Abstract
Objective
Patients with aneurysmal subarachnoid hemorrhage (aSAH) experience high mortality and morbidity. Neuroinflammation causes brain damage expansion after aSAH. Due to the complexity of the inflammatory response multiple biomarkers are needed to evaluate its' progression. We studied inflammatory process after aSAH by measuring two inflammatory biomarkers, interleukin-6 (IL-6) and high-mobility group box 1 (HMGB1) at simultaneous time-points after aSAH.
Methods
In this prospective population-based study, IL-6 and HMGB1 were measured in aSAH patients (n = 47) for up to five days. Plasma concentrations of IL-6 and HMGB1 were measured at 0, 12 and 24 h after hospital admission, and thereafter daily for up to five days or until the patient was transferred from the intensive care unit (ICU). The patients' neurological outcomes were evaluated with the modified Rankin Scale at six months after aSAH.
Results
A high IL-6 level during the first day after aSAH was associated with a severe initial clinical presentation (p = 0.002) and infection during follow-up (p = 0.031). The HMGB1 level did not associate with these parameters. There was no correlation between IL-6 and HMGB1 levels at any time point during the follow-up. The concentrations of IL-6 and HMGB1 were not associated with neurological outcome.
Conclusions
High initial IL-6 values seem to reflect the intensity of the inflammatory response but not the brain damage per se. An early inflammatory response might even be beneficial since although elevated IL-6 levels were observed in patients with a more severe initial clinical presentation, they were not associated with neurological outcome. The lack of correlation between IL-6 and HMGB1 questions the role of macrophages in the process of the secretion of these inflammatory markers after aSAH, instead pointing to the activation of alternative pro-inflammatory pathways.
Keywords: Aneurysmal subarachnoid hemorrhage, Biomarkers, Neuroinflammation, Inflammatory response, Il-6, HMGB1
Highlights
-
•
IL-6 reflects the intensity of inflammation after aSAH without association to neurological outcome.
-
•
Early inflammatory response might even be beneficial after aSAH.
-
•
Lack of correlation between IL-6 and HMGB1 questions the role of macrophages in inflammatory response after aSAH.
1. Introduction
Despite recent advances in the management of aneurysmal subarachnoid hemorrhage (aSAH), it remains a devastating disease with a mortality approaching 50% and fewer than 60% of the survivors achieving functional independence [1]. The brain injury developing after aSAH occurs in multiple phases. There is evidence suggesting that after the primary insult, an additional brain injury is evoked by early brain injury (EBI) and delayed cerebral ischemia (DCI). There are reports that the development of DCI doubles the risk of poor outcome in aSAH [2], [3].
Early brain injury refers to the acute effects of blood in the subarachnoid space and the transient global ischemia caused by acute elevation in the intracranial pressure. Delayed cerebral ischemia is a multifactorial phenomenon involving angiographic vasospasm, microcirculatory vasoconstriction, microvascular thrombosis, cortical spreading depolarization and blood-brain barrier dysfunction. It is also thought that processes activated during EBI contribute to the development of DCI [2], [4], [5].
Various biomarkers have been examined in aSAH patients but thus far, there are no clinically reliable biomarkers for predicting DCI or prognosis [6]. Recent findings from our group support the hypothesis that UCH-L1 could be useful in predicting patient outcome after aSAH although no association between UCH-L1 and DCI was found [7]. Nevertheless, there is accumulating evidence that inflammation contributes to the development of DCI [2], [8], [9], [10]. Knowledge about the neural control mechanisms of inflammation is accumulating rapidly [11]. A number of cytokines and proinflammatory markers have been associated with a poor outcome in aSAH [6]. Interleukin-6 (IL-6) and high-mobility group box 1 (HMGB1) are two important inflammatory biomarkers; their peripheral blood concentrations have been associated with a poor outcome in aSAH [12], [13], [14].
It has been postulated that the inflammatory response displays biphasic features in EBI and DCI [2], [15]. The biphasic response refers to the double-edged effects of the inflammatory response which can be detrimental at some phase of the disease but alleviating at another. The biphasic properties of multiple molecular pathways have been proposed to contribute to the secondary brain injury also in other types of acute strokes [16], [17]. The putative biphasic effects of the inflammatory response highlight the need for a better understanding of the mechanisms and timescale of the response in aSAH. The development of reliable biomarkers acting as surrogates for the response could help to achieve this goal.
The aim of the present study was to analyze the role of two biomarkers in a group of patients with recent aSAH. We evaluated whether there was any correlation between IL-6 and HMGB1 levels. By comparing the concentrations of these two inflammatory biomarkers at the same time points, we wanted to determine whether the changes in their concentrations could offer more specific information about the inflammatory pathways and possible mechanisms leading to the neural injury developing after aSAH. In addition, we assessed their association with neurological outcome and selected clinical conditions.
2. Methods
Following the approval of the institutional ethics committee, we conducted a prospective, observational, single-center clinical study in Tampere University Hospital (Tampere, Finland). The hospital is one of five tertiary referral centers in Finland serving a population of approximately 1 million inhabitants and thus providing care for all patients suffering from subarachnoid hemorrhage in the area.
The study cohort consisted of 61 consecutive aSAH patients admitted to our center from March 2013 to December 2013. Written informed consent was obtained from each patient or from their next of kin. The time of the onset of symptoms associated with aSAH was registered from the patient records. We excluded patients with an unknown time of onset of symptoms as well as patients whose samples for the IL-6 and HMGB1 assays were not collected within the first 24 h after the onset of symptoms. All patients were treated according to standardized in-house guidelines. In total, 47 patients were considered eligible and were thus included in the study.
The severity of the initial clinical presentation was evaluated according to the World Federation of Neurological Surgeons (WFNS) and Hunt & Hess grading scales. The extent of the primary hemorrhage on the CT scan was graded with Fisher scale. WFNS and Hunt & Hess were dichotomized into non-severe (WFNS 1–3/Hunt & Hess 1–3) or severe (WFNS 4–5 / Hunt & Hess 4–5). The Fisher grade was dichotomized into non-severe (Fisher 1–2) or severe (Fisher 3–4). The neurological outcome was evaluated with the modified Rankin Scale (mRS) six months after aSAH based on a structured interview performed by telephone or during an outpatient clinic visit. mRS was dichotomized into a favorable outcome (mRS 0–2) or an unfavorable outcome (mRS 3–6). Detailed categorizations of WFNS, Hunt & Hess, Fisher and mRS are presented in a Supplementary table. Additionally, we assessed the incidence of infection, which was defined as the need for antimicrobial medication during the follow-up period.
Plasma concentrations of IL-6 and HMGB1 were measured in the samples collected at 0, 12 and 24 h after the admission, and thereafter at every 24 h for up to five days or until the patient was transferred from the ICU. Before the statistical analysis, the IL-6 and HMGB1 measurements were divided into consecutive 24 h intervals starting from the onset of symptoms. If IL-6 and HMGB1 were measured more than once per interval, the mean concentration was used. In a subgroup of 22 patients who had up to five days' follow-up, we also checked if the patient had been treated for DCI. Treatment of DCI was initiated based on the clinical evaluation.
Blood samples were collected into EDTA-containing tubes from an arterial cannula that had been routinely inserted for invasive blood pressure monitoring as well as for blood sampling. After collection, the sample was immediately delivered to the laboratory where it was centrifuged for 10 min at 2000g (room temperature). After centrifugation, the plasma was collected and kept at - 70 °C. IL-6 and HMBG1 concentrations in the plasma samples were measured by ELISA with reagents from eBioscience Inc. (San Diego, CA, USA) and IBL International (Hamburg, Germany), respectively. Assay protocols are described in the following datasheets:
http://www.ebioscience.com/media/pdf/tds/88/88-7066.pdf (IL-6)
The absorbance was measured with Victor3 Multilabel Counter (Perkin Elmer, Finland) and the results were calculated against a standard curve using the smoothed spline method with MultiCalcTM (Perkin Elmer, Finland).
Statistical analyses were performed with R (version 3.3.0 for Mac OS X). Fisher's exact test was used for the categorical variables and Mann-Whitney U test for continuous variables. Linear regression was used for the time interval analysis. Spearman's correlation was used to estimate whether IL-6 and HMGB1 levels correlated
3. Results
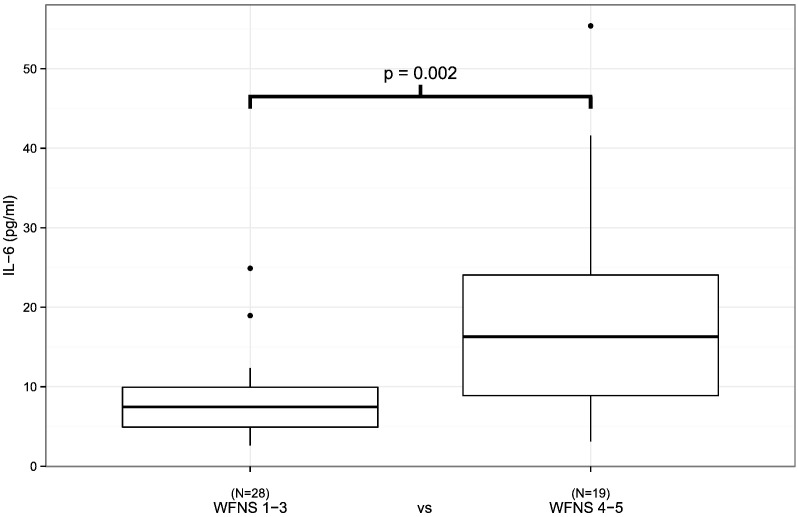
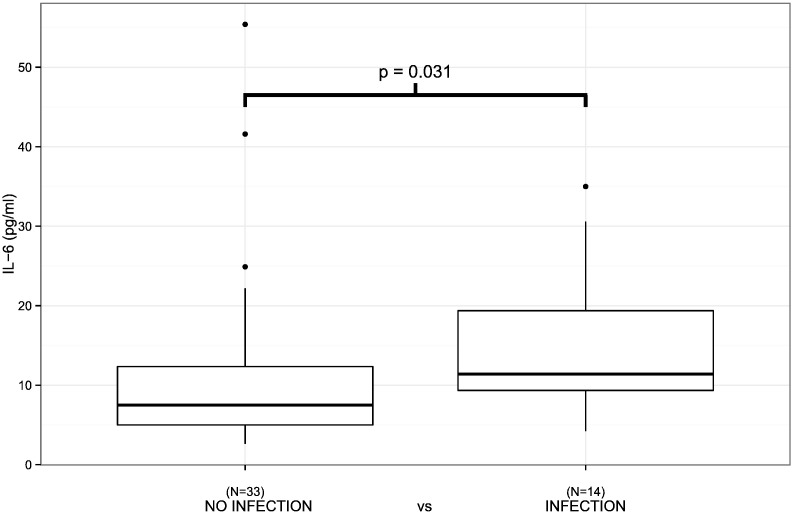
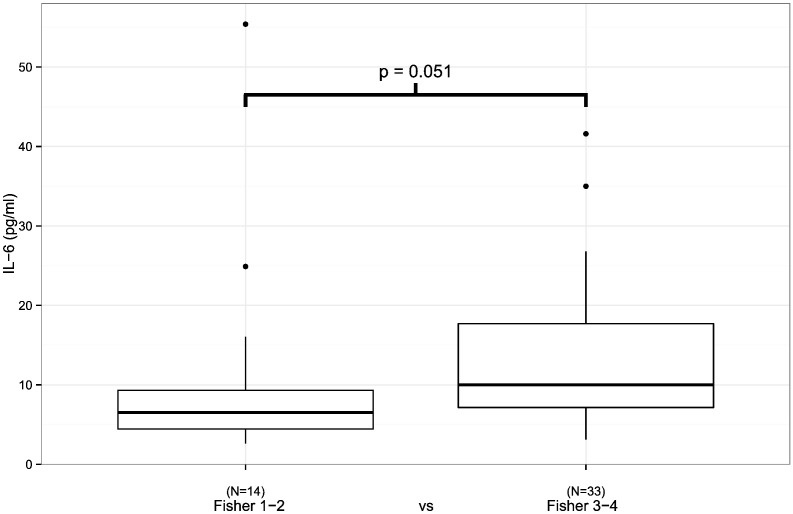
The patients with a severe initial clinical presentation of aSAH (WFNS 4–5/Hunt & Hess 4–5) had a higher plasma IL-6 concentration during the first 24 h following aSAH than the patients with a non-severe (WFNS 1–3, p = 0.002/Hunt & Hess 1–3, p = 0.019) presentation (Fig. 1, Table 1). In addition, the administration of antimicrobial medication was associated with elevated plasma IL-6 levels (Fig. 2, Table 1, p = 0.031). Finally, plasma IL-6 concentrations tended to be higher in patients with severe aSAH according to Fisher scale (Fig. 3, p = 0.051). The plasma HMGB1 concentration during the first 24 h after aSAH was not associated with the severity of aSAH (Table 2).
Fig. 1.
IL6 levels measured within the first 24 h after aSAH in relation to the initial clinical presentation. Patients with more severe clinical presentations had significantly higher IL-6 levels (p = 0.002). Black circles represent outliers.
Table 1.
IL-6 concentration during the first 24 h after aSAH (n = 47).
| IL-6 (pg/ml) | Mean | SD | Median | IQR | p-Value | |
|---|---|---|---|---|---|---|
| mRS | 0.334 | |||||
| 0–2 | (n = 16) | 9.92 | 6.51 | 8.93 | 4.84–13.28 | |
| 3–6 | (n = 31) | 13.96 | 12.20 | 8.85 | 6.30–18.35 | |
| WFNS | 0.002 | |||||
| 1–3 | (n = 28) | 8.19 | 4.77 | 7.45 | 4.93–9.93 | |
| 4–5 | (n = 19) | 19.07 | 13.58 | 16.30 | 8.88–24.05 | |
| Hunt & Hess | 0.019 | |||||
| 1–3 | (n = 35) | 11.21 | 11.04 | 7.70 | 5.13–10.63 | |
| 4–5 | (n = 12) | 16.93 | 9.33 | 16.18 | 9.90–21.10 | |
| Fisher | 0.051 | |||||
| 1–2 | (n = 14) | 11.27 | 14.03 | 6.53 | 4.44–9.30 | |
| 3–4 | (n = 33) | 13.15 | 9.14 | 10.00 | 7.15–17.70 | |
| Infection | 0.031 | |||||
| Yes | (n = 14) | 14.84 | 8.63 | 11.40 | 9.34–19.38 | |
| No | (n = 33) | 11.63 | 11.46 | 7.50 | 5.00–12.35 |
Mann-Whitney U test was used.
Fig. 2.
IL6 levels measured within the first 24 h after aSAH in relation to clinically suspected infection during ICU follow-up. Early IL-6 levels are significantly higher in patients with infection during follow-up (p = 0.031). Black circles represent outliers.
Fig. 3.
IL6 levels measured within the first 24 h after aSAH in relation to the severity of aSAH in primary CT. There is a trend towards higher IL-6 levels in patients with more severe CT findings but statistical significance is not reached by a narrow margin (p = 0.051). Black circles represent outliers.
Table 2.
HMGB1 concentration during the first 24 h after aSAH (n = 47).
| HMGB1 (ng/ml) | Mean | SD | Median | IQR | p-Value | |
|---|---|---|---|---|---|---|
| mRS | 0.486 | |||||
| 0–2 | (n = 16) | 7.87 | 4.13 | 6.85 | 5.59–8.67 | |
| 3–6 | (n = 31) | 6.85 | 2.94 | 6.27 | 4.55–8.61 | |
| WFNS | 0.502 | |||||
| 1–3 | (n = 28) | 6.83 | 2.78 | 6.04 | 5.39–7.72 | |
| 4–5 | (n = 19) | 7.73 | 4.13 | 6.95 | 5.04–8.73 | |
| Hunt & Hess | 0.097 | |||||
| 1–3 | (n = 35) | 6.61 | 2.70 | 5.83 | 4.55–7.87 | |
| 4–5 | (n = 12) | 8.87 | 4.62 | 8.04 | 6.34–10.10 | |
| Fisher | 0.771 | |||||
| 1–2 | (n = 14) | 6.95 | 2.34 | 6.85 | 5.52–8.78 | |
| 3–4 | (n = 33) | 7.30 | 3.76 | 6.27 | 4.61–8.59 | |
| Infection | 0.693 | |||||
| Yes | (n = 14) | 6.84 | 3.11 | 6.33 | 4.38–8.32 | |
| No | (n = 33) | 7.35 | 3.53 | 6.62 | 5.44–8.62 |
Mann-Whitney U test was used.
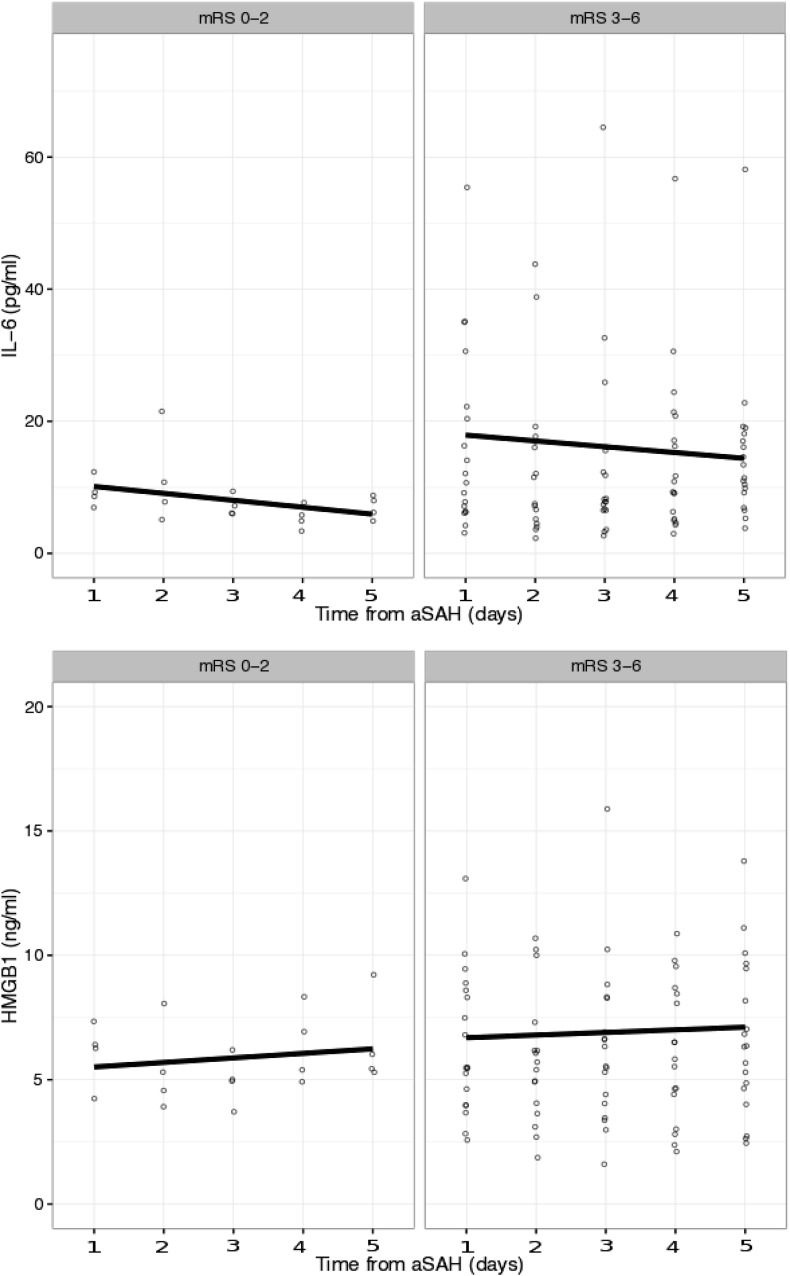
IL-6 and HMGB1 concentrations measured during the first 24 h were not associated with neurological outcome. The descriptive statistics for IL-6 and HMGB1 concentrations measured during the first 24 h after aSAH are presented in Table 1, Table 2. The linear regression analysis revealed no association between neurological outcome and IL-6 or HMGB1 concentrations during the five-day follow-up (Fig. 4).
Fig. 4.
IL-6 and HMGB1 levels for patients (n = 22) with the ICU follow-up lasting for five days (n = 22). Circles represent individual patient values. Values are grouped into favorable and non-favorable neurological outcome. Linear regression shows no significant change between baseline values for IL-6 (mRS 0–2: p-value 0.084, beta − 1.048/mRS 3–6: p-value 0.515, beta − 0.879) or HMGB1 (mRS 0–2: p-value 0.454, beta 0.183/mRS 3–6: p-value 0.776, beta 0.106) during the follow-up. Outliers are not shown.
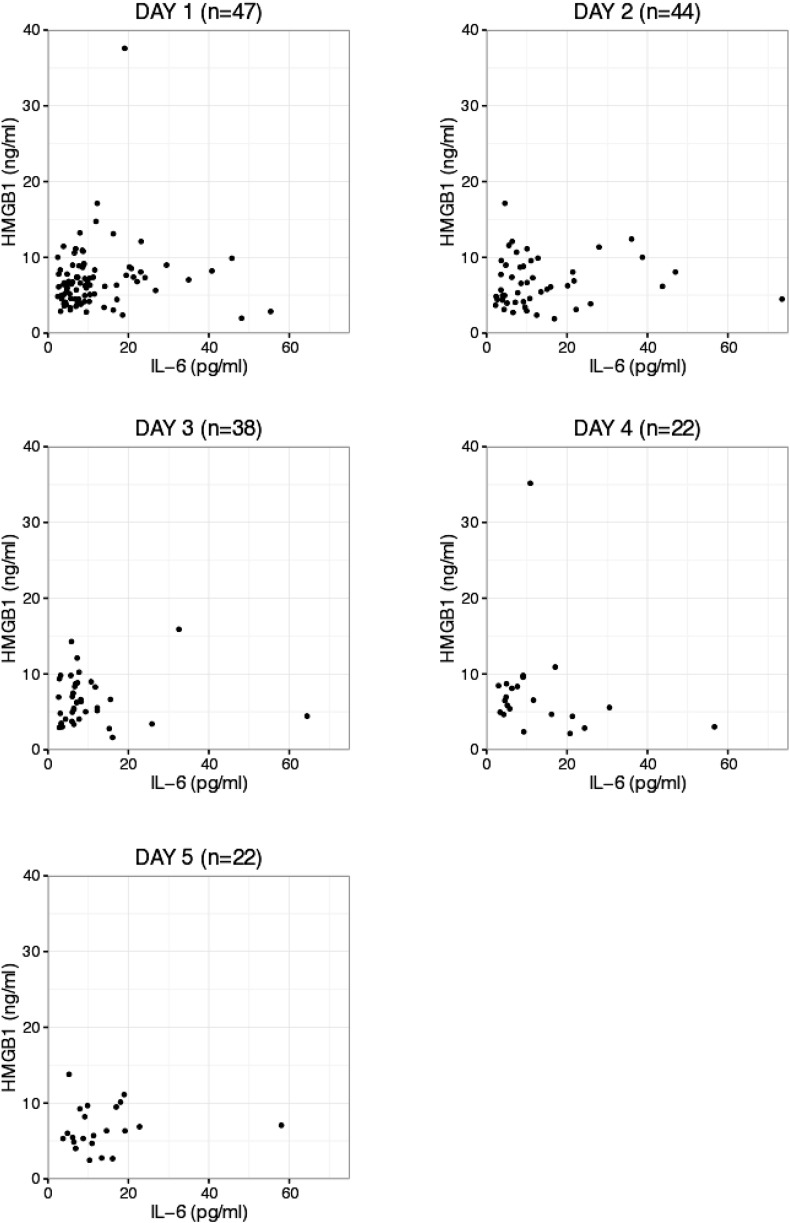
Plasma IL-6 and HMGB1 concentrations did not correlate with each other at any of the time points during the five-day follow-up (Fig. 5, Table 3). In the subgroup of those patients followed for up to five days, IL-6 or HMGB1 concentrations during the first 24 h did not predict the development of DCI. In addition, the presentation of DCI did not associate with IL-6 or HMGB1 concentrations measured on day five (Table 4).
Fig. 5.
Scatter plots displaying the correlation of individual patient values of IL-6 against those of HGMB1 at different time points during the ICU follow-up. Outliers are not shown.
Table 3.
Spearman's correlation coefficients for levels of IL-6 against HMGB1 during ICU follow-up.
| Timepoint | Number of patients in follow-up | Spearman's correlation |
|---|---|---|
| Day 1 | 47 | 0.171 |
| Day 2 | 44 | 0.114 |
| Day 3 | 38 | − 0.034 |
| Day 4 | 22 | − 0.306 |
| Day 5 | 22 | 0.212 |
Table 4.
Descriptive statistics of IL-6 and HMGB1 levels in patients suffering DCI (n = 16) and those without DCI (n = 6) in a subgroup of patients (n = 22) with at least five days' ICU follow-up.
| Mean | SD | Median | IQR | p-Value | ||
|---|---|---|---|---|---|---|
| IL-6 (pg/ml) | ||||||
| Day 1 | DCI | 12.07 | 8.87 | 8.93 | 6.33–12.79 | 0.155 |
| NO DCI | 21.73 | 17.56 | 18.35 | 10.94–21.75 | ||
| Day 5 | DCI | 14.48 | 13.08 | 11.65 | 6.43–17.50 | 0.914 |
| NO DCI | 11.48 | 3.94 | 10.70 | 9.50–11.30 | ||
| HMGB-1 (ng/ml) | ||||||
| Day 1 | DCI | 6.03 | 2.46 | 5.48 | 4.17–7.59 | 0.197 |
| NO DCI | 7.67 | 3.42 | 7.14 | 6.49–8.95 | ||
| Day 5 | DCI | 6.92 | 3.04 | 6.33 | 5.20–9.29 | 0.507 |
| NO DCI | 6.07 | 2.70 | 5.50 | 4.83–7.56 | ||
Mann-Whitney U test was used.
4. Discussion
In the present study, we examined the prognostic potential and interdependence of two inflammatory biomarkers IL-6 and HMGB1 after aSAH.
Our study suggests that a high plasma IL-6 concentration during the first 24 h after aSAH is strongly associated with a severe clinical presentation as well as with the development of infection during the five-day follow-up period. However, neither IL-6 nor HMGB1 levels were associated with neurological outcome in patients suffering from aSAH. Thus, our study failed to support the findings of some previous studies i.e. that there would be an association between increased concentrations of these biomarkers and poor neurological outcome. Zhu et al. claimed that the early plasma HMGB1 concentration was associated with one-year mortality and poor neurological outcome as determined by the Glasgow Outcome Scale. These investigators applied stricter exclusion criteria, for example, patients with systemic diseases such as hypertension, diabetes and chronic heart or lung disease were excluded from the study [13]. Höllig et al. described an association between early serum and cerebrospinal fluid IL-6 concentration with poor neurological outcome at discharge, but not at six months after aSAH. They considered this lack of correlation at six months to be caused partly by the high drop-out rate (27%) after discharge [14]. Kao et al. observed an association between early IL-6 concentration measured from the aneurysmal orifice and peripheral veins with a poor neurological outcome at one month after aSAH. They included only patients who had undergone endovascular coiling while patients with surgically ligated aneurysm were excluded from the study [12]. The discrepant findings between these previous studies and the present study may be partly explained by the differences in the study protocols, the patient selection and the different time-points used to measure neurological outcome. As stated by Höllig et al. [14], it is also essential to appreciate that an elevated IL-6 concentration is a non-specific finding which can reflect the inflammatory response to various pathologies occurring after aSAH. For example, pneumonia is common after aSAH since patients with depressed level of consciousness are susceptible to aspiration of gastric contents until the airway is secured. Invasive mechanical ventilation predisposes patients to ventilator-associated pneumonia and need for ventriculostomy creates a possibility for ventriculitis. All of these infections may lead to increased levels of inflammatory biomarkers.
The secretion of IL-6 and HMGB1 as a part of the inflammatory response is a complex process. One well known mechanism involves the release of these biomarkers or rather, mediators, from monocytes and macrophages [18], [19], [20]. In this study, an elevated IL-6 concentration was associated with the clinical severity after aSAH. Furthermore, a nearly statistically significant trend for elevations in IL-6 was observed in patients with more severe aSAH on the primary CT. On the other hand, the HMGB1 level was not associated with either clinical severity or the CT findings. Furthermore, the IL-6 and HMGB1 concentrations did not correlate with each other at any time point during the five-day follow-up. Based on these findings, it would be tempting to speculate that the secretion of IL-6 after aSAH is mediated through a pathway unrelated to their secretion by monocytes and macrophages.
Inflammation has been associated with DCI [2], [9] and poor outcome after aSAH [12], [14]. In our study, high concentrations of IL-6 immediately after aSAH were associated with severe initial clinical presentation and head CT findings. On the other hand, linear regression did not reveal any mass increase in plasma IL-6 concentrations during follow-up and there was no difference between IL-6 concentrations in patients with favorable and non-favorable neurological outcomes. Moreover, IL-6 levels were not associated with the development of DCI. Thus, our findings raise questions about the biphasic properties of IL-6 in inflammatory response after aSAH, suggesting that the early inflammatory response might not be detrimental to the patient's outcome. Plasma HMGB1, as a late phase cytokine, was not associated with the initial severity of aSAH or with the neurological outcome. In addition to secretion from macrophages during inflammatory response, HGMB1 is also released from cells if they die by necrosis but not when they undergo apoptosis [21]. Apoptosis has been associated with EBI and DCI [2], [4]. The relatively low concentrations of HMGB1 suggest that there had not been extensive tissue necrosis in our patients and support the hypothesis that cell death during EBI and DCI occurs by apoptosis.
This study has some limitations. The sample size is relatively small to make it possible to draw definite conclusions about the prognostic potential of the studied biomarkers. However, the study is well powered to rule out the possibility that there would be a significant correlation between IL-6 and HMGB1 levels during the five-day follow-up. We measured IL-6 and HMGB1 in peripheral blood so the specific mechanisms involved in their secretion remain elusive. Still, the lack of correlation between the studied biomarkers raises interesting questions for future studies investigating more specific cellular and molecular mechanisms of inflammatory response during aSAH. Here, we defined infection as the use of antimicrobial medication during follow-up. Although, extreme caution is adopted in our unit with respect to the use of antimicrobial medication in order to avoid unnecessary treatment, it is still possible that in a small number of patients, antibiotic therapy had been initiated for prophylaxis or simply based on strong clinical suspicion for infection.
High initial IL-6 concentrations in patients with a severe clinical presentation after aSAH seem to reflect the intensity of the “inflammatory reflex” [11] rather than the neuronal injury itself. Thus, the expression of IL-6 differs from that of UCH-L1, which in our previous study was shown to correlate with the neuronal injury caused by aSAH [7]. According to recent evidence, inflammation seems to be a significant contributing factor to DCI. It is plausible that inflammation can exert both detrimental or beneficial effects depending on the phase of the secondary injury process in aSAH [2]. The putative biphasic effects of the inflammatory response highlight the need for clarifying the specific inflammatory pathways and timescales in which they are activated after aSAH. This knowledge would be of paramount importance for targeting anti-inflammatory therapies to appropriate patients at valid time points. As stated by Höllig et al. [14], a “silver-bullet” or “brain troponin” might not exist due to the extreme complexity of physiological processes involved in aSAH. Our findings highlight the complexity of inflammatory response and pathophysiology of DCI in aSAH . Therefore, future studies should test multiple biomarkers or preferably panels of diverse biomarkers measured at the same time-points in order to gain a better understanding of pathophysiology underpinning the inflammatory response in aSAH.
5. Conclusion
High initial IL-6 values seem to reflect the intensity of the inflammatory response associated with initial clinical presentation but not the eventual brain damage per se. An early inflammatory response might even be beneficial since although elevated IL-6 levels were observed in patients with a more severe initial clinical presentation, they were not associated with neurological outcome. The lack of correlation between IL-6 and HMGB1 raises questions about the role of macrophages in the process of the secretion of these inflammatory markers after aSAH, instead pointing to the activation of alternative pro-inflammatory pathways.
Funding statement
The study was financially supported by Academy of Finland (Grant number 138402) and the Competitive State Research Financing of the Expert Responsibility area of Tampere University Hospital.
Ethics approval
The study was conducted with the approval of the Tampere University Hospital Ethics Committee.
Conflict of interest
None.
Acknowledgements
Ms. Meiju Kukkonen and Terhi Salonen are acknowledged for their excellent technical assistance.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ensci.2016.11.010.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Zacharia B.E., Hickman Z.L., Grobelny B.T. Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurg. Clin. N. Am. 2010;21:221–233. doi: 10.1016/j.nec.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2013;10:44–58. doi: 10.1038/nrneurol.2013.246. [DOI] [PubMed] [Google Scholar]
- 3.Dorhout Mees S.M., Kerr R.S., Rinkel G.J.E. Occurrence and impact of delayed cerebral ischemia after coiling and after clipping in the International Subarachnoid Aneurysm Trial (ISAT) J. Neurol. 2011;259:679–683. doi: 10.1007/s00415-011-6243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill W.J., Calvert J.H., Zhang J.H. Mechanisms of early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2006;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald R.L., Pluta R.M., Zhang J.H. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat. Clin. Pract. Neurol. 2007;3:256–263. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- 6.Hong C.M., Tosun C., Kurland D.B. Biomarkers as outcome predictors in subarachnoid hemorrhage – a systematic review. Biomarkers. 2014;19:95–108. doi: 10.3109/1354750X.2014.881418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiiski H., Tenhunen J., Ala-Peijari M. Increased plasma UCH-L1 after aneurysmal subarachnoid hemorrhage is associated with unfavorable neurological outcome. J. Neurol. Sci. 2016;361:144–149. doi: 10.1016/j.jns.2015.12.046. [DOI] [PubMed] [Google Scholar]
- 8.Sarrafzadeh A., Schlenk F., Gericke C. Relevance of cerebral interleukin-6 after aneurysmal subarachnoid hemorrhage. Neurocrit. Care. 2010;13:339–346. doi: 10.1007/s12028-010-9432-4. [DOI] [PubMed] [Google Scholar]
- 9.Carr K.R., Zuckerman S.L., Mocco J. Inflammation, cerebral vasospasm, and evolving theories of delayed cerebral ischemia. Neurol. Res. Int. 2013;2013:506584-12. doi: 10.1155/2013/506584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucke-Wold B., Logsdon A., Manoranjan B. Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int. J. Mol. Sci. 2016;17:497-17. doi: 10.3390/ijms17040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg B.E., Sundman E., Terrando N. Neural control of inflammation: implications for perioperative and critical care. Anesthesiology. 2016;124:1174–1189. doi: 10.1097/ALN.0000000000001083. [DOI] [PubMed] [Google Scholar]
- 12.Kao H.-W., Lee K.-W., Kuo C.-L. Interleukin-6 as a prognostic biomarker in ruptured intracranial aneurysms. PLoS ONE. 2015;10:e0132115–e0132118. doi: 10.1371/journal.pone.0132115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu X.-D., Chen J.-S., Zhou F. Relationship between plasma high mobility group box-1 protein levels and clinical outcomes of aneurysmal subarachnoid hemorrhage. J. Neuroinflammation. 2012;9:194. doi: 10.1186/1742-2094-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Höllig A., Remmel D., Stoffel-Wagner B. Association of early inflammatory parameters after subarachnoid hemorrhage with functional outcome: a prospective cohort study. Clin. Neurol. Neurosurg. 2015;138:177–183. doi: 10.1016/j.clineuro.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Hanafy K.A. The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J. Neuroinflammation. 2013;10:83. doi: 10.1186/1742-2094-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo E.H. A new penumbra: transitioning from injury into repair after stroke. Nat. Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 17.Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nat. Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erlandsson Harris H., Andersson U. Mini-review: the nuclear protein HMGB1 as a proinflammatory mediator. Eur. J. Immunol. 2004;34:1503–1512. doi: 10.1002/eji.200424916. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi M.E., Manfredi A.A. How macrophages ring the inflammation alarm. Proc. Natl. Acad. Sci. U. S. A. 2014;111:2866–2867. doi: 10.1073/pnas.1324285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012;33:571–577. doi: 10.1016/j.it.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Raucci A., Palumbo R., Bianchi M.E. HMGB1: a signal of necrosis. Autoimmunity. 2009;40:285–289. doi: 10.1080/08916930701356978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables