Abstract
The perception of music can be impaired after a stroke. This dysfunction is called amusia and amusia patients often also show deficits in visual abilities, language, memory, learning, and attention. The current study investigated whether deficits in music perception are selective for musical input or generalize to other perceptual abilities. Additionally, we tested the hypothesis that deficits in working memory or attention account for impairments in music perception. Twenty stroke patients with small infarctions in the supply area of the middle cerebral artery were investigated with tests for music and visual perception, categorization, neglect, working memory and attention. Two amusia patients with selective deficits in music perception and pronounced lesions were identified. Working memory and attention deficits were highly correlated across the patient group but no correlation with musical abilities was obtained. Lesion analysis revealed that lesions in small areas of the putamen and globus pallidus were connected to a rhythm perception deficit. We conclude that neither a general perceptual deficit nor a minor domain general deficit can account for impairments in the music perception task. But we find support for the modular organization of the music perception network with brain areas specialized for musical functions as musical deficits were not correlated to any other impairment.
Keywords: Music perception, Stroke, Visual abilities, Working memory, Attention, Lesion analysis
Highlights
-
•
Acquired amusia is not necessarily linked to other cognitive or perceptual deficits.
-
•
Amusia and language impairments are connected to each other.
-
•
Small lesions in right basal ganglia are related to rhythm perception deficit.
-
•
Selective deficits support modular view of music perception network.
1. Introduction
The perception, recognition, and joyful sensation of music can be affected by a stroke – a condition called acquired amusia. Impairments of music perception are widely reported in the literature [2], [30], [32], [41], [55], and can occur after lesions to temporal, frontal, and parietal areas [4], [10], [11], [18], [25], [35], [39], [40], [44], [45], [46], [47], [50], but also after subcortical lesions [19].
These patient studies showed a double dissociation between melody [18], [36], [47], [59] and rhythm perception [10], [36], [47], [56]. Melody perception refers to the perception of pitch height, intervals and the contour of a melody (e.g. whether the melody is ascending or descending in pitch). Rhythm perception involves the temporal organization of the melody including the length of different tones also with respect to the underlying beat of that sequence. Patients with deficits in melody perception cannot perceive sequences of single tones as a melody but are able to recognize the rhythmic structure. Patients with deficits in rhythm perception on the other hand can perceive melodic parts of a musical sequence (like single pitch height) and the ongoing melodic structure (like the contour of a melody), but have problems identifying the rhythm of that sequence. In both types of amusia the comparison of musical sequences with either differing melody or differing rhythm cannot be differentiated.
This double dissociation was supported by recent models for music perception suggesting a highly complex and distributed network of temporal, frontal, and parietal areas, additional to subcortical and limbic structures [7], [16], [27], [38], [51]. Melodic information is supposed to be mainly processed in superior temporal and frontal areas; the cerebellum and basal ganglia are thought to be involved in processing rhythmic material. Other studies strengthen the role of premotor and supplementary motor areas in beat perception [14], [17], [58]. Furthermore, functions of pitch and contour processing as well as rhythm perception are attributed to the parietal lobe [15], [29], [49], [52].
Hemispheric lateralization has also been addressed by these models: it was suggested that the right hemisphere processes melodic information and that rhythm is processed in both hemispheres [1], [7], [16], [51]. Johnsrude, Penhune, & Zatorre [26] found that patients with right (but not left) temporal lobe removal overlapping with the Heschl's gyrus showed significantly higher thresholds in judging direction of pitch changes but not in pitch discrimination. In the context of lateralization, the relationship of music and language perception comes into mind. Although the feature extraction for language perception seems to be similar to that for music, speech perception also needs segmentation of phonetic information [27]. Long time it was thought that language is processed in the left hemisphere while music is solely processed in the right hemisphere. However, a number of functional magnetic resonance imaging studies showed that music and speech perception share similar processes and brain structures [12], [13], [27]. A positron emission tomography study demonstrated that primary motor cortex, supplementary motor area, Broca's area, anterior insula, primary and secondary auditory cortices, the temporal pole, basal ganglia, ventral thalamus and posterior cerebellum were all activated while generating melodic and linguistic phrases [6]. Behavioral and electroencephalography studies determined the beneficial relationship between music and language functions (for a review see [23], [31], [34]). Additionally, music and language impairments seem to be correlated [11], [24], [35]. This knowledge about neuroanatomical correlates of music processing is further complicated by individual differences in the representation of music perception [47], [53].
In addition, the music perception network widely overlaps with areas usually responsible for domain-general attention and working memory [22]. This knowledge is expanded by findings that amusia patients often show deficits in visual-spatial abilities, executive functions, memory, learning, and attention [10], [18], [44], [45]. Furthermore music perception problems seem to be highly correlated with aphasia [44], [45], [47], [48], [49], [50], [51] and to visuo-spatial neglect [44], [45]. Conclusively, a direct link between music perception and other cognitive functions, as well as even visual abilities, has been suggested.
Särkärmö and colleagues [44], [45] measured a large group of amusia patients after stroke who presented several cognitive deficits, primarily in attention, working memory (WM), and executive functions. Their work underlined the close relationship between amusia and other cognitive deficits. However, the question whether these connected deficits arise because music perception and the other cognitive functions are accomplished by shared neural processes or whether they involve functionally different but anatomically close areas could not be answered. Lesions in the amusic group were significantly larger than in the non-amusic group and therefore might have mediated the results. Additionally, deficits in attention and WM may have accounted for the poor performance in the music perception task of the amusic patients. The Montreal Battery of Evaluation of Amusia [37] was used in this study and the selected tasks require relatively good WM, attention, and executive abilities [44], [45].
In the current study we wanted to investigate whether symptoms of amusia are specific for musical material or whether a general perceptual deficit can explain the symptoms. Furthermore we were interested in the question whether or not impairments in general domain specific functions like WM or attention could account for poor performances in the MBEA. For this aim we specifically measured stroke patients with small cerebral artery infarctions in order to control for lesions possibly damaging a large array of areas and functions. We applied a large battery of neuropsychological and psychophysical tests including the Montreal Battery of Evaluation of Amusia and tests for visual perception, categorization, neglect, and cognitive functions of attention and WM. Our sample of patients suffering subacute stroke in supply areas of the middle cerebral artery showed a variety of initial symptoms including aphasia, paresis, sensory deficits, and also visual symptoms. Performances in different tests were compared via correlation analysis. Results of healthy control subjects served as cut-off values to determine impaired performances across patient groups. Furthermore, a lesion analysis based on MRI images obtained in the stroke unit was conducted.
2. Material and methods
2.1. Ethical approval
This study was approved by the local ethics committee of the University of Bremen. Subjects were informed about the aim and procedure of the experiment and had to sign a written consent form according to the Declaration of Helsinki. They were free to withdraw from the study at any time.
2.2. Subjects
Patients (n = 20) were ten female and ten male volunteers suffering a subacute stroke in supply areas of the medial cerebral artery. Patients were tested one to six days after the stroke onset in the stroke-unit of the central hospital in Bremen. The mean age was 52 years (± 9.8) and all of them were right-handed.
Data from age-matched healthy control subjects (n = 20) with a mean age of 58 (± 7.7) years were acquired in this study as well. Age differences between both groups were not significant (determined by two-tailed two-sample t-test).
Exclusion criteria were previous neurological, psychiatric or ophthalmological disorders and auditory defects. Further exclusion criteria for the stroke patients were bleedings, bilateral and previous lesions.
2.3. Clinical investigations
All patients underwent a series of neuropsychological tests, including assessment of visual neglect and extinction, visual fields, stereoscopic vision, colour vision, and hearing.
The visual neglect tests included: a line bisection test [57], the apple test [3], the clock task [20], and a copying task (target: flower). For assessment of visual field defects static perimetry of 30° of the visual field was conducted with the contralesional eye. The Lang Test [28] and the Ishihara Colour Vision test [21] served as measures for stereoscopic and colour vision. An audiometry with 8 frequencies for each ear was applied for assessment of hearing.
Furthermore, patients were asked for impairments in the following domains: memory deficits, anomia, reading deficits, visual field defects, spatial orienting disorder and auditory impairments in relation to loudness, sound, voice, and music perception.
All following computer-based tests were performed at 60 cm distance from the screen and subjects wore headphones when required (Sennheiser HD 201). Spatial resolution of the monitor (Samsung Sync Master 1100 MB) was 1600 × 1200 pixels (2041 × 1617 arcmin) and the temporal resolution was 75 Hz. The fixation dot in each test had a size of 5 arcmin. Response time was ‘infinite’, i.e. the next trial started only after a response was given (enforced response).
2.4. Attention test
The D2 Concentration Endurance Test [5] is a test for assessing sustained attention and visual scanning ability. It is a paper and pencil task, where subjects are required to cross out targets and leave non-targets untagged with a time constraint of 20 s for each row (14 rows and 47 characters per row). To measure the quality of performance (correctly processed characters) for each subject the overall number of processed characters, omissions, and errors were evaluated.
2.5. Montreal battery of evaluation of amusia
2.5.1. Stimuli
In order to compute a computer-based version of the Montreal Battery of Evaluation of Amusia ([37]; MBEA) stimuli were taken from the original version. The subtests ‘scale’ and ‘rhythm’ with thirty trials each were used for this experiment. Each trial consisted of a target melody and a comparison melody and both subtests included 15 same and 15 different trials. In ‘different’ trials, one tone was in a different scale or the rhythm of two subsequent tones was changed (for further information see [37]). Särkämö and colleagues [44], [45] showed that the subtests ‘scale’ assessing melody perception and ‘rhythm’ assessing rhythmic perception are sufficient to adequately assess music perception skills. Therefore only these two subtests were administered in this experiment. These two different tests are needed because of the double dissociation between melody and rhythm perception.
2.5.2. Experimental procedure
Two practice trials were completed in advance, in case of difficulties the examples were played for several times until the subjects understood the procedure of the test. Subjects were told to listen to the melody pairs and to decide whether the melody pair was identical or different (two-alternative-forced choice). Each trial began with a 3-s inter-trial interval while the word ‘break’ was displayed on the computer screen. Then a melody pair with a 2-s silent interval was played to the subjects while a note was shown on the screen. Answers were given via button press; buttons were held in both hands (one for same, one for different; button position permuted across subjects). Subjects were allowed to press the button while the music was still playing (e.g. as soon as they heard the different tone they were allowed to press the button for ‘different’) or after the trial.
2.6. Visual gestalt perception task
Some of the reported cases of amusic patients showed visual deficits as well. To test similar visual abilities comparable to the MBEA a visual Gestalt perception task was developed in-house.
2.6.1. Stimuli
The Gestalt images consisted of (68 × 43) Gabor elements distributed over the entire screen while 31–33 of them yielded a Gestalt shape by aligned elements. Five Gestalts had been produced by patterns used in the L-POST test [54]. Picture size was 1600 × 1200 px and the Gestalt shapes extended over 6.5 × 6.5° of visual angle, placed centrally in an area of 10.5 × 10.5° of visual angle (Fig. 1). There were two levels of difficulty: easy (perfectly aligned) and difficult (Gabor elements rotated by up to 15°). The whole task consisted of 40 trials: 20 same and 20 different trials, each containing ten difficult and ten easy comparisons. For each comparison it was ensured that the Gestalt was not placed at the exact same position on both pictures.
Fig. 1.
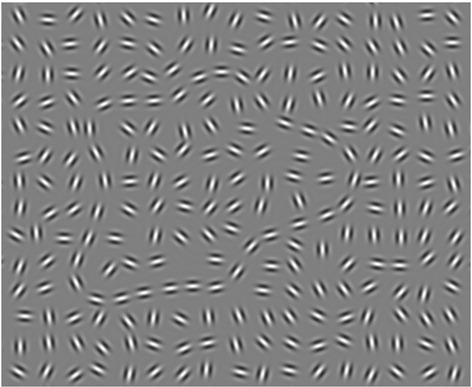
Example image for Gestalt perception task (Gabor shape cropped out for visualization).
2.6.2. Experimental procedure
Before the test, five practice trials were completed to familiarize the subjects with the task. Each trial started with 1000 ms fixation (red dot on grey background). Then target and comparison pictures were shown for 100 ms with a 1000 ms inter-stimulus interval. After that a green fixation point was shown to indicate that the answer was expected. Subjects were instructed to carefully watch the presented pairs of shapes and to decide whether or not the shapes were identical (two-alternative-forced choice). Answers were given via button press (as for the MBEA task).
2.7. Categorization
Because of the reported deficits in visual abilities and language [10], [18], [44], [47], [51], a categorization task consisting of visual and language-related material was invented. In order to investigate whether deficits were present in only one modality or in several ones, the test consisted of four different elements: visual and auditory material as well as verbal and nonverbal stimuli.
2.7.1. Stimuli
The categorization task consisted of 56 stimuli (28 animals and means of transportation each). The task was repeated four times in both visual and auditory modalities (written words, spoken words, images, sounds). Chosen stimuli were controlled for word length, number of syllables and frequency in German language.
Sounds were animals and means of transportation sounds cut to the duration of 700 ms. Only the sound of the corresponding animal/means of transportation was presented to the subjects and loudness was adjusted for all trials. Spoken words had the duration of 295–912 ms (mean: 528 ms).
Images were extracted pictures of animals or means of transportation on a square grey background (11.4 × 11.4°). The extracted pictures were placed centrally in an area of 5.7 × 5.7° of visual angle (Fig. 2). Written words were presented in black on a white background (11.4 × 11.4°) and the words were placed centrally in an area of 8.5 × 0.95° of visual angle which corresponds to a font size of 48 pt. (Fig. 3).
Fig. 2.

Example image for categorization and two-back task.
Fig. 3.
Example image for a word in the categorization task, with black border for visualization.
2.7.2. Experimental procedure
Each trial started with a 1000 ms fixation where a red fixation dot on a grey background had appeared. Stimulus presentation was different for each modality (images: 50 ms; written words: 60 ms; sounds: 700 ms; spoken words: 295–912 ms depending on the word length). A visual mask was applied for 300 ms after stimulus presentation for the visual trials. During auditory stimulation a grey screen was displayed to the subject. Subjects were instructed to decide whether the seen or heard stimulus belongs to the category ‘animal’ or ‘means of transportation’ and to indicate the answer via button press (green: animal, red: means of transportation; button position permuted across subjects). The inter-trial-interval was 500 ms.
2.8. Two-back task (WM)
2.8.1. Stimuli
The two-back task consisted of a visual subtest (pictures, Fig. 2) and an auditory subtest (sounds). Stimuli were chosen from the categorization stimuli, but only five animals and five means of transportation were included in the task (different ones for each modality).
2.8.2. Experimental procedure
Each trial started with 500 ms fixation where a red fixation dot on a grey background was shown. Stimulus presentation of 500 ms and response time of 1000 ms followed. During response time a grey screen was shown. The inter-trial-interval was 500 ms. Subjects were instructed to carefully listen to/look at the presented stimuli and to press a button whenever the presented one was the same as the second last (two-back) one (Go-No Go task). They were allowed to press the button during stimulus presentation or during the response time. The whole experiment consisted of 64 trials with 20 target trials.
2.9. Data analysis
Performance (number correct of answers) of all computer-based tasks and the attention test were analyzed using IBM SPSS Statistics 23. The analysis of the relationships between music and Gestalt perception, attention, categorization, and WM was based on a correlation analysis (Pearson, two-tailed, Bonferroni-corrected).
To determine cut-off values for normal performance on newly designed cognitive tests (visual Gestalt perception, categorization and working memory tasks) the method suggested by Crawford [8], [9] was used. By this method a test score of one individual patient can be compared to a group of healthy controls to determine a significant difference between the patient and the control group.
2.9.1. Lesion analysis
Lesion analysis was performed with MRI images obtained when patients were admitted to the stroke unit. MRIcron [43] and the clinical toolbox of SPM [42] served to delineate and normalize lesions of nineteen patients (Patient P12 only had a cCT measurement). The MNI Flair template brain was used for normalization.
3. Results
3.1. Basic investigations
Initial symptoms reported by the patients are displayed in Table 1. Aphasia and Paresis were the most common symptoms in this patient population. In the measurement session a few patients reported still existing anomia (8), memory deficits (5), reading disorders (4) and auditory deficits concerning loudness (2). No visual or spatial orienting disorders were reported.
Table 1.
Initial symptoms of patients in decreasing order of frequency.
| All patients | Left-hemispheric | Right-hemispheric | |
|---|---|---|---|
| N | 20 | 13 | 7 |
| Aphasia | 10 | 9 | 1 |
| Paresis | 9 | 6 | 3 |
| Nausea | 7 | 5 | 2 |
| Headache | 6 | 5 | 1 |
| Sensory impairments | 6 | 3 | 3 |
| Confusion | 3 | 3 | 0 |
| Amnesia | 3 | 2 | 1 |
| Visual symptoms | 2 | 2 | 0 |
Extinction was not present in the group of patients, but one neglect patient (P13) was identified showing abnormal neglect-typical responses in three of four neglect tests (clock task was normal). Colour vision was normal in the patient sample, but six patients showed problems in stereoscopic vision (three minor and three major).
3.2. Clinical investigations
Norm-values for the D2 test and the MBEA were available. For the newly invented tasks results from age-matched healthy control subjects were acquired in order to determine cut-off values for normal performance. Mean values, standard deviation and cut-off values determined by the method suggested by Crawford can be seen in Table 2. Results of stroke patients for the attention test (D2), MBEA and visual Gestalt tasks, categorization, and WM tests can be seen in Table 3 (correct number of answers).
Table 2.
Mean values, standard deviation and cut-off values of twenty healthy age-matched control subjects for newly invented tests of visual Gestalt perception (Gestalt), categorization (Cat) and working memory (WM).
| Test | Gestalt | Cat sounds | Cat pictures | Cat auditory words | Cat visual words | WM visual | WM auditory |
|---|---|---|---|---|---|---|---|
| Mean | 34.3 | 46.9 | 50.9 | 54.8 | 53.5 | 17.1 | 15.1 |
| Standard deviation | 4.4 | 3.1 | 3.3 | 1.3 | 2.5 | 3.3 | 3.2 |
| Cut-off value | 26 | 41 | 45 | 52 | 49 | 11 | 9 |
Table 3.
Number of correct answers for the attention test (D2), MBEA and visual Gestalt tasks, categorization (cat) and WM tests for all patients. Impaired performances are highlighted in bold (below 75% correct or below cut-off value determined by healthy controls). MBEA 1: scale task; MBEA 2: rhythm task.
| Patient | Attention D2 | MBEA 1 | MBEA 2 | Gestalt | Cat sounds | Cat pictures | Cat auditory words | Cat visual words | WM visual | WM auditory |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 315 | 24 | 26 | 37 | 51 | 47 | 54 | 54 | 7 | 7 |
| 2 | 310 | 27 | 24 | 29 | 52 | 44 | 55 | 32 | 19 | 15 |
| 3 | 333 | 28 | 30 | 35 | 48 | 52 | 54 | 53 | 15 | 9 |
| 4 | 327 | 29 | 28 | 33 | 48 | 54 | 56 | 55 | 20 | 13 |
| 5 | 316 | 13 | 21 | 36 | 50 | 51 | 56 | 55 | 16 | 15 |
| 6 | 379 | 26 | 20 | 32 | 45 | 46 | 54 | 49 | 20 | 17 |
| 7 | 207 | 23 | 24 | 33 | 54 | 49 | 56 | 55 | 12 | 8 |
| 8 | 287 | 28 | 25 | 18 | 48 | 55 | 55 | 52 | 12 | 13 |
| 9 | 451 | 26 | 26 | 35 | 49 | 49 | 56 | 52 | 16 | 15 |
| 10 | 262 | 24 | 30 | 22 | 50 | 49 | 56 | 55 | 17 | 10 |
| 11 | 390 | 26 | 29 | 29 | 52 | 55 | 56 | 56 | 18 | 16 |
| 12 | 108 | 23 | 28 | 39 | 50 | 41 | 55 | 49 | 8 | 5 |
| 13 | 331 | 27 | 28 | 20 | 48 | 47 | 55 | 48 | 16 | 14 |
| 14 | 367 | 29 | 25 | 34 | 47 | 53 | 56 | 54 | 14 | 14 |
| 15 | 99 | 25 | 26 | 30 | 51 | 51 | 55 | 44 | 15 | 13 |
| 16 | 453 | 26 | 24 | 32 | 51 | 52 | 55 | 55 | 19 | 18 |
| 17 | 477 | 27 | 30 | 37 | 53 | 54 | 56 | 55 | 15 | 17 |
| 18 | 372 | 27 | 27 | 33 | 47 | 42 | 54 | 55 | 13 | 16 |
| 19 | 406 | 25 | 27 | 35 | 49 | 52 | 56 | 52 | 18 | 16 |
| 20 | 408 | 25 | 25 | 34 | 51 | 54 | 55 | 54 | 18 | 16 |
Five patients showed deficits in attention, two in musical perception, three in visual Gestalt perception, five in categorization and four in the two-back task. The two patients with amusic symptoms (P5 and P6) did not show any other impairment in the applied tests except for P6 showing slight impairments in visual word categorization. Both of them reported aphasia as initial symptoms, one (P5) still displayed anomia, reading disorder and reported auditory deficits. Thus, both amusia patients showed impairments in language related skills, but not in general perceptual abilities nor in attention or working memory. The neglect patient (P13) did not show amusic symptoms but deficits in the visual Gestalt task.
A correlation analysis (Pearson correlation coefficient, two-tailed, Bonferroni-corrected) for the patient group (of data in Table 2) revealed two significant correlations: auditory WM correlated with visual memory (r = 0.728, p = 0.000), and with the attention test (r = 0.713, p = 0.000).
3.3. Lesion data
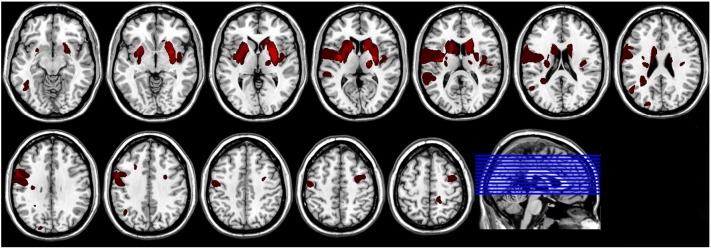
Lesion overlap in the patient sample was relatively small, except for lesions in the basal ganglia (Fig. 4F).
Fig. 4.
Lesion overview: normalized lesions of nineteen patients (left on left and right on right side). Bright red areas are associated with maximum lesion overlap.
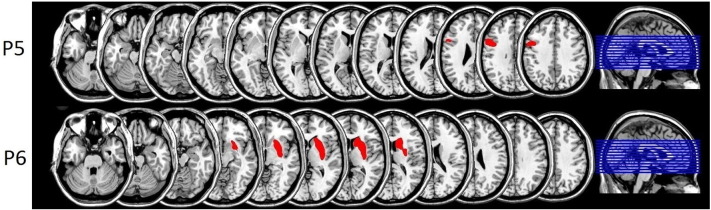
Amusia patients presented lesions in the left frontal lobe (P5) and the right basal ganglia (P6) and both are shown separately in Fig. 5.
Fig. 5.
Lesion overview: normalized lesions of amusia patients P5 and P6 (left on left and right on right side).
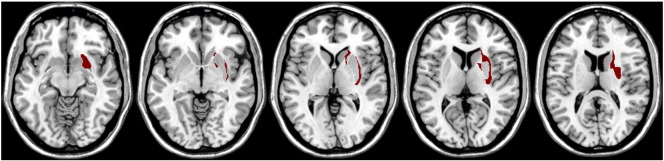
Patient P6 showed amusia symptoms and a lesion in the right basal ganglia. Two other patients with right basal ganglia infarction but without amusia symptoms were identified: patients P11 and P19. A subtraction plot revealed relatively small and circumscribed areas of the putamen and the globus pallidus of the basal ganglia associated with rhythm deficit amusia (Fig. 6). The caudate nucleus seems not to be connected to the music perception deficit.
Fig. 6.
Basal ganglia lesion subtraction plot: Amusia patient P6 minus two non-amusic patients with right basal ganglia infarction.
4. Discussion
This study was carried out in order to investigate whether symptoms of amusia are music selective or whether they can be explained by (1) general perceptual deficits or (2) impairments in attention or WM.
4.1. Music perception deficits
We present data from twenty patients suffering middle cerebral artery infarction with typical initial symptoms and subjective impairments. In this sample, only two amusia patients were identified and they did not show any other deficits in the applied assessment. We infer that amusia is not necessarily connected with impairments in domain general cognitive functions or with other perceptual deficits per se, as we present two cases with selective deficits in the MBEA and relatively pronounced cortical lesions (frontal lobe and basal ganglia).
Both amusia patients presented initial aphasic symptoms, the more severe amusia patient showed still an existing reading disorder and anomia as subjective impairments during testing. Aphasia and music perception deficits seem to be connected in our study as well, in line with previous literature [44], [45], [47], [48], [49], [50], [51]. Furthermore, the double dissociation was also visible in our sample, which shows that it is very important to test both rhythmic and melodic abilities ([10], [18], [36], [47], [56], [59]). Our patient group did comprise one patient suffering hemi-spatial neglect and he did not show any impairment in music perception. Thus, neglect is not always associated with amusia which is in contrast to [44] and [45].
Thus, both amusia patients presented difficulties in language related skills, but the impaired music perception cannot be explained by a general perceptual deficit nor by poor attention or working memory skills.
4.2. Anatomical correlates of amusia
Patient P6 presented a right basal ganglia lesion and rhythm perception deficits. This is in line with a study investigating stroke patients with damage in the basal ganglia who showed difficulties detecting beat or rhythm-based differences in melodies [48]. The lesion analysis of patient P6 and other stroke patients with lesions in the right basal ganglia revealed that the putamen and globus pallidus were associated with a deficit in rhythm perception, while the caudate nucleus was not. Lesions in the left basal ganglia did not lead to amusic symptoms although the lesions were distributed relatively similar on both hemispheres. Two possible conclusions come to mind: 1) Either only right hemispheric basal ganglia lesions of the specific regions lead to amusic symptoms or 2) individual differences in the representation of music perception complicate this view about the music perception network and its dysfunctions. Other studies indeed showed results supporting highly individual representations of the music perception network [47], [53]. In contrast, Schwartze et al. [48] presented a group of patients with basal ganglia lesions in both hemispheres. Group results showed a significant impairment compared to healthy controls. Unfortunately, this study only presented results on the group level and no further distinction between lesion sites of their patients was made. Therefore, one cannot exclude that only a few patients in the patient group presented deficits, severe enough to induce significant differences compared to the control sample. A lesion analysis of this patient group and their behavioral results may further contribute to this issue. Other studies also present a clear relationship between basal ganglia damage and rhythm perception deficits, e.g. in Parkinson's patients [17], [33]. Thus, there is clear evidence that the basal ganglia engage in rhythm perception. But at present, no final differentiation between both possible explanations (lateralization vs. individualization) can be made. Additional work about basal ganglia infarctions and music perception deficits are needed to further investigate this issue.
Patient P5 presented deficits in rhythm and melody perception and had a left frontal infarction (Fig. 5). The stroke was located in the primary motor area, near Broca's area. It seems that the stroke affects two different musical areas as both melodic and rhythmic perception are impaired. But a subtraction plot was not possible with other patients examined in this study, as no other patient presented a similar lesion. It is known that frontal areas engage in processing melodic information while premotor and supplementary motor areas are involved in beat perception [14], [17], [58]. However, further studies with patients presenting similar lesions need to differentiate the roles of specific areas in the frontal lobe in processing diverse musical attributes.
4.3. Other deficits
Generally, we found only few deficits in categorization abilities, and a few more patients suffering from visual Gestalt perception deficits. Attention and WM were impaired in five and four patients, respectively, and a strong correlation between both abilities was shown by correlation statistics. On the other hand, no correlations between performances of other tasks were found.
This shows that deficits in attention and WM, which occurred relatively often, were connected to each other but cannot account for low performances in the MBEA. Our patients with low performance in attention and WM tests were still able to solve the MBEA. Additionally, visual perception deficits were not associated with musical deficits or vice versa. Therefore, the hypothesis that deficits in the MBEA may be explained by a general perceptual dysfunction or domain general deficits have to be rejected. Although cognitive load of the MBEA is relatively high [44], [45], it is not sensitive to minor impairments in domain general cognitive functions (like those in our patients). Whether or not major impairments influence performance in the MBEA remains to be investigated.
4.4. Conclusion
Our study shows that amusia is not necessarily connected to other deficits in perceptual or cognitive functions or to neglect. Previous results may have been mediated by increased lesion size of amusic patients [44]. In contrast the lesions of our patient sample were relatively pronounced. One may infer that the increased lesions damaged several areas responsible for different functions and that in our study the small lesions damaged exactly the specific area important for music perception (functionally distinct but anatomical close).
Important regions seem to be the putamen and the globus pallidus as a lesion in these areas induced rhythm perception deficits. The question whether this deficit is associated with lesions only in right hemispheric infarctions or whether individual differences account for the results cannot finally be answered.
Our findings of patients with selective deficits and pronounced lesions support the view of a modular organization of the music perception network [38], [40]. We found patients with selective deficits not connected to other deficits supporting the theory of specific sub-modules in distinct brain areas that are specialized for musical functions.
4.5. Limitations
For this study twenty-five stroke patients were screened with the MBEA to look for music perception deficits. Only two amusic stroke patients were identified. Full data were only available for the twenty subjects presented here. The lesion overlap in this study was small. However, the study was intended to specifically test patients with small lesions to avoid the danger of large lesions that have an increased risk to damage a large array of functions. The mean age of stroke patients in the study by [44], [45] was 56 and 60 years for both groups. Our patient group had a mean age of 52 years. The younger age may have induced less severe deficits or faster recovery, which we could not control for. Additionally, differences between patients may be due to demographic or clinical values we did not access.
Nevertheless, the results can make a significant contribution to what is already known about the music perception network and acquired amusia.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The first author received a PhD grant from the German National Academic Foundation (Studienstiftung des Deutschen Volkes).
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors would like to thank Dennis Trenner for writing Matlab programs for stimuli presentation and data analysis.
References
- 1.Alossa N., Castelli L. Amusia and musical functioning European. Neurology. 2009;61(5):269–277. doi: 10.1159/000206851. [DOI] [PubMed] [Google Scholar]
- 2.Ayotte J., Peretz I., Rousseau I. Patterns of music agnosia associated with middle cerebral artery infarcts. Brain. 2000;123(9):1926–1938. doi: 10.1093/brain/123.9.1926. [DOI] [PubMed] [Google Scholar]
- 3.Bickerton W.L., Samson D., Williamson J. Separating forms of neglect using the apples test: validation and functional prediction in chronic and acute stroke. Neuropsychology. 2011;25(5):567–580. doi: 10.1037/a0023501. [DOI] [PubMed] [Google Scholar]
- 4.Botez M.I., Wertheim N. Expressive aphasia and amusia following right frontal lesion in a right-handed man. Brain. 1959;82(2):186–202. doi: 10.1093/brain/82.2.186. [DOI] [PubMed] [Google Scholar]
- 5.Brickenkamp R. 8. Auflage. Hogrefe; Göttingen, Germany: 1994. D2 Aufmerksamkeits- Belastungs-Test [D2 Concentration Endurance Test] [Google Scholar]
- 6.Brown S., Martinez M.J., Parsons L.M. Music and language side by side in the brain: a PET study of the generation of melodies and sentences. Eur J Neurosci. 2006;23(10):2791–2803. doi: 10.1111/j.1460-9568.2006.04785.x. [DOI] [PubMed] [Google Scholar]
- 7.Clark C.N., Golden H.L., Warren J.D. Acquired amusia. Handb. Clin. Neurol. 2015;129:607–631. doi: 10.1016/B978-0-444-62630-1.00034-2. [DOI] [PubMed] [Google Scholar]
- 8.Crawford J.R., Garthwaite P.H. Investigation of the single case in neuropsychology: Confidence limits on the abnormality of test scores and test score differences. Neuropsychologia. 2002;40(8):1196–1208. doi: 10.1016/s0028-3932(01)00224-x. S002839320100224X. [DOI] [PubMed] [Google Scholar]
- 9.Crawford J.R., Howell D.C. Comparing an individual's test score against norms derived from small samples. Clin. Neuropsychol. 1998;12:482–486. [Google Scholar]
- 10.Di Pietro M., Laganaro M., Leemann B. Receptive amusia: temporal auditory processing deficit in a professional musician following a left temporo-parietal lesion. Neuropsychologia. 2004;42(7):868–877. doi: 10.1016/j.neuropsychologia.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Eustache F., Lechevalier B., Viader F. Identification and discrimination disorders in auditory perception: a report on two cases. Neuropsychologia. 1990;28(3):257–270. doi: 10.1016/0028-3932(90)90019-k. [DOI] [PubMed] [Google Scholar]
- 12.Fadiga L., Craighero L., D'Ausilio A. Broca's area in language, action, and music. Ann. N. Y. Acad. Sci. 2009;1169:448–458. doi: 10.1111/j.1749-6632.2009.04582.x. [DOI] [PubMed] [Google Scholar]
- 13.Fedorenko E., Patel A., Casasanto D. Structural integration in language and music: evidence for a shared system. Mem. Cogn. 2009;37(1):1–9. doi: 10.3758/MC.37.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Fedorenko E., McDermott J.H., Norman-Haignere S. Sensitivity to musical structure in the human brain. J. Neurophysiol. 2012;108(12):3289–3300. doi: 10.1152/jn.00209.2012. 26-2202-12-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster N.E.V., Zatorre R.J. A role for the intraparietal sulcus in transforming musical pitch information. Cereb. Cortex. 2009 doi: 10.1093/cercor/bhp199. [DOI] [PubMed] [Google Scholar]
- 16.García-Casares N., Berthier Torres M.L., Froudist Walsh S. Model of music cognition and amusia. Neurología (English Edition) 2013;28(3):179–186. doi: 10.1016/j.nrl.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Grahn J.A., Brett M. Impairment of beat-based rhythm discrimination in Parkinson's disease. Cortex. 2009;45(1):54–61. doi: 10.1016/j.cortex.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths T. Spatial and temporal auditory processing deficits following right hemisphere infarction. A psychophysical study. Brain. 1997;120(5):785–794. doi: 10.1093/brain/120.5.785. [DOI] [PubMed] [Google Scholar]
- 19.Hochman M.S., Abrams K.J. Amusia for pitch caused by right middle cerebral artery infarct. J Stroke Cerebrovasc Dis. 2014;23(1):164–165. doi: 10.1016/j.jstrokecerebrovasdis.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Ishiai S., Sugishita M., Ichikawa T. Clock-drawing test and unilateral spatial neglect. Neurology. 1993;43(1):106–110. doi: 10.1212/wnl.43.1_part_1.106. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara S. Kanehara & Co., LTD.; Tokyo, Japan: 1986. Ishihara's Tests for Colour-Blindness. [Google Scholar]
- 22.Janata P., Tillmann B., Bharucha J.J. Listening to polyphonic music recruits domain-general attention and WM circuits. Cogn Affect Behav Neurosci. 2002;2(2):121–140. doi: 10.3758/cabn.2.2.121. [DOI] [PubMed] [Google Scholar]
- 23.Jentschke S., Koelsch S. Musical training modulates the development of syntax processing in children. NeuroImage. 2009;47(2):735–744. doi: 10.1016/j.neuroimage.2009.04.090. [DOI] [PubMed] [Google Scholar]
- 24.Jentschke S., Koelsch S., Sallat S. Children with specific language impairment also show impairment of music-syntactic processing. J. Cogn. Neurosci. 2008;20(11):1940–1951. doi: 10.1162/jocn.2008.20135. [DOI] [PubMed] [Google Scholar]
- 25.Johkura K., Matsumoto S., Hasegawa O. Defective auditory recognition after small hemorrhage in the inferior colliculi. J. Neurol. Sci. 1998;161(1):91–96. doi: 10.1016/s0022-510x(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 26.Johnsrude I.S., Penhune V.B., Zatorre R.J. Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain. 2000;123(1):155–163. doi: 10.1093/brain/123.1.155. [DOI] [PubMed] [Google Scholar]
- 27.Koelsch S. Toward a neural basis of music perception - a review and updated model. Front. Psychol. 2011;2:110. doi: 10.3389/fpsyg.2011.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang J. Ein neuer Stereosehtest [A new test for stereoscopic vision] Klin. Monatsbl. Augenheilkd. 1983;182:373–375. [Google Scholar]
- 29.Lee Y., Janata P., Frost C. Investigation of melodic contour processing in the brain using multivariate pattern-based fMRI. NeuroImage. 2011;57(1):293–300. doi: 10.1016/j.neuroimage.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Liegeois-Chauvel C., Peretz I., Babai M. Contribution of different cortical areas in the temporal lobes to music processing. Brain. 1998;121:1853–1867. doi: 10.1093/brain/121.10.1853. [DOI] [PubMed] [Google Scholar]
- 31.Magne C., Schon D., Besson M. Musician children detect pitch violations in both music and language better than nonmusician children: behavioral and electrophysiological approaches. J. Cogn. Neurosci. 2006;18(2):199–211. doi: 10.1162/089892906775783660. [DOI] [PubMed] [Google Scholar]
- 32.Mendez M.F., Geehan G.R. Cortical auditory disorders: clinical and psychoacoustic features. J. Neurol. Neurosurg. Psychiatry. 1988;51(1):1–9. doi: 10.1136/jnnp.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merchant H., Luciana M., Hooper C. Interval timing and parkinson's disease: heterogeneity in temporal performance. Exp. Brain Res. 2008;184(2):233–248. doi: 10.1007/s00221-007-1097-7. [DOI] [PubMed] [Google Scholar]
- 34.Milovanov R., Tervaniemi M. The interplay between musical and linguistic aptitudes: a review. Front. Psychol. 2011;2:321. doi: 10.3389/fpsyg.2011.00321. ( http://doi.org/10.3389/fpsyg.2011.00321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel A.D., Peretz I., Tramo M. Processing prosodic and musical patterns: a neuropsychological investigation. Brain Lang. 1998;61(1):123–144. doi: 10.1006/brln.1997.1862. [DOI] [PubMed] [Google Scholar]
- 36.Peretz I. Processing of local and global musical information by unilateral brain-damaged patients. Brain. 1990;113(4):1185–1205. doi: 10.1093/brain/113.4.1185. [DOI] [PubMed] [Google Scholar]
- 37.Peretz I., Champod A.S., Hyde K. Varieties of musical disorders. Ann. N. Y. Acad. Sci. 2003;999(1):58–75. doi: 10.1196/annals.1284.006. [DOI] [PubMed] [Google Scholar]
- 38.Peretz I., Coltheart M. Modularity of music processing. Nat. Neurosci. 2003;6(7):688–691. doi: 10.1038/nn1083. [DOI] [PubMed] [Google Scholar]
- 39.Peretz I., Kolinsky R., Tramo M. Functional dissociations following bilateral lesions of auditory cortex. Brain. 1994;117(6):1283–1301. doi: 10.1093/brain/117.6.1283. [DOI] [PubMed] [Google Scholar]
- 40.Piccirilli M., Sciarma T., Luzzi S. Modularity of music: evidence from a case of pure amusia. J. Neurol. Neurosurg. Psychiatry. 2000;69(4):541–545. doi: 10.1136/jnnp.69.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quensel F., Pfeifer R.A. Ein fall von reiner sensorischer amusie [a case of pure sensory amusia] Dtsch Z Nervenheilkd. 1923;77(1):156–163. [Google Scholar]
- 42.Rorden C., Bonilha L., Fridriksson J. Age-specific CT and MRI templates for spatial normalization. NeuroImage. 2012;61(4):957–965. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rorden C., Karnath H.O., Bonilha L. Improving lesion-symptom mapping. J. Cogn. Neurosci. 2007;19(7):1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 44.Särkämö T., Tervaniemi M., Soinila S. Cognitive deficits associated with acquired amusia after stroke: a neuropsychological follow-up study. Neuropsychologia. 2009;47(12):2642–2651. doi: 10.1016/j.neuropsychologia.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Särkämö T., Tervaniemi M., Soinila S. Amusia and cognitive deficits after stroke. Ann. N. Y. Acad. Sci. 2009;1169(1):441–445. doi: 10.1111/j.1749-6632.2009.04765.x. [DOI] [PubMed] [Google Scholar]
- 46.Satoh M., Takeda K., Murakami Y. A case of amusia caused by the infarction of anterior portion of bilateral temporal lobes. Cortex. 2005;41(1):77–83. doi: 10.1016/s0010-9452(08)70180-1. [DOI] [PubMed] [Google Scholar]
- 47.Schuppert M., Münte T.F., Wieringa B.M. Receptive amusia: evidence for cross-hemispheric neural networks underlying music processing strategies. Brain. 2000;123(3):546–559. doi: 10.1093/brain/123.3.546. [DOI] [PubMed] [Google Scholar]
- 48.Schwartze M., Keller P.E., Patel A.D. The impact of basal ganglia lesions on sensorimotor synchronization, spontaneous motor tempo, and the detection of tempo changes. Behav. Brain Res. 2011;216(2):685–691. doi: 10.1016/j.bbr.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 49.Schwenzer M., Mathiak K. Numeric aspects in pitch identification: an fMRI study. BMC Neurosci. 2011;12 doi: 10.1186/1471-2202-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinke W.R., Cuddy L.L., Jakobson L.S. Dissociations among functional subsystems governing melody recognition after right-hemisphere damage. Cogn Neuropsychol. 2001;18(5):411–437. doi: 10.1080/02643290125702. [DOI] [PubMed] [Google Scholar]
- 51.Stewart L., von Kriegstein K., Warren J.D. Music and the brain: disorders of musical listening. Brain. 2006;129(Pt 10):2533–2553. doi: 10.1093/brain/awl171. [DOI] [PubMed] [Google Scholar]
- 52.Thaut M.H., Trimarchi P.D., Parsons L.M. Human brain basis of musical rhythm perception: common and distinct neural substrates for meter, tempo, and pattern. Brain Sci. 2014;4(2):428–452. doi: 10.3390/brainsci4020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toiviainen P., Alluri V., Brattico E. Capturing the musical brain with lasso: dynamic decoding of musical features from fMRI data. NeuroImage. 2014;88:170–180. doi: 10.1016/j.neuroimage.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 54.Torfs K., Vancleef K., Lafosse C. The Leuven perceptual organization screening test (L-POST), an online test to assess mid-level visual perception. Behav. Res. Methods. 2014;46(2):472–487. doi: 10.3758/s13428-013-0382-6. [DOI] [PubMed] [Google Scholar]
- 55.Tramo M.J., Bharucha J.J., Musiek F.E. Music perception and cognition following bilateral lesions of auditory cortex. J. Cogn. Neurosci. 1990;2(3):195–212. doi: 10.1162/jocn.1990.2.3.195. [DOI] [PubMed] [Google Scholar]
- 56.Vignolo L.A. Music agnosia and auditory agnosia. Ann. N. Y. Acad. Sci. 2003;999(1):50–57. doi: 10.1196/annals.1284.005. [DOI] [PubMed] [Google Scholar]
- 57.Wilson B.A., Cockburn J., Halligan P.W. Pearson Assessments; Oxford, UK: 1987. Behavioural Inattention Test (BIT) [Google Scholar]
- 58.Zatorre R.J., Chen J.L., Penhune V.B. When the brain plays music: Auditory-motor interactions in music perception and production. Nat. Rev. Neurosci. 2007;8(7):547–558. doi: 10.1038/nrn2152. (doi:nrn2152) [DOI] [PubMed] [Google Scholar]
- 59.Zatorre R.J. Discrimination and recognition of tonal melodies after unilateral cerebral excisions. Neuropsychologia. 1985;23(1):31–41. doi: 10.1016/0028-3932(85)90041-7. [DOI] [PubMed] [Google Scholar]