Abstract
Background: There is a paucity of evidence regarding long-term outcomes of late preterm (34-36 weeks) and early term (37-38 weeks) delivery. The objective of this systematic review was to assess long-term cognitive outcomes of children born at these gestations.
Methods: Four electronic databases (Medline, Embase, clinicaltrials.gov and PsycINFO) were searched. Last search was 5 th August 2016. Studies were included if they reported gestational age, IQ measure and the ages assessed. The protocol was registered with the International prospective register of systematic reviews (PROSPERO Record CRD42015015472). Two independent reviewers assessed the studies. Data were abstracted and critical appraisal performed of eligible papers.
Results: Of 11,905 potential articles, seven studies reporting on 41,344 children were included. For early term births, four studies (n = 35,711) consistently showed an increase in cognitive scores for infants born at full term (39-41 weeks) compared to those born at early term (37-38 weeks) with increases for each week of term (difference between 37 and 40 weeks of around 3 IQ points), despite differences in age of testing and method of IQ/cognitive testing. Four studies (n = 5644) reporting childhood cognitive outcomes of late preterm births (34 – 36 weeks) also differed in study design (cohort and case control); age of testing; and method of IQ testing, and found no differences in outcomes between late preterm and term births, although risk of bias was high in included studies.
Conclusion: Children born at 39-41 weeks have higher cognitive outcome scores compared to those born at early term (37-38 weeks). This should be considered when discussing timing of delivery. For children born late preterm, the data is scarce and when compared to full term (37-42 weeks) did not show any difference in IQ scores.
Keywords: Labour, Labour induction, Prematurity, Preterm labour
Introduction
Globally, preterm birth rates are rising with 10% of neonates born less than 37 weeks gestation 1. Late preterm births (34–36 weeks) account for three quarters of all preterm births 2. Early term births (37–38 weeks gestation) have also increased and contribute substantially to an overall decrease in gestational age at delivery. In the US, the average gestational age at delivery has decreased from 40 weeks in 1994 to 39 weeks of gestation in 2004 3.
Early term delivery is associated with increased short term adverse physical morbidity, including respiratory distress syndrome, transient tachypnoea of the neonate and ventilator use, as well as an increased risk of infant mortality at 37 weeks compared to full-term delivery 4– 6. It is for this reason that both the Royal College of Obstetricians and Gynaecologists UK (RCOG) 7 and the American college of Obstetricians and Gynecologists (ACOG) 8 endorse the policy of elective birth after 39 weeks in order to reduce the risk of adverse outcome in infants born before full term (39–40 weeks gestation). There is a paucity of evidence regarding the long-term morbidity of this group, in particular the impact on cognitive function. Advanced gestational age is associated with a lower risk of having special educational need at school 9. Davis et al. 10 has also shown that even amongst the weeks of term advanced gestational age is associated with better neurodevelopment as demonstrated by magnetic resonance imaging (MRI). As obstetric efforts worldwide continue to attempt to reduce stillbirth amongst term deliveries, induction of labour at an earlier gestational age is becoming more common, despite the guidance above, and therefore it is imperative to consider the long-term outcomes of deliveries before term to guide clinicians and parents on optimum timing of delivery.
The association between preterm birth and long-term neurological morbidity is better established with the risk increasing with decreasing gestational age, with extremely preterm babies (≤ 26 weeks) having the worst neurological outcomes 11, 12. The aetiology of this is hypothesized to be due to the disruption of the pathways of dendritic arborisation, synaptogenesis and the thickening of the developing cortex 13. However, there is less evidence regarding long-term cognitive outcomes of late preterm/early term infants, and given they account for the largest proportion of singleton preterm births more research is necessary. A systematic review of 29,375,675 late preterm infants (34–36 weeks) 14, found increased risks of cerebral palsy (RR 3.1, 95% CI 2.3-4.2) and lower likelihood of finishing school in the late preterm born infants (RR 0.96, 95% CI 0.95-0.97), but we could find no prior reviews on cognitive outcomes for early term births.
The aims of this systematic review are to describe the objectively measured cognitive outcomes in childhood up to the age of 18 years i) within each gestational week of term (37–42 weeks) and ii) of late-preterm (34–36 weeks) compared to term (37–42 weeks) deliveries. The results are necessary for informed decision making regarding timing of delivery.
Methods
This systematic review of the literature was conducted according to the STROBE guidelines 15 and reported according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 16 (see Supplementary File 1). The study protocol was registered with the University of York Centre for Reviews and Dissemination International prospective register of systemic reviews (PROSPERO Record CRD42015015472). MEDLINE (1946–2016), EMBASE (1947–2016) and PsycINFO (1945–2016) were searched using a search strategy developed and tested in collaboration with a librarian experienced in literature searching ( Supplementary File 2). The searches were supplemented with a manual search through the reference lists of selected primary articles. A forward citation search was performed on all included studies. The first search date was 12 th January 2015 and the last search was 5th of August 2016. A subsequent search on Clinicaltrials.gov was performed on 2 nd June 2017.
Study selection
One reviewer (JL) screened all titles and abstracts and a second reviewer (BG) independently screened through a 10% sample of the 10,882 articles, by reading the title and abstracts of the first 100 articles of every 1000. The search was updated in August 2016, which yielded an additional 1023 titles and abstracts screened independently by two reviewers (SM, KM). After a consensus was reached, the full texts were retrieved and critically appraised by both reviewers independently (SM, KM). We contacted the individual authors of the included studies to obtain the data necessary to complete the results table. Reasons for exclusion were recorded.
Late-preterm birth was defined as a live birth from 34 to 36+6 completed weeks of gestation. The primary outcome was the results of standardised general intelligence quotient (IQ) tests before age 18 rather than specific domains of cognition. General cognitive ability of a physically and neurologically normal, healthy population of individuals was the key outcome measure recorded. Term birth was defined as a live birth from 37 to 42 completed weeks of gestation.
Studies were included if they reported the range of the participants gestational age, assessment of IQ using a validated score; and the age when IQs was assessed. There were no restrictions by study design, language or method of gestational age assessment. Preterm participants were included as long as there was a clear subgroup of gestational age of 34–36 weeks. Excluded studies included those with: unclear method of cognitive testing; if only selected domains of cognition (e.g. verbal intelligence) were tested; if educational outcomes rather than IQ reported; studies involving high-risk or atypical groups as controls (e.g. multiple births, intra-uterine growth restriction, those with bronchopulmonary dysplasia or brain haemorrhage). Full details of inclusion and exclusion criteria are presented in Supplementary File 3.
The quality of studies was assessed based on the representativeness of the general population, the method of measurement of gestational age and the recording of intelligence testing using the Risk of Bias Assessment Tool for nonrandomized studies (RoBANS) tool 17) ( Supplementary File 4). Two independent reviewers extracted data from each paper on study location, design, population, IQ score used and the main results.
The studies differed widely in outcome measures of cognition used, and due to the large heterogeneity between the study designs and methods a meta-analysis was not possible.
Results
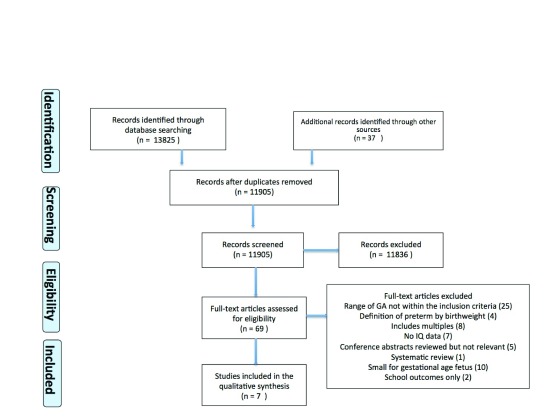
The database and additional record search identified 11,905 articles after removal of duplicates. After exclusions (see flow diagram, Figure 1), six studies and one conference abstract (which reported on both late preterm and outcomes within term and is therefore included in both groups), reporting on 41,344 children/adolescents, were included in the review; four studies comparing the outcomes within term (37–42 weeks) and three studies comparing the outcomes of late preterm delivery (34–36 weeks) with term delivery.
Figure 1. Flowchart showing the study selection process (adapted from the PRISMA flow diagram).
The studies comparing the outcomes within term differed in a number of ways, including: age of testing (1 year, 4 years and 6 years); method of IQ testing (Bayley Scales of Infant Development (BSID), the Stanford-Binet general IQ test and the Wechsler Abbreviated Scale of Intelligence (WASI); details in Supplementary File 5); and the categories of gestational age investigated (37–41 weeks in one study, 37–42 weeks in two studies and 37–43 weeks in one study).
The studies comparing the outcomes of late preterm delivery (34–36 weeks) also differed in a number of ways, including: study design (three prospective cohort studies and one case control study); age of testing (2 years and 13–14 years); and the method of IQ testing (Bayley Scales of Infant Development (BSID) and the Wechsler Intelligence Scale for Children (WISC); details in Supplementary File 5).
Table 1 provides details on the characteristics of the included studies. Three studies 18– 20 and one study, which was only available as a conference abstract (on contacting the author no further information was available as it is not yet published) 21, reporting on term deliveries (35,711 children/adolescents) and three studies 22– 24 (as well as the conference abstract) on late preterm deliveries (5,644 children/adolescents).
Table 1. Characteristics of the included studies, ordered by gestational age categories and age of participant testing.
| Author, Year
(country) |
Study design,
total n |
GA (n) | Control
(n) |
Age
assessed (yrs) |
Exclusions | Exposure
measurement |
Cognitive outcomes * | Adjustment |
|---|---|---|---|---|---|---|---|---|
| Comparison within term (37-42 weeks) | ||||||||
| Espel
et al. 2014
18
(USA) |
Longitudinal
prospective study N = 232 |
37-41
(232) |
- | 1 | Preterm birth (<37 weeks), Post
term birth (>41 weeks) |
GA measured from LMP
And Ultrasound |
BSID II assessed by
trained clinicians, no blinding |
Maternal ethnicity
Marital status Fetal Sex Obstetric complications Maternal education Parity Socio-economic status Birth weight percentile |
| Rose
et al. 2013
19
(Chile) |
Prospective
cohort from an RCT N = 1,562 |
37-42
(1562) |
- | 1 | Infants born at >42 weeks
gestation, birth weight <3kg |
GA based on LMP | BSID II performed by
trained psychologists, blinding not reported |
Fetal sex
Socio-economic status Birthweight |
| Gyamfi
Bannerman et al. 2014 21 (USA) |
Prospective
cohort study N = 20,093 |
37-42
(18,242) |
4 | Parents with mental retardation or
alcohol or drug use |
Not reported | Full-scale IQ scores from
the Stanford-Binet IQ |
Maternal age
Maternal education Socio-economic status Smoking |
|
| Yang
et al. 2009
20
(Belarus) |
Prospective
cohort from an RCT N = 13,824 |
37 (469)
38 (2100) 42 (171) 43 (10) |
39-41
(11, 074) |
6.5 | Birth weight <2500g | GA from hospital
records, 94% confirmed by ultrasound |
Full-scale IQ from WASI
scores performed by trained pediatricians |
Maternal age
Marital status Maternal education Parity Smoking Maternal height Parental occupation |
|
Late preterm vs
term (34 – 36+6) |
||||||||
| Nepomnyaschy
et al. 2011 22 (USA) |
Prospective
cohort study N = 5450 |
34-36
(400) |
37-41
(5050) |
2 | Mothers <15 yrs, congenital
anomalies, multiple births |
GA measured by
maternal estimated LMP |
General, BSID II
(converted to a percentile) mental ability, Language by trained interviewer blinded to GA |
Maternal ethnicity
Maternal age Marital status Fetal sex Obstetric complications Maternal Education Parity Socio-economic status Smoking |
| Romeo
et al.
2015 23 (Italy) |
Prospective
cohort study N -= 119 |
34-36
(71) |
37-41
(48) |
2 | Abnormal cranial Ultrasound,
congenital malformations, SGA |
Not reported | MDI of BSID II at 2 yrs
Blinding and interviewer training not reported |
Fetal sex |
| Narberhaus
et al.
2007 24 (Spain) |
Case Control
study N = 64 |
34-36
(11) |
37-41
(53) |
13-14 | Mental or physical disability,
perinatal complications/hypoxic risk |
Not reported | General WISC full scale
assessed by the same psychologist |
Matched by:
Maternal ethnicity Maternal age Fetal sex Maternal education Socio-economic status |
*Cognitive Outcomes (details in Supplementary File 4): BSID-II = Bayley Scales of Infant Development (2 nd Edition); MDI = Mental Developmental Index, PDI = Psychomotor Developmental Index, WISC = Wechsler Intelligence Scale for Children, WASI = Wechsler Abbreviated Scale of Intelligence
Table 2 shows the cognitive outcomes of each week of gestation among term births (range 37–43 weeks). In general, although the studies differed in age at assessment and the IQ test used, all four studies(18–21) showed an increase in cognitive outcome scores for Infants born at full term (39–41 weeks) compared to those children born at early term (37–38 weeks), with statistically significant increases for each week of term. Three of the studies were classed at moderate risk of bias 18– 20 and one was at high risk because it did not have any clear information on how gestational age was measured 21. Yang et al. 20 was the only study to measure outcomes up to post-term (43 weeks gestation) and found an inverse U-shaped relationship of IQ score and gestational age. In this large study (n = 13,824), with a moderate risk of bias, full-term (39–41 weeks) was used as a reference group with mean differences in IQ scores reported at early term (37–38 weeks) weeks that were lower than full term and also post term (42–43 weeks), which had a higher mean difference from full term than early term (for full risk of bias see Supplementary File 4). The effect size cannot be summarised due to differences between the studies, but the IQ difference between 37 and 40 weeks was approximately 3 IQ points. This may not be clinically significant at an individual level, but would have an impact at a population level.
Table 2. Results of individual studies comparing cognitive outcomes of children born within term (37-42 weeks gestation), ordered by participant age at cognitive testing.
| Study (n) | Cognitive test
and overall scores |
Age at
testing (years) |
Main findings | Effect size (95%CI)/significance
level |
Risk of
bias a |
|||
|---|---|---|---|---|---|---|---|---|
| Scores | ||||||||
| Espel
et al. 2014
18
(n = 232) |
BSID-II (Bayley
scores of infant development) Range 50-150 MDI and PDI € |
1 | GA
b
37-38 39-40 41 |
MDI mean ± SD
c
92±NA e 95±NA 96±NA |
PDI mean±SD
d
92.5±NA 97±NA 106±NA |
Gestational age as a continuous
variable Adj MDI f b = 2.0 (0.45-3.51), p <0.05 Adj PDI g b = 3.9 (1.52 - 6.05), p <0.01 |
Moderate | |
| Rose
et al. 2013
19
(n = 1562) |
Original BSID
(Bayley scores of infant development) MDI and PDI |
1 | GA (n)
37 (45) 38 (260) 39 (469) 40 (604) 41 (184) |
MDI mean ± SD
102.6±11.4 103.4±12.3 103.3±12.9 105.1±11.5 105.4±12.2 |
PDI mean (SD)
94.4±14.9 94.4±14.6 96.6±15.4 98.5±15.1 97.6±14.8 |
Gestational age as a continuous
variable Adj MDI h b = 0.8 (0.2-1.4), p = 0.025 Adj PDI i b = 1.4 (0.6-2.1), p = 0.036 |
Moderate | |
| Gyamfi-Bannerman
et al. 2014 21 (n = 20,093) |
Stanford-Binet IQ
Full scale intelligence Quotient (IQ) Range 40-160 |
4 | GA (n)
37 (1290) 38 (2361) 39 (4040) 40 (4816) 41 (3782) 42 (1953) |
Mean IQ Scores (95%CI)
95.9 (93.6 - 95.3) 97.6 (95.2 - 96.5) 98.6 (97.1 - 98.1) 99.8 (98.1 - 99.0) 99.8 (99.3 - 100.4) 98.1 (99.0 - 100.5) |
Test of linear trend
P <0.001 |
High | ||
| Yang
et al. 2009
20
(n = 13,824) |
WASI (Wechsler
Abbreviated Scale of Intelligence) Full scale intelligence quotient (IQ) Range 40-160 |
6.5 | GA (n)
37 (469 38 (2100) 39 (11074) 42 (171) 43 (10) |
IQ scores
NA NA NA NA NA |
Unadj mean diff
(95%CI) -2.6 (-3.7 - -1.4) 0.6 (-1.1- -0.01) REF k -1.4 (-3.5 - 0.6) -5.8 (-14 - -2.5) |
Adj
j mean diff
(95%CI) -1.7 (-2.7 - -0.7) -0.4 (-1.1 - 0.2) REF -0.4 (-2.5 - 1.7) -5.9 (-15 - 3.3) |
Moderate | |
bGA = Gestational age
cMDI = Mental Developmental Index, SD = standard deviation
dPDI = Psychomotor Developmental Index
eNA = not available
f,g β coefficients represent the actual change in score with each additional week of term, Adj = adjusted for factors ethnicity, parity, maternal age, obstetric risk, birth weight percentile
h,iMeans difference coefficients represent the actual change in score with each additional week of term, Adj = adjusted for factors fetal sex, birth weight centile, socio-economic status
kREF = Reference category
jAdjusted for cluster, birth weight, sex, maternal age, maternal height, parental education, and parental occupation
Table 3 shows the results of the three studies included reporting childhood cognitive outcomes of late preterm birth (34–36 weeks) compared to term birth (37–42 weeks). The abstract by Gyamfi-Bannerman et al. 21 did not specifically compare late preterm and term deliveries statistically; however, the results were available for each week of gestation and have therefore been recorded in the table. This was the only study that showed a difference in IQ scores between late preterm (mean IQ 92.5) compared to term born children (mean IQ 98.3); however this was not statistically analysed in the published abstract and standard deviations were not available on contacting the authors. This was a large study (n = 20,093), but was assessed as having a high risk of bias as there was no information on the method of gestational age measurement. The two studies using the Bayley scores of infant development did not show a difference in scores between late preterm and term born infants; however this was only done at age 2 and there was no further follow up of the infants. The study by Romeo et al. 23 was assessed as having a high risk of bias as there was no mention of how gestational age was measured. The study by Narberhaus et al. 24 provided the longest follow up of the late preterm-born children, testing IQ using the WISC score (Wechsler Intelligence Scale for Children 25) at ages 13–14. No statistically significant difference was found in the IQ scores between late preterm (mean IQ 112.7, SD [standard deviation] 13.8) and term born children (mean IQ 113.6, SD 11.5). However, these results should be interpreted with caution as the risk of bias was high (no way to determine selective outcome reporting, only some mentioning blinding of outcome assessments and no clear indication of how gestational age was calculated).
Table 3. Results of individual studies comparing cognitive outcomes of children born late preterm (34-36 weeks gestation) to term-born infants (37-41 weeks gestation), ordered by participant age at cognitive testing.
| Study (n) | Cognitive test and
overall scores |
Age at
testing (years) |
Main findings
Late preterm Term Mean±SD Mean±SD |
Difference in means (95%CI)/
significance level |
Risk of
bias |
|---|---|---|---|---|---|
| Nepomnyaschy
et al. 2011 22 n = 5,450 (400 late preterm, 5050 term) |
BSID-II (Bayley scores
of infant development) Bayley short form – mental ability (MDI) Bayley short form –psychomotor ability (PDI) Scores standardised as short form only used (unadjusted range 50-150) |
2 | MDI 48.9±10 50.3±10
(Range 92.3 – 174.14) PDI 50.2±9.9 49.9±10 (Range 56.43 – 108.53) |
Unadj MDI -1.43 (-2.70 - -0.16), p <0.05
Adj MDI-0.35 (-1.52 - 0.83), p >0.10 Unadj PDI -0.38 (-1.61 - 0.85), p >0.10 Adj PDI -0.33 (-1.58 - 0.91), p >0.10 |
Moderate |
| Romeo
et al. 2015
23
n = 119 (71 late preterm, 48 term) |
BSID-II (Bayley scores of
infant development) MDI only (range 50-150) |
2 | 96.7±9.3 97.1±6.5 | p > 0.05 | High |
| Gyamfi-Bannerman
et al. 2014 21 n = 20,093 (1,951 late preterm, 18,242 term) |
Stanford-Binet IQ+
Full scale intelligence Quotient (IQ) Range 40-160 |
4 | 92.5±NA 98.3±NA | NA | High |
| Naberhaus
et al.
2007 24 n = 64 (11 late preterm, 53 term) |
WISC (Wechsler
Intelligence Scale for Children) Full scale intelligence quotient Range 40-160 |
13-14 | 112.7±13.8 113.6±11.5 | p > 0.05 | High |
1 Risk of bias is a summary using the RoBANs tool, full details in Supplementary File 4
2MDI = Mental Developmental Index, PDI = Psychomotor Developmental Index
3 Coefficient represent the actual change in score associated with being late-preterm versus full term and Adj. = adjusted for race/ethnicity, age, education, marital birth, father co-residence, household residence, household below poverty
4 Coefficient represents the actual change in score associated with being late-preterm versus full term and Adj. = adjusted for race/ethnicity, age, education, marital birth, father co-residence, household residence, household below poverty
5 NA = not available
Discussion
Main findings
In this systematic review of seven studies (reporting on 41,433 children), the four studies investigating IQ scores within term deliveries found that children born at early term (37–38 weeks) had lower IQ scores at ages one, four and six compared to those born at full term (39–41 weeks). One study (n = 13,824) 20 found a decrease in IQ score at >42 weeks. In the four studies comparing late-preterm deliveries (34–36 weeks gestation) to their term counterparts there were no differences in cognitive outcomes at ages two, four and 14. Studies were heterogeneous and several were at high risk of bias, and therefore summary effect sizes cannot be reported. No studies were identified comparing outcomes between the ages of four and 14. However, it is useful to consider individual study effect sizes and the potential effect on clinical practice. For example, a three point difference in the Stanford-Binet IQ test between children born at 37 weeks and those born at 41 weeks 21 may not be important at an individual level, but this can have important implications at a population level and should be considered along with other factors (estimated birthweight, obstetric risk factors) when clinicians are discussing timing of delivery with parents.
Strengths and limitations
The strengths of this review include the comprehensive and extensive search strategy, with no language restriction, combined with a detailed pre-defined eligibility criteria for study selection. At the screening stage, to reduce reporter bias, two reviewers independently screened a selected sample to check for accuracy and agreement regarding inclusion of studies. Two reviewers critically appraised all included studies independently. A wide range of cognitive assessments was used in the included studies providing a good overview of the various tests available, but this does make comparison between studies more difficult.
Despite the comprehensive nature of the search, the possibility of missing relevant papers cannot be excluded. We did not have the resources to translate the papers in foreign languages; however we did non-expertly translate to see if any papers fitted the inclusion criteria and none were thought to be relevant. Another limitation was the problems encountered with categorisations of gestational age. A number of studies (22 studies reporting cognitive outcomes of 3,357 infants) only listed <37 weeks of gestational age (all preterm births), which inevitably included those <34 weeks and therefore the whole study was excluded. This may have potentially exacerbated the risk of publication bias as we excluded these studies. Due to limited resources, attempts to contact the individual authors of these studies to see if data was available for 34–36 weeks of gestation was not performed. At delivery, birthweight and gestational age are highly correlated. There is a small but statistically significant correlation between birthweight and cognition in childhood and adulthood (each 1kg increase is associated with 0.13 standard deviation test score increase) 26. Some studies did account for birthweight in analyses, and some did not. This may not be appropriate due to their high correlation, and birth weight may be a mediator of the relationship between gestational age and cognition, rather than a confounder. Future studies should report both birth weight and gestational age as a continuous measure, to allow their relative contributions to be measured. Structural equation modelling or similar techniques could be used to model the potential competing causal pathways. We excluded studies with intra-uterine growth restriction (IUGR) because we wanted to study normal healthy singletons, appropriate for gestational age and IUGR may be associated with adverse cognitive outcomes. This review is based on observational data with high levels of between-study heterogeneity, and therefore statistical analysis of the studies was not possible given that the studies were not directly comparable. Limited conclusions can be made regarding the mechanism of action of gestational age on long-term cognitive outcomes because of the nature of the observational data. There were a number of potential sources of bias across the included studies. Although most studies stated how the participants in the studies were chosen in an attempt to reduce selection bias, it is difficult to determine generalizability of the results outwith the populations that were studied. There was a large variation in the number of confounders ( Table 1) adjusted for the various studies, and many did not account for indication for delivery and some did not account for socio-economic factors (strongly associated with cognitive outcomes); therefore there is a risk of residual confounding among the studies.
Interpretation
Comparing the outcomes within the weeks of term (37–42 weeks), this review has shown that cognitive scores in childhood differ throughout the weeks of term delivery and are lowest in those individuals born in early term gestation (37–38 weeks) when measured at ages one, four and six. Although this review specifically set out to look at cognitive outcomes, the findings are in line with those studies of school performance of individuals born within the different weeks of term. Two large population based studies 27, 28 have recently published school outcomes of individuals born within the weeks of term. Smithers et al. 28 (n = 12,601) showed that children born at 40–41 weeks gestation had the lowest risk of vulnerability at school aged five compared to those born early term (37–39 weeks) or post term (42–45 weeks). Figlio et al. 27 (n = 1,536,482) showed that children born late term (41 weeks) performed better in school at the age of five through to 18 compared to those born at full term (39 or 40 weeks). Only one of the studies in the review looked at the effect of post-term delivery (>42 weeks) on cognitive outcomes and found those individuals to have a lower score compared to full term (39–40 weeks). This U-shaped relationship has previously been described in the study by MacKay et al. (n = 407,503), which found the lowest risk of special educational need at school in those born at 41 weeks gestation compared to those born <41 weeks and >41 weeks 9. We identified a previous systematic review published in 2015 that also found a reduction in long term cognitive outcomes of children born early term compared to those born full term, but we were unable to reconcile data included in this previous review with source data 29.
The mechanism of early term (37–38 week) delivery leading to lower cognitive outcome scores compared to full term deliveries (39–41 weeks) is likely to be multifactorial. Vohr et al. described how brain weight increases rapidly in the last trimester of pregnancy with brain weight at 38 weeks 90% of the weight at full term, which may account for the increased vulnerability of early term infants at school 5. For those born post term (>42 weeks), it is thought the increased vulnerability at school age is due to poorer placental perfusion 30.
Only three studies were included in the review comparing the cognitive outcomes of children born late preterm (34–36 weeks) with those born at term (37–41 weeks). There were no (statistically or clinically) significant differences in cognition found between these groups at ages two, four and 13. However, the quality of evidence from these observational studies is poor due to high risk of bias (high chance of residual confounding, no outcome assessor blinding and no way to ascertain if selective outcome reporting took place). We therefore do not make any clinical recommendations relating to the timing of delivery, as these observational data cannot be used for this purpose. The included studies all used term deliveries as the control group, but as this includes early term (which, as described above, have lower cognitive outcomes than 39+ weeks) there is a possibility that differences between <37 weeks and later were masked. The conference abstract, although it did not specifically compare the results for late preterm versus term delivery, if we compared late preterm deliveries (mean IQ 92.5) with only full term deliveries (39–41 weeks, mean IQ 98.3) the difference is large and provides further evidence of a partial dilution of the results in treating term deliveries as a continuum. This was a large study; however the data was taken from an old cohort study performed between 1959 and 1966, and many of the variables, including method of gestational age measurement, were not available. Three out of four of the studies had a high risk of bias, and they all assumed homogeneity between the term cases, which, as shown above, is not the case. Although a previous systematic review has shown a clear increase in physical morbidity associated with late preterm delivery 14 (34–36 weeks) compared to 37+ weeks, there remains a paucity of evidence regarding long term cognitive outcomes in this group. Future studies should use a full-term delivery group (39–41 weeks) as the control group and adopt uniform gestational age categorizations, ideally with similar outcome measures, to allow for easy comparison between studies. Individual level data should be made available as soon as possible to allow large scale individual participant meta-analysis.
Conclusion
Overall, this systematic review has found that children born at full term (39–41 weeks) have the highest cognitive outcome scores compared to those born at early term (37–38 weeks). Given the high prevalence of early term deliveries (the fastest growing proportion of singleton births in the US), small differences at an individual level in cognitive outcomes are likely to have a large impact at a population level. Further research is required to look at the potential reasons for this, and to consider outcomes of late-preterm delivery using a suitable control group of full term (39–41 weeks). The findings from this review have important implications for clinicians and the long-term cognitive outcomes based on gestation at delivery should be discussed with parents regarding optimum timing of delivery.
Acknowledgements
The authors would like to thank Sheila Fisken for her contribution to the database search strategies and the corresponding authors who provided more information about their studies (Lisa Smithers, David Figlio, Cynthia Gyamfi-Bannerman and Lenna Nepomnyaschy).
Funding Statement
This work was supported by a Wellcome Trust PhD studentship [104490] to Sarah Murray.
[version 1; referees: 1 approved, 2 approved with reservations]
Supplementary material
Supplementary File 1: PRISMA checklist.
.
Supplementary File 2: Search strategies.
.
Supplementary File 3: Eligibility criteria for study selection.
.
Supplementary File 4: Study quality assessed using the RoBANS tool.
.
Supplementary File 5: Description of the cognitive tests included in the review.
.
References
- 1. Blencowe H, Cousens S, Oestergaard MZ, et al. : National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–72. 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- 2. Blencowe H, Cousens S, Chou D, et al. : Born too soon: the global epidemiology of 15 million preterm births. Reprod Health.BioMed Central;2013;10 Suppl 1:S2. 10.1186/1742-4755-10-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gyamfi-Bannerman C: The scope of the problem: the epidemiology of late preterm and early-term birth. Semin Perinatol. 2011;35(5):246–8. 10.1053/j.semperi.2011.05.013 [DOI] [PubMed] [Google Scholar]
- 4. Reddy UM, Ko CW, Raju TN, et al. : Delivery indications at late-preterm gestations and infant mortality rates in the United States. Obstet Gynecol Surv. 2009;64(12):780–1. 10.1097/01.ogx.0000363249.47533.8e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vohr B: Long-term outcomes of moderately preterm, late preterm, and early term infants. Clin Perinatol. 2013;40(4):739–51. 10.1016/j.clp.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 6. Martínez-Nadal S, Demestre X, Raspall F, et al. : [Neonatal morbidity in early-term newborns]. An Pediatr (Barc). 2014;81(1):39–44. 10.1016/j.anpedi.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 7. Royal College of Obstetricians and Gynaecologists: Greentop Guideline No. 7.: Antenatal Corticosteroids to Reduce Neonatal Morbidity and Mortality. London: RCOG. 2010. Reference Source [Google Scholar]
- 8. American College of Obstetricians and Gynecologists: ACOG committee opinion no. 559: Cesarean delivery on maternal request. Obstet Gynecol. 2013;121(4):904–907. 10.1097/01.AOG.0000428647.67925.d3 [DOI] [PubMed] [Google Scholar]
- 9. MacKay DF, Smith GC, Dobbie R, et al. : Gestational age at delivery and special educational need: retrospective cohort study of 407,503 schoolchildren.Lau TK, editor. PLoS Med.Public Library of Science;2010;7(6):e1000289. 10.1371/journal.pmed.1000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis EP, Buss C, Muftuler LT, et al. : Children's Brain Development Benefits from Longer Gestation. Front Psychol. 2011;2:1. 10.3389/fpsyg.2011.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore T, Hennessy EM, Myles J, et al. : Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. British Medical Journal Publishing Group; 2012;345:e7961. 10.1136/bmj.e7961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costeloe KL, Hennessy EM, Haider S, et al. : Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ. British Medical Journal Publishing Group; 2012;345:e7976. 10.1136/bmj.e7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huttenlocher PR, Dabholkar AS: Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–78. [DOI] [PubMed] [Google Scholar]
- 14. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. : A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205(4):374.e1–374.e9. 10.1016/j.ajog.2011.07.015 [DOI] [PubMed] [Google Scholar]
- 15. von Elm E, Altman DG, Egger M, et al. : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, W64. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 17. Kim SY, Park JE, Lee YJ, et al. : Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66(4):408–14. 10.1016/j.jclinepi.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 18. Espel EV, Glynn LM, Sandman CA, et al. : Longer gestation among children born full term influences cognitive and motor development. PLoS One. 2014;9(11):e113758. 10.1371/journal.pone.0113758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rose O, Blanco E, Martinez SM, et al. : Developmental scores at 1 year with increasing gestational age, 37–41 weeks. Pediatrics. 2013;131(5):e1475–81. 10.1542/peds.2012-3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang S, Platt RW, Kramer MS: Variation in child cognitive ability by week of gestation among healthy term births. Am J Epidemiol. 2010;171(4):399–406. 10.1093/aje/kwp413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gyamfi-Bannerman C, Son M, Ananth C: 696: Long-term neurodevelopmental outcomes of late preterm and early term neonates. Am J Obstet Gynecol. 2014;210(1):S342–3. 10.1016/j.ajog.2013.10.729 [DOI] [Google Scholar]
- 22. Nepomnyaschy L, Hegyi T, Ostfeld BM, et al. : Developmental outcomes of late-preterm infants at 2 and 4 years. Matern Child Health J. 2012;16(8):1612–24. 10.1007/s10995-011-0853-2 [DOI] [PubMed] [Google Scholar]
- 23. Romeo DM, Brogna C, Sini F, et al. : Early psychomotor development of low-risk preterm infants: Influence of gestational age and gender. Eur J Paediatr Neurol. 2016;20(4):518–23. 10.1016/j.ejpn.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 24. Narberhaus A, Segarra D, Caldú X, et al. : Gestational age at preterm birth in relation to corpus callosum and general cognitive outcome in adolescents. J Child Neurol. 2007;22(6):761–5. 10.1177/0883073807304006 [DOI] [PubMed] [Google Scholar]
- 25. Benson DF: Neuropsychological Assessment By Muriel Lezak. Oxford University Press: London. 1976. Psychol Med. 1978;8(02):347–349. 10.1017/S0033291700014422 [DOI] [Google Scholar]
- 26. Grove BJ, Lim SJ, Gale CR, et al. : Birth weight and cognitive ability in adulthood: A systematic review and meta-analysis. Intelligence. 2017;61:146–58. 10.1016/j.intell.2017.02.001 [DOI] [Google Scholar]
- 27. Figlio DN, Guryan J, Karbownik K, et al. : Long-term Cognitive and Health Outcomes of School-Aged Children Who Were Born Late-Term vs Full-Term. JAMA Pediatr.American Medical Association;2016;170(8):758–64. 10.1001/jamapediatrics.2016.0238 [DOI] [PubMed] [Google Scholar]
- 28. Smithers LG, Searle AK, Chittleborough CR, et al. : A whole-of-population study of term and post-term gestational age at birth and children's development. BJOG. 2015;122(10):1303–11. 10.1111/1471-0528.13324 [DOI] [PubMed] [Google Scholar]
- 29. Chan E, Leong P, Malouf R, et al. : Long-term cognitive and school outcomes of late-preterm and early-term births: a systematic review. Child Care Health Dev. 2016;42(3):297–312. 10.1111/cch.12320 [DOI] [PubMed] [Google Scholar]
- 30. Link G, Clark KE, Lang U: Umbilical blood flow during pregnancy: evidence for decreasing placental perfusion. Am J Obstet Gynecol. 2007;196(5):489.e1–7. 10.1016/j.ajog.2006.11.017 [DOI] [PubMed] [Google Scholar]