Abstract
The association of dementia related pathologies with cognition is hypothesized to decrease as age advances. We examined this in 413 persons without cognitive impairment at baseline who completed annual cognitive evaluations during a mean of 10.4 years. After death, neuropathologic examinations quantified beta-amyloid plaque load, neurofibrillary tangles, and TDP-43 pathology, and identified Lewy bodies, hippocampal sclerosis, and gross and microscopic cerebral infarcts. We tested whether age at death modified associations of these neuropathologies with the nonlinear trajectory of cognitive decline using mixed-effects change point models. The rate of global cognitive decline was gradual at first and then increased approximately 10-fold in the last 3 years of life. After adjustment for all other pathologic indices, tangle density, gross infarcts, Lewy bodies, and TDP-43 were associated with global cognitive decline. However, the deleterious association of dementia related pathologies with cognitive decline did not systematically vary by age. This suggests that the neuropathologic mechanisms underlying late-life cognitive decline do not substantially differ across the spectrum of age.
Keywords: Neuropathologies, Aging, Dementia, Cognitive decline, Mixed-effects change point model
1. INTRODUCTION
The proportion of persons aged 65 years or older is increasing in much of the world population, particularly those aged 85 years or older, underscoring the need for strategies to treat or delay age related cognitive impairment. Many factors contribute to cognitive decline in old age. Clinical-pathologic studies demonstrate that much of late-life cognitive decline is driven by common neurodegenerative and cerebrovascular pathologies (Driscoll et al., 2006, Schneider et al., 2007, Sonnen et al., 2007), including beta amyloid plaques and neurofibrillary tangles associated with Alzheimer’s disease (AD), cerebral infarcts and Lewy bodies. Importantly, although these pathologies account for most cases of dementia, they also are common and related to cognitive decline among older persons without dementia or overt cognitive impairment (Bennett et al., 2012c, Boyle et al., 2013b, Rahimi and Kovacs, 2014). Further, recent data suggest that other less well studied pathologies such as transactive DNA binding protein 43 (TDP 43), hippocampal sclerosis, and cerebral amyloid angiopathy are more common than previously recognized and independently related to decline (Boyle et al., 2015, Nag et al., 2015, Wilson et al., 2016). Thus, cognitive decline in old age begins well before the onset of clinical dementia and is driven by a complex constellation of pathologies with relatively independent effects.
Interestingly, some studies suggest that the association of neuropathology with cognition may vary by age. Specifically, the relation of Alzheimer’s disease (AD) pathology (i.e., neuritic plaques and neurofibrillary tangles) with clinical dementia is attenuated in very old persons compared to younger old persons (Haroutunian et al., 2008, James et al., 2012, Middleton et al., 2011, Savva et al., 2009). This observation implies that the pathologic processes that impair cognition in the beginning of old age differ from the processes impairing cognition toward the end of old age, which could have important implications for the development and implementation of interventions for cognitive decline in old age. Importantly, however, prior studies did not account for several of the pathologies now known to be related to cognitive decline. Further, the hallmark of age related dementia is cognitive decline and we are not aware of prior studies that have examined whether the association of common brain pathologies with cognitive decline is attenuated at advanced ages.
Here, we examine whether the neuropathologic basis for late-life cognitive decline shifts with advancing age. We use data from two clinical-pathologic studies to test the hypothesis that the associations of pathology with cognition varies by age. Older persons without cognitive impairment at baseline completed annual cognitive assessments for up to 20 years prior to death (mean=10.4). After death, they underwent detailed neuropathologic examinations that quantified seven brain pathologies. Mixed-effects change point models were used to test whether a continuous measure of age at death modified the association of each pathologic marker with components of trajectories of cognitive change in global cognition and subsequently 5 specific cognitive domains.
2. METHODS
2.1 Participants
Participants in two longitudinal clinical-pathologic studies agreed to annual cognitive evaluation and brain autopsy at death. The Religious Orders Study (ROS) began in 1994 and followed older Catholic nuns, priests and brothers from more than 40 groups across the United States (Bennett et al., 2012a). The Rush Memory and Aging Project (MAP) started in 1997 and included older persons from retirement communities and subsidized housing facilities in the Chicago area (Bennett et al., 2012b). Participants in both studies underwent uniform clinical, cognitive and neuropathologic evaluations. Annual follow-up of survivors exceeds 95% and the current autopsy rate is 87%. Written informed consent and Anatomic Gift Act were obtained from all participants. The studies were approved by the Institutional Review Board of Rush University Medical Center.
Inclusion criteria for these analyses were absence of cognitive impairment at baseline, to allow examination of incipient cognitive changes and pathology-cognition associations across the whole spectrum of cognitive aging, and completion of at least 5 annual clinical evaluations to make it possible to characterize nonlinear trajectories of cognitive decline. At the time of the present analyses, 3088 persons had completed their baseline evaluation. We excluded 968 persons with cognitive impairment at baseline (777 with mild cognitive impairment [MCI] and 191 with dementia). This left 2120 individuals without cognitive impairment at baseline. There were 230 deaths during the next 4 years and 304 persons who had enrolled within the last 4 years, leaving 1586 individuals eligible to meet the criterion of at least 5 annual follow-ups and 1430 (90.2%) met the criterion. At the time of these analyses, 636 individuals had died of whom 588 (92.5%) underwent a brain autopsy, the results of which were complete in 562. There were 106 individuals missing data on at least one pathologic measure, leaving 456 with complete pathologic data. We excluded an additional 43 individuals whose last clinical evaluation did not occur within 2 years of death, leaving a total of 413 participants for these analyses. Characteristics of the 413 participants in our sample are shown in Table 1. Participants were on average aged 89.1 (SD: 6.3; range: 73.3–103.7) years at the time of death and the average length of participation was 10.4 (SD: 3.9; range: 3.7–20.0) years. As shown it e-table 1, the 413 individuals in the analytic group were older and more likely to be male compared to the 1707 persons who were cognitively healthy at baseline but not eligible for analyses.
Table 1.
Descriptive characteristics (n=413)
| Characteristic | Mean (SD; range) or no. (%) |
|---|---|
| Age at death, years | 89.1 (6.3; 71.3–103.7) |
| Follow-up time, years | 10.4 (3.9; 3.7–20.0) |
| Annual cognitive evaluations, count | 10.2 (3.7; 5–20) |
| Interval between last evaluation and death, months | 8.8 (5.4; 0.13–23.3) |
| Post mortem interval, hours | 8.7 (6.6; 0.0–61.5) |
| Education, years | 16.4 (3.7; 5–28) |
| Female sex | 280 (67.8%) |
| MMSE baseline | 28.5 (1.6; 18–30) |
| MMSE proximate to death | 23.3 (7.2; 0–30) |
MMSE=mini mental state examination.
2.2 Cognitive evaluation
From baseline until death, participants annually underwent detailed cognitive evaluations consisting of episodic memory measures: word list memory, recall, and recognition, immediate and delayed recall of the East Boston story, and story A from Logical Memory; semantic memory measures: verbal fluency, a 15-item form of the Boston Naming Test, and a measure of reading recognition; working memory measures: digit span forward, digit span backward, and digit ordering; perceptual speed measures: number comparison and Symbol Digit Modalities Test; and measures of visuospatial ability: short forms of Judgment of Line Orientation and Standard Progressive Matrices. The primary outcome was a composite measure of global cognition based on all 17 tests, chosen because much of late-life change in cognitive function is global (Lindenberger and Ghisletta, 2009, Wilson et al., 2002, Wilson et al., 2012) and making use of all cognitive data minimizes random variability, which is critical for analyses such as those done here. Cognitive change in old age can also be selective. The 17 tests used here have been hypothesized to assess 5 cognitive domains, and this hypothesis has been consistently supported in earlier analyses of these (Wilsonet al., 2002) and other (Wilson et al., 2009, Wilson et al., 2003) cohorts. For the purposes of the present study, we re-examined the hypothesized cognitive domains using baseline data from 2 groups: the 2120 individuals without cognitive impairment and the subset of 413 individuals on whom analyses are based. In each group, we followed a 2-step process as in previous research (Wilson et al., 2002; Wilson et al., 2003; Wilson et al., 2009). We first conducted a principal components analysis of the 17 tests followed by a varimax rotation. Second, we used Rand’s statistic (Rand, 1971; Wilson et al., 2002) to estimate the concordance of the hypothesized test groupings with the factor analytically based groupings, with +1 indicating perfect agreement and −1 indicating perfect disagreement. We used a permutation test to determine what fraction of all possible test clusters gave agreement with the hypothesized target clusters as high in the factor analytically based clusters (Wilson et al., 2002). The overall agreement of the factor analytically obtained test clusters with the hypothesized test clusters was 0.57 in the group of 2120 individuals (p=0.01) and 0.56 in the subset of 413 in the analytic group (p=0.02), supporting the hypothesized 5 cognitive domains used in previous research. The agreement between the factor analytic results in the analytic group of 413 and the group of 2120 was 0.78 (p<0.01).
Scores on individual tests were converted to z-scores using the mean and SD of the pooled ROS and MAP cohorts at baseline. Composite measures of global cognition and specific cognitive domains were calculated by averaging the z-scores of component tests.
Cognitive testing also supported clinical classification of MCI and dementia which was done in 3 steps. First, an algorithm rated impairment in 5 cognitive domains based on educationally adjusted cutoff scores on 11 tests and rules for deriving domain ratings from test ratings, as previously described (Bennett et al., 2012a and 2012b). Second, after reviewing all cognitive test data, education, and occupation, a neuropsychologist either agreed with each cognitive domain rating or revised it. Third, after review of the history, neurological examination, and cognitive data, an experienced clinician diagnosed dementia in those with a history of cognitive decline and impairment in at least two cognitive domains (McKhann et al., 1984). Those who did not meet dementia criteria but were impaired in at least one cognitive domain were classified as MCI. These MCI criteria have previously been associated with cognitive decline (Bennett et al., 2002, Boyle et al., 2006) and dementia related pathology (Bennett et al., 2006).
2.3 Neuropathologic assessment
Neuropathologists were blinded to clinical data. A standard protocol (Bennett et al., 2006) was followed for brain removal and weighing, tissue sectioning and preservation, and quantification of pathologic findings.
During gross examination, the age, volume, and anatomic location of all macroscopic infarcts were identified in both hemispheres. Hemispheres were cut coronally into 1-cm slabs and fixed for at least 3 days in 4% paraformaldehyde. Nine brain regions of interest (i.e., midfrontal, midtemporal, inferior parietal, anterior cingulate, and entorhinal cortices, midhippocampus at the level of the lateral geniculate body, basal ganglia, thalamus and midbrain) from a single hemisphere were dissected, processed using standard techniques and embedded in paraffin. Hematoxylin and eosin stained sections (6µm) were used to detect and age microscopic infarcts. Only chronic (older than 6 months) macro- and microinfarcts in the cortex and white matter were included in the analyses and were treated as dichotomous variables (present/absent). Hippocampal sclerosis was evaluated unilaterally in a coronal section of the midhippocampus, and graded as absent or present based on severe neuronal loss and gliosis in CA1 and/or subiculum.
Tissue blocks from eight brain regions (i.e., entorhinal, superior frontal, dorsolateral prefrontal, inferior temporal, anterior cingulate, and occipital cortices, midhippocampus and angular gyrus) were cut into 20µm sections for immunohistochemistry to identify and assess average amyloid load and tangle density. One of 3 N-terminus–directed monoclonal antibodies (4G8; Covance Labs, Madison, WI; 1:9,000 or 6F/3D; Dako North America Inc., Carpinteria, CA; 1:50 or 10D5; Elan Pharmaceuticals, San Francisco, CA; 1:600) was used to detect β-amyloid–immunoreactive plaques. Neurofibrillary tangles were labeled with an antibody specific for phosphorylated tau (AT8; Innogenetics, San Ramon, CA; 1:1,000). Computer-assisted sampling and image analysis were used to quantify the percentage of each area occupied by amyloid or tangles. Regional measures were averaged to yield composite measures of amyloid and tangle density. Because the distribution of the amyloid density measure was skewed, the square root was used in the analyses.
Intracytoplasmic Lewy bodies were localized in sections (6-µm) of the substantia nigra, limbic sites (entorhinal and anterior cingulate cortices and amygdala), and neocortical sites (midfrontal, middle temporal and inferior parietal cortices) with one of 2 antibodies to α-synuclein (LB509; Zymed Laboratories, San Fransisco, CA; 1:100 or phosphorylated anti- α-synuclein antibody, Wako Chemicals, USA, Richmond, VA; 1:20,000). Neocortical Lewy bodies were used in the analyses as a dichotomous variable (present/absent).
TDP-43 immunohistochemistry was performed on 6-µm sections of the amygdala, hippocampus (CA1 and dentate), and midfrontal, midtemporal, and entorhinal cortices using a monoclonal antibody to phosphorylated TDP-43 (pS409/410; Ascenion, Munich, Germany; 1:100) (Neumann et al., 2009). The severity was rated on a 6-point scale based on the number of inclusions in a 0.25-mm2 area of greatest density within each region and averaged across regions. Grading was as follows: none, sparse [1–2 inclusions], sparse to moderate [3–5 inclusions], moderate [6–12 inclusions], moderate to severe [13–19 inclusions], and severe [20 or more inclusions].
2.4 Statistical analysis
Because the trajectory of late life cognitive decline is nonlinear and can become steeper in the years prior to death (i.e., terminal decline), mixed-effects change point models (Hall et al., 2000, Laird and Ware, 1982) were used to determine when the rate of cognitive decline accelerated prior to death and to characterize rates of change before and after the change point. The change point model used is a piecewise linear model hierarchical structure such that
where k=1, …, number of covariates +1, i=1, …, N subjects, j=1, …, ni repeated observations per subject.
Thus, the mixed-effects change point model included 4 random trajectory components that allow for between-person variation: linear slope of decline prior to the change point (b1i, slope1), change point (b2i), linear slope of decline after the change point (b3i, slope 2), and level at death (b4i). Time (tij) was represented in years prior to death given our interest in terminal decline. First, we explored cognitive decline in a model including only terms for age at death (continuous variable; centered at the mean of 89 years), sex and education (centered at the mean of 16 years). Sex and education were not significantly associated with the trajectory components and therefore omitted from further analyses. Second, we added terms for pathologies to the model. Third, we added the interactions of each pathology measure with age at death to the models to examine whether the association of each pathologic index with cognitive decline was modified by age at death. Finally, we added terms for cohort (MAP or ROS) and cohort by pathology interactions to examine whether the associations varied by cohort. The primary outcome was the composite measure of global cognition.
Therefore, the saturated change point model can be represented by
Given that k equal 1 to 16 terms that are respectively mean, age at death, tangles, amyloid, gross infarcts, microinfarcts, Lewy bodies, TDP-43, hippocampal sclerosis, and the interaction of each pathology and age at death (7 additional terms).
All models were repeated using each of the 5 cognitive domains as outcome measures in separate analyses. Parameters were estimated using a Bayesian Monte Carlo Markov Chain (MCMC) approach (Hall et al., 2003) implemented in OpenBUGS software (Lunn et al., 2009). We apriori selected non-informative priors; specifically, each coefficient for the fixed effects assumes normal prior with mean 0 and variance 10000; inverse of variance covariance matrix for random effects assumes Wishart (R, 4), where R is a 4 by 4 diagonal matrix of 0.1; and inverse of residual variance assumes gamma with parameters of 0.001s. We imposed a limit on the random change point component such that it must occur after the first cognitive assessment and before the last assessment (i.e., prior to death); thus, the estimated time delays are positive. Posterior means from the sampling chain were used as the point estimates and posterior 2.5 and 97.5 quantiles were used to construct 95% credible intervals. To assess model convergence, we 1) monitored the trace plot for evidence of a stable simulation process, 2) ran multiple chains simultaneously and examined whether the trace plots of individual chains overlapped and whether the BGR diagnostic ratios were approaching 1, and 3) visually inspected the autocorrelations (which should be small) (Cowles et al., 1996). All model inspections supported convergence.
3. RESULTS
3.1 Trajectories of change in global cognition
At baseline, scores on the composite measure of global cognition (mean: 0.23, SD: 0.38) were approximately normally distributed. We first examined the mean trajectory of global cognitive decline using a mixed-effects model that allowed the rate of cognitive decline to accelerate beginning at a person-specific time before death, including only age at death as predictor. At the mean age at death, global cognition declined at a rate of −0.03 (CI: −0.04 to −0.027) standard unit per year before the change point. A change-point occurred at a mean of 2.92 (CI: −3.18 to −2.67) years before death, when the rate of decline increased approximately 10-fold to −0.31 (CI: −0.35 to −0.28) standard unit per year. Older age at death was associated with an earlier change point, indicating a longer terminal period in those who lived longer. Notably, 91 (22%) of the participants had 1 cognitive measurement after their estimated change point, 176 (43%) had two, and 146 (35%) had 3 or more assessments after the estimated change point. Plots of the predicted cognitive trajectories in 20 randomly selected individuals showed good agreement with raw scores, suggesting adequate model fit (e-figure 1).
3.1.1 Neuropathology and global cognition
At neuropathologic examination, the tau tangle density ranged from 0 to 30.5 (mean: 4.8, SD: 5.1), β-amyloid plaque burden ranged from 0 to 22.9 (mean: 4.6, SD: 4.5), and severity of TDP-43 pathology ranged from 0 to 4.5 (mean: 0.5, SD: 0.8). Further, 95 (23.0%) participants had Lewy bodies, 132 (32.0%) had at least one chronic gross infarct, 128 (31.0%) had at least one chronic microinfarct, and 39 (9.4%) had hippocampal sclerosis. e-table 2 shows the correlations among age at death and the pathologic indices. Tau tangle density, beta-amyloid plaque burden, gross infarcts, TDP-43 pathology and hippocampal sclerosis were correlated with age at death, while microinfarcts and Lewy Bodies were not.
To determine the association of these neuropathologic indices with the trajectory of global cognition, we added terms for these markers to the model (Table 2). Higher tangle density was associated with faster decline before the change point and with an earlier change point. Gross infarcts were related to faster decline before and after the change point. The presence of Lewy bodies was related to an increased rate of decline after the change point only. TDP-43 pathology was associated with faster decline before the change point and an earlier change point. Amyloid load, microinfarcts and hippocampal sclerosis were unrelated to any of the global cognition trajectory components. The trajectories predicted by this model showed good fit with the lots of the raw data (e-figure 1). Further, it is noteworthy that adding the neuropathologic indices to the model markedly reduced the association of age at death with the trajectory of global cognition.
Table 2.
Association of the pathologic indices with trajectory of global cognition
| Model term | Global cognition | |||
|---|---|---|---|---|
| Est. | 95% CI | |||
| Mean | Slope 1 | −0.0138 | −0.0233 | −0.0044 |
| Change point | −2.2640 | −2.6160 | −1.9350 | |
| Slope 2 | −0.2629 | −0.3172 | −0.2101 | |
|
| ||||
| Age | Slope 1 | 0.0004 | −0.0007 | 0.0015 |
| Change point | −0.0201 | −0.0531 | 0.0133 | |
| Slope 2 | 0.0042 | −0.0014 | 0.0099 | |
|
| ||||
| Tangles | Slope 1 | −0.0022 | −0.0038 | −0.0007 |
| Change point | −0.1185 | −0.1637 | −0.0724 | |
| Slope 2 | 0.0002 | −0.0060 | 0.0063 | |
|
| ||||
| Amyloid | Slope 1 | −0.0021 | −0.0078 | 0.0035 |
| Change point | −0.1151 | −0.3058 | 0.0755 | |
| Slope 2 | −0.0189 | −0.0500 | 0.0116 | |
|
| ||||
| Gross infarcts | Slope 1 | −0.0147 | −0.0242 | −0.0054 |
| Change point | −0.2242 | −0.5060 | 0.0608 | |
| Slope 2 | −0.0507 | −0.0953 | −0.0061 | |
|
| ||||
| Microinfarcts | Slope 1 | 0.0021 | −0.0072 | 0.0116 |
| Change point | −0.1188 | −0.4326 | 0.1925 | |
| Slope 2 | 0.0102 | −0.0417 | 0.0588 | |
|
| ||||
| Lewy bodies | Slope 1 | −0.0125 | −0.0267 | 0.0017 |
| Change point | −0.2148 | −0.6353 | 0.2148 | |
| Slope 2 | −0.1074 | −0.1814 | −0.0345 | |
|
| ||||
| TDP-43 | Slope 1 | −0.0142 | −0.0240 | −0.0040 |
| Change point | −0.3482 | −0.6170 | −0.0776 | |
| Slope 2 | −0.0352 | −0.0778 | 0.0066 | |
|
| ||||
| HS | Slope 1 | −0.0026 | −0.0293 | 0.0246 |
| Change point | −0.5094 | −1.2440 | 0.2257 | |
| Slope 2 | 0.0299 | −0.0846 | 0.1433 | |
The population-level estimates (95% credible interval) were generated from a single multivariate mixed-effects change point model with terms for age at death (centered at 89 years) and all 7 pathologic indices (continuous measures centered at the median: amyloid at 1.8, tangles at 3.3; other pathologies at 0). The mean indicates the trajectory of cognitive decline at age 89 and no pathology. Each significant pathology term characterizes the difference from the mean due to the particular pathologic index. Statistically significant estimates in bold. Change point in years before death.
TDP-43= transactive response DNA-binding protein 43. HS=hippocampal sclerosis.
The participants for these analyses were drawn from two cohorts. To test whether clinical-pathologic correlations varied by cohort, we repeated the analysis with terms added for cohort (i.e., ROS or MAP) and the interaction of cohort with each pathologic marker. In this analysis, there was no main effect for cohort and no cohort by pathology interactions.
3.1.2 Age, neuropathology and global cognition
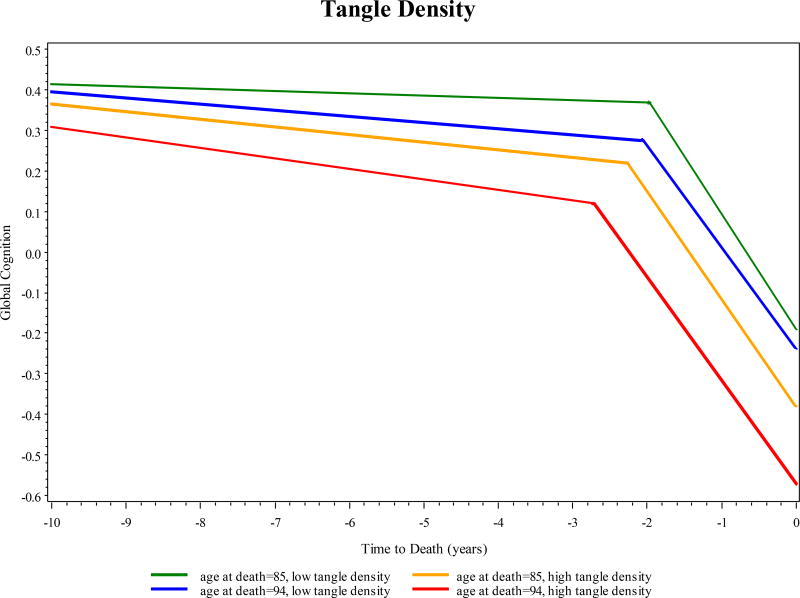
To test the hypothesis that age modifies the association of dementia related pathologies with the rate of change in cognitive function, we augmented the previous model by adding terms for the interaction of age at death with each postmortem pathologic marker. As shown in Table 3, relatively few of these interactions were significant, and when age did interact with pathology, there was little consistency in the direction of the association or the trajectory component affected. For example, tangle density was associated with an earlier change point with advancing age, but the rates of tangle related decline before and after the change point were similar at varying ages (Figure 1), and there was no evidence of age interactions with amyloid load. Whereas the association of gross infarcts with cognition did not vary by age, microinfarcts were associated with slower decline before the change point at older ages, but the associations with the change point and rate of decline thereafter did not vary by age. The presence of Lewy bodies was associated with slower decline after the change point at advanced age, but the association of Lewy bodies with other trajectory components did not vary by age. The association of TDP-43 with global cognitive decline prior to the change point was stronger at younger ages and the opposite relation was found for hippocampal sclerosis, while the relation of both TDP-43 and hippocampal sclerosis with the change point and decline thereafter did not differ by age.
Table 3.
Association of the pathologic indices with trajectory of global cognitive decline across age
| Model term | Global cognition | |||
|---|---|---|---|---|
| Est. | 95% CI | |||
| Mean | Slope 1 | −0.0146 | −0.0240 | −0.0052 |
| Change point | −2.2020 | −2.5610 | −1.8670 | |
| Slope 2 | −0.2630 | −0.3212 | −0.2078 | |
|
| ||||
| Age | Slope 1 | −0.0010 | −0.0026 | 0.0006 |
| Change point | −0.0263 | −0.0727 | 0.0205 | |
| Slope 2 | 0.0025 | −0.0054 | 0.0107 | |
|
| ||||
| Tangles | Slope 1 | −0.0024 | −0.0042 | −0.0007 |
| Change point | −0.0893 | −0.1398 | −0.0391 | |
| Slope 2 | 0.0014 | −0.0061 | 0.0088 | |
|
| ||||
| Amyloid | Slope 1 | −0.0018 | −0.0074 | 0.0039 |
| Change point | −0.0898 | −0.2794 | 0.0972 | |
| Slope 2 | −0.0294 | −0.0623 | 0.0038 | |
|
| ||||
| Gross infarcts | Slope 1 | −0.0149 | −0.0244 | −0.0055 |
| Change point | −0.2448 | −0.5165 | 0.0327 | |
| Slope 2 | −0.0533 | −0.1041 | −0.0041 | |
|
| ||||
| Microinfarcts | Slope 1 | 0.0021 | −0.0070 | 0.0115 |
| Change point | −0.0679 | −0.3878 | 0.2339 | |
| Slope 2 | 0.0015 | −0.0535 | 0.0573 | |
|
| ||||
| Lewy bodies | Slope 1 | −0.0128 | −0.0275 | 0.0020 |
| Change point | −0.0989 | −0.5456 | 0.3416 | |
| Slope 2 | −0.1558 | −0.2365 | −0.0768 | |
|
| ||||
| TDP-43 | Slope 1 | −0.0185 | −0.0296 | −0.0073 |
| Change point | −0.4603 | −0.7526 | −0.1673 | |
| Slope 2 | −0.0364 | −0.0868 | 0.0117 | |
|
| ||||
| HS | Slope 1 | 0.0116 | −0.0172 | 0.0400 |
| Change point | −0.5696 | −1.3380 | 0.1828 | |
| Slope 2 | 0.0534 | −0.0745 | 0.1800 | |
|
| ||||
| Tangles × Age | Slope 1 | 0.0001 | −0.0002 | 0.0003 |
| Change point | −0.0082 | −0.0161 | −0.0002 | |
| Slope 2 | −0.0005 | −0.0016 | 0.0007 | |
|
| ||||
| Amyloid × Age | Slope 1 | −0.0002 | −0.0011 | 0.0007 |
| Change point | 0.0018 | −0.0256 | 0.0305 | |
| Slope 2 | 0.0028 | −0.0021 | 0.0076 | |
|
| ||||
| Gross infarcts × Age | Slope 1 | −0.0008 | −0.0022 | 0.0006 |
| Change point | 0.0194 | −0.0235 | 0.0607 | |
| Slope 2 | 0.0016 | −0.0057 | 0.0094 | |
|
| ||||
| Microinfarcts × Age | Slope 1 | 0.0019 | 0.0003 | 0.0034 |
| Change point | −0.0444 | −0.0884 | 0.0050 | |
| Slope 2 | −0.0018 | −0.0105 | 0.0063 | |
|
| ||||
| Lewy bodies × Age | Slope 1 | 0.0018 | −0.0006 | 0.0041 |
| Change point | 0.0214 | −0.0469 | 0.0924 | |
| Slope 2 | 0.0159 | 0.0028 | 0.0284 | |
|
| ||||
| TDP-43 × Age | Slope 1 | 0.0028 | 0.0007 | 0.0050 |
| Change point | 0.0030 | −0.0518 | 0.0597 | |
| Slope 2 | 0.0036 | −0.0054 | 0.0129 | |
|
| ||||
| HS × Age | Slope 1 | −0.0089 | −0.0144 | −0.0034 |
| Change point | 0.0877 | −0.0610 | 0.2383 | |
| Slope 2 | −0.0152 | −0.0390 | 0.0087 | |
The population-level estimates (95% credible interval) were generated from a single multivariate mixed-effects change point model with terms for age at death (centered at 89 years), all 7 pathologic indices (continuous measures centered at the median: amyloid at 1.8, tangles at 3.3; other pathologies at 0) and interactions between age and pathologic indices. Statistically significant estimates in bold. Change point in years before death.
TDP-43= transactive response DNA-binding protein 43. HS=hippocampal sclerosis.
Figure 1. Predicted trajectory of global cognition by age at death and tangle density.
Older age at death: 75th percentile (94 years) and younger age at death: 25th percentile (85 years).
3.2 Trajectories of change in specific cognitive domains
Since cognition is not a unitary construct, in secondary analyses we examined whether the relation of the pathologic indices to the trajectory components varied across age in specific cognitive domains. We therefore repeated the initial series of analyses using composite measures of specific cognitive domains in place of the global cognition measure. Associations of the pathologic indices with trajectory components in each cognitive domain are reported in e-table 3. After accounting for the pathologic indices, age at death was no longer associated with decline in any of the domains. Further, analyses examining whether age at death modified the relation of the pathologic indices to cognitive domains (e-table 4) again revealed little evidence of systematic modification. Specifically, of the 105 age-by-pathology interactions (7 pathologies, 5 cognitive domains, 3 trajectory components) tested, 16 (15.2%) were significant, with 9 suggesting a reduced association of the pathologic indices with cognition at older ages and 7 suggesting an increased association. We also did not identify a consistently affected trajectory component in these cases. Most notably, the associations of tangle density and amyloid load with cognition did not vary by age in any cognitive domain. In general, these results indicate that the deleterious association of dementia related pathologies with cognitive decline does not vary systematically by age.
4. DISCUSSION
We examined the associations of age and multiple pathologies with trajectories of cognitive decline in more than 400 initially cognitively healthy persons who were followed annually for up to 20 years and died between 71 and 103 years of age. Pathologies were robustly related to late-life trajectories of cognitive decline. However, the deleterious impact of these pathologies did not systematically vary by age. These results suggest that the neuropathologic mechanisms underlying late-life cognitive decline do not substantially differ across the spectrum of age and that age should not be considered a modulating factor in the development or implementation of interventions to prevent cognitive decline.
In contrast to the present findings, including a prior study from these cohorts (James et al., 2012), most (Haroutunian et al., 2008, James et al., 2012, Middleton et al., 2011, Savva et al., 2009) but not all (Dolan et al., 2010) previous studies have suggested that the association of neuropathology with dementia is attenuated in the oldest old. Prior work was cross-sectional, however, involving comparisons of postmortem pathologic findings in persons with and without dementia in different age groups (Dolan et al., 2010, Middleton et al., 2011, Savva et al., 2009). Further, AD pathology was of main interest in these studies while other (non-AD) neuropathologies were not always controlled for or examined.
Specifically, the studies reporting an attenuated association of neuritic plaques (Haroutunian et al., 2008, Savva et al., 2009), tangles (Haroutunian et al., 2008, Middleton et al., 2011, Savva et al., 2009) or a combination of the two (James et al., 2012) with dementia in very old persons compared to younger older persons looked at individual pathologies (Haroutunian et al., 2008, Savva et al., 2009) or controlled for Lewy bodies and vascular pathologies only (James et al., 2012, Middleton et al., 2011). We also adjusted for TDP-43 pathology and hippocampal sclerosis. These often unrecognized pathologies tend to increase with age and are known to increase the likelihood of dementia in persons with AD pathology (Nag et al., 2015, Wilson et al., 2013). Higher burdens of coexisting pathologies at older ages may therefore have accounted for the age related reduction in the association between clinical dementia and AD pathology reported in previous studies. Further, all prior studies looked at the cross-sectional diagnosis of dementia proximate to death as outcome, rather than examining associations with cognitive decline. Dementia is characterized by slowly progressive cognitive decline. Thus, we focused on the association of neuropathology with the continuous rate of change in cognitive function rather than the binary, cross-sectional diagnosis of dementia in order to more precisely capture differences in the relation of pathology with cognition across the spectrum of age as well as to minimize limitations such as inaccurate diagnosis at older ages and differences in disease severity between age groups. Also, since previous research has relied on binary cognitive outcomes (i.e., dementia) mortality could not be accounted for. It is possible is that the correlation of pathology with cognition has previously been found reduced in very old persons because terminal cognitive change is more pronounced in this age group, and the neurobiological basis of terminal decline remains unclear (Wilson et al., 2010, Wilsonet al., 2012). A novel feature of this study is that we let rate of cognitive decline accelerate proximate to death to separate terminal from preterminal cognitive change. Consistent with previous research (Wilson et al., 2003), there was a marked acceleration in rate of cognitive decline beginning a mean of about 3 years before death. However, we found no evidence that age systematically modified the relation of pathology to the onset or rate of terminal cognitive decline.
Consistent with earlier findings (Wilson et al., 2010), no evidence of age related cognitive decline was found after accounting for the seven neuropathologic indices. This suggests that cognitive decline in old age is mainly due to pathologic rather than normal developmental processes and efforts to mitigate this decline should focus on factors that prevent or protect against the deleterious effects of neuropathology. Tau tangles, gross infarcts, Lewy bodies and TDP-43 pathology were in particular associated with the rate of global cognitive decline while amyloid load, microinfarcts and hippocampal sclerosis were not. The finding that amyloid load does not predict cognitive function after accounting for tau tangle pathology is consistent with previous results, as was the lack of finding for microinfarcts. Still, we previously reported that common pathologies account for less than half of the variance in late life cognition (Boyle et al., 2013a), suggesting that other factors are important. A better understanding of individual differences in neuropathology related cognitive decline may aid the development of strategies for reducing cognitive decline.
The current study confirms that age should not be considered a modulating factor in the development of such strategies.
Strengths of this study are that all participants underwent detailed cognitive evaluations and postmortem evaluations in which a wide array of pathologies was assessed by trained and experienced examiners. The low dropout rate and high autopsy rate reduced chances of missing data affecting the results. In addition, the long follow-up time and cognitive evaluations proximate to death allowed us to reliably apply change point models. However, findings are also based on a selected cohort consisting primarily of highly educated non-Latino Whites and therefore generalizability remains to be determined. Also, it must be noted that participants were 71 years or older at death indicating that our conclusions apply to persons at older ages and might differ if a broader distribution of age had been studied. Further, although the use of changepoint models allowed us to examine components of the cognitive trajectory in detail, these models are complex and the choice of priors can be difficult. Finally, although methodologically challenging, postmortem pathological indices were used to predict cognitive decline before death as it is not yet possible to measure neuropathology in vivo. In the future it may prove possible to measure the evolution of neuropathology in vivo and correlate this to longitudinal cognitive change.
5. CONCLUSIONS
In conclusion, the neuropathologic basis for cognitive decline in younger older persons is not substantially different from that in very old persons. Age should not be considered a modulating factor in the development of strategies targeted at reducing cognitive decline.
Supplementary Material
Highlights.
Dementia related neuropathologies are associated with late-life trajectories of cognitive decline
The deleterious impact of these pathologies did not systematically vary by age
The pathologic basis for cognitive decline does not substantially differ across the spectrum of age
Acknowledgments
The authors are grateful to Donna Esbjornson, MS, for statistical programming, the participants of the Religious Orders Study and the Rush Memory and Aging Project, and the faculty and staff of the Rush Alzheimer's Disease Center. Thanks are also expressed to Manuela Neumann and Elisabeth Kremmer for providing the phosphorylation specific TDP-43, ID3 antibody.
Study funding
This research was supported by the National Institute on Aging (R01AG17917, P30AG10161, R01AG15819, R01AG34374) and the Illinois Department of Public Health. The funding source had no role in study design, collection, analysis and interpretation of data, in writing of the report or the decision to submit for publication.
Disclosures
W. Jansen receives research support from Biogen. R. Wilson serves as a consulting editor for Aging, Neuropsychology, and Cognition, Psychology and Aging, and Neuropsychology; has served as a consultant for Pain Therapeutics, Inc.; and receives research support from the NIH (P30AG010161 [coinvestigator], RF1AG015819 [coinvestiga- tor], R01AG017917 [coinvestigator], R01AG034374 [coinvestigator], R01AG039478 [coinvestigator], R01AG036042 [coinvestigator], R01AG036836 [coinvestigator], R01AG041797 [coinvestigator], R01AG042210 [coinvestigator], and R01NR013151 [coinvestigator]), the Alzheimer’s Association (NIRGD-11-205469), and Zinfandel Pharmaceuticals. PJ. Visser received grants from EU/EFPIA Innovative Medicines Initiative Joint Undertaking, EU Joint Programme–Neurodegenerative Disease Research (JPND), ZonMw; receives research support from Biogen; has served as member of the advisory board of Roche Diagnostics and Eli Lilly; and has received nonfinancial support from GE Healthcare. S. Nag receives research support from the NIH (P30AG010161 [neuropathologist], R01AG017917 [neuropatholo- gist], R01NS078009 [neuropathologist], R01AG043379 [neuropatholo- gist], and R01NS082416 [neuropathologist]). J. Schneider has served as a consultant to Navidea Biopharma- ceuticals and Genentech USA; and receives research support from NIH (R01AG042210 [principal investigator], P30AG010161 [core leader], R01HL096944 [neuropathologist], R01AG039478 [neuropathologist], R01AG017917 [neuropathologist], RF1AG015819 [neuropathologist], R01AG022018 [neuropathologist], R01AG036042 [neuropathologist], R01AG040039 [neuropathologist], R01AG036836 [neuropathologist], R01AG034374 [neuropathologist], U01AG046152 [neuropathologist], R01AG043379 [neuropathologist], R01NS078009 [neuropathologist], R01AG043975 [neuropathologist], R01AG033678 [neuropathologist], R21ES021290 [neuropathologist], R01NS084965 [neuropathologist], R01AG043617 [neuropathologist], R56NS08967 [neuropathologist], R01AG038651 [neuropathologist], R01DK099269 [neuropathologist], and P01AG014449 [neuropathologist]). B. James is funded by National Institute on Aging (NIA) grants R01AG17917, R01AG033678, and P30 AG10161; and serves as a consultant for the Alzheimer's Association and Partner's Health Care System. S. Leurgans received support from the NIH (P20MD006886 [biostatistician], P30AG010161 [biostatistician], R01AG036042 [biostatistician], R01NS078009 [biostatistician], R01AG047976 [biostatistician], R01AG036836 [biostatistician], and R01AG042210 [biostatistician]). A. Capuano receives research support from NIH (R01AG022018 [coinvestigator], R01AG017917 [coinvestigator], R01AG015819 [coinvestiga- tor], P30AG010161 [coinvestigator], R01AG043379 [coinvestigator], R01HL096944 [coinvestigator], and R01AG040039 [coinvestigator]). D. Bennett serves on the editorial board of Neurology®; has served as a consultant to Schering-Plough Corp., Mediation, Inc., and the Gerson Lehrman Group; and receives research support from Danone Inc., the NIH (R01AG017917 [principal investigator], R01AG015819 [principal investigator], R01AG036042 [principal investigator], U01AG032984 [co-principal investigator, leader of epidemiologic cohort studies], R01HL096944 [coinvestigator], R01AG033678 [coinvestigator], R01AG034374 [coinvestigator], R01AG022018 [coinvestigator], R01AG034119 [coinvestigator], P30AG010161 [principal investigator—administrative core leader, Religious Orders Study core leader], U01AG046152 [coinvestiga- tor], R01AG040039 [coinvestigator], R01AG039478 [coinvestiga- tor], R01AG038651 [coinvestigator], R01AG036836 [coinvestigator], R01AG041797 [coinvestigator], R01AG042210 [coinvestigator], P20MD006886 [coinvestigator], R01NS078009 [coinvestigator], R01AG043617 [coinvestigator], R01AG043975 [coinvestigator], U18NS082140 [coinvestigator], R01NS082416 [coinvestigator], R01NS084965 [coinvestigator], R01NS086736 [coinvestigator], R01DK099269 [coinvestigator], R01AG046174[coinvestigator], R01AG48015 [coinvestigator], and P01AG014449 [coinvestigator]), and the Illinois Department of Public Health. P. Boyle receives research support from the NIH (R01AG034374 [principal investigator], R01AG034119 [coinvestigator], R01AG033678 [principal investigator], and R01AG040039 [coinvestigator]).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res. 2012a;9(6):628–45. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012b;9(6):646–63. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol. 2012c;72(4):599–609. doi: 10.1002/ana.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67(3):441–5. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. 2013a;74(3):478–89. doi: 10.1002/ana.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Yu L, Nag S, Leurgans S, Wilson RS, Bennett DA, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85(22):1930–6. doi: 10.1212/WNL.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Yu L, Wilson RS, Schneider JA, Bennett DA. Relation of neuropathology with cognitive decline among older persons without dementia. Frontiers in aging neuroscience. 2013b;5:50. doi: 10.3389/fnagi.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles Mary Kathryn, Bradley PCarlin. Markov Chain Monte Carlo Convergence Diagnostics: A Comparative Review. Journal of the American Statistical Association. 1996;91(434):883–904. [Google Scholar]
- Dolan D, Troncoso J, Resnick SM, Crain BJ, Zonderman AB, O'Brien RJ. Age, Alzheimer's disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain. 2010;133(Pt 8):2225–31. doi: 10.1093/brain/awq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Resnick SM, Troncoso JC, An Y, O'Brien R, Zonderman AB. Impact of Alzheimer's pathology on cognitive trajectories in nondemented elderly. Annals of Neurology. 2006;60(6):688–95. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer's disease. Statistics in medicine. 2000;19(11–12):1555–66. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hall CB, Ying J, Kuo L, Lipton RB. Bayesian and profile likelihood change point methods for modeling cognitive function over time. Comput Stat Data An. 2003;42(1–2):91–109. [Google Scholar]
- Haroutunian V, Schnaider-Beeri M, Schmeidler J, Wysocki M, Purohit DP, Perl DP, et al. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol. 2008;65(9):1211–7. doi: 10.1001/archneur.65.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA. Dementia from Alzheimer disease and mixed pathologies in the oldest old. Jama. 2012;307(17):1798–800. doi: 10.1001/jama.2012.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–74. [PubMed] [Google Scholar]
- Lindenberger U, Ghisletta P. Cognitive and sensory declines in old age: gauging the evidence for a common cause. Psychol Aging. 2009;24(1):1–16. doi: 10.1037/a0014986. [DOI] [PubMed] [Google Scholar]
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: Evolution, critique and future directions. Statistics in medicine. 2009;28(25):3049–67. doi: 10.1002/sim.3680. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Wang L. Modeling age-based turning points in longitudinal life-span growth curves of cognition. In: Cohen P, editor. Applied data analytic techniques for turning points research. Routledge; 2008. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Middleton LE, Grinberg LT, Miller B, Kawas C, Yaffe K. Neuropathologic features associated with Alzheimer disease diagnosis: age matters. Neurology. 2011;77(19):1737–44. doi: 10.1212/WNL.0b013e318236f0cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol. 2015;77(6):942–52. doi: 10.1002/ana.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117(2):137–49. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther. 2014;6(9):82. doi: 10.1186/s13195-014-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand WM. Objective criteria for the evaluation of clustering methods. Journal of the American Statistical Association. 1971;66:846–850. [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C, et al. Age, Neuropathology, and Dementia. New England Journal of Medicine. 2009;360(22):2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62(4):406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2009;66(6):767–72. doi: 10.1001/archneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179–93. [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Bienias JL, Evans DA, Bennett DA. Terminal decline in cognitive function. Neurology. 2003;60(11):1782–7. doi: 10.1212/01.wnl.0000068019.60901.c1. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Capuano AW, Bennett DA, Schneider JA, Boyle PA. Temporal course of neurodegenerative effects on cognition in old age. Neuropsychology. 2016;30(5):591–9. doi: 10.1037/neu0000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75(12):1070–8. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Hizel LP, Boyle PA, Bennett DA. Terminal dedifferentiation of cognitive abilities. Neurology. 2012;78(15):1116–22. doi: 10.1212/WNL.0b013e31824f7ff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Trojanowski JQ, Chen EY, Boyle PA, Bennett DA, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA neurology. 2013;70(11):1418–24. doi: 10.1001/jamaneurol.2013.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.