Abstract
Objective
Measurement of plasma levels of protein-conjugated acrolein (PC-Acro) together with IL-6 and CRP can be used to identify silent brain infarction (SBI) with high sensitivity and specificity. The aim of this study was to determine how these biomarkers vary during stroke.
Methods
Levels of PC-Acro, IL-6 and CRP in plasma were measured on day 0, 2, 7 and 14 after the onset of ischemic or hemorrhagic stroke.
Results
After the onset of stroke, the level of PC-Acro in plasma was elevated corresponding to the size of stroke. It returned to near control levels by day 2, and remained similar through day 14. The degree of the decrease in PC-Acro on day 2 was greater when the size of brain infarction or hemorrhage was larger. An increase in IL-6 and CRP occurred after the increase in PC-Acro, and it was well correlated with the size of the injury following infarction or hemorrhage. The results suggest that acrolein becomes a trigger for the production of IL-6 and CRP, as previously observed in a mouse model of stroke and in cell culture systems. The increase in IL-6 and CRP was also correlated with poor outcome judging from mRS.
Conclusion
The results indicate that the degree of the decrease in PC-Acro and the increase in IL-6 and CRP from day 0 to day 2 was correlated with the size of brain infarction, and the increase in IL-6 and CRP with poor outcome at discharge.
Abbreviations: PC-Acro, protein-conjugated acrolein; IL-6, interleukin-6; CRP, C-reactive protein; NIHSS, NIH stroke scale; mRS, modified Rankin Scale; SBI, silent brain infarction; WMH, white matter hyperintensity
Keywords: Brain infarction, Brain hemorrhage, Biomarkers, Protein-conjugated acrolein (PC-Acro), Interleukin-6, C-reactive protein
1. Introduction
Polyamines (putrescine, spermidine and spermine) are synthesized from ornithine and S-adenosylmethione at the order of mM in cells, and mainly exist as a polyamine-RNA complex, where they stimulate several kinds of protein synthesis that are important for cell growth [1], [2]. However, when cells are damaged, polyamines are released from RNA and the toxic compound acrolein (CH2 CH—CHO) is produced by polyamine oxidases (spermine oxidase and acetylpolyamine oxidase) [3], [4]. We examined whether the levels of polyamine oxidases and protein-conjugated acrolein (PC-Acro) in plasma are correlated with pathologies that involve tissue damage, and found that the levels of polyamine oxidases and PC-Acro in plasma are well correlated with the severity of chronic renal failure [5] and stroke [6]. It was also found that the induction of brain infarction in mice was correlated with increases in PC-Acro at the locus of infarction and in plasma [7].
It is thought that the major factor responsible for cell damage is reactive oxygen species (ROS) such as superoxide anion radical (O2•−), hydrogen peroxide (H2O2) and hydroxyl radical (·OH) [8]. However, when the toxicity of acrolein and ROS was compared, acrolein was more toxic than H2O2[9] and slightly more toxic than hydroxyl radical [10]. This finding supports an idea that PC-Acro becomes a biomarker for diseases accompanied with tissue or cell damage like renal failure [5], stroke [6], and Alzheimer's disease [11], because acrolein interacts efficiently with cysteine, lysine and histidine residues of proteins resulting in the inactivation of proteins.
There are reports that silent brain infarction (SBI) increases the risk of subsequent stroke [12], [13], [14], dementia [14] and mild cognitive impairment [15]. It has been also reported that carotid atherosclerosis (CA) is a risk factor for stroke and SBI [16], [17] and that SBI and marked white matter hyperintensity (WMH) are risk factors for stroke [18]. We looked for biomarkers to estimate SBI and marked WMH, and found that measurement of PC-Acro together with interleukin-6 (IL-6) and C-reactive protein (CRP) makes it possible to identify SBI and marked WMH with high sensitivity and specificity [19]. Therefore, we studied how these three markers change during stroke. An increase in all three markers was seen after the onset of stroke in the order PC-Acro > IL-6 > CRP as observed in thrombosis model mice [20], and these three biomarkers were correlated with the size of stroke, and IL-6 and CRP with the outcome at discharge.
2. Materials and methods
2.1. Patients
Plasma samples were collected from 44 patients with brain infarction (28 males, 16 females; 73.5 ± 8.5 years of age), and 35 patients with intracerebral hemorrhage (21 males, 14 females; 65.0 ± 8.0 years of age), who were admitted to the Atsugi Municipal Hospital within 24 h after the onset of stroke.Stroke patients were defined as having focal infarcts or hemorrhage detected by magnetic resonance imaging (MRI) or computed tomography (CT) and managed according to the Japanese Guideline for the Management of Stroke (2009) [21]. In brief, patients were treated with anticoagulants and/or antiplatelets with or without edaravone, a free radical scavenger for 7 to 14 days, and dextran for 5 days after the stroke. None of the patients received immunodepressive medicines. Blood was collected from patients 4 times (day 0 on admission, day 2, 7 and 14) using procedures approved by the ethics committees of Atsugi Municipal Hospital and Graduate School of Pharmaceutical Sciences, Chiba University.Informed consent was given by patients or by their relatives as legally required.Clinical investigations were conducted in accordance with the Declaration of Helsinki principles.Blood containing 3 U/ml heparin was centrifuged at 1500 x g for 10 min at 4 °C. The plasma was carefully collected to avoid contamination by erythrocytes, and kept at − 80 °C.
2.2. Measurement of PC-Acro, IL-6 and CRP
PC-Acro [Nε-(3-formyl-3,4-dehydropiperidino)-lysine (FDP-lysine) in protein] was determined by the method of Uchida et al. [22] using ACR-LYSINE ADDUCT ELISA SYSTEM (NOF Corporation) and 0.01 ml plasma.IL-6 and CRP were measured using human IL-6 ELISA kit (R & D Systems) and N-assay LA CRP-S kit (Nittobo), respectively, according to the manufacturer's protocol.
2.3. Imaging, NIH stroke scale (NIHSS), modified Rankin Scale (mRS) and assessment of outcome
All patients underwent T1- and T2-weighted MRI, fluid-attenuated inversion recovery (FLAIR), diffusion weighted image (DWI), and CT. MRI was performed in 7-nm thickness with 1- to 2-nm slice gap with a 1.5-T MRI unit (Signa: GE Medical Systems). A standard head coil with a receive-transmit birdcage design was used. The maximum size of focal infarcts were measured using 5 or 10 nm length calibration accompanied in each image [6]. The volume of intracerebral hemorrhage was measured using the CT image according to the method of Broderick et al. [23]. NIHSS was evaluated on admission according to the method of Brott et al. [24]. Modified RS (score 0–6, 0, no symptoms at all; 1, no significant disability despite symptoms; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; 6, dead) was assessed according to the method described by Shinohara et al. [25] at the day of discharge. Assessment of the outcome was performed according to Glassgow Outcome Scale: Good outcome, good recovery; Poor outcome, moderately disabled, severely disabled and persistent vegetative state [26].
2.4. Statistics
Statistical calculations were performed with GraphPad Prism® Software (GraphPad Software). Values are indicated as median ± interquartile deviation or median with interquartile range. Groups were compared using Wilcoxon rank sum test, Wilcoxon signed-rank test, Kruskal-Wallis test, chi-test or Fisher's exact test. Correlations between each factor were examined by Spearman's rank correlation analysis to obtain the correlation coefficient (rs) and p value. False Discovery Rate (FDR) correction [27] was used for multiple comparison.
3. Results
3.1. Time course of the levels of PC-Acro, IL-6 and CRP during brain infarction and hemorrhage
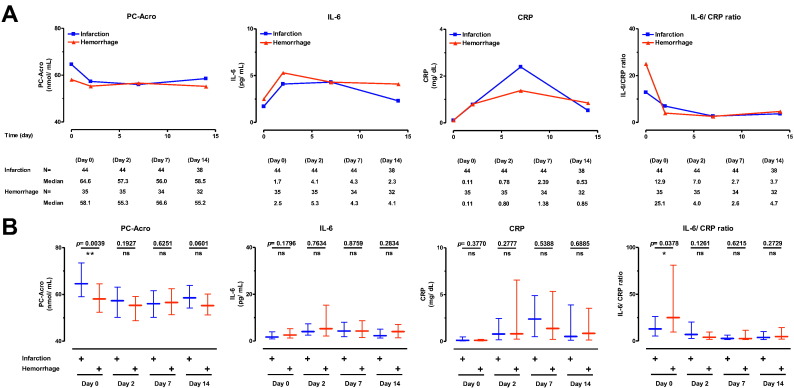
As shown in Table 1, the number of brain infarction and hemorrhage patients was 44 (73.5 ± 8.5 years of age) and 35 (65.0 ± 8.0 years old), respectively, at the onset of the study. Three biomarkers (PC-Acro, IL-6 and CRP) were followed for 15 days (days 0, 2, 7 and 14) after the onset of the stroke (Fig. 1). The level of PC-Acro on day 0 was higher in both brain infarction and hemorrhage. However, unexpectedly, the level of PC-Acro in brain infarction patients was reduced on day 2 compared to day 0, and the level remained similar through day 14. Although the level of PC-Acro in patients with brain hemorrhage was lower on day 0 than that in patients with brain infarction, a similar reduction on day 2 compared to day 0 was observed. In both groups of patients, the level of IL-6 on day 2 increased about 2-fold compared to that on day 0, and gradually decreased from day 2 to day 14 (Fig. 1A). The level of CRP was maximal on day 7 and subsequently decreased by day 14 in both groups of patients (Fig. 1A). The results suggest that production of PC-Acro at the locus of brain stroke may be a trigger for the production of IL-6 and CRP, as has been observed in a mouse model of stroke and in cell culture systems [20]. PC-Acro was higher in brain infarction, and the IL-6/CRP ratio was higher in brain hemorrhage on day 0, suggesting that the orderly increase in PC-Acro, IL-6 and CRP may be rapid in brain infarction than hemorrhage. The results strongly suggest that three biomarkers, measured early after a stroke, may be useful biomarkers to differentiate brain infarction from brain hemorrhage.
Table 1.
Baseline sociodemographic variables.
| Fig. 1 | Fig. 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Infarction | Hemorrhage | Infarction |
Hemorrhage |
|||||||
| Small | Large | Small | Large | |||||||
| N | 44 | 35 | 22 | 21 | 18 | 17 | ||||
| Age (years) | 73.5 ± 8.5 | 65.0 ± 8.0 | p = 0.0057 | 72.0 ± 8.0 | 76.0 ± 10.0 | p = 0.2636 | 64.0 ± 8.8 | 68.0 ± 8.3 | p = 0.2282 | |
| (37–95) | (34–86) | ** | (48–92) | (37–95) | ns | (34–79) | (44–86) | ns | ||
| Female, N (%) | 16 (36.4) | 14 (40.0) | p = 0.7408 | 7 (31.8) | 9 (42.9) | p = 0.6650 | 7 (38.9) | 7 (41.2) | p = 0.8359 | |
| ns | ns | ns | ||||||||
| non-CEBI/CEBI | 29/15 | – | 17/5 | 11/10 | – | – | ||||
| Hypertension, N (%) | 26 (59.1) | 28 (80.0) | p = 0.0816 | 13 (59.1) | 12 (57.1) | p = 0.8573 | 16 (88.9) | 12 (70.6) | p = 0.2285 | |
| ns | ns | ns | ||||||||
| Hyperglycemia, N (%) | 16 (36.4) | 4 (11.4) | p = 0.0231 | 9 (40.7) | 7 (33.8) | p = 0.8429 | 2 (11.1) | 2 (11.8) | p = 1.0000 | |
| * | ns | ns | ||||||||
| Atrial fibrillation, N (%) | 15 (34.1) | 2 (5.7) | p = 0.0023 | 5 (22.7) | 10 (47.6) | p = 0.1640 | 1 (5.6) | 1 (5.9) | p = 1.0000 | |
| ** | ns | |||||||||
| NIHSS on admission | 4.0 ± 3.3 | 5.0 ± 8.0 | p = 0.0512 | 3.0 ± 2.0 | 7.0 ± 3.8 | p = 0.0058 | 3.5 ± 5.8 | 16.0 ± 9.0 | p = 0.0693 | |
| ns | ** | ns | ||||||||
| mRS at discharge | 2.0 ± 1.5 | 4.0 ± 1.5 | p = 0.3096 | 1.0 ± 1.0 | 4.0 ± 1.3 | p = 0.0026 | 2.0 ± 2.0 | 4.0 ± 0.5 | p = 0.1781 | |
| ns | ** | ns | ||||||||
| Hospitalization period (day) | 26.0 ± 12.0 | 27.5 ± 13.5 | p = 0.3917 | 18.0 ± 8.5 | 37.0 ± 8.5 | p = 0.0012 | 24.5 ± 15.0 | 34.0 ± 17.3 | p = 0.0977 | |
| ns | ** | ns | ||||||||
| Infarction size (cm2) | 7.3 ± 17.5 | – | 1.0 ± 6.9 | 36.0 ± 29.0 | p < 0.0001 | – | – | |||
| *** | ||||||||||
| Hemorrhage volume (cm3) | – | 12.4 ± 16.9 | – | – | 4.0 ± 1.9 | 37.6 ± 10.8 | p < 0.0001 | |||
| *** | ||||||||||
| Location of infarct | ||||||||||
| Cortex, N (%) | 15 (34.1) | – | 6 (27.3) | 9 (42.9) | – | – | ||||
| Perforator, N (%) | 16 (36.4) | – | 13 (59.1) | 3 (14.3) | – | – | ||||
| Both, N (%) | 12 (27.3) | – | 3 (13.6) | 9 (42.9) | – | – | ||||
| Unknown, N (%) | 1 (2.3) | – | 1 (4.5) | 0 (0) | – | – | ||||
| Location of hematoma | ||||||||||
| Subcortical, N (%) | – | 9 (25.7) | – | – | 0 (0) | 9 (52.9) | ||||
| Cerebeller, N (%) | – | 4 (11.4) | – | – | 3 (16.7) | 1 (5.9) | ||||
| Brain stem, N (%) | – | 4 (11.4) | – | – | 4 (22.2) | 0 (0) | ||||
| Putaminal, N (%) | – | 15 (42.9) | – | – | 8 (44.4) | 7 (41.2) | ||||
| Thalamic, N (%) | – | 3 (8.6) | – | – | 3 (16.7) | 0 (0) | ||||
| Free radical scavenger | edaravone, N (%) | 35 (79.5) | – | 16 (72.7) | 19 (90.5) | – | – | |||
| Thrombolytic therapy | t-PA, N (%) | 2 (4.5) | – | 1 (4.5) | 1 (4.8) | – | – | |||
| Hemodilution therapy | dextran, N (%) | 32 (72.7) | – | 14 (63.6) | 18 (85.7) | – | – | |||
| Anticoagulant drug | heparin, N (%) | 13 (29.5) | – | 5 (22.7) | 8 (38.1) | – | – | |||
| argatroban, N (%) | 7 (15.9) | – | 2 (9.1) | 5 (23.8) | – | – | ||||
| rivaroxaban, N (%) | 6 (13.6) | – | 3 (13.6) | 3 (14.3) | – | – | ||||
| warfarin, N (%) | 5 (11.4) | – | 3 (13.6) | 2 (9.5) | – | – | ||||
| apixaban, N (%) | 1 (2.3) | – | 0 (0) | 1 (4.8) | – | – | ||||
| Antiplatelet drug | ozagrel, N (%) | 5 (11.4) | – | 3 (13.6) | 2 (9.5) | – | – | |||
| clopidgrel, N (%) | 11 (25.0) | – | 6 (27.3) | 5 (23.8) | – | – | ||||
| cilostazol, N (%) | 10 (22.7) | – | 5 (22.7) | 5 (23.8) | – | – | ||||
| aspirin, N (%) | 7 (15.9) | – | 5 (22.7) | 2 (9.5) | – | – | ||||
| Hemorrhage surgery | ||||||||||
| Conservative, N (%) | – | 18 (51.4) | – | – | 14 (77.8) | 4 (23.5) | ||||
| Evacuation, N (%) | – | 7 (20.0) | – | – | 1 (5.6) | 6 (35.3) | ||||
| Stereo, N (%) | – | 7 (20.0) | – | – | 1 (5.6) | 6 (35.3) | ||||
| AVM resection, N (%) | – | 1 (2.9) | – | – | 0 (0) | 1 (5.9) | ||||
| Clipping, N (%) | – | 0 (0) | – | – | 0 (0) | 0 (0) | ||||
| Unknown, N (%) | – | 2 (5.7) | – | – | 2 (11.1) | 0 (0) | ||||
The values are shown in median ± interquatile deviation or percentages. CEBI, cardioembolic infarction. ns, p > 0.05; * p < 0.05; ** p < 0.01; *** p < 0.001.
Fig. 1.
Comparison of three biomarkers of brain infarction and hemorrhage during 15 days. A. The levels of PC-Acro, IL-6 and CRP, and the IL-6/CRP ratio on day 0, 2, 7 and 14 after the incidence of brain infarction and hemorrhage are shown. B. The difference of three biomarkers and IL-6/CRP ratio between brain infarction and hemorrhage was compared on the corresponding days. Data are shown as median with interquartile range. ns, p ≥ 0.05; *p < 0.05; **p < 0.01.
Similar results were obtained with age-matched patients of brain infarction (37 patients, 72.0 ± 6.0 years old) and hemorrhage (35 patients, 65.0 ± 8.0 years old) (data not shown).
3.2. Dependency of the increase in three biomarkers on the size of brain infarction and hemorrhage
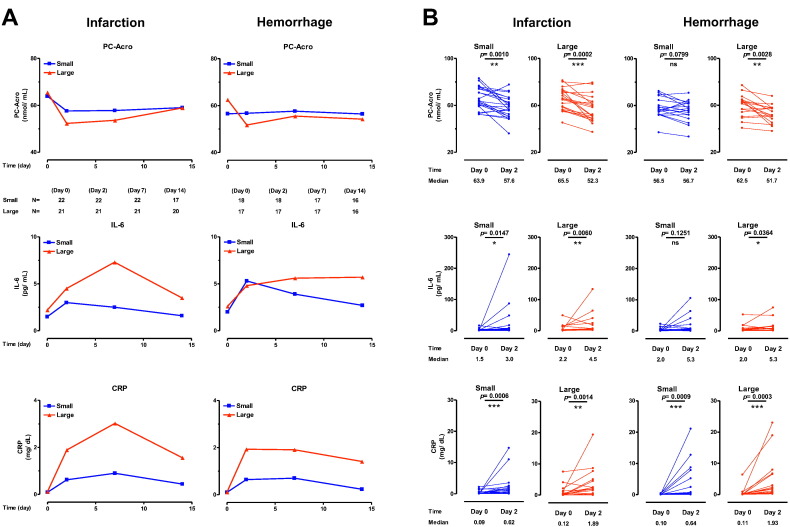
It was then determined whether levels of the biomarkers were correlated with the size of brain damage following infarction and hemorrhage. For this analysis, patients with brain infarction and hemorrhage were classified into two groups in which numbers were nearly equal in small and large groups. As shown in Fig. 2AB, the degree of the decrease in PC-Acro from day 0 to day 2 and that of the increase in IL-6 and CRP from day 0 to day 2 or 7 were correlated with the size of brain infarction and hemorrhage. These results indicate that measurements of PC-Acro together with IL-6 and CRP at the early period of brain stroke contribute to the judgement of the infarct size and hemorrhage volume.
Since the size of cardioembolic (CE) infarction was bigger than that of atheroembolic including lacnar (non-CE) infarction (Supplementary Table 1S), it was determined whether there were differences in the three biomarkers in patients with CE and non-CE brain infarctions (i.e., subsets of the “infarction” group presented in Supplementary Table 1S).In both groups, PC-Acro was elevated on day 0 but had returned toward control levels by day 2 (Supplementary Fig. 1S).The level of IL-6 was initially higher in CE infarction than in non-CE infarction, but it became nearly equal on day 7 and 14 (Supplementary Fig.1S).Although the level of CRP was higher in CE infarction than in non-CE infarction on day 2 and 7, the difference was not statistically significant.The higher level of three biomarkers (PC-Acro on day 0, IL-6 on day 2, and CRP on day 7) in CE than non-CE infarction was parallel with the size of brain infarction.The significant difference between CE and non-CE brain infarctions was observed in IL-6 and PC-Acro/IL-6 ratio on day 0.
3.3. Correlation between biomarkers and mRS at discharge
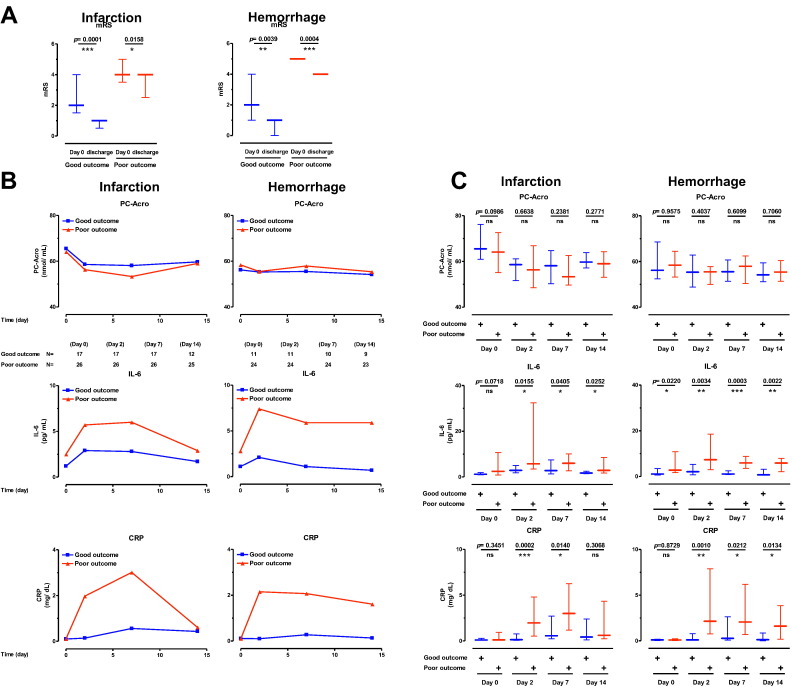
It was then tested whether three biomarkers were correlated with the change of mRS at day 0 and the day of discharge. As shown in Table 1, the average hospitalization periods of patients with small and big infarction and hemorrhage size were 18.0, 37.0, 24.5 and 34.0 days, respectively. In the case of poor outcome judging from mRS, there was a tendency that increase in IL-6 and CRP, but not decrease in PC-Acro, from day 0 to day 2 was more significant than those in good outcome (Fig. 3). However, when correlation between biomarkers and mRS was analyzed by Spearman's rank correlation analysis, it was slightly better in PC-Acro/IL-6 and PC-Acro/CRP ratio rather than IL-6 and CRP itself in brain infarction, but it was nearly equal in PC-Acro/IL-6 and IL-6 itself, and in PC-Acro/CRP and CRP itself in brain hemorrhage (Table 2). The results also confirm that measurements of PC-Acro together with IL-6 and CRP contribute to judgement of good and poor outcome, especially in brain infarction.
Fig. 3.
Correlation between assessment of outcome, mRS, and three biomarkers in brain infarction and hemorrhage. A. Correlation between mRS, and good and poor outcome in brain infarction and hemorrhage was shown. B and C. The levels of PC-Acro, IL-6 and CRP on day 0, 2, 7 and 14 in good and poor outcome patients were compared. ns, p ≥ 0.05; *p < 0.05; **p < 0.01; ***p < 0.001.
Table 2.
Correlation between biomarkers and mRS.
| Biomarker | Infarction |
Hemorrhage |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Time | Day 0 | Day 2 | Day 7 | Day 14 | Day 0 | Day 2 | Day 7 | Day 14 | |
| N = | 44 | 44 | 44 | 38 | 35 | 35 | 34 | 32 | |
| PC-Acro | rs | − 0.1696 | − 0.0568 | − 0.0462 | − 0.0156 | − 0.0277 | − 0.1683 | 0.0802 | − 0.0186 |
| p = | 0.2710 | 0.7143 | 0.7657 | 0.9261 | 0.8744 | 0.3337 | 0.6519 | 0.9197 | |
| IL-6 | rs | 0.2720 | 0.4713 | 0.5171 | 0.6530 | 0.3883 | 0.5960 | 0.6530 | 0.6467 |
| p = | 0.0741 | 0.0012 | 0.0003 | p < 0.0001 | 0.0212 | 0.0002 | p < 0.0001 | p < 0.0001 | |
| CRP | rs | 0.2434 | 0.5870 | 0.4566 | 0.4506 | 0.1999 | 0.6287 | 0.4871 | 0.5711 |
| p = | 0.1113 | p < 0.0001 | 0.0018 | 0.0045 | 0.2495 | p < 0.0001 | 0.0035 | 0.0006 | |
| PC-Acro/IL-6 ratio | rs | − 0.3237 | − 0.4983 | − 0.5402 | − 0.6655 | − 0.4171 | − 0.5877 | − 0.6267 | − 0.6329 |
| p = | 0.0321 | 0.0006 | 0.0002 | p < 0.0001 | 0.0127 | 0.0002 | p < 0.0001 | 0.0001 | |
| PC-Acro/CRP ratio | rs | − 0.2852 | − 0.6209 | − 0.5027 | − 0.4493 | − 0.1987 | − 0.6286 | − 0.4737 | − 0.5513 |
| p = | 0.0606 | p < 0.0001 | 0.0005 | 0.0047 | 0.2525 | p < 0.0001 | 0.0047 | 0.0011 | |
| IL-6/CRP ratio | rs | − 0.0423 | − 0.2351 | − 0.1506 | 0.6554 | 0.0928 | − 0.2280 | − 0.1322 | 0.6393 |
| p = | 0.7853 | 0.1244 | 0.3293 | p < 0.0001 | 0.5959 | 0.1877 | 0.4562 | p < 0.0001 | |
Correlation between the outcome of stroke damage, i.e. mRS, and biomarkers on day 0, 2, 7 and 14 were examined by Spearman's rank correlation analysis to obtain correlation coefficient (rs) and p value. Bolded measures indicate measures that remained significant difference after False Discovery Rate (FDR) correction for multiple comparison [27].
4. Discussion
We have previously shown that enzymes (polyamine oxidases) that produce acrolein and PC-Acro in plasma are increased after the onset of stroke [6] and that measurement of PC-Acro together with IL-6 and CRP made it possible to detect SBI with approximately 84% sensitivity and specificity [19]. Furthermore, we found that increased acrolein led to an increase in IL-6 production and subsequently CRP production in an animal model of thrombosis and in cultured cells [20].Both IL-6 and CRP functioned as protective factors against acrolein toxicity [20].
This study was a prospective clinical trial assessing the levels of PC-Acro, IL-6 and CRP after stroke.We confirmed that the time course of increases in these three biomarkers after stroke is in the order PC-Acro > IL-6 > CRP.However, generation of IL-6 and then CRP, following the increase in PC-Acro, was relatively slow compared with changes seen in a mouse model of ischemic stroke [20], requiring 1 to 3 days in human subjects but occurring within 1 day in the model mice.Since young mice were used in our experiments, the cellular responses to protect against brain damage and/or the synthesis and metabolism of these compounds may be faster at a younger age, or there may be species- or pathology-dependent differences in the underlying mechanisms.An earlier increase in IL-6 and CRP in stroke patients has also been previously reported [28], [29], [30].However, it was not mentioned which is a trigger of IL-6 and CRP synthesis.Our results in this study and the previous study [20] indicate that acrolein becomes a trigger of IL-6 and CRP synthesis.
An unexpected finding was that PC-Acro in plasma decreased rapidly beyond 1 day after a stroke, in particular with larger infarction or hemorrhage (Fig. 2).PC-Acro generated after a stroke may accumulate at the locus of infarction or hemorrhage, as observed in a mouse model [7], perhaps due to reduced blood flow and reduced clearance at the locus of the insult.Alternatively, PC-Acro in plasma may be degraded more rapidly than acrolein unconjugated proteins.In the case of IL-6 and CRP, they may have been largely produced outside the locus of infarction or hemorrhage, depending on the increase in acrolein production on day 0.Since an increase in IL-6 and CRP was clearly observed on day 2, it may be better to estimate the seriousness of stroke using the values of PC-Acro on day 0 and IL-6 and CRP on day 2.In addition, the degree of decrease in PC-Acro from day 0 to day 2 was parallel with the size of infarction or the volume of hemorrhage (Fig. 2).It was also shown that PC-Acro/IL-6 and PC-Acro/CRP ratios as well as IL-6 and CRP themselves contributed to judgement of poor and good outcome together with mRS (Fig. 3, and Table 2).
Fig. 2.
Change of three biomarkers in small and large infarctions and hemorrhage (A), and comparison of these biomarkers on day 0 and day 2 (B). B. Small infarction, 1.0 ± 6.9 cm2; large infarction, 36.0 ± 29.0 cm2; small hemorrhage, 4.0 ± 1.9 cm3; large hemorrhage, 37.6 ± 10.8 cm3. ns, p ≥ 0.05; *p < 0.05; **p < 0.01; ***p < 0.001.
In patients with hemorrhage, the increase in PC-Acro in plasma on day 0 was not significant (Fig. 1).This may be explained as follows: All patients with hemorrhage were accompanied with the perifocal edema.So, PC-Acro may be accumulated at the locus of edema.
It has been reported that lactate dehydrogenase (LDH) is high in cerebrospinal fluid of stroke patients [31] and in plasma of SBI patients [32].Although we showed that adiponectin was high in plasma of SBI patients [32], different results were reported on adiponectin [33], [34].Through a different analysis, other useful biomarkers for brain stroke may be found.At present, the measurements of PC-Acro, IL-6 and CRP together are one of the most reliable biomarkers to find silent brain infarction [19] as well as the judgement of seriousness of brain stroke evaluated in this study.
5. Conclusions
Seriousness of stroke was thus far judged by MRI or CT, because there were no effective biomarkers.In this study, we found that the measurements of PC-Acro, IL-6 and CRP become sensitive biomarkers of brain stroke.Since MRI and CT analysis is not carried out so often, it is thought that a follow-up of biomarkers contributes to the suitable treatment of the patients.
The following are the supplementary data related to this article.
Comparison of three biomarkers of cardioembolic (CE) and non-CE infarction during 15 days. A. The levels of PC-Acro, IL-6 and CRP, and the PC-Acro/IL-6 ratio on day 0, 2, 7 and 14 after the incidence of brain infarction are shown. B. The difference of three biomarkers and PC-Acro/IL-6 ratio between CE and non-CE brain infarction was compared on the corresponding days. Data are shown as median with interquatile range. ns, p ≥ 0.05; *p < 0.05.
Baseline sociodemographic variables.
Conflict of interest
None.
Acknowledgements
We thank Drs. A. J. Michael and K. Williams for their help in preparing the manuscript. This study was supported by JSPS KAKENHI Grant Number 2390038, and a Grant from Chiba Prefecture Genki Zukuri Fund Support Programs for SMEs.
References
- 1.Igarashi K., Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39–51. doi: 10.1016/j.biocel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Igarashi K., Kashiwagi K. Modulation of protein synthesis by polyamines. IUBMB Life. 2015;67:160–169. doi: 10.1002/iub.1363. [DOI] [PubMed] [Google Scholar]
- 3.Igarashi K., Kashiwagi K. Protein-conjugated acrolein as a biochemical marker of brain infarction. Mol Nutr Food Res. 2011;55:1332–1341. doi: 10.1002/mnfr.201100068. [DOI] [PubMed] [Google Scholar]
- 4.Pegg A.E. Toxicity of polyamines and their metabolic products. Chem Res Toxicol. 2013;26:1782–1800. doi: 10.1021/tx400316s. [DOI] [PubMed] [Google Scholar]
- 5.Sakata K., Kashiwagi K., Sharmin S., et al. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem Biophys Res Commun. 2003;305:143–149. doi: 10.1016/s0006-291x(03)00716-2. [DOI] [PubMed] [Google Scholar]
- 6.Tomitori H., Usui T., Saeki N., et al. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke. 2005;36:2609–2613. doi: 10.1161/01.STR.0000190004.36793.2d. [DOI] [PubMed] [Google Scholar]
- 7.Saiki R., Nishimura K., Ishii I., et al. Intense correlation between brain infarction and protein-conjugated acrolein. Stroke. 2009;40:3356–3361. doi: 10.1161/STROKEAHA.109.553248. [DOI] [PubMed] [Google Scholar]
- 8.Giorgio M., Trinei M., Migliaccio E., et al. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 9.Sharmin S., Sakata K., Kashiwagi K., et al. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem Biophys Res Commun. 2001;282:228–235. doi: 10.1006/bbrc.2001.4569. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida M., Tomitori H., Machi Y., et al. Acrolein toxicity: comparison with reactive oxygen species. Biochem Biophys Res Commun. 2009;378:313–318. doi: 10.1016/j.bbrc.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 11.Waragai M., Yoshida M., Mizoi M., et al. Increased protein-conjugated acrolein and amyloid-β40/42 ratio in plasma of patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2012;32:33–41. doi: 10.3233/JAD-2012-120253. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi S., Okada K., Koide H., et al. Subcortical silent brain infarction as a risk factor for clinical stroke. Stroke. 1997;28:1932–1939. doi: 10.1161/01.str.28.10.1932. [DOI] [PubMed] [Google Scholar]
- 13.Vermeer S.E., Hollander M., van Dijk E.J., et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 14.Vermeer S.E., Longstreth W.T., Jr., Koudstaal P.J. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 15.Lopez O.L., Jagust W.J., Dulberg C., et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 16.O'Leary D.H., Polak J.F., Kronmal R.A., et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 17.Inoue K., Matsumoto M., Shono T., et al. Increased intima media thickness and atherosclerotic plaques in the carotid artery as risk factors for silent brain infarcts. J Stroke Cerebrovasc Dis. 2007;16:14–20. doi: 10.1016/j.jstrokecerebrovasdis.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Bokura H., Kobayashi S., Yamaguchi S., et al. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: a prospective cohort study. J Stroke Cerebrovasc Dis. 2006;15:57–63. doi: 10.1016/j.jstrokecerebrovasdis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida M., Higashi K., Kobayashi E., et al. Correlation between images of silent brain infarction, carotid atherosclerosis and white matter hyperintensity, and plasma levels of acrolein, IL-6 and CRP. Atherosclerosis. 2010;211:475–479. doi: 10.1016/j.atherosclerosis.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Saiki R., Hayashi D., Ikuo Y., et al. Acrolein stimulates the synthesis of IL-6 and C-reactive protein (CRP) in thrombosis model mice and cultured cells. J Neurochem. 2013;127:652–659. doi: 10.1111/jnc.12336. [DOI] [PubMed] [Google Scholar]
- 21.Shinohara Y., Nagayama M., Origasa H. Postpublication external review of the Japanese guidelines for the management of stroke 2004. Stroke. 2009;40:1439–1443. doi: 10.1161/STROKEAHA.108.535070. [DOI] [PubMed] [Google Scholar]
- 22.Uchida K., Kanematsu M., Morimitsu Y., et al. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 23.Broderick J.P., Brott T.G., Duldner J.E., et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 24.Brott T., Adams H.P., Jr., Olinger C.P., et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 25.Shinohara Y., Minematsu K., Amano T., et al. Modified Rankin scale with expanded guidance scheme and interview questionnaire: interrater agreement and reproducibility of assessment. Cerebrovasc Dis. 2006;21:271–278. doi: 10.1159/000091226. [DOI] [PubMed] [Google Scholar]
- 26.Jennett B., Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y., Hochberg Y. Controling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B (Methodological) 1995;57:289–300. [Google Scholar]
- 28.Waje-Andreassen U., Krakenes J., Ulvestad E., et al. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand. 2005;111:360–365. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 29.Worthmann H., Tryc A.B., Goldbecker A., et al. The temporal profile of inflammatory markers and mediators in blood after acute ischemic stroke differs depending on stroke outcome. Cerebrovasc Dis. 2010;30:85–92. doi: 10.1159/000314624. [DOI] [PubMed] [Google Scholar]
- 30.Shenhar-Tsarfaty S., Ben Assayag E., Bova I., et al. Interleukin-6 as an early predictor for one-year survival following an ischaemic stroke/transient ischaemic attack. Int J Stroke. 2010;5:16–20. doi: 10.1111/j.1747-4949.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- 31.Lampl Y., Paniri Y., Eshel Y., et al. Cerebrospinal fluid lactate dehydrogenase levels in early stroke and transient ischemic attacks. Stroke. 1990;21:854–857. doi: 10.1161/01.str.21.6.854. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida M., Tomitori H., Machi Y., et al. Acrolein, IL-6 and CRP as markers of silent brain infarction. Atherosclerosis. 2009;203:557–562. doi: 10.1016/j.atherosclerosis.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Chen M.P., Tsai J.C., Chung F.M., et al. Hypoadiponectinemia is associated with ischemic cerebrovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:821–826. doi: 10.1161/01.ATV.0000157784.25920.a7. [DOI] [PubMed] [Google Scholar]
- 34.Soderberg S., Stegmayr B., Stenlund H., et al. Leptin, but not adiponectin, predicts stroke in males. J Intern Med. 2004;256:128–136. doi: 10.1111/j.1365-2796.2004.01351.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of three biomarkers of cardioembolic (CE) and non-CE infarction during 15 days. A. The levels of PC-Acro, IL-6 and CRP, and the PC-Acro/IL-6 ratio on day 0, 2, 7 and 14 after the incidence of brain infarction are shown. B. The difference of three biomarkers and PC-Acro/IL-6 ratio between CE and non-CE brain infarction was compared on the corresponding days. Data are shown as median with interquatile range. ns, p ≥ 0.05; *p < 0.05.
Baseline sociodemographic variables.