 were used for the assessment of hazard ratios (HRs) for disease‐related mortality. As covariates for multivariate analysis, we used the US fatty liver grades and liver fibrosis grades (NFS and FIB‐4 categories).
Statistical significance was defined as P < 0.05. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria).21 More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.
Results
PATIENT CHARACTERISTICS AND CAUSES OF DEATH
The characteristics of all 4,073 patients are summarized in Table 2. Of the study patients with US fatty liver scores, 231 (5.7%), 1,034 (25.4%), 1,033 (25.4%), 1,029 (25.3%), 531 (13.0%), and 215 (5.3%) had scores of 1, 2, 3, 4, 5, and 6, respectively. US fatty liver scores were then used to group study patients based on their fatty liver grade as follows: 1 or 2 points, low‐grade fatty liver group (n = 1,265; 31.1%); 3 or 4 points, intermediate‐grade fatty liver group (n = 2,062; 50.6%); and 5 or 6 points, high‐grade fatty liver group (n = 746; 18.3%). The median NFS was –1.83 (−2.85 to −0.81). The numbers of patients in the low, intermediate, and high NFS groups were 2,451 (60.2%), 1,462 (35.9%), and 160 (3.9%), respectively. The median follow‐up duration was 7.1 (4.8‐9.2) years. Of the 4,073 patients, 179 (4.4%) died during follow‐up; causes of death are shown in Table 2. In 5.0% (9/179) of patients, mortality was due to liver‐related diseases, including primary liver cancer. Conversely, 95.0% (170/179) of deaths were due to non‐liver‐related diseases; among these were a variety of malignancies (48.8%, 83/170), including hematologic malignancies (primary liver cancer was excluded). Deaths due to non‐liver‐related diseases other than malignancy were caused by cerebrovascular and cardiovascular diseases in 24.7% (42/170) of patients and by benign diseases, excluding liver‐related, cerebrovascular, and cardiovascular diseases, in 26.5% (45/170) of patients.
Table 2.
Patient Characteristics (n = 4,073)
| Age (years)a | 61.0 (52.0 ‐ 69.0) |
| Sex (female/male) | 1,871/2,202 |
| Smoking (yes/no/unknown) | 1,059/2,726/288 |
| BMI (kg/m2)a | 25.0 (23.1 ‐ 27.4) |
| AST (IU/L)a | 24 (19 ‐ 33) |
| ALT (IU/L)a | 26 (18 ‐ 49) |
| Gamma‐glutamyl transpeptidase (IU/L) | 32 (22 ‐ 55) |
| Albumin (g/dL)a | 4.4 (4.2 ‐ 4.6) |
| Total bilirubin (mg/dL)a | 0.6 (0.5 ‐ 0.8) |
| Total cholesterol (mg/dL)a | 204 (179 ‐ 228) |
| Triglycerides (mg/dL)a | 133 (94 ‐ 191) |
| Low‐density lipoprotein cholesterol (mg/dL)a | 124 (103 ‐ 146) |
| High‐density lipoprotein cholesterol (mg/dL)a | 47 (40 ‐ 56) |
| Platelet count (×104/mm3)a | 24.5 (20.6 ‐ 29.4) |
| Fasting plasma glucose (mg/dL)a | 92.0 (85.0 ‐ 100.0) |
| Hemoglobin A1c (%)a | 5.6 (5.2 ‐ 7.0) |
| Systolic blood pressure (mmHg)a | 146 (132 ‐ 163) |
| Diastolic blood pressure (mmHg)a | 77 (68 ‐ 87) |
| Diabetes mellitus (yes/no) | 1,254/2,819 |
| Follow‐up duration (years)a | 7.1 (4.8 ‐ 9.2) |
| US fatty liver scores (1/2/3/4/5/6) | 231/1,034/1,033/1,029/531/215 |
| NFSa | −1.83 (−2.85 to −0.81) |
| FIB‐4 indexa | 1.16 (0.81 ‐ 1.62) |
| Deaths | 179 |
| Causes | |
| Liver‐related diseases | 9/179 (5.0%) |
| Primary liver cancer | 5 |
| Liver failure | 4 |
| Non‐liver‐related diseases | 170/179 (95.0%) |
| Malignancies | 83/170 (48.8%) |
| Digestive malignancies | 36 |
| Respiratory malignancies | 19 |
| Hematological malignancies | 13 |
| Other | 15 |
| Nonmalignant diseases | 87/170 (51.2%) |
| Digestive diseases | 4 |
| Respiratory diseases | 27 |
| Cardiovascular disease | 33 |
| Cerebrovascular diseases | 9 |
| Injury | 3 |
| Other | 11 |
Values are expressed as medians (interquartile range).
Abbreviation: BMI, body mass index.
PATIENT CHARACTERISTICS ACCORDING TO THE SEVERITY OF LIVER FIBROSIS
The baseline characteristics of the 4,073 study patients grouped by NFS are summarized in Table 3. There were significant differences among the three groups in all parameters except for total bilirubin levels.
Table 3.
Characteristics Of Patients Stratified By Nfs Grades (n = 4,073)
| Low NFS (n = 2,451) | Intermediate NFS (n = 1,462) | High NFS (n = 160) | P Value | |
|---|---|---|---|---|
| Age (years)a | 57.0 (45.0 ‐ 64.0) | 67.0 (61.0 ‐ 73.0) | 73.5 (68.0 ‐ 77.3) | <0.001 |
| Sex (female/male) | 1,095/1,356 | 685/777 | 91/69 | 0.008 |
| Smoking (yes/no/unknown) | 693/1,574/184 | 335/1,032/95 | 31/120/9 | <0.001 |
| BMI (kg/m2)a | 24.8 (22.9 ‐ 27.1) | 25.4 (23.3 ‐ 27.7) | 25.9 (23.9 ‐ 28.9) | <0.001 |
| AST (IU/L)a | 23 (19 ‐ 31) | 24 (19 ‐ 33) | 28 (21 ‐ 49) | <0.001 |
| ALT (IU/L)a | 28 (19 ‐ 46) | 24 (17 ‐ 38) | 23 (14 ‐ 42) | <0.001 |
| Gamma‐glutamyl transpeptidase (IU/L) | 33 (22 ‐ 57) | 31 (21 ‐ 50) | 36 (22 ‐ 73) | <0.001 |
| Albumin (g/dL)a | 4.5 (4.3 ‐ 4.7) | 4.3 (4.1 ‐ 4.5) | 4.1 (3.7 ‐ 4.3) | <0.001 |
| Total bilirubin (mg/dL)a | 0.6 (0.5 ‐ 0.8) | 0.6 (0.5 ‐ 0.8) | 0.6 (0.5 ‐ 0.9) | 0.150 |
| Total cholesterol (mg/dL)a | 208 (182 ‐ 231) | 198 (175 ‐ 221) | 183 (155 ‐ 214) | <0.001 |
| Triglycerides (mg/dL)a | 136 (96 ‐ 193) | 130 (94 ‐ 188) | 121 (85 ‐ 176) | 0.041 |
| Low‐density lipoprotein cholesterol (mg/dL)a | 128 (107 ‐ 150) | 120 (97 ‐ 141) | 110 (85 ‐ 136) | <0.001 |
| High‐density lipoprotein cholesterol (mg/dL)a | 48 (41 ‐ 57) | 46 (39 ‐ 55) | 43 (35 ‐ 51) | <0.001 |
| Platelet count (×104/mm3)a | 27.4 (23.7 ‐ 32.0) | 21.2 (18.4 ‐ 24.0) | 16.1 (12.8 ‐ 18.7) | <0.001 |
| Fasting plasma glucose (mg/dL)a | 92 (86 ‐ 99) | 93 (84 ‐ 104) | 89 (78 ‐ 100) | <0.001 |
| Hemoglobin A1c (%)a | 5.4 (5.1 ‐ 5.9) | 6.3 (5.5 ‐ 8.0) | 6.9 (6.0 ‐ 8.1) | <0.001 |
| Systolic blood pressure (mmHg)a | 143 (130 ‐ 159) | 152 (136 ‐ 167) | 154 (138 ‐ 173) | <0.001 |
| Diastolic blood pressure (mmHg)a | 80 (78 ‐ 88) | 75 (66 ‐ 84) | 71 (62 ‐ 83) | <0.001 |
| Diabetes mellitus (yes/no) | 393/2,058 | 750/712 | 111/49 | <0.001 |
| Follow‐up duration (years)a | 6.7 (4.5 ‐ 8.7) | 7.6 (5.5 ‐ 9.6) | 6.5 (4.5 ‐ 9.4) | <0.001 |
| US fatty liver scores (1/2/3/4/5/6) | 128/616/610/616/331/150 | 93/374/372/374/189/60 | 10/44/51/39/11/5 | 0.038 |
| NFSa | −2.61 (−3.44 to −2.00) | −0.67 (−1.07 to −0.18) | 1.06 (0.89 to −1.44) | <0.001 |
| Deaths | 57 (2.3%) | 97 (6.6%) | 25 (15.6%) | <0.001 |
| Causes | ||||
| Liver‐related diseases | 3/57 (5.3%) | 4/97 (4.1%) | 2/25 (8.0%) | 0.728 |
| Primary liver cancer | 2 | 2 | 1 | |
| Liver failure | 1 | 2 | 1 | |
| Non‐liver‐related diseases | 54/57 (94.7%) | 93/97 (95.6%) | 23/25 (92.0%) | |
| Malignancies | 29/54 (53.7%) | 44/93 (47.3%) | 10/23 (43.5%) | |
| Digestive malignancies | 13 | 19 | 4 | |
| Respiratory malignancies | 10 | 6 | 3 | |
| Hematological malignancies | 2 | 8 | 3 | |
| Other | 4 | 11 | 0 | |
| Nonmalignant diseases | 25/54 (46.3%) | 49/93 (52.7%) | 13/23 (56.5%) | |
| Digestive diseases | 1 | 2 | 1 | |
| Respiratory diseases | 7 | 18 | 2 | |
| Cardiovascular disease | 9 | 17 | 7 | |
| Cerebrovascular diseases | 4 | 4 | 1 | |
| Injury | 1 | 1 | 1 | |
| Other | 3 | 7 | 1 |
Values are expressed as medians (interquartile range).
Abbreviation: BMI, body mass index.
The mortality rate from all causes increased as NFS increased: 2.3% (n = 57) in the low NFS group, 6.6% (n = 97) in the intermediate NFS group, and 15.6% (n = 25) in the high NFS group (P < 0.001). Mortality was due to liver‐related diseases, malignancies, cerebrovascular and cardiovascular diseases, and benign diseases, respectively, in 3 (5.3%), 29 (50.9%), 13 (22.8%), and 12 (21.1%) low NFS patients; in 4 (4.1%), 44 (45.4%), 21 (21.6%), and 28 (28.9%) intermediate NFS patients; and in 2 (8.0%), 10 (40.0%), 8 (32.0%), and 5 (20.0%) high NFS patients. The mortality rates for each disease category did not differ among the three groups.
CUMULATIVE MORTALITY RATES FOR LIVER‐RELATED AND NON‐LIVER‐RELATED DISEASES
The 3‐, 5‐, and 10‐year cumulative mortality rates for liver‐related diseases were 0.1% (95% confidence interval [CI], 0.0‐0.2), 0.1% (95% CI, 0.0‐0.2), and 0.4% (95% CI, 0.2‐0.9), respectively (Figure 2). Conversely, the 3‐, 5‐, and 10‐year cumulative mortality rates for non‐liver‐related diseases were 0.8% (95% CI, 0.6‐1.1), 2.0% (95% CI, 1.6‐2.5), and 6.9% (95% CI, 5.8‐8.1), respectively.
Figure 2.
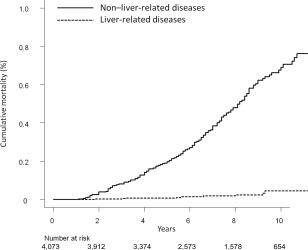
Cumulative mortality rates for liver‐related and non‐liver‐related diseases. The 3‐, 5‐, and 10‐year cumulative mortality rates for liver‐related diseases were 0.1%, 0.1%, and 0.4%, respectively (dotted line). The 3‐, 5‐, and 10‐ cumulative mortality rates for non‐liver‐related diseases were 0.8%, 2.0%, and 6.9%, respectively (solid line).
CUMULATIVE MORTALITY FROM CATEGORIZED NON‐LIVER‐RELATED DISEASES BASED ON THE SEVERITY OF FATTY LIVER
Mortality curves for malignancies in study patients according to fatty liver severity are shown in Fig. 3A. The respective 3‐, 5‐, and 10‐year cumulative mortality rates were 0.4% (95% CI, 0.2‐0.9), 1.0% (95% CI, 0.5‐1.7), and 3.6% (95% CI, 2.2‐5.4) in the low‐grade fatty liver group; 0.5% (95% CI, 0.2‐0.8), 1.3% (95% CI, 0.9‐1.9), and 3.6% (95% CI, 2.6‐4.8) in the intermediate‐grade fatty liver group; and 0.2% (95% CI, 0.0‐0.8), 0.3% (95% CI, 0.1‐1.1), and 1.0% (95% CI, 0.4‐2.3) in the high‐grade fatty liver group. Cumulative mortality differed significantly between the low‐ and high‐grade groups (P = 0.038) and between the intermediate‐ and high‐grade groups (P = 0.038). Conversely, the cumulative mortality did not differ between the low‐ and intermediate‐grade groups (P = 0.988).
Figure 3.
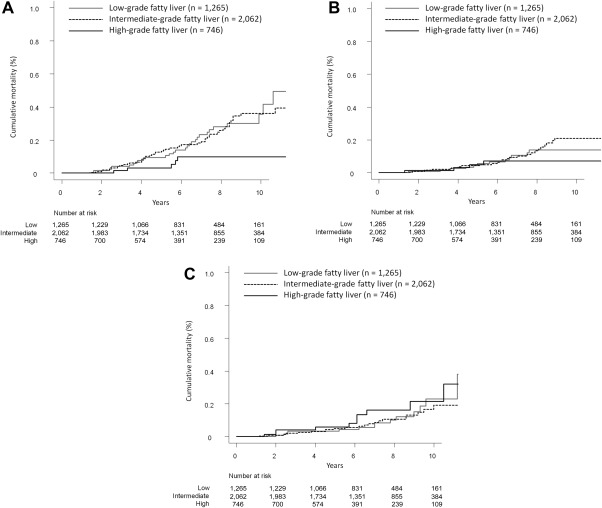
Cumulative mortality from categorized non‐liver‐related diseases based on the severity of fatty liver. (A) Malignancies. The respective 3‐, 5‐, and 10‐year cumulative mortality rates were 0.4%, 1.0%, and 3.6% in the low‐grade fatty liver group (solid gray line); 0.5%, 1.3%, and 3.6% in the intermediate‐grade fatty liver group (dotted line); and 0.2%, 0.3%, and 1.0% in the high‐grade fatty liver group (solid black line). (B) Cerebrovascular and cardiovascular diseases. The respective 3‐, 5‐, and 10‐year cumulative mortality rates were 0.1%, 0.6%, and 1.4% in the low‐grade fatty liver group (solid gray line); 0.2%, 0.4%, and 2.1% in the intermediate‐grade fatty liver group (dotted line); and 0.1%, 0.5%, and 0.7% in the high‐grade fatty liver group (solid black line). (C) Benign diseases (excluding cerebrovascular and cardiovascular diseases). The respective 3‐, 5‐, and 10‐year cumulative mortality rates were 0.3%, 0.3%, and 2.3% in the low‐grade fatty liver group (solid gray line); 0.2%, 0.5%, and 1.9% in the intermediate‐grade fatty liver group (dotted line); and 0.4%, 0.6%, and 2.2% in the high‐grade fatty liver group (solid black line).
Mortality curves for cerebrovascular and cardiovascular disease in study patients according to fatty liver severity are shown in Fig. 3B. The respective 3‐, 5‐, and 10‐year cumulative mortality rates were 0.1% (95% CI, 0.0‐0.4), 0.6% (95% CI, 0.2‐1.2), and 1.4% (95% CI, 0.7‐2.4) in the low‐grade fatty liver group; 0.2% (95% CI, 0.1‐0.5), 0.4% (95% CI, 0.2‐0.8), and 2.1% (95% CI, 1.4‐3.1) in the intermediate‐grade fatty liver group; and 0.1% (95% CI, 0.0‐0.7), 0.5% (95% CI, 0.1‐1.4), and 0.7% (95% CI, 0.2‐1.7) in the high‐grade fatty liver group. Cumulative mortality did not differ between the low‐ and intermediate‐grade groups (P = 0.900), the low‐ and high‐grade groups (P = 0.900), or the intermediate‐ and high‐grade groups (P = 0.580).
Mortality curves for benign diseases in study patients according to fatty liver severity are shown in Fig. 3C. The respective 3‐, 5‐, and 10‐year cumulative mortality rates were 0.3% (95% CI, 0.1‐0.8), 0.3% (95% CI, 0.1‐0.8), and 2.3% (95% CI, 1.2‐4.1) in the low‐grade fatty liver group; 0.2% (95% CI, 0.1‐0.5), 0.5% (95% CI, 0.2‐0.9), and 1.9% (95% CI, 1.1‐3.0) in the intermediate‐grade fatty liver group; and 0.4% (95% CI, 0.1‐1.2), 0.6% (95% CI, 0.2‐1.5), and 2.2% (95% CI, 1.0‐4.2) in the high‐grade fatty liver group. Cumulative mortality did not differ between the low‐ and intermediate‐grade groups (P = 0.950), the low‐ and high‐grade groups (P = 0.950), or the intermediate‐ and high‐grade groups (P = 0.720).
CUMULATIVE MORTALITY FROM CATEGORIZED NON‐LIVER‐RELATED DISEASES BASED ON THE SEVERITY OF LIVER FIBROSIS
Mortality curves for malignancies in study patients according to NFS are shown in Fig. 4A. The respective 3‐, 5‐, and 10‐year cumulative mortality rates were 0.2% (95% CI, 0.1‐0.5), 0.6% (95% CI, 0.5‐1.2), and 2.0% (95% CI, 1.3‐3.0) in the low NFS group; 0.6% (95% CI, 0.3‐1.1), 1.3% (95% CI, 0.8‐2.0), and 4.2% (95% CI, 3.0‐5.7) in the intermediate NFS group; and 1.3% (95% CI, 0.3‐4.2), 2.7% (95% CI, 0.9‐6.4), and 8.9% (95% CI, 4.4‐15.3) in the high NFS group. Cumulative mortality differed significantly between the low and intermediate NFS groups (P = 0.002), the low and high NFS groups (P < 0.001), and the intermediate and high NFS groups (P = 0.019).
Figure 4.
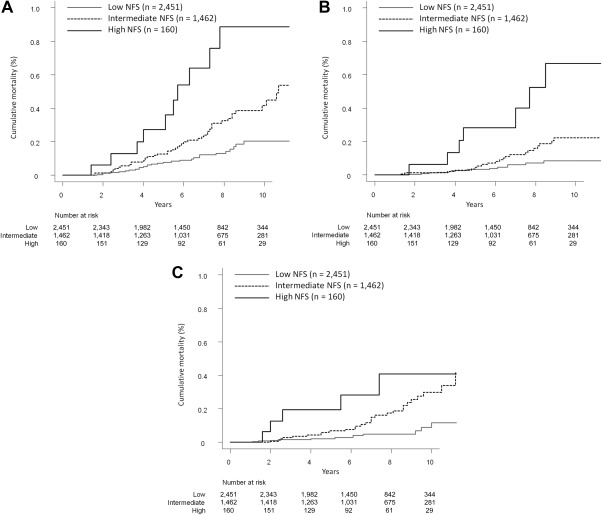
Cumulative mortality from categorized non‐liver‐related diseases based on the severity of liver fibrosis. (A) Malignancies. The respective 3‐, 5‐, and 10‐year cumulative mortality rates were 0.2%, 0.6%, and 2.0% in the low‐grade fatty liver group (solid gray line); 0.6%, 1.3%, and 4.2% in the intermediate‐grade fatty liver group (dotted line); and 1.3%, 2.7%, and 8.9% in the high‐grade fatty liver group (solid black line). (B) Cerebrovascular and cardiovascular diseases. The respective 3‐, 5‐, and 10‐year cumulative mortality rates were 0.1%, 0.3%, and 0.8% in the low‐grade fatty liver group (solid gray line); 0.1%, 0.5%, and 2.2% in the intermediate‐grade fatty liver group (dotted line); and 0.6%, 2.8%, and 6.7% in the high‐grade fatty liver group (solid black line). (C) Benign diseases (excluding cerebrovascular and cardiovascular diseases). The respective 3‐, 5‐, and 10‐year cumulative mortality rates were 0.2%, 0.2%, and 1.2% in the low‐grade fatty liver group (solid gray line); 0.3%, 0.7%, and 3.0% in the intermediate‐grade fatty liver group (dotted line); and 2.0%, 2.0%, and 4.1% in the high‐grade fatty liver group (solid black line).
Mortality curves for cerebrovascular and cardiovascular diseases in study patients according to NFS are shown in Fig. 4B. The respective 3‐, 5‐, and 10‐year cumulative mortality rates were 0.1% (95% CI, 0.0‐0.4), 0.3% (95% CI, 0.1‐0.7), and 0.8% (95% CI, 0.5‐1.5) in the low NFS group; 0.1% (95% CI, 0.0‐0.5), 0.5% (95% CI, 0.2‐1.0), and 2.2% (95% CI, 1.4‐3.4) in the intermediate NFS group; and 0.6% (95% CI, 0.1‐3.2), 2.8% (95% CI, 0.9‐6.6), and 6.7% (95% CI, 2.8‐12.9) in the high NFS group. Cumulative mortality differed significantly between the low and intermediate NFS groups (P = 0.018), the low and high NFS groups (P < 0.001), and the intermediate and high NFS groups (P = 0.002).
Mortality curves for benign diseases in study patients according to NFS are shown in Fig. 4C. The respective 3‐, 5‐, and 10‐year cumulative mortality rates were 0.2% (95% CI, 0.1‐0.4), 0.2% (95% CI, 0.1‐0.5), and 1.2% (95% CI, 0.5‐2.3) in the low NFS group; 0.3% (95% CI, 0.1‐0.7), 0.7% (95% CI, 0.3‐1.3), and 3.0% (95% CI, 1.9‐4.4) in the intermediate NFS group; and 2.0% (95% CI, 0.5‐5.2), 2.0% (95% CI, 0.5‐5.2), and 4.1% (95% CI, 1.4‐8.9) in the high NFS group. Cumulative mortality differed significantly between the low and intermediate NFS groups (P < 0.001) and the low and high NFS groups (P < 0.001). Conversely, mortality did not differ between the intermediate and high NFS groups (P = 0.261).
FACTORS ASSOCIATED WITH PATIENT MORTALITY FROM CATEGORIZED NON‐LIVER‐RELATED DISEASES
In the analysis of mortality from malignancies, multivariate Fine and Gray proportional hazards modeling using the covariates of US fatty liver grades (low, intermediate, and high) and NFS (low, intermediate, and high) showed that intermediate NFS (HR, 2.163) and high NFS (HR, 4.814) were independent factors associated with mortality (Table 4).
Table 4.
Multivariate Analysis Of Factors Related To Mortality From Categorized Non‐Liver‐Related Diseases Adjusted By Us Fatty Liver Grades and NFS Categories
| Categorized Diseases | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Malignancies | |||
| Low NFS (n = 2,451) | 1 | ||
| Intermediate NFS (n = 1,462) | 2.163 | 1.354 ‐ 3.457 | 0.001 |
| High NFS (n = 160) | 4.814 | 2.323 ‐ 9.977 | <0.001 |
| Cerebrovascular and cardiovascular diseases | |||
| Low NFS (n = 2,451) | 1 | ||
| Intermediate NFS (n = 1,462) | 2.265 | 1.141 ‐ 4.497 | 0.002 |
| High NFS (n = 160) | 8.482 | 3.558 ‐ 20.220 | <0.001 |
| Benign diseasesa | |||
| Low NFS (n = 2,451) | 1 | ||
| Intermediate NFS (n = 1,462) | 3.216 | 1.641 ‐ 6.303 | 0.001 |
| High NFS (n = 160) | 5.558 | 1.923 ‐ 16.070 | 0.002 |
Excluding cerebral and cardiovascular diseases
In the analysis of mortality from cerebrovascular and cardiovascular diseases, multivariate Fine and Gray proportional hazards modeling using the covariates of US fatty liver grades and NFS grades showed that intermediate NFS (HR, 2.265) and high NFS (HR, 8.482) were independent factors associated with mortality (Table 4).
In the analysis of mortality from benign diseases, multivariate Fine and Gray proportional hazards modeling using the covariates of US fatty liver grades and NFS grades showed that intermediate NFS (HR, 3.216) and high NFS (HR, 5.558) were independent factors associated with mortality (Table 4).
In the additional analysis of mortality from each categorized non‐liver‐related disease, multivariate Fine and Gray proportional hazards modeling using the covariates of US fatty liver grades (low, intermediate, and high) and FIB‐4 (low and high) are shown in Table 5.
Table 5.
Multivariate Analysis Of Factors Related To Mortality From Categorized Non‐Liver‐Related Diseases Adjusted By US Fatty Liver Grades and Fib‐4 Categories
| Categorized Diseases | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Malignancies | |||
| Low FIB‐4 (n = 2,346) | 1 | ||
| High FIB‐4 (n = 1,727) | 1.750 | 1.132 ‐ 2.704 | 0.012 |
| Cerebrovascular and cardiovascular diseases | |||
| Low FIB‐4 (n = 2,346) | 1 | ||
| High FIB‐4 (n = 1,727) | 1.958 | 1.057 ‐ 3.626 | 0.033 |
| Benign diseasesa | |||
| Low FIB‐4 (n = 2,346) | 1 | ||
| High FIB‐4 (n = 1,727) | 2.610 | 1.400 ‐ 4.866 | 0.003 |
Excluding cerebral and cardiovascular diseases
Discussion
In the present study, only 5.0% of mortality in patients with NAFLD was due to liver‐related diseases; the remaining 95.0% was due to non‐liver‐related diseases. In addition, the mortality rate from all causes was positively associated with degree of liver fibrosis in patients with NAFLD. Among patients who died from non‐liver‐related causes, multivariate analysis showed that progression of liver fibrosis was independently associated with risk of mortality from all categorized diseases (i.e., malignancies, cerebrovascular and cardiovascular diseases, and benign diseases). Conversely, multivariate analysis indicated that progression of liver steatosis did not confer a risk of mortality from any categorized diseases.
NFS is one of the complex markers used to distinguish the presence and absence of advanced fibrosis in patients with NAFLD and is based on six variables: age, body mass index, hyperglycemia/diabetes, AST/ALT ratio, platelet count, and albumin.14 NFS has been externally validated in 13 studies, with more than 3,000 patients.22 Angulo et al.14 reported that this scoring system had areas under the receiver operating characteristic curve of 0.88 and 0.82 for predicting the presence or absence of advanced fibrosis in patients with NAFLD in the estimation and validation groups, respectively. By applying a low NFS cutoff score (−1.455), advanced fibrosis could be excluded with high accuracy (negative predictive values of 93% and 88% in the estimation and validation groups, respectively). By applying a high cutoff score (0.676), the presence of advanced fibrosis could be diagnosed with high accuracy (positive predictive values of 90% and 82% in the estimation and validation groups, respectively). In the present study, therefore, we used this scoring system to categorize liver fibrosis severity in 4,073 patients with NAFLD. More than half of patients with NAFLD (60.2%) in this study had NFS consistent with lack of significant fibrosis (NFS < −1.455), whereas 4.4% had a score indicative of advanced fibrosis (NFS > 0.676).
Hamaguchi et al.17 reported the usefulness of the US fatty liver scoring system for the diagnosis of NAFLD. They reported that this scoring system had an area under the receiver operating characteristic curve of 0.98, a sensitivity of 91.7%, and a specificity of 100%. In addition, they reported that the correlation coefficient between scores derived using this system and liver steatosis as determined by liver biopsy was 0.87, which indicates a strong correlation. In the present study, therefore, we used this US scoring system for the imaging diagnosis of NAFLD and for categorization of liver steatosis severity.
Kim et al.23 reported an association between noninvasive fibrosis markers and mortality among patients with NAFLD. In their report, they used the NFS, AST to platelet ratio index, and FIB‐4 index as noninvasive fibrosis markers. They found that the presence of NAFLD was not associated with higher mortality (age and sex‐adjusted HR, 1.05; 95% CI, 0.93‐1.19) compared to the absence of NAFLD. In contrast, they showed that compared to patients with NAFLD without fibrosis, patients with NAFLD with a high probability of advanced fibrosis had a 69% increase in mortality (for NFS: HR, 1.69; 95% CI, 1.09‐2.63) after adjusting for other known predictors of mortality. In addition, they reported that the mortality from cardiovascular disease increased in patients with NAFLD with a high probability of advanced fibrosis (for NFS: HR, 3.46; 95% CI, 1.91‐6.25). They also reported that the mortality from liver disease, malignancy, and diabetic complications was not associated with the degree of liver fibrosis in patients with NAFLD. Consistent with these findings, we found that progression of liver fibrosis, evaluated by both NFS and FIB‐4 index, in patients with NAFLD was an independent risk factor for mortality from cerebrovascular and cardiovascular diseases (approximately 80% [33/42] of these deaths were due to cardiovascular causes) in this study. In addition, we demonstrated that progression of liver fibrosis, evaluated by both NFS and FIB‐4 index, in patients with NAFLD was an independent risk factor for mortality from malignancies (excluding primary liver cancer) and benign diseases (excluding liver‐related, cerebrovascular, and cardiovascular diseases). Further, we showed that the severity of liver steatosis was not associated with the mortality from each category of non‐liver‐related disease. Recently, Angulo et al.24 also reported that the fibrosis stage but no other histologic feature of steatohepatitis was independently associated with long‐term overall mortality in patients with NAFLD. Seko et al.25 reported that patients with NAFLD and elevated type IV collagen 7s levels as a liver fibrosis marker are at increased risk for extrahepatic cancer and overall mortality. They also reported that the incidence of extrahepatic cancer in patients with NAFLD was higher than that of hepatocellular carcinoma. Compared to the report of Kim et al.,23 the advantages of our study are its detailed investigations of causes of death and its analysis of mortality based on not only severity of liver fibrosis but also severity of liver steatosis. In addition, we used the competing risk method to analyze mortality in our study and not Cox's proportional hazards regression analysis as used by Kim et al. The competing risk method is the most suitable technique for assessing the mortality of subjects who may die of a variety of causes. However, the usual approaches for analyzing the time to an event, such as the Kaplan‐Meier method, produce biased results when applied in the context of competing risks.20 There is a strong relation between NAFLD and systemic diseases, including diabetes.26 Diabetes itself is associated with numerous disease types, including infectious, vascular, renal, neurologic, skin, mental, and malignant.27, 28, 29, 30 Nakamura et al.31 reported that the most frequent causes of death in 45,708 Japanese patients with diabetes were malignancies (38.3%), followed by infections (17.0%), then vascular diseases (14.9%), including renal failure (3.5%), ischemic heart diseases (4.8%), and cerebrovascular diseases (6.6%). The formula for calculating NFS, which was used in this study as a liver fibrosis marker for NAFLD, includes the presence or absence of diabetes. It also includes age, which is associated with various causes of mortality. In fact, the prevalence of diabetes and the mean age were significantly higher in the intermediate and high NFS groups than in the low NFS group, with these values being greatest in the high NFS group. Therefore, it is possible that use of the NFS to assess liver fibrosis severity in the present study was reflective of mortality from all causes, especially non‐liver‐related diseases. Further studies are warranted to investigate prediction of liver fibrosis in patients with NAFLD using noninvasive tools (e.g., US elastography) that do not include age or diabetes.
The main limitations of this study were its hospital‐based subject population and its retrospective nature. In addition, the follow‐up duration was relatively short. Although our hospital is the only general hospital serving a large number of nearby patients with various diseases, including NAFLD, further prospective studies with community‐based subjects and longer follow‐up durations are warranted. Another limitation was that we used the US fatty liver scoring system to assess liver steatosis severity in this study. The diagnostic ability of US to evaluate fatty liver depends on individual ultrasound machines and examiners. Several complex scores for predicting liver steatosis have recently been developed,32, 33, 34 and further studies using these steatosis scores are warranted.
In conclusion, the majority of deaths in patients with NAFLD were due to non‐liver‐related causes. In addition, progression of liver fibrosis severity was associated with mortality from cerebrovascular and cardiovascular diseases as well as malignancies and benign diseases (excluding liver‐related, cerebrovascular, and cardiovascular diseases). Further studies are warranted to confirm these findings in other populations.
Potential conflict of interest: Nothing to report.
Supported by the Research on Hepatitis of Ministry, Labour and Welfare in Japan (H28‐kansei‐ippan‐001, to J.T.).
REFERENCES
- 1. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686‐690. [DOI] [PubMed] [Google Scholar]
- 2. Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol 2007;17:863‐869. [DOI] [PubMed] [Google Scholar]
- 3. Suzuki A, Angulo P, Lymp J, St Sauver J, Muto A, Okada T, et al. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology 2005;41:64‐71. [DOI] [PubMed] [Google Scholar]
- 4. Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long‐term follow‐up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865‐873. [DOI] [PubMed] [Google Scholar]
- 5. Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology 1999;29:664‐669. [DOI] [PubMed] [Google Scholar]
- 6. Mendes FD, Suzuki A, Sanderson SO, Lindor KD, Angulo P. Prevalence and indicators of portal hypertension in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2012;10:1028‐1033.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 2011;54:1208‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non‐alcoholic fatty liver disease: an emerging menace. J Hepatol 2012;56:1384‐1391. [DOI] [PubMed] [Google Scholar]
- 9. Agopian VG, Kaldas FM, Hong JC, Whittaker M, Holt C, Rana A, et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg 2012;256:624‐633. [DOI] [PubMed] [Google Scholar]
- 10. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology 2005;129:113‐121. [DOI] [PubMed] [Google Scholar]
- 11. Dam‐Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long‐term, clinical follow‐up in fatty liver patients. Scand J Gastroenterol 2009;44:1236‐1243. [DOI] [PubMed] [Google Scholar]
- 12. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology 2017;65:1557‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta‐analysis. Hepatology 2011;54:1082‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846‐854. [DOI] [PubMed] [Google Scholar]
- 15. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005‐2023. [DOI] [PubMed] [Google Scholar]
- 16. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33 (Suppl. 1):S62‐S69. Erratum in: Diabetes Care 2010;33:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007;102:2708‐2715. [DOI] [PubMed] [Google Scholar]
- 18. McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non‐invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non‐alcoholic fatty liver disease. Gut 2010;59:1265‐1269. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization . Classification of Diseases (ICD). Published 2017. http://www.who.int/classifications/icd/en/. Accessed March 7, 2017.
- 20. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496‐509. [Google Scholar]
- 21. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Machado MV, Cortez‐Pinto H. Non‐invasive diagnosis of non‐alcoholic fatty liver disease. A critical appraisal. J Hepatol 2013;58:1007‐1019. [DOI] [PubMed] [Google Scholar]
- 23. Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013;57:1357‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397.e.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seko Y, Sumida Y, Tanaka S, Taketani H, Kanemasa K, Ishiba H, et al. Predictors of malignancies and overall mortality in Japanese patients with biopsy‐proven non‐alcoholic fatty liver disease. Hepatol Res 2015;45:728‐738. [DOI] [PubMed] [Google Scholar]
- 26. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62 (Suppl.):S47‐S64. [DOI] [PubMed] [Google Scholar]
- 27. American Diabetes Association . Standards of medical care in diabetes–2012. Diabetes Care 2012;35 (Suppl. 1):S11‐S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta‐analyses of observational studies. BMJ. 2015;350:g7607. [DOI] [PubMed] [Google Scholar]
- 29. de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta‐analysis. Psychosom Med. 2001;63:619‐630. [DOI] [PubMed] [Google Scholar]
- 30. Baena‐Díez JM, Peñafiel J, Subirana I, Ramos R, Elosua R, Marín‐Ibañez A, et al.; FRESCO Investigators . Risk of cause‐specific death in individuals with diabetes: a competing risks analysis. Diabetes Care 2016;39:1987‐1995. [DOI] [PubMed] [Google Scholar]
- 31. Nakamura J, Kamiya H, Haneda M, Inagaki N, Tanizawa Y, Araki E, et al. Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001‐2010: Report of the Committee on Causes of Death in Diabetes Mellitus. J Diabetes Investig 2017;8:397‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol 2005;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non‐alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009;137:865‐872. [DOI] [PubMed] [Google Scholar]


