Abstract
Ferric sulfate (FS) has been commonly used as a local hemostatic agent for more than three decades in dentistry. Even though the hemostatic mechanism of FS is questioned, it seems that agglutination of blood proteins occurs due to the reaction of blood with ferric and sulfate ions in acidic pH. FS has gained widespread importance as a pulpotomy medicament in contemporary dentistry; nevertheless, it has several applications in different fields of dentistry which was paid little attention. Hence, the purpose of this paper is to review the various applications of FS in dentistry, along with restorative dentistry and endodontics.
Key words: Applications, dentistry, ferric sulfate, hemostasis
INTRODUCTION
Ferric sulfate or FS [Fe2(SO4)3] is a sulfate of trivalent iron. It is yellow in color with a rhombic crystalline salt and is soluble in water at room temperature. It is produced on a large scale by the reaction of sulfuric acid, a hot solution of ferrous sulfate, with an oxidizing agent. It was first introduced in the field of dermatology as Monsel's solution in 1856.[1] In dentistry, 15%–20% FS is used as an astringent and styptic.[2,3] Hemostatic action of FS is due to the agglutination of blood proteins resulting from the reaction of blood with ferric and sulfate ions in acidic pH.[4] The agglutinated proteins form plugs that occlude the capillary orifices.[5]
FS is available in two chemical forms.
Ferric subsulfate [Fe4(OH)2(SO4)5] (Monsel's solution)
Monsel's solution, 20% FS, is used as an effective styptic agent in skin and mucosal biopsies.[1,4,6] However, the ferric and ferrous salts are corrosive and injurious to the soft, as well as hard tissues, causing subsequent staining of the teeth due to its high acidity (pH <1).[4,7] The postoperative complications associated with the soft tissues are delayed reepithelialization and dyspigmentation.[8,9] It also causes reactive and degenerative changes.[1,10] Armstrong et al.[9] observed inflammation, higher incidence of wound infection, and delayed reepithelialization rate in the punch biopsy wounds treated by Monsel's solution compared to collagen matrix at 4 weeks.
Ferric sulfate [Fe2(SO4)3]
As a 15.5% solution, FS is a coagulative and hemostatic agent which forms ferric ion-protein complex on contact with blood. It seals the damaged vessels mechanically, thus producing hemostasis, and the capillary orifices are occluded by the agglutinated protein complex, which prevents blood clot formation.[1] It causes a local and reversible inflammatory response to the oral soft tissues.[11] The recommended application time is 1–3 min and should be placed directly against the damaged tissue due to its quick action. Solutions of FS above 15% are highly acidic and may cause considerable tissue irritation and postoperative root sensitivity.
FS is available commercially with different names [Table 1].[2,12,13,14,15]
Table 1.
Commercial forms of ferric sulfate available and their concentration

APPLICATIONS OF FERRIC SULFATE IN DENTISTRY
Pulpotomy medicament
FS has been commonly used as a pulpotomy medicament to control pulpal bleeding in vital pulp therapy since three decades. It induces hemostasis by forming a sealing membrane at the damaged vessels of pulpal tissue by agglutinating the blood proteins with ferric and sulfate ions.[12]
The nonaldehyde form of FS is most preferred as a pulpotomy agent due to its mechanism of controlling hemorrhage, believed to be associated with physiological clot formation. This might minimize the chances of inflammation and internal resorption when placed on the amputated pulp tissue for 5 min.[16,17]
The most important form of FS is minimal devitalization and preservation of the pulp tissue.[13] The metal-protein clot at the surface of the amputated pulp stumps may probably act as a barrier to the irritative components of the sub-base and functions exclusively in a passive manner.[18] FS showed superior quality of minimizing inflammation and internal resorption compared to calcium hydroxide because of physiological clot formation by the agglutinated proteins.[5,16]
Landau and Johnsen[19] reported the application of FS as a pulpotomy medicament in monkey teeth. Fei et al.[12] published the first human clinical trial using FS with 100% clinical success, compared to formocresol (77%) with 1-year follow-up. Similarly, Fuks et al.[13] reported a study employing FS showing high radiographic success rate of 74.5%.
Antibacterial agent
FS not only exhibited hemostatic action but also has antimicrobial activity. Antibacterial efficacy of FS is similar to 0.2% chlorhexidine digluconate but better than Ankaferd blood stopper® on oral microorganisms such as Staphylococcus aureus, Enterococcus faecalis, Candida albicans, Candida albicans, Porphyromonas gingivalis, Lactobacillus acidophilus, Lactobacillus salivarius, Streptococcus mutans, Streptococcus sobrinus, and Aggregatibacter actinomycetemcomitans under in vitro condition.[20] This might be due to its acidic pH[4] and cytotoxicity.[5] In addition, the occlusion of capillary orifices by agglutinated proteins prevents the ingress of bacteria.[20]
During restorative procedures
FS in a concentration of 15.5%–20%[21] is one of the most common chemical hemostatic reagents used in the restorative dentistry.[22,23,24] It is chemically impregnated into retraction cords in chemomechanical gingival retraction technique in which FS is used in 15%–25% concentration for 3–10 min[24] and offers greater sulcus displacement due to combined physical and chemical effect.[21] Nevertheless, high acidity of gingival retraction fluids and the high affinity of ferric ions for hard tooth structures results in the interaction with bacterial byproducts and precipitation of insoluble ferric sulfide in the porous demineralized dentin. This is the reason for internal discoloration of the dentin. Conrad and Holtan[25] observed that use of FS gingival retraction fluid in combination with translucent porcelain restorations resulted in black internal discoloration of the dentin under porcelain crowns.
Dentinal exposure to such highly acidic solutions of FS (pH range of 0.7–2.0), for 30 s, results in rapid removal of superficial smear layer and peritubular dentin was also lost after prolonged exposure.[26] Furthermore, removal of smear layer by hemostatic agents has been suggested to negatively affect the bonding mechanism of self-etching adhesive,[25,27] which may further promote marginal microleakage and discoloration.[28] Hence, cavity disinfection-associated resin composite restorations is material specific.[29] Cohesive failures were observed in primary dentin bonded with self-etch adhesive systems after application of FS.[30] Ebrahimi et al.[31] observed that dentin surfaces contaminated with ViscoStat® for 60 s before applying adhesives showed reduced shear bond strength of adhesive to dentin. Most of the failures observed were adhesive.
To achieve better outcomes during impression making or application of bonding agents, the hemostatic agents applied before or during etching should be rinsed off properly to create a dentin smear layer. However, rinsing alone cannot eliminate surfaces contamination and thus the remnant FS interfered with diffusion of adhesive in dentinal tubules.[31,32] Because of the weak acidity of the primer in self-etch adhesives, they could not dissolve the contamination by ViscoStat®;[33] hence, it is impossible to penetrate deeper into dentin.[32] The suggested mechanism was that hemostatic agent causes derangement in bonding procedure because of dentinal tubules obturation and dentinal surface was demineralized in different values.[28] It is preferably recommended that FS should be used with etch-and-rinse adhesive systems only.[24] Use of self-etching adhesive significantly lowered the bond strength of dentin contaminated with the hemostatic agent compared to normal dentin. Kuphasuck et al.[28] found that the hemostatic agent does not have any effect on dentin bond strength of the total-etching adhesive.
For gingival displacement in prosthodontics
Fischer (1987) first used FS as a coagulative and hemostatic agent during crown and bridge impressions.[4] It is used for tissue displacement and it would be maintained for at least 30 min.[4,14] Nonetheless, its use for gingival displacement in implantology is not clearly understood as a result of its ability to interrupt the setting reaction of polyether and polyvinyl siloxane impression materials.[25,34] Owing to its iron content, FS stains the gingival tissue yellow-brown to black color for a few days after its application. It has been shown to interfere with surface details of impression materials, as well as discolor dentin by precipitating ferric sulfide in an anaerobic environment.[25] Irrigation with water for at least 10 s eliminates the staining and discoloration effect of ferric compounds on gingiva and esthetic restorations. Furthermore, two studies reported that chlorhexidine gluconate helps achieve hemostasis in a shorter time due to its surfactant effect.[34,35] Shaw et al.[36] observed reversible damage to the connective tissue adjacent to the sulcular gingiva after application of FS. Nevertheless, when used within the gingival sulcus for <10 min, they cause minimal tissue damage.[21,22,23]
Management of postextraction hemorrhage
FS is not widely used to control postextraction hemorrhage; however, it may offer assistance with mucosal tears or uncontrolled postextraction hemorrhage in gingival tissues.[37]
As hemostatic agent used in periradicular and endodontic surgery
Control of bleeding during any surgical procedure is imperative and can be achieved by practical and effective systemic or topical approaches. Epinephrine pellets used either alone or in combination with a FS-soaked pellet are effective topical hemostats when applied in the bony crypt under light pressure.[38] Aluminum chloride alone or in combination with FS (Stasis®) appeared to be the most efficient hemostatic agent to control the bleeding during periapical surgery.[15,39]
Vickers et al.[2] reported that in one-third of the cases where FS was used some oozing of blood occurred in the bony crypt and suction was required to maintain the dryness of the root-end preparation. However, FS was found to be less effective than aluminum chloride in controlling the bleeding. Kim and Kratchman[40] recommended the application of FS on small bleeding points of cortical bone surface only.
An intense inflammatory response including foreign body reaction and delayed osseous healing was documented histologically after 18 and 46 days, when FS was left in situ for maximum exposure.[5] However, when adequately curetted and irrigated from the surgical site before closure, FS neither causes persistent inflammation nor delays osseous repair.[41] Adequate hemostasis can be achieved following the application of 20% FS (Viscostat®) for 5 s during the endodontic surgeries such as root-end resection, root-end preparation, and root-end filling.[2]
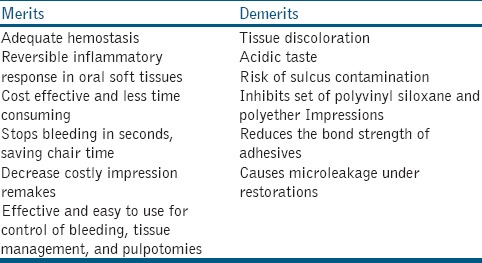
Merits and demerits
FS has several merits and a few demerits, which are shown in Table 2.[4,8,9,12,25,28,31]
Table 2.
Merits and demerits of ferric sulphate
Toxicity
As FS is highly biocompatible, no concerns about toxic or harmful effects have been reported in the dental literature till date.[1,11,14]
CONCLUSION
The FS has been equally beneficial as a local hemostatic agent compared to other chemical hemostatic agents. FS is a commonly used astringent solution (15.5%) that has multiple uses in dentistry. It is widely used in dentistry as hemostatic agent. The use in restorative dentistry and endodontics, pediatric dentistry, prosthodontics, and oral surgery has not been clearly documented. Hence, further research is needed regarding the effective application of FS in the various fields of the dentistry.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Epstein E, Maibach HI. Monsel's solution; history, chemistry, and efficacy. Arch Dermatol. 1964;90:226–8. doi: 10.1001/archderm.1964.01600020094022. [DOI] [PubMed] [Google Scholar]
- 2.Vickers FJ, Baumgartner JC, Marshall G. Hemostatic efficacy and cardiovascular effects of agents used during endodontic surgery. J Endod. 2002;28:322–3. doi: 10.1097/00004770-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Azargoon H, Williams BJ, Solomon ES, Kessler HP, He J, Spears R, et al. Assessment of hemostatic efficacy and osseous wound healing using HemCon dental dressing. J Endod. 2011;37:807–11. doi: 10.1016/j.joen.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Fischer DE. Tissue management: A new solution to an old problem. Gen Dent. 1987;35:178–82. [PubMed] [Google Scholar]
- 5.Lemon RR, Steele PJ, Jeansonne BG. Ferric sulfate hemostasis: Effect on osseous wound healing. 1. left in situ for maximum exposure. J Endod. 1993;19:170–3. doi: 10.1016/s0099-2399(06)80681-3. [DOI] [PubMed] [Google Scholar]
- 6.Larson PO. Topical hemostatic agents for dermatologic surgery. J Dermatol Surg Oncol. 1988;14:623–32. doi: 10.1111/j.1524-4725.1988.tb03390.x. [DOI] [PubMed] [Google Scholar]
- 7.Palm MD, Altman JS. Topical hemostatic agents: A review. Dermatol Surg. 2008;34:431–45. doi: 10.1111/j.1524-4725.2007.34090.x. [DOI] [PubMed] [Google Scholar]
- 8.Amazon K, Robinson MJ, Rywlin AM. Ferrugination caused by Monsel's solution. Clinical observations and experimentations. Am J Dermatopathol. 1980;2:197–205. doi: 10.1097/00000372-198000230-00002. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong RB, Nichols J, Pachance J. Punch biopsy wounds treated with monsel's solution or a collagen matrix. A comparison of healing. Arch Dermatol. 1986;122:546–9. [PubMed] [Google Scholar]
- 10.Olmstead PM, Lund HZ, Leonard DD. Monsel's solution: A histoologic nuisance. J Am Acad Dermatol. 1980;3:492–8. doi: 10.1016/s0190-9622(80)80115-0. [DOI] [PubMed] [Google Scholar]
- 11.Casas MJ, Kenny DJ, Johnston DH, Judd PL. Long-term outcomes of primary molar ferric sulfate pulpotomy and root canal therapy. Pediatr Dent. 2004;26:44–8. [PubMed] [Google Scholar]
- 12.Fei AL, Udin RD, Johnson R. A clinical study of ferric sulfate as a pulpotomy agent in primary teeth. Pediatr Dent. 1991;13:327–32. [PubMed] [Google Scholar]
- 13.Fuks AB, Holan G, Davis JM, Eidelman E. Ferric sulfate versus dilute formocresol in pulpotomized primary molars: Long-term follow up. Pediatr Dent. 1997;19:327–30. [PubMed] [Google Scholar]
- 14.Christensen GJ, Christensen R. Astringedent by ultradent. Clin Res Assoc Newsletter. 1979;3:2. [Google Scholar]
- 15.von Arx T, Jensen SS, Hänni S, Schenk RK. Haemostatic agents used in periradicular surgery: An experimental study of their efficacy and tissue reactions. Int Endod J. 2006;39:800–8. doi: 10.1111/j.1365-2591.2006.01152.x. [DOI] [PubMed] [Google Scholar]
- 16.Schröder U. Effect of an extra-pulpal blood clot on healing following experimental pulpotomy and capping with calcium hydroxide. Odontol Revy. 1973;24:257–68. [PubMed] [Google Scholar]
- 17.Havale R, Anegundi RT, Indushekar K, Sudha P. Clinical and radiographic evaluation of pulpotomies in primary molars with formocresol, glutaraldehyde and ferric sulphate. Oral Health Dent Manag. 2013;12:24–31. [PubMed] [Google Scholar]
- 18.Ranly DM. Pulpotomy therapy in primary teeth: New modalities for old rationales. Pediatr Dent. 1994;16:403–9. [PubMed] [Google Scholar]
- 19.Landau MJ, Johnsen DC. Pulpal responses to ferric sulfate in monkeys. J Dent Res. 1988;67:215. [Google Scholar]
- 20.Cinar C, Odabaş ME, Akca G, Işik B. Antibacterial effect of a new haemostatic agent on oral microorganisms. J Clin Exp Dent. 2012;4:e151–5. doi: 10.4317/jced.50750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenstiel SF. Tissue management and impression making. In: Rosenstiel SF, Land M, Fujimoto J, editors. Contemporary Fixed Prosthodontics. 4th ed. St. Louis, MO: Mosby-Elsevier; 2006. pp. 431–65. [Google Scholar]
- 22.Morgano SM, Malone WF, Gregoire SE, Goldenberg BS. Tissue management with dental impression materials. Am J Dent. 1989;2:279–84. [PubMed] [Google Scholar]
- 23.Akca EA, Yildirim E, Dalkiz M, Yavuzyilmaz H, Beydemir B. Effects of different retraction medicaments on gingival tissue. Quintessence Int. 2006;37:53–9. [PubMed] [Google Scholar]
- 24.Tarighi P, Khoroushi M. A review on common chemical hemostatic agents in restorative dentistry. Dent Res J (Isfahan) 2014;11:423–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Conrad HJ, Holtan JR. Internalized discoloration of dentin under porcelain crowns: A clinical report. J Prosthet Dent. 2009;101:153–7. doi: 10.1016/S0022-3913(09)00025-0. [DOI] [PubMed] [Google Scholar]
- 26.Land MF, Rosenstiel SF, Sandrik JL. Disturbance of the dentinal smear layer by acidic hemostatic agents. J Prosthet Dent. 1994;72:4–7. doi: 10.1016/0022-3913(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 27.O'Keefe KL, Pinzon LM, Rivera B, Powers JM. Bond strength of composite to astringent-contaminated dentin using self-etching adhesives. Am J Dent. 2005;18:168–72. [PubMed] [Google Scholar]
- 28.Kuphasuck W, Harnirattisai C, Senawongse P, Tagami J. Bond strength of two adhesive systems to dentin contaminated with a hemostatic agent. Oper Dent. 2007;32:399–405. doi: 10.2341/06-121. [DOI] [PubMed] [Google Scholar]
- 29.Sharma V, Nainan MT, Shivanna V. The effect of cavity disinfectants on the sealing ability of dentin bonding system: An in vitro study. J Conserv Dent. 2009;12:109–13. doi: 10.4103/0972-0707.57634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prabhakar AR, Bedi S. Effect of glutaraldehyde and ferric sulfate on shear bond strength of adhesives to primary dentin. J Indian Soc Pedod Prev Dent. 2008;26(Suppl 3):S109–13. [PubMed] [Google Scholar]
- 31.Ebrahimi SF, Shadman N, Abrishami A. Effect of ferric sulfate contamination on the bonding effectiveness of etch-and-rinse and self-etch adhesives to superficial dentin. J Conserv Dent. 2013;16:126–30. doi: 10.4103/0972-0707.108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harnirattisai C, Kuphasuk W, Senawongse P, Tagami J. Bond strengths of resin cements to astringent-contaminated dentin. Oper Dent. 2009;34:415–22. doi: 10.2341/08-107. [DOI] [PubMed] [Google Scholar]
- 33.Anil A, Sekhar A, Thomas MS, Ginjupalli K. Haemostatic agents on the shear bond strength of self-adhesive resin. J Clin Exp Dent. 2015;7:e356–60. doi: 10.4317/jced.52284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prasad KD, Hegde C, Agrawal G, Shetty M. Gingival displacement in prosthodontics: A critical review of existing methods. J Interdis Dent. 2011;1:80–6. [Google Scholar]
- 35.Wassell RW, Barker D, Walls AW. Crowns and other extracoronal restorations: Impression materials and technique. Br Dent J. 2002;192:679. doi: 10.1038/sj.bdj.4801456. [DOI] [PubMed] [Google Scholar]
- 36.Shaw DH, Krejci RF, Kalkwarf KL, Wentz FM. Gingival response to retraction by ferric sulfate (Astringedent) Oper Dent. 1983;8:142–7. [PubMed] [Google Scholar]
- 37.McCormick NJ, Moore UJ, Meechan JG. Haemostasis. Part 1: The management of post-extraction haemorrhage. Dent Update. 2014;41:290. doi: 10.12968/denu.2014.41.4.290. [DOI] [PubMed] [Google Scholar]
- 38.Jang Y, Kim H, Roh BD, Kim E. Biologic response of local hemostatic agents used in endodontic microsurgery. Restor Dent Endod. 2014;39:79–88. doi: 10.5395/rde.2014.39.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maestre-Ferrin L, Penarrocha-Diago M. Hemostatic agents used in apical surgery: A review. J Clin Exp Dent. 2011;3:e310–3. [Google Scholar]
- 40.Kim S, Kratchman S. Modern endodontic surgery concepts and practice: A review. J Endod. 2006;32:601–23. doi: 10.1016/j.joen.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Jeansonne BG, Boggs WS, Lemon RR. Ferric sulfate hemostasis: Effect on osseous wound healing. II. With curettage and irrigation. J Endod. 1993;19:174–6. doi: 10.1016/S0099-2399(06)80682-5. [DOI] [PubMed] [Google Scholar]


