Abstract
Background:
Recent studies hypothesize that dyslipidemia can predict glycated hemoglobin (HbA1c) and could be important contributing factor to the pathogenesis of type 2 diabetes mellitus (DM2). Therefore, we aimed to evaluate the influence of lipid parameters on long-term glycemic control in DM2.
Materials and Methods:
A total of 275 sedentary DM2 (mean [±standard deviation] age 60.6 [±10.0] years) who volunteered to participate in this cross-sectional study were enrolled. Anthropometric (body weight, body hight, and waist circumference), biochemical parameters (fasting glucose, HbA1c, lipid parameters, creatinine), as well as blood pressure were obtained.
Results:
Total cholesterol (odds ratio [OR] =1.30, 95% confidence interval [CI] [1.02–1.66], P = 0.032), triglycerides (OR = 1.34, 95% CI (1.07–1.67), P = 0.010), and low density lipoprotein cholesterol (OR = 1.42, 95% CI [1.10–1.83], P = 0.006) were the independent predictors of higher HBA1c, and as they increased by 1 mmol/L each, probabilities of higher HBA1c increased by 30%, 34%, and 42%, respectively. Low level of high-density lipoprotein cholesterol (HDL-c) was found to be the independent predictor of higher HBA1c (OR = 0.44, 95% CI [0.20–0.67], P = 0.039), and increase in HDL-c by 1 mmol/L, reduced the probability of higher HBA1c by 56%.
Conclusion:
Unfavorable lipid profile can predict HbA1c level in DM2 patients. Early diagnosis of dyslipidemia, as well as its monitoring and maintaining good lipids control can be used as a preventive measure for optimal long-term glycemic control.
Keywords: Diabetes mellitus, dyslipidemia, glycated hemoglobin, glycemic control
INTRODUCTION
Diabetes epidemic grows worldwide,[1] accounting for 382 million people around the world (8.3% of adults), to have diabetes. It is estimated that by 2035, one adult in 10 will have this metabolic disorder.[2]
The prevalence of diabetes in adults in Montenegro is 10.1%.[2] However, according to the estimation of International Diabetes Federation this figure is supposed to be even higher. Patients with type 2 diabetes mellitus (DM2) have a greatly increased risk of cardiovascular events, suggesting the importance of attaining optimal glycemic control in reducing the risk of cardiovascular disease (CVD) and all-cause mortality.[3]
Glycated hemoglobin (HbA1c) is well-established marker for long-term glycemic control.[4] Not only that HbA1c predicts the risk of diabetic complications development[4] but is also an independent risk factor of CVD in both diabetic and nondiabetic population.[5]
Diabetic dyslipidemia (e.g., characterized by high-plasma level of triglycerides [TG] and low-density lipoprotein cholesterol [LDL-c], but low level of high-density lipoprotein cholesterol [HDL-c]) is tightly associated with glycemic control.[6] A growing body of evidence suggests that dyslipidemia is secondary to insulin resistance or factors closely related to insulin resistance, such as adiposity. Increased free fatty acid flux secondary to insulin resistance and increased proinflammatory adipokines and cytokines from enlarged adipose tissue may be the underlying determinants of this interrelationship.[7,8]
However, some studies hypothesize that dyslipidemia could be important contributing factor to the pathogenesis of DM2.[9,10]
Although previous studies put emphasis on achieving good glycemic control with antihyperglycemic therapy and showed that HbA1c can be used as a valuable biomarker for prognosticating serum lipid status in DM2 patients,[6,11,12,13] we hypothesized that dyslipidemia can predict HbA1c level, suggesting that screening of dyslipidemia and its better control could be of great benefit in optimizing HbA1c.
Therefore, considering the high prevalence of DM2 in Montenegro and regarding the need for better glycemic control, we aimed to evaluate the influence of anthropometric and lipid parameters on long-term glycemic control in this population group.
MATERIALS AND METHODS
Study population
A total of 275 sedentary DM2 (mean ± standard deviation [SD] age 60.6 ± 10.0 years) who volunteered to participate in this cross-sectional study were enrolled. Diabetic patients were consecutively recruited by the endocrinologist in the Center of Laboratory Diagnostics of the Primary Health Care Center in Podgorica, Montenegro, for their regular checkup in a period from October 2012 to June 2013. All the participants completed a self-administered questionnaire including demographic characteristics, somatic illnesses, and lifestyle habits [(e.g., information about diabetes duration (years), physical activity (sedentary patients were regarded with <90 min of weekly exercise), cigarette smoking (all participants answered the question: “have you smoked a cigarette in the last month?” participants answering “yes” are classified as current smokers, and those who answered “no” are classified as never smokers), alcohol consumption (patients with ethanol consumption >20 g/day were excluded from the study)].
Participants that were eligible for the study were: sedentary patients with DM2, without acute inflammatory disease, with no history or the presence of malignancy. Diabetes cases were defined as self-reported diabetes, or with at least two elevated plasma glucose levels (fasting glucose ≥7.0 mmol/L, a random plasma glucose level of ≥11.1 mmol/L, or a plasma glucose level ≥11.1 mmol/L 2 h after an oral glucose tolerance test), or HBA1c ≥6.5% on different occasions in the absence of symptoms; or treatment with insulin or oral antihyperglycemic agents.[4]
Participants that were excluded from the study were: participants with DM1, estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2, patients with renal disease other than diabetic nephropathy, with anemia, hepatic dysfunction, with a recent (6 months) history of acute myocardial infarction or stroke, pregnancy, with history of alcohol abuse as well as participants who were unwilling to enter the study.
All the participants provided written informed consent. The study protocol was approved by the Ethical Committee of Primary Health Care Center in Podgorica, Montenegro (number 317/2) and the research was carried out in compliance with the Declaration of Helsinki.
Anthropometric measurements
Anthropometric measurements: body height (cm), body weight (kg), and waist circumference (WC) (cm) were obtained, and body mass index (BMI) was calculated.[14] Waist-to-height ratio (WHtR) was calculated as waist (cm) divided by height (cm). Visceral adiposity index (VAI) was calculated using the formula:
([WC/36.58 + (1.89 × BMI)] × (TG/0.81) × (1.52/HDL-c)) for females, and ([WC/39.68 + (1.88 × BMI)] × (TG/1.03) × (1.31/HDL-c)) for males,[15]
Where WC is expressed in cm, BMI in kg/m2, TG and HDL-c in mmol/L.
Lipid accumulation product (LAP) was calculated by the following equation: ([WC-58] × TG) for females, and ([WC-65] × TG) for males,[15]
Where WC is expressed in cm, and TG in mmol/L.
Biochemical analyses
Venous blood samples (10 mL) were obtained from each participant. The blood samples were taken between 7–9 o’clock a.m., after 12–14 h an overnight fast. Samples were left to clot for 30 min and then centrifuged at 3000 rpm for 10 min.
Another aliquot was collecting as a whole blood in K2 EDTA for determination of HbA1c. Serum levels of glucose, creatinine, total cholesterol (TC), LDL-c, HDL-c, and TG were measured spectrophotometrically using enzymatic procedures, while HbA1c was measured with immunoturbidimetric assay (Roche Cobas 400, Mannheim, Germany).
Glomerular filtration rate was estimated using creatinine in the Modification of Diet in Renal Disease Study equation (eGFRMDRD):
eGFRMDRD (mL/min/1.73 m2) = 186 × (serum creatinine [μmol/L]/88.4) −1.154 × (age [years]) − 0.203 × 0.742 (if female).[15]
Blood pressure was measured as described previously.[14]
Statistical analysis
The distribution of variables was tested by Kolmogorov–Smirnov test. Distributions of HDL-c, LAP and VAI achieved normality after logarithmic transformation. Distributions of age, body weight, body height, BMI, duration of diabetes, TC, TG, glucose, creatinine, and eGFRMDRD were skewed even after logarithmic transformation.
Data with normal and log-normal distributions were tested by the one-way ANOVA according to long-term glycemic control. Differences between two HBA1c tertile subgroups were analysed with Bonferroni post hoc test. Data not-normally distributed were subjected to the nonparametric Kruskal–Wallis and Mann–Whitney tests, depended on number of compared groups. The results were expressed as the arithmetic mean ± SD for normally distributed variables. Log-normally distributed variables were expressed as the geometrical mean and the 95% confidence interval (CI) for geometrical mean.[16] Variables that were not normally distributed after logarithmic transformation were presented as median (interquartile range). Categorical variables were tested with Chi-square test and presented as absolute frequencies. Spearman's correlation analysis was conducted to determine relationships between long-term glycemic control and anthropometric and lipid status parameters. Data were given as coefficient correlation (ρ). To estimate possible predictions of lipid parameters and calculated indexes (LAP and VAI) with long-term glycemic control (HBA1c), ordinal regression, analysis was employed. HBA1c (dependent variable) was ranked and divided by tertiles. The lipid status parameters (TC, HDL-c, LDL-c, and TG) and calculated indexes (LAP and VAI) were used as independent variables (covariates). Categorical variables such as gender and smoking status were set as factors. In ordinal logistic regression analysis models which included independent variables showing no multicollinearity, were used to predict the dependent variable. For internal validation of the models and to determine if our data approximate the true population data, the bootstrap method with 10000 permutations was used. Data are presented as the estimated odds ratio (95% CI).
Statistical analyses were performed using PASW® Statistic version 18 (Chicago, Illinois, USA). Two-tailed P < 0.05 were considered as statistically significant.
RESULTS
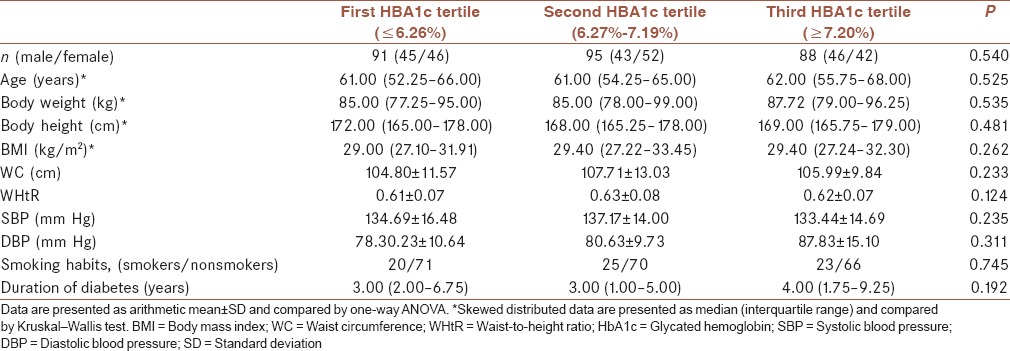
Table 1 shows general and anthropometric characteristics of DM2 participants. There were no differences between the groups with respect to demographic characteristics of study participants between HBA1c tertile groups [Table 1]. Chi-square analysis demonstrated equal distribution of both gender (males/females: 45/46; 43/52; 46/42; P = 0.540) and smoking habits (smokers/nonsmokers: 20/71; 25/70; 23/66; P = 0.745) across tertile HBA1c groups.
Table 1.
General and anthropometric characteristics of diabetic patients according to glycemic control
As expected, significantly higher TC, LDL-c, TG (5.75 [4.94–6.38]; 3.48 ± 1.09; 2.02 [1.54–3.17]; P = 0.029, P = 0.018, and P = 0.001, respectively) were found in the highest HBA1c tertile group, as compared with the lowest (5.16 [4.40–5.70]; 3.06 ± 0.92; 1.59 [1.14–2.04], respectively) and intermediate HBA1c (5.32 [4.84–6.29]; 3.30 ± 0.97; 1.87 [1.74–2.11], respectively) tertile group.
In addition, significantly higher calculated indexes (VAI, LAP) were found in the highest HBA1c tertile group (3.23 [1.82–5.62] and 87.78 [76.10–98.91]; P < 0.001, respectively), as compared with the lowest (2.30 [2.00–2.65] and 67.06 [59.72–75.32], respectively) and intermediate HBA1c (2.95 [2.60–3.35] and 87.89 [77.98–99.07], respectively) tertile group.
On the other hand, significantly lower HDL-c (1.07 [1.00–1.14]) was found in the highest HBA1c tertile group (P = 0.015), as compared with the lowest (1.20 [1.14–1.27]) and intermediate (1.18 [1.12–1.25]) HBA1c tertile group [Table 2].
Table 2.
Biochemical parameters in diabetic patients according to glycemic control
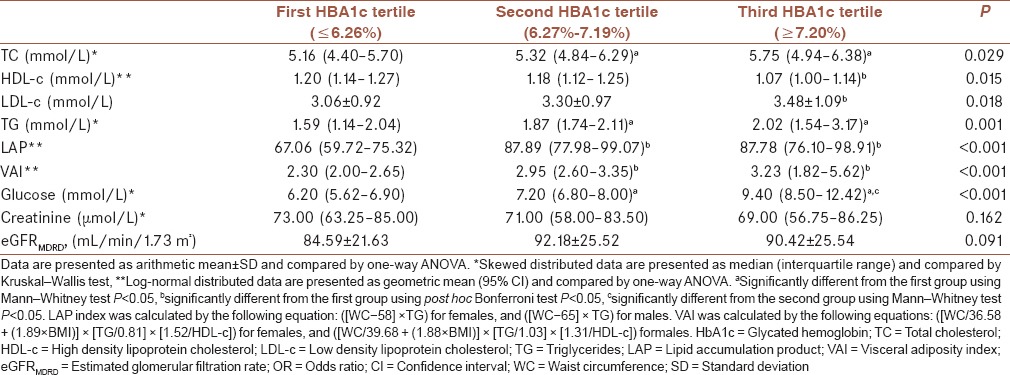
Bonferroni post hoc test showed significantly higher TC and TG concentrations in the second (5.32 [4.84–6.29] and 1.87 [1.74–2.11], respectively) and in the third group (5.75 [4.94–6.38] and 2.02 (1.54–3.17), respectively] than in the first one (5.16 [4.40–5.70] and 1.59 [1.14–2.04], respectively; P for both = 0.025). LDL-c concentration was significantly higher in the third (3.48 ± 1.09) than in the first tertile group (3.06 ± 0.92, P = 0.015). On the contrary, HDL-c concentration was significantly higher in the first (1.20 [1.14–1.27]) than in the third group (1.07 [1.00–1.14]; P = 0.022). Both indexes (VAI and LAP) were higher in the second (2.95 [2.60–3.35]; 87.89 [77.98–99.07]) and third (3.23 [1.82–5.62]; 87.78 [27.10–31.91]) than those in the first group (2.30 [2.00–2.65]; 67.06 [59.72–75.32]; P for both < 0.001, respectively).
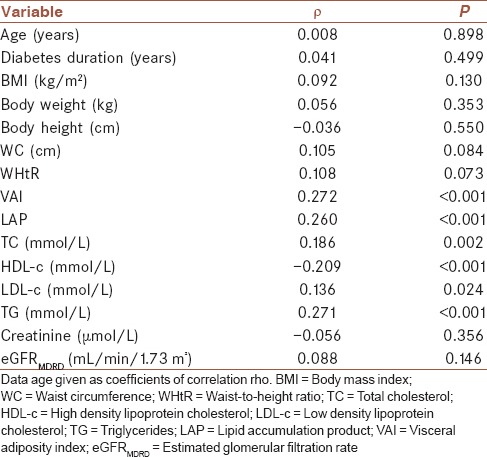
Thereafter, we performed Spearman's correlation analysis to test the association between lipid parameters and calculated indexes with long-term glycemic control, and showed that TG, LAP, and VAI highly positively correlated with HBA1c (ρ =0.271; ρ =0.260; ρ =0.272; P < 0.001 for all). In addition, we found significant positive correlations between TC and LDL-c with HBA1c (ρ = 0.186; P = 0.002 and ρ = 0.136; P = 0.024, respectively). Only, HDL-c highly negatively correlated with HBA1c (ρ = −0.209; P < 0.001) [Table 3].
Table 3.
Associations between glycated hemoglobin and clinical parameters using Spearman's correlation analysis
In addition, ordinal logistic regression analysis was performed to analyze predictive roles of lipid status parameters and calculated indexes on long-term glycemic control presented as HBA1c [Table 4].
Table 4.
Estimated odds ratios after ordinal regression analysis for glycemic control risk
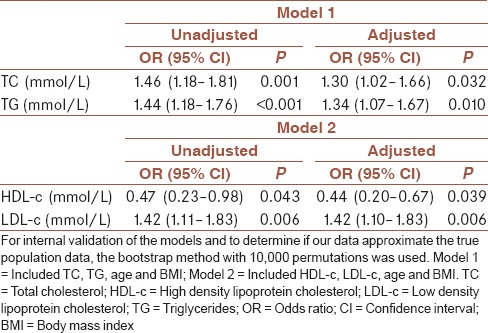
The analysis showed that as TC (OR = 1.46, 95% CI [1.18–1.81], P = 0.001), TG (OR = 1.44, 95% CI [1.18–1.76], P < 0.001), and LDL-c (OR = 1.42, 95% CI [1.11–1.83], P = 0.006), increased by 1 mmol/L each, probabilities of higher HBA1c increased by 46%, 44% and 42%, respectively. High TC and TG were the independent predictors of higher HBA1c, when beside them were included age and BMI as covariates in Model 1 ([OR = 1.30, 95% CI (1.02–1.66), P = 0.032], [OR = 1.34, 95% CI (1.07–1.67), P = 0.010], respectively), but adjusted odds were less than unadjusted (30% and 34%, respectively).
After testing model 2 for prediction of LDL-c, estimated adjusted odds for higher HBA1c remained significant (OR = 1.42, 95% CI [1.10–1.83], P = 0.006).
On the other hand, increase in HDL-c (OR = 0.47, 95% CI [0.23–0.98], P = 0.043), by 1 mmol/L reduced the probability of higher HBA1c by 53%. We also found low HDL-c to be the independent predictor of higher HBA1c. Adjusted odds given in Model 2, demonstrated that rise in HDL-c by 1 mmol/L reduced the probability of higher HBA1c by even 56% (OR = 0.44, 95% CI [0.20–0.67], P = 0.039).
Furthermore, VAI which was calculated by WC, BMI, TG, and HDL-c, also gave significant odds for higher HBA1c (OR = 1.16, 95% CI [1.07–1.26], P = 0.001). Rise in VAI by 1 unit, increased probability of higher HBA1c by 16%. Although VAI was correlated with all lipid parameters, we were not able to include it in any model given in Table 4. On the contrary, LAP, which was calculated by WC and TG, had not a predictive influence on HBA1c (OR = 1.01, 95% CI [1.00–1.01], P = 0.864) [data not presented in Table 4].
DISCUSSION
Previous studies have confirmed the importance of attaining optimal glycemic control in reducing the risk of CVD and all-cause mortality.[3] A reduction in HbA1c for only 1% reduces myocardial infarction by 14%, risk of microvascular complications by 37%, and diabetes-related deaths by 21%,[17] emphasizing the need for achieving, and maintaining of HBA1c goals <7% for many adults.
Indeed, hyperglycemia is regarded to be a promoting factor of increased LDL glycation and other pathology responsible for atherosclerosis and CVD.[18]
The finding of the current study reports also predictive role of traditional lipid status parameters on long-term glycemic control (presented as HBA1c) in DM2 patients.
In our study, we found significant increase in all examined lipid parameters and calculated indexes (VAI, LAP) across HBA1c tertile groups, with exception of HDL-c that decreased along with increment in HBA1c tertiles [Table 2]. Moreover, HBA1c showed association with all lipid parameters and calculated indexes [Table 3].
Correlation of HBA1c with TC and TG, but not with HDL-c and LDL-c was shown by previous studies[6,19] whereas on the contrary, Babikr et al.[11] reported association of HBA1c with HDL-c and LDL-c, but not with TC and TG.
However, we hypothesized that screening of dyslipidemia and its better control could be of great benefit in optimizing HbA1c, in addition to previous studies showing that HbA1c can be used as a valuable biomarker for prognosticating serum lipid status in diabetic patients.[6,11,12,13] Ordinal regression analysis reported predictive roles of lipid status parameters on long-term glycemic control presented as HbA1c. Namely, the analysis showed that as TC, TG, and LDL-c increased by 1 mmol/L each, probabilities of higher HBA1c increased by 46%, 44%, and 42%, respectively [Table 4].
Moreover, TC, TG, and LDL-c were the independent predictors of higher HBA1c, even after adjustment for confounding factors (30%, 34%, and 42%, respectively), such as age and BMI, taking into account that adiposity may be confounding factor for the relationship between dyslipidemia and DM2.[20] In line with our results, Gupta et al.[21] showed that higher TG levels predict incident DM2 independently of BMI. In the large study that included more than 14,000 participants, they found that increase in TG by 1 mmol/L increased the risk of DM2 by 12%.[21]
Contrary to our findings, prospective studies have shown that LDL-c levels are not an independent risk factors for DM2.[21,22] Surprisingly, a most recent findings support the notion that high LDL-c levels, although regarded as an established predictor of CVD,[23] may be associated with low risk of DM2.[23,24] Namely, a recent cross-sectional study[24] reported the prevalence of DM2 to be 50% lower in patients with familiar hypercholesterolemia, compared to unaffected relatives.
In line with this, Andersson et al.[25] conducted a study comprised of participants not treated with hypolipemics, and during a mean follow-up of 4.5 years, showed the association between low LDL-c levels and increased risk of incident DM2. Their results are in line with recent randomized-controlled trials showing that lipid-lowering treatment (e.g., statins) was associated with increased risk of DM2.[26]
Nevertheless, we reported high LDL-c to be the predictor of higher HBA1c level, even after adjustment for age and BMI. This is contrary to previously reported investigations that low LDL-c level might be associated with increased DM2 risk both in those participants who used hypolipemic medications and those who did not.[24,25]
Rütti et al.[27] also raised the question whether unfavorable lipid profile contributes to manifestation and progression of DM2. They showed that prolonged exposure to LDL-c decreased insulin secretion and proliferation in human and murine islet beta cells, whereas contrary to LDL-c, HDL-c decreased beta-cell apoptosis.
We found lower HDL-c to be the independent predictor of higher HBA1c. Namely, increase in HDL-c by 1 mmol/L, reduced the probability of higher HBA1c by 53%, and after adjustment for confounding factors by even 56% [Table 4].
Indeed, low levels of HDL-c were the independent risk factors for poor glycemic control and DM2,[21,22,28] since HDL-c exerts a wide spectrum of favorable actions, such as antioxidative, anti-inflammatory, and antiapoptotic properties, thus protecting beta cells from cholesterol-induced dysfunction, islet inflammation, and stress-induced apoptosis.[9,10]
In addition, recent genetic investigations reported that lipid-related genetic loci may affect glycemic metabolism,[29] suggesting potentially causal relationship between genetically determined low HDL-c or high TG levels and increased risk of DM2.[22]
Taken all these discrepant reports together when considering the causalty between dyslipidemia and DM2, there is an urgent need for carefully designing the clinical trials of the lipid-modifying agents to improve insulin sensitivity and reduce the risk of DM2, its complications, as well as CVD.
Of note, the underlying mechanisms potentially linking lipid-modifying drugs with glycemic control are of increasing interest, especially when novel lipid-lowering agents are emerging[8,23] to achieve target lipid levels in people with diabetes.
The cross-sectional design and a small number of participants are the limitations of the current study. However, our results show the novel finding that unfavorable lipid profile could predict HbA1c level in DM2 patients.
CONCLUSION
Early diagnosis of dyslipidemia, as well as its monitoring and maintaining good lipids control can be used as a preventive measure for the optimal long-term glycemic control. In that sense, therapeutic implications of dyslipidemia, either through lifestyle intervention or pharmacologically may be of paramount importance.
Financial support and sponsorship
This work was financially supported in part by a grant from the Ministry of Education, Science and Technological Development, Republic of Serbia (Project number 175035).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank to all patients that participated in the study.
REFERENCES
- 1.Maghsoudi Z, Azadbakht L. How dietary patterns could have a role in prevention, progression, or management of diabetes mellitus? Review on the current evidence. J Res Med Sci. 2012;17:694–709. [PMC free article] [PubMed] [Google Scholar]
- 2.6th ed. Brussels, Belgium: International Diabetes Federation; 2013. [Last accessed on 2017 Feb 17]. International Diabetes Federation. IDF Diabetes Atlas; p. 30. Available from: http://www.idf.org/diabetesatlas . [Google Scholar]
- 3.Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Erratum. Classification and diagnosis of diabetes. Sec 2. In standards of medical care in diabetes-2016. Diabetes care. 2016;39(Suppl 1):S13–S22. doi: 10.2337/dc16-er09. Diabetes Care 2016;39:1653. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda F, Doi Y, Ninomiya T, Hirakawa Y, Mukai N, Hata J, et al. Haemoglobin A1c even within non-diabetic level is a predictor of cardiovascular disease in a general Japanese population: The hisayama study. Cardiovasc Diabetol. 2013;12:164. doi: 10.1186/1475-2840-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullugeta Y, Chawla R, Kebede T, Worku Y. Dyslipidemia associated with poor glycemic control in type 2 diabetes mellitus and the protective effect of metformin supplementation. Indian J Clin Biochem. 2012;27:363–9. doi: 10.1007/s12291-012-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faghihimani Z, Mirmiran P, Sohrab G, Iraj B, Faghihimani E. Effects of pomegranate seed oil on metabolic state of patients with type 2 diabetes mellitus. Int J Prev Med. 2016;7:124. doi: 10.4103/2008-7802.194883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chehade JM, Gladysz M, Mooradian AD. Dyslipidemia in type 2 diabetes: Prevalence, pathophysiology, and management. Drugs. 2013;73:327–39. doi: 10.1007/s40265-013-0023-5. [DOI] [PubMed] [Google Scholar]
- 9.Kruit JK, Brunham LR, Verchere CB, Hayden MR. HDL and LDL cholesterol significantly influence beta-cell function in type 2 diabetes mellitus. Curr Opin Lipidol. 2010;21:178–85. doi: 10.1097/MOL.0b013e328339387b. [DOI] [PubMed] [Google Scholar]
- 10.Pétremand J, Puyal J, Chatton JY, Duprez J, Allagnat F, Frias M, et al. HDLs protect pancreatic β-cells against ER stress by restoring protein folding and trafficking. Diabetes. 2012;61:1100–11. doi: 10.2337/db11-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babikr WA, Alshahrani AS, Hamid HG, Abdelraheem AH, Shalayel MH. The correlation of HbA1c with body mass index and HDL-cholesterol in type 2 diabetic patients. Biomed Res. 2016;27:1280–3. [Google Scholar]
- 12.Khan HA, Sobki SH, Khan SA. Association between glycaemic control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia. Clin Exp Med. 2007;7:24–9. doi: 10.1007/s10238-007-0121-3. [DOI] [PubMed] [Google Scholar]
- 13.Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. 2016;11:95–104. doi: 10.4137/BMI.S38440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klisic A, Kavaric N, Jovanovic M, Soldatovic I, Gligorovic-Barhanovic N, Kotur-Stevuljevic J. Bioavailable testosterone is independently associated with fatty liver index in postmenopausal women. Arch Med Sci. 2017;5:1188–96. doi: 10.5114/aoms.2017.68972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavaric N, Klisic A, Ninic A. Are visceral adiposity index and lipid accumulation product reliable indices for metabolic disturbances in patients with type 2 diabetes mellitus?? J Clin Lab Anal. 2017 doi: 10.1002/jcla.22283. doi:10.1002/jcla.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ. 1996;312:1079. doi: 10.1136/bmj.312.7038.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Glycemic targets. Sec. 5. standards of medical care in diabetes-2016. Diabetes Care. 2016;39(Suppl 1):S39–46. doi: 10.2337/dc16-S008. [DOI] [PubMed] [Google Scholar]
- 18.Singh AK, Singh SK, Singh N, Agrawal N, Gopal K. Obesity and dyslipidemia. Int J Biol Med Res. 2011;2:824–8. [Google Scholar]
- 19.Zadhoush F, Sadeghi M, Pourfarzam M. Biochemical changes in blood of type 2 diabetes with and without metabolic syndrome and their association with metabolic syndrome components. J Res Med Sci. 2015;20:763–70. doi: 10.4103/1735-1995.168383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papaetis GS, Papakyriakou P, Panagiotou TN. Central obesity, type 2 diabetes and insulin: Exploring a pathway full of thorns. Arch Med Sci. 2015;11:463–82. doi: 10.5114/aoms.2015.52350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta AK, Dahlof B, Dobson J, Sever PS, Wedel H, Poulter NR, et al. Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the anglo-scandinavian cardiac outcomes trial – Blood pressure lowering arm and the relative influence of antihypertensive medication. Diabetes Care. 2008;31:982–8. doi: 10.2337/dc07-1768. [DOI] [PubMed] [Google Scholar]
- 22.Qi Q, Liang L, Doria A, Hu FB, Qi L. Genetic predisposition to dyslipidemia and type 2 diabetes risk in two prospective cohorts. Diabetes. 2012;61:745–52. doi: 10.2337/db11-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chogtu B, Magazine R, Bairy KL. Statin use and risk of diabetes mellitus. World J Diabetes. 2015;6:352–7. doi: 10.4239/wjd.v6.i2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besseling J, Kastelein JJ, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA. 2015;313:1029–36. doi: 10.1001/jama.2015.1206. [DOI] [PubMed] [Google Scholar]
- 25.Andersson C, Lyass A, Larson MG, Robins SJ, Vasan RS. Low-density-lipoprotein cholesterol concentrations and risk of incident diabetes: Epidemiological and genetic insights from the framingham heart study. Diabetologia. 2015;58:2774–80. doi: 10.1007/s00125-015-3762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 27.Rütti S, Ehses JA, Sibler RA, Prazak R, Rohrer L, Georgopoulos S, et al. Low- and high-density lipoproteins modulate function, apoptosis, and proliferation of primary human and murine pancreatic beta-cells. Endocrinology. 2009;150:4521–30. doi: 10.1210/en.2009-0252. [DOI] [PubMed] [Google Scholar]
- 28.Firouzi S, Barakatun-Nisak MY, Azmi KN. Nutritional status, glycemic control and its associated risk factors among a sample of type 2 diabetic individuals, a pilot study. J Res Med Sci. 2015;20:40–6. [PMC free article] [PubMed] [Google Scholar]
- 29.White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, et al. Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiol. 2016;1:692–9. doi: 10.1001/jamacardio.2016.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]


