Abstract
Aims:
The aim of this study was to evaluate the specific chromatic alterations in tooth crowns induced by two different endodontic restorative materials.
Settings and Design:
This in vitro study was conducted at the Department of Conservative Dentistry, KMCT Dental College, Kozhikode, Kerala.
Subjects and Methods:
Forty-five freshly extracted, fully developed, single-rooted teeth were prepared and randomly assigned to two experimental groups (n = 15 each) and one negative control group (n = 15). Group 1 consists of white mineral trioxide aggregate (WMTA), Biodentine formed Group 2, and controls formed Group 3. Double-beam ultraviolet spectrophotometer equipment was used to assess the coronal discoloration as determined by CIE L*, a*, and b* and their corresponding total values.
Results:
At baseline, no significant difference was detected for CIE values between the groups. Group 1 showed a significant decrease in L*, a*, and b* values over time. The color change with WMTA led to clinically perceptible crown discoloration after 6 weeks which exceeded the perceptible threshold for the human eye, i.e., ΔE > 3.3. No changes were observed with Biodentine.
Conclusions:
Materials used in endodontics may stain teeth. WMTA induced clinically perceptible crown discoloration, whereas Biodentine demonstrated color stability.
Key words: Biodentine, endodontic restorative materials, spectrophotometry, tooth discoloration, white mineral trioxide aggregate
INTRODUCTION
Tooth discoloration induced by endodontic materials is a common finding and may impair the esthetic outcome of endodontically treated teeth.[1] A major etiological factor for the occurrence of local intrinsic staining, especially in the cervical and middle third of the crown, is the presence of root canal filling materials in contact with the coronal dentin of the pulp chamber. Any change to the optical and chromatic properties of the dentinal structure is likely to cause an alteration in the outward appearance of the crown caused by its light transmitting and reflecting properties.[2]
Mineral trioxide aggregate (MTA) is a biomaterial that has been investigated for endodontic applications since the early 1990s. MTA materials are derived from a Portland cement parent compound and have been demonstrated to be biocompatible endodontic repair materials, with its biocompatible nature strongly suggested by its ability to form hydroxyapatite when exposed to physiologic solutions. With some exceptions, MTA materials provide better microleakage protection than traditional endodontic repair materials, which is demonstrated by using dye, fluid filtration, and bacterial penetration leakage models.[3] In both animal and human studies, MTA materials have been shown to have excellent potential as pulp-capping and pulpotomy medicaments, but studies with long-term follow-up are limited. Even though high success rates were reported, a number of cases of discoloration have also been noted.
Calcium silicate-based materials have gained popularity in recent years due to their resemblance to MTA and their applicability in cases where MTA is indicated. Although various calcium silicate-based products have been launched to the market recently, one among these has been the focus of attention and the topic of a variety of investigations. This material is the “Biodentine” calcium silicate-based product, which became commercially available in 2009.[4] The material is actually formulated using the MTA-based cement technology and the improvement of some properties of these types of cements such as physical qualities and handling.[5] It is indicated for crown and root dentin repair treatment, indirect and direct pulp capping, pulpotomy, repair of perforations or resorptions, apexification, and root-end fillings.[6,7] Several studies reveal severe discoloration produced by MTA.[8] However, there are limited studies documenting discoloration caused by biodentine.[9]
SUBJECTS AND METHODS
Forty-five freshly extracted, fully developed, single-rooted teeth that were free of cracks, fractures, caries, and abrasions, and intrinsic discolouration was selected. Soft tissue was removed, and teeth were sectioned in the coronal third of the root complex, 1 mm below the cementoenamel junction. Pulps were extirpated with an excavator, and the internal axial walls of the pulp chambers were chemomechanically debrided with Hedstrom files (sizes 60–80) and 2.5% w/w sodium hypochlorite (10 ml) through the access. After the final irrigation, the pulp chambers were washed with sterile saline (5 ml). The teeth (n = 45) were randomly assigned to two experimental (n = 15 each) and one negative control group (n = 15). White MTA (WMTA) (Angelus TM, Londrina, Brazil) (Group 1) and Biodentine (Septodont, France) (Group 2) were the materials under study. Materials were prepared according to the manufacturer's instructions and placed into the pulp chambers with a Dovgan carrier (Quality Aspirators, Duncanville, TX, USA) through the access. A slight vertical pressure was applied with finger pluggers to fill the pulp chamber with the material, up to the cervical sectioning level. Negative controls (n = 15) were instrumented and kept unfilled (Group 3). All crowns were transferred and completely immersed in individual tubes containing distilled water and placed in an incubator at 37 ± 1°C. Measurements were carried out by the same operator before material placement, i.e., at baseline and at 2, 4, and 6 weeks after the material placement. Teeth samples were immersed in distilled water and placed in incubator in between the readings.
Assessment of coronal discoloration
A double-beam ultraviolet (UV) spectrophotometer equipped with an integrating sphere (colorflex, Hunterlab, USA) was used in this study. The spectrophotometer was equipped with a black bakelite sample assembly. The cylindrical inner frame (diameter = 2 cm, inner height = 0.2 cm) of the sample assembly was filled with black, monochromatic, thermo-plasticized silicone. The specimens were positioned in the circular opening of the integrating sphere's aperture mask according to a reference system to ensure the reproducibility of specimen's position. The spectrophotometer was connected to a computer, which recorded the spectral reflectance curves of the measured surfaces in the visual spectrum (380–780 nm). The double-beam integrating sphere was set to measure the intensity of spectral reflectance (R). The emitted light was directed toward the specimen. The reflected portion was collected in the integration sphere, normalized to the source light of the reflectance, and calibrated with the measurement of a pure white standard (100% reflection) over the entire wavelength spectrum of visible light. The color appearance of the buccal surface of each crown was measured to simulate its appearance clinically.
Reflectance spectra were related to color using the CIE L*, a*, and b* color space. Using appropriate software (Shimadzu Corp, Color Analysis UV-2401PC), the spectral curves were transformed into L*, a*, and b* values of the color space. L* values represent lightness on a continuous scale from black (0) to white (100), a* values represent red (+80a*) to green (−80a*), and b* values represent yellow (+80b*) to blue (−80b*) variations. Total color differences (ΔE) were calculated according to the following equation:
ΔE* = ([ΔL*] 2 + [Δa*] 2 + [Δb*] 2) × 1/2
The proposed limit for color matching adopted in this study was set at 3.3ΔE units (perceptibility threshold). Differences beyond this were considered clinically perceptible.[10]
RESULTS
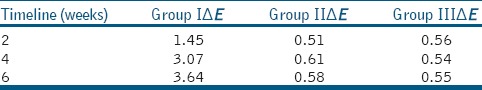
The mean values of the CIE L*, a*, and b* parameters for Groups 1–3 for all time intervals are presented in Table 1. At baseline, no significant difference was detected for L*, a*, and b* parameters between groups. WMTA group showed significant decreases in L*, a*, and b* values. The color change with WMTA led to clinically perceptible crown discoloration after 6 weeks which exceeded the perceptible threshold for the human eye, i.e., ΔE > 3.3 presented in Table 2.
Table 1.
CIE laboratory values of the groups (mean±standard deviation)
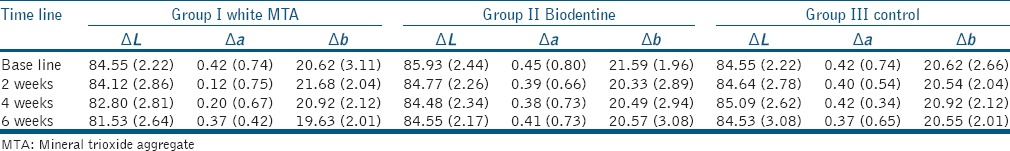
Table 2.
The mean values for color change (ΔE)
Statistical analysis
One-way ANOVA with repeated measurements was used for data analysis of CIE L*, a*, and b* chromatic parameters and total color differences (ΔE). Pairwise between-group and within-group comparisons were conducted with Bonferroni's method. The overall analysis was performed with SPSS software version 16.0 (SPSS Inc, Chicago). The level of statistical significance was set at P < 0.05 [Tables 3 and 4].
Table 3.
The mean values for color change (ΔE) inter group comparison at various intervals
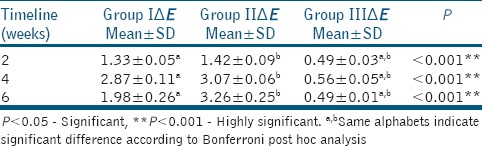
Table 4.
Comparison of each groups (intragroup) at various intervals

DISCUSSION
The results of this study indicate that MTA formulations induced significant color changes in WMTA group. ΔE values ranged between 3 and 3.6 units, in the last 6 weeks, and slightly exceeded the perceptibility threshold, i.e. ΔE > 3.3.[10] Direct comparisons cannot be performed because of the different experimental methodologies, including the colorimetric method of measurement, the material application method, and the sample selection.
The alterations observed in the WMTA group were significantly greater compared with the Biodentine group (intergroup at 6 weeks, P < 0.001 and intragroup at various intervals, P < 0.001). In terms of visual perception, the descriptive analysis of L* values indicates a darkening effect in tooth crowns. When WMTA was applied in the esthetic zone for conservative pulp therapy, apexification, treatment of horizontal root fractures, internal root resorption, and revascularization procedures; a dark tooth stain in the neck of the crowns was a common macroscopic finding.[11] Regarding a* and b* chromatic parameters, a significant decrease was also measured in both experimental groups.[12] The alterations observed in the WMTA group were significantly greater compared with the other two groups. In terms of visual perception, the descriptive analysis of a* and b* values indicates a reduction in redness and yellowness, thus an increasing tendency toward green and blue, respectively. The mechanism of MTA-induced discoloration has not been elucidated. MTA is a bioactive silicate that consists of hydrophilic particles, whose principal components are tricalcium silicate, dicalcium silicate – tricalcium aluminate, tricalcium oxide, bismuth, and iron compounds.
MTA application (white) for pulp capping or pulpotomy can lead to tooth discoloration due to the presence of bismuth oxide.[13] It has been reported that Bi2O3 undergoes thermal dissociation at high temperature, which yields metallic bismuth and oxygen. The reduced dark crystals of bismuth atoms are responsible for the darkening of the sample, and the presence of these crystals has been identified by X-ray diffraction.
After contamination of the specimens with blood, all Portland cement-based materials showed an increased discoloration. It was demonstrated that the microstructure of the materials shows pH-dependent porosities.[14] These porosities may uptake blood components and may be responsible for the observed discoloration. This is of clinical relevance because Portland cement-based materials are usually placed in direct contact to vital, vascularized tissue.[15] Thus, the development of biocompatible materials with a reduced porosity level may be beneficial.[8]
Biodentine does not contain bismuth oxide.[16] Thus, in esthetic area, Biodentine may be more suitable than MTA.[17] One study evaluated Biodentine from this perspective where Biodentine, along with four different materials, was exposed to different oxygen and light conditions and spectrophotometric analysis was performed at different periods until 5 days. Favorable results were obtained for Portland cement and Biodentine and these two materials demonstrated color stability over a period of 5 days.[18] Based on their results, the authors suggested that Biodentine could serve as an alternative for use under light-cured restorative materials in areas that are esthetically sensitive.[19]
The results of this study indicate that WMTA is associated with chromogenic effects. The application of WMTA in pulp chamber in the esthetic zone should be avoided while Biodentine should be used with caution. Further investigation is required for the determination of the etiology of MTA-induced discoloration in association with its chemical constitution.
CONCLUSION
Within the limitations of this study, it may be concluded that WMTA caused perceptible color changes while Biodentine resulted in no perceptible color change.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lenherr P, Allgayer N, Weiger R, Filippi A, Attin T, Krastl G. Tooth discoloration induced by endodontic materials: A laboratory study. Int Endod J. 2012;45:942–9. doi: 10.1111/j.1365-2591.2012.02053.x. [DOI] [PubMed] [Google Scholar]
- 2.van der Burgt TP, Mullaney TP, Plasschaert AJ. Tooth discoloration induced by endodontic sealers. Oral Surg Oral Med Oral Pathol. 1986;61:84–9. doi: 10.1016/0030-4220(86)90208-2. [DOI] [PubMed] [Google Scholar]
- 3.Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 4.Rajasekharan S, Martens LC, Cauwels RG, Verbeeck RM. Biodentine™ material characteristics and clinical applications: A review of the literature. Eur Arch Paediatr Dent. 2014;15:147–58. doi: 10.1007/s40368-014-0114-3. [DOI] [PubMed] [Google Scholar]
- 5.Butt N, Talwar S, Chaudhry S, Nawal RR, Yadav S, Bali A. Comparison of physical and mechanical properties of mineral trioxide aggregate and Biodentine. Indian J Dent Res. 2014;25:692–7. doi: 10.4103/0970-9290.152163. [DOI] [PubMed] [Google Scholar]
- 6.Bronnec F. Biodentine: A dentin substitute for the repair of root perforations, apexification and retrograde root filling. Journal of Endodontics. 2010;36:400–13. [Google Scholar]
- 7.Camilleri J. Investigation of Biodentine as dentine replacement material. J Dent. 2013;41:600–10. doi: 10.1016/j.jdent.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Ioannidis K, Mistakidis I, Beltes P, Karagiannis V. Spectrophotometric analysis of coronal discolouration induced by grey and white MTA. Int Endod J. 2013;46:137–44. doi: 10.1111/j.1365-2591.2012.02098.x. [DOI] [PubMed] [Google Scholar]
- 9.Vallés M, Roig M, Duran-Sindreu F, Martínez S, Mercadé M. Color stability of teeth restored with Biodentine: A 6-month in vitro study. J Endod. 2015;41:1157–60. doi: 10.1016/j.joen.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Fontes ST, Fernández MR, de Moura CM, Meireles SS. Color stability of a nanofill composite: Effect of different immersion media. J Appl Oral Sci. 2009;17:388–91. doi: 10.1590/S1678-77572009000500007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felman D, Parashos P. Coronal tooth discoloration and white mineral trioxide aggregate. J Endod. 2013;39:484–7. doi: 10.1016/j.joen.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 12.Bortoluzzi EA, Araújo GS, Guerreiro Tanomaru JM, Tanomaru-Filho M. Marginal gingiva discoloration by gray MTA: A case report. J Endod. 2007;33:325–7. doi: 10.1016/j.joen.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Marciano MA, Costa RM, Camilleri J, Mondelli RF, Guimarães BM, Duarte MA. Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J Endod. 2014;40:1235–40. doi: 10.1016/j.joen.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 14.Namazikhah MS, Nekoofar MH, Sheykhrezae MS, Salariyeh S, Hayes SJ, Bryant ST, et al. The effect of pH on surface hardness and microstructure of mineral trioxide aggregate. Int Endod J. 2008;41:108–16. doi: 10.1111/j.1365-2591.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 15.Vallés M, Mercadé M, Duran-Sindreu F, Bourdelande JL, Roig M. Influence of light and oxygen on the color stability of five calcium silicate-based materials. J Endod. 2013;39:525–8. doi: 10.1016/j.joen.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Fridland M, Rosado R. Mineral trioxide aggregate (MTA) solubility and porosity with different water-to-powder ratios. J Endod. 2003;29:814–7. doi: 10.1097/00004770-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Kohli MR, Yamaguchi M, Setzer FC, Karabucak B. Spectrophotometric analysis of coronal tooth discoloration induced by various bioceramic cements and other endodontic materials. J Endod. 2015;41:1862–6. doi: 10.1016/j.joen.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Keskin C, Demiryurek EO, Ozyurek T. Color stabilities of calcium silicate-based materials in contact with different irrigation solutions. J Endod. 2015;41:409–11. doi: 10.1016/j.joen.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Malkondu Ö, Karapinar Kazandag M, Kazazoglu E. A review on biodentine, a contemporary dentine replacement and repair material. Biomed Res Int. 2014;2014:160951. doi: 10.1155/2014/160951. [DOI] [PMC free article] [PubMed] [Google Scholar]


