Abstract
Aim:
This study was undertaken to evaluate the efficacy of smear layer removal and nanostructural and chemical changes caused by chitosan and ethylenediaminetetraacetic acid (EDTA) on tooth surface using atomic force microscopic analysis and energy-dispersive X-ray (EDX) analysis.
Methodology:
Forty single-rooted premolars were decoronated to a standard length of 15 mm and enlarged to Protaper F3 with irrigation of 1 mL 1% NaOCl and deionized water. Specimens were then divided into 4 groups with 10 samples each and subjected to final rinse with 17% EDTA solution, 0.2% and 0.5% chitosan solution for 1 min. Samples were sectioned into 2 halves. One half of sample from each group were subjected to EDX analysis to check the calcium/phosphate (Ca/P) ratio. The second half of sample from each group subjected to atomic force microscopy (AFM) analysis to study the smear layer removal and nanostructural changes. Statistical analysis was done using ANOVA and Chi-square test.
Results:
The AFM images showed no difference in the elimination of smear layer. The quantitative analysis using AFM showed EDTA group had significantly higher surface alteration than chitosan. EDX analysis showed that the Ca/P ratio of root dentine in EDTA group is significantly lower than chitosan group.
Conclusion:
Chitosan is an effective chelating agent with less alteration in radicular dentine.
Key words: Atomic force microscope, calcium/phosphate ratio, chitosan, energy-dispersive X-ray analysis, nanostructural changes, smear layer
INTRODUCTION
Irrigation and instrumentation play a critical role in root canal therapy. Currently used rotary instrumentation produces smear layer that covers canal walls and the openings of the dentinal tubules[1] which contains organic and inorganic substances.[1,2,3]
Sodium hypochlorite is not able to remove the smear layer thoroughly because it is effective only on organic debris.[3,4,5] Ethylenediaminetetraacetic acid (EDTA) effectively removes the inorganic portion of smear layer.[4,6] Therefore, to obtain a complete removal of the smear layer combination of sodium hypochlorite and a chelating agent like EDTA is recommended,[1,3,7] use of which may cause changes in biomechanical properties of dentin. Any change in the calcium phosphate (Ca/P) ratio alters the proportion of organic and inorganic components and consequently affects the characteristic hardness, permeability, and solubility of dentin.[8,9,10] Therefore, the search for more biocompatible agent continues.
Chitosan, a biocompatible natural polysaccharide, has a high chelating ability.[9] The antifungal effect of a 2% chitosan gel has been demonstrated.[11] However, studies on the chelating ability of chitosan for smear layer removal is scarce in literature.
Conventionally, smear layer has been inspected under scanning electron microscope (SEM) which is easy to operate.[12] However, SEM gives a low-resolution 2-dimensional image of a sample.[13] Furthermore, it may not clearly differentiate smear layer with sclerotic dentine.[12] Moreover, the sample preparation is complicated and invasive changing the primary structure of samples.[14]
Atomic force microscopy (AFM) has the ability to reconstruct real-time three-dimensional (3D) surfaces of samples on a computer screen with high spatial resolution of structural details of different materials.[15] Therefore, AFM should be ideally suited to study the effect of irrigants on surface topography of root dentine surface.[14,16,17]
Surface alteration in root dentine may be due to the chemical changes brought by the irrigants due to change in Ca/P.[10] Energy dispersive X-ray (EDX) analysis is used for elemental analysis of tooth material.[18]
Hence, the purpose of the study was to compare the in vitro chelating ability of chitosan solution with EDTA visualized under atomic force microscope and Ca/P ratio using EDX with the following null hypothesis. (1) There is no significant difference in the score of smear layer removal efficiency between the groups. (2) There is no significant difference in the mean surface roughness parameters between the groups. (3) There is no significant difference in the mean Ca/P ratio value between the groups.
METHODOLOGY
Forty permanent intact single canal premolar teeth were decoronated to a length of 15 mm and prepared to Protaper F3 (Dentsply Maillefer, Ballaigues, Switzerland) with the standardized working length of 1 mm short of apex and irrigation with 1 mL of 1% sodium hypochlorite (DEOR, Kochi). Canals were dried with paper points to receive final irrigation and divided into 4 groups with 10 samples: Group 1: 17% EDTA solution (Smear off–DEOR, Kochi), Group 2: 0.2% chitosan solution (Everest-Biotech, Bengaluru), Group 3: 0.5% chitosan solution (Everest-Biotech, Bengaluru), and Group 4: Control group (Samples without any treatment). Each group received 5 mL of their respective chelating solution for 1 min.
Longitudinal grooves were made on the buccolingual surfaces using a diamond disc without penetrating the canal, then split into two halves with chisel. The samples were subjected to EDX analysis to determine the alteration in Ca/P ratio caused by the various irrigants. The other halves were preserved for AFM analysis.
Atomic force microscopy analysis
Atomic force microscope requires an absolute flat surface of samples.[14,16] Therefore, external surface of 2nd halves of samples from each group were mounted on acrylic resin block and denuded of its cementum layer with help of cast trimmer and the surface made flat. Then, all samples were polished with help of 600 grit emery paper for 60 s to create a uniform smear layer. Then, each group was subjected to treatment with the respective irrigating solutions.
Smear layer remained on dentine surface from 3D images from AFM was scored according to criteria described by Takeda et al.[19] was used but with modifications. Briefly:
Score 1 = No smear layer, with all tubules cleaned and opened
Score 2 = Few areas covered by smear layer, with most tubules cleaned and opened
Score 3 = Smear layer covering almost all the surface, with few tubules opened
Score 4 = Smear layer covering all the surfaces.
The results of AFM images were subjected to statistical analysis using (SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc.) (α = 0.05) by means of Chi-square tests. Results of EDX analysis and roughness parameters from AFM were subjected to statistical analysis by means of ANOVA and post hoc test. Multiple comparison between groups were done by Tukey's honest significant difference analysis.
RESULTS OF ATOMIC FORCE MICROSCOPIC ANALYSIS
Evaluation of smear layer removal under atomic force microscopy
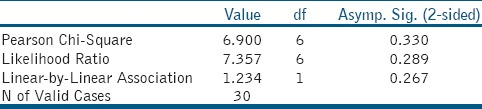
Figures 1–3 are representative images of samples treated with 17% EDTA, 0.2% chitosan, and 0.5% chitosan under AFM which shows the smear layer removal efficiency of test samples. Table 1 and showed no statistically significant difference between any of the group (P > 0.05).
Figure 1.
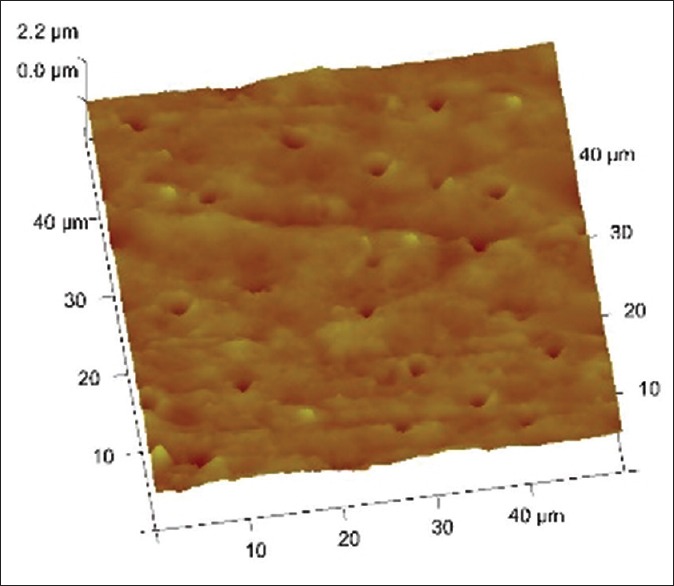
3 D images of tooth treated with 17% edta
Figure 3.
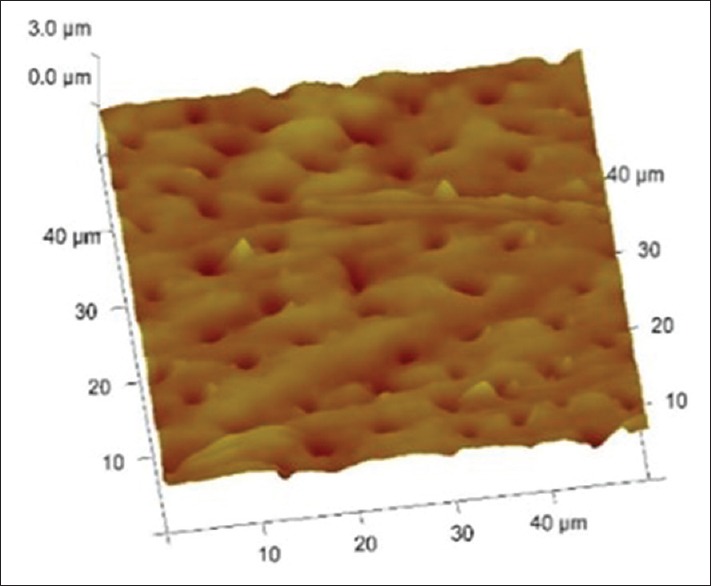
3D images of tooth treated with 0.5% chitosan
Table 1a.
Score distribution with regard to smear layer removal in each experimental group (n=10/group)
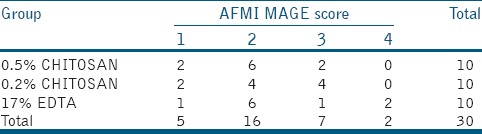
Figure 2.
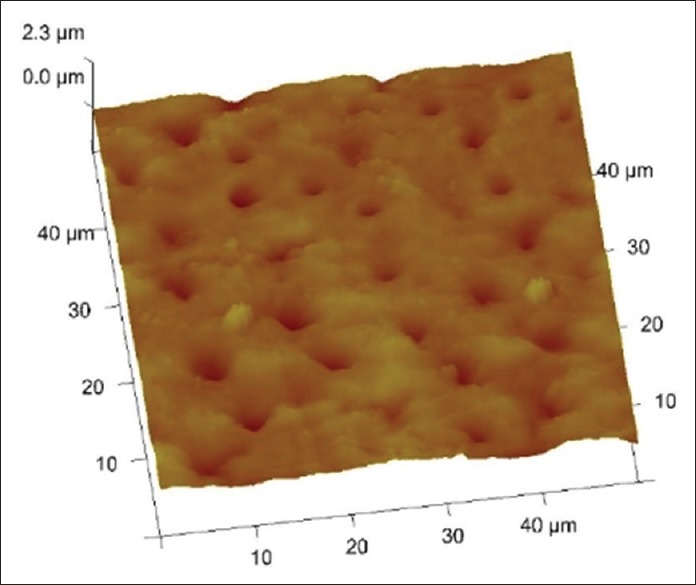
3 D images of tooth treated with 0.2% chitosan
Evaluation of surface roughness parameters using atomic force microscopy
All the tested tooth surface showed the topographic irregularities and nanostructural changes at nanometric scale. Table 2, Graphs 1 and 2 shows roughness parameters of all the test sample. The roughness average (Ra), root mean square (rms) average, and R max value are highest for the 17% EDTA samples than both the groups of chitosan. Roughness parameters between groups are compared, and the difference between groups are statically significant (P = 0.001 for Ra and Rq and P = 0.007 for Rmax).
Table 2.
Comparison of mean roughness parameters of different irrigation treatment
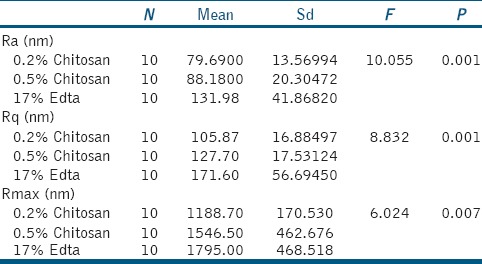
Graph 1.
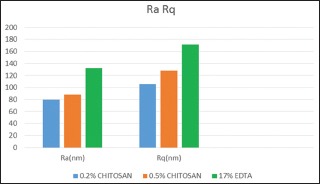
Roughness parameters (the roughness average [Ra]& roughness quotient [Rq]of all the test samples.
Graph 2.
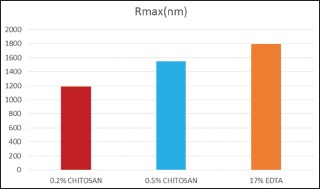
Roughness parameters (Rmax value) of all the test samples.
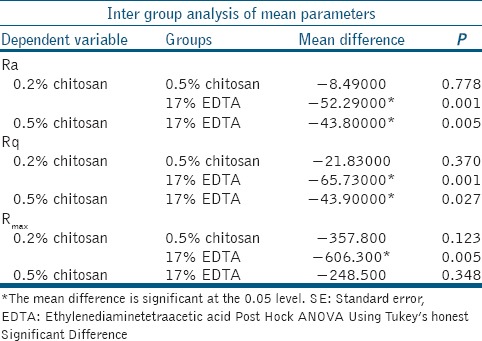
Table 3 shows the Ra, Rq, and Rmax value of both 0.2% chitosan and 0.5% chitosan group is lower than 17% EDTA group and is statistically significant. This indicates that the roughness average produced by EDTA on tooth surface is higher than chitosan group.
Table 3.
Tukey's honest significant difference analysis in which multiple comparisons between groups are done
Energy dispersive X-ray analysis
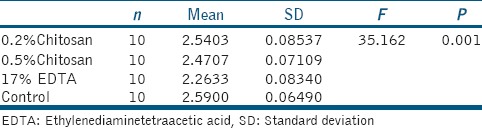
Table 4 and Graph 3 shows the total Ca/P ratio of entire root canal from all the test group. It is found that Ca/P ratio of the control group on root dentine is highest followed by 0.2% chitosan, 0.5% chitosan, and 17% EDTA and is statistically significant (P = 0.001).
Table 4.
Total Ca/P ratio of all the test group
Graph 3.
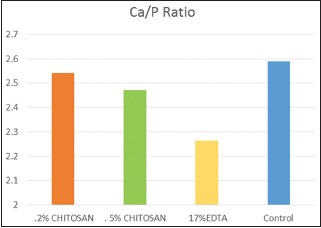
One-way comparison of total Calcium/Phosphate ratio of all the test samples
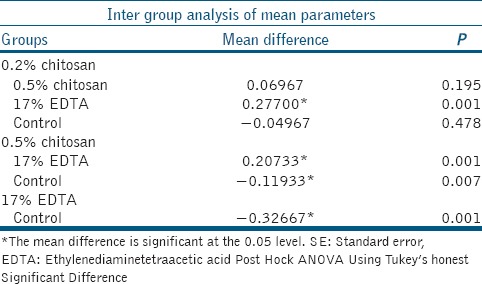
Table 5 shows multiple comparison of total Ca/P ratio using Tukey's analysis. 0.2% chitosan group and 0.5% chitosan group showed statistically significant (P = 0.001) high Ca/P ratio compared to EDTA group. Even though a difference is there among Ca/P ratio of 0.2% chitosan, 0.5% chitosan, and control group, it does not hold any statistical significance.
Table 5.
Tukey HSD analysis: Multiple comparison of total Ca/P ratio
DISCUSSION
The main goal of instrumentation is to facilitate effective irrigation, disinfection, and filling. Root canal instrumentation produces smear layer on the root canal walls which the irrigants eliminates. There is no single irrigating solution that alone sufficiently covers all the functions required from an irrigant. Optimal irrigation is based on the combined use of 2 or several irrigating solutions.[5]
EDTA is the most common decalcifying agent, and hence, it is used as a final irrigant.[3] Crumpton et al. reported that the removal of smear layer was effective using 1 mL of 17% EDTA for 1 min.[20] However, the application of EDTA for more than 1 min led to the erosion[3] and change in biomechanical properties of dentine.[1,3,8] Any change in the Ca/P ratio alters the proportion of organic and inorganic components and consequently affects the characteristic hardness, permeability, and solubility of dentin.[8,10] Hence, in the current study, 5 mL of 17% EDTA was used for 1 min to make valid comparisons among the three final irrigants.
Chitosan, a biocompatible material with chelating property is a natural copolymer of glucosamine and N-acetylglucosamine obtained from shells of crustaceans and shrimps. It is abundantly available waste product recycled from the food industry.[21] On the other hand, studies on the chelating ability of chitosan on smear layer removal are scarce in literatures.
AFM provides a topographical 3D image in real time. The greatest advantage of AFM is special construction of AFM liquid scanner which enables to work directly with biological samples even in liquid environments. Artifacts caused by dehydration of samples are eliminated this way.[15,16]
In our study, the AFM images of all test groups showed an effective cleaning action and opening of dentinal tubules [Table 1] with no statistically significant difference (P > 0.05). A study by PV Silva et al. concluded that 15% EDTA, 0.2% chitosan, and 10% citric acid effectively removed smear layer from the middle and apical thirds of the root canal. They also found out that 15% EDTA and 0.2% chitosan were associated with the greatest effect on root dentine demineralization.[9] Darrag had proved that the final irrigation with 0.2% chitosan solution was more efficient in smear layer removal than 17% EDTA, 10% CA, MTAD.[22] In an another in vitro study, Silva et al. concluded that 0.2% chitosan for 3 min removed the smear layer adequately and caused less erosion than EDTA.[23]
Table 1b.
Chi-Square Tests
Chitosan acts on the inorganic portion of the smear layer favoring its removal by the formation of complexes with metal ions due to adsorption, ionic exchange, and chelation and is responsible for the elimination of dentin calcium ions.[9,24] Most researchers have analyzed only its ability to remove the smear layer. However, their effects on dentin are also important. Table 2, Graphs 1 and 2] shows roughness parameters of all the test sample with greater roughness for 17% EDTA group. It is also shown that the roughness parameters between groups are compared and the difference between groups are statically significant (P = 0.001 for Ra and Rq and P = 0.007 for Rmax). Table 3, multiple comparison between groups, showing that surface roughness caused by both chitosan groups is lower than 17% EDTA group and is statistically significant.
Change in Ca/P ratio gives an idea about the chemical changes brought by the different irrigants on root dentine[10] which might result in surface changes observed under AFM. EDX analysis is used to detect change in Ca/P ratio of root dentine.[17] From the energy-dispersive X-ray spectroscopy (EDS) [Tables 4–5 and Graph 3] analysis, it is clear that Ca/P ratio of EDTA group is lower than all the other test group in the coronal third, middle-third, and apical third. Control group shows the highest Ca/P ratio than any other groups.
Chitosan polymer is hydrophilic and adsorbed to canal wall which favors intimate contact. In addition, it has a large number of free hydroxyl and amino groups that make it cationic in nature that is responsible for the ionic interaction between the dentin calcium ions and the chelating agent.[25]
Hence, from the result of this study, we can conclude that both the groups of chitosan have the capacity to remove smear layer as effectively as done by 17% EDTA. This was in accordance to the study conducted by Silva et al., in which 0.2% chitosan, 15% EDTA, and 10% citric acid were associated with similar smear layer removal patterns[9] but with chitosan group causing less alteration in surface structure and Ca/P ratio.
In our study during instrumentation, 17% EDTA was used as lubricant. Therefore, it can be speculated that the chemical and structural changes within chitosan group might be on account of cumulative effect of both lubricant as well as the final rinse. Hence, further studies are required to evaluate the use of chitosan as a lubricant as well as final rinse.
CONCLUSION
Within the limitations of this in vitro study, it can be concluded that:
No statistically significant difference in smear layer removal between 0.2% and 0.5% chitosan and 17% EDTA group
Evaluation of roughness parameters using AFM showed that 0.2% and 0.5% chitosan group cause significantly less surface alteration on root dentine than 17% EDTA group
EDS analysis showed that 0.2% and 0.5% chitosan group cause significantly less alteration in Ca/P ratio on root dentine than 17% EDTA when used as a final irrigant for 1 min.
Our study concludes that chitosan is an effective chelating agent with less chemical and physical changes in radicular dentine and can be considered as a less invasive alternative or replacement to 17% EDTA.
Further in situ and in vivo studies for the physical, chemical, and biological properties of various concentrations of chitosan solution, their bioactivity and durability to verify the benefits of their use as root canal chelating agents and its effect on biomechanical properties of dentine such as permeability, solubility, and hardness have to be assessed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Adigüzel O, Yiǧit-Özer S, Kaya S, Uysal İ, Ganidaǧli-Ayaz S, Akkuş Z, et al. Effectiveness of ethylenediaminetetraacetic acid (EDTA) and MTAD on debris and smear layer removal using a self-adjusting file. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:803–8. doi: 10.1016/j.tripleo.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 2.Violich DR, Chandler NP. The smear layer in endodontics – A review. Int Endod J. 2010;43:2–15. doi: 10.1111/j.1365-2591.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 3.Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:658–66. doi: 10.1067/moe.2002.128962. [DOI] [PubMed] [Google Scholar]
- 4.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am. 2010;54:291–312. doi: 10.1016/j.cden.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Ram Z. Chelation in root canal therapy. Oral Surg Oral Med Oral Pathol. 1980;49:64–74. doi: 10.1016/0030-4220(80)90032-8. [DOI] [PubMed] [Google Scholar]
- 7.Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: Part 3. J Endod. 1983;9:137–42. doi: 10.1016/S0099-2399(83)80032-6. [DOI] [PubMed] [Google Scholar]
- 8.Calt S, Serper A. Time-dependent effects of EDTA on dentin structures. J Endod. 2002;28:17–9. doi: 10.1097/00004770-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Silva PV, Guedes DF, Nakadi FV, Pécora JD, Cruz-Filho AM. Chitosan: A new solution for removal of smear layer after root canal instrumentation. Int Endod J. 2013;46:332–8. doi: 10.1111/j.1365-2591.2012.02119.x. [DOI] [PubMed] [Google Scholar]
- 10.Barón M, Llena C, Forner L, Palomares M, González-García C, Salmerón-Sánchez M, et al. Nanostructural changes in dentine caused by endodontic irrigants. Med Oral Patol Oral Cir Bucal. 2013;18:e733–6. doi: 10.4317/medoral.18713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senel S, Ikinci G, Kaş S, Yousefi-Rad A, Sargon MF, Hincal AA, et al. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int J Pharm. 2000;193:197–203. doi: 10.1016/s0378-5173(99)00334-8. [DOI] [PubMed] [Google Scholar]
- 12.Lottanti S, Gautschi H, Sener B, Zehnder M. Effects of ethylenediaminetetraacetic, etidronic and peracetic acid irrigation on human root dentine and the smear layer. Int Endod J. 2009;42:335–43. doi: 10.1111/j.1365-2591.2008.01514.x. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Vilchis LE, Contreras-Bulnes R, Olea-Mejìa OF, Sánchez-Flores I, Centeno-Pedraza C. Morphological and structural changes on human dental enamel after Er:YAG laser irradiation: AFM, SEM, and EDS evaluation. Photomed Laser Surg. 2011;29:493–500. doi: 10.1089/pho.2010.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neri JR, Passos VF, de Alencar Viana FB, Rodrigues LKA, Saboia VPA, Santiago SL, et al. Efficacy of smear layer removal by cavity cleaning solutions: An atomic force microscopy study. Rev Odonto Cienc. 2011;26:253–7. [Google Scholar]
- 15.Radmacher M, Tillamnn RW, Fritz M, Gaub HE. From molecules to cells: Imaging soft samples with the atomic force microscope. Science. 1992;257:1900–5. doi: 10.1126/science.1411505. [DOI] [PubMed] [Google Scholar]
- 16.Kubinek R, Zapletalova Z, Vujtek M, Novotný R, Kolarova H, Chmelickova H, et al. Examination of dentin surface using AFM and SEM. Mod Res Educ Top Microsc. 2007:593–8. [Google Scholar]
- 17.Jandt KD. Atomic force microscopy of biomaterials surfaces and interfaces. Surf Sci. 2001;491:303–32. [Google Scholar]
- 18.Russ JC. Fundamentals of Energy Dispersive X-ray Analysis. London: Butterworths; 1984. [Google Scholar]
- 19.Takeda FH, Harashima T, Kimura Y, Matsumoto K. Efficacy of Er:YAG laser irradiation in removing debris and smear layer on root canal walls. J Endod. 1998;24:548–51. doi: 10.1016/S0099-2399(98)80075-7. [DOI] [PubMed] [Google Scholar]
- 20.Crumpton BJ, Goodell GG, McClanahan SB. Effects on smear layer and debris removal with varying volumes of 17% REDTA after rotary instrumentation. J Endod. 2005;31:536–8. doi: 10.1097/01.don.0000148871.72896.1d. [DOI] [PubMed] [Google Scholar]
- 21.Jeon YJ, Shahid F, Kin SK. Preparation of chitin and chitosan oligomers and their applications in physiological functional foods. Food Rev Int. 2000;16:159–76. [Google Scholar]
- 22.Darrag AM. Effectiveness of different final irrigation solutions on smear layer removal in intraradicular dentin. Tanta Dent J. 2014;11:93–9. [Google Scholar]
- 23.Silva PV, Guedes DF, Pécora JD, da Cruz-Filho AM. Time-dependent effects of chitosan on dentin structures. Braz Dent J. 2012;23:357–61. doi: 10.1590/s0103-64402012000400008. [DOI] [PubMed] [Google Scholar]
- 24.Rhazi M, Desbrie'res J, Tolaimate A, Rinaudob M, Votterod P, Alaguic A, El Meraye M, et al. Influence of the nature of the metal ions on the complexation with chitosan. Application to the treatment of liquid waste. European Polymer Journal. 2002;38:1523–30. [Google Scholar]
- 25.Zhang J, Xia Z, Liu P, Cheng Q, Tahirou T, Gu W, et al. Chitosan modification and pharmaceutical/biomedical applications. Mar Drugs. 2010;8:1962–87. doi: 10.3390/md8071962. [DOI] [PMC free article] [PubMed] [Google Scholar]


