Abstract
Background
Epidemiology of myocarditis in childhood is largely unknown. Men are known to have a higher incidence of myocarditis than women in adults aged <50 years, but whether this is true by sex in pediatric age groups is unknown. We set out to study the occurrence and potential sex differences of myocarditis in a general pediatric population.
Methods and Results
Data of all hospital admissions with myocarditis in Finland occurring in patients aged ≤15 years from 2004 to 2014 were collected from a mandatory nationwide registry. All patients with myocarditis as a primary, secondary, or tertiary cause of admission were included. Total and age‐ and sex‐specific incidence rates were calculated using corresponding population data. There were 213 admissions with myocarditis in pediatric patients. Myocarditis was the primary cause of admission in 86%. The overall incidence rate of myocarditis was 1.95/100 000 person‐years. Of all patients, 77% were boys, but sex differences in incidence rates were age‐dependent. In children aged 0 to 5 years, there was no sex difference in the occurrence of myocarditis. Boys aged 6 to 10 years had a higher incidence rate compared with girls (72% boys; incidence rate ratio: 2.46; 95% confidence interval, 1.03–5.89; P=0.04). Sex difference further increased in children aged 11 to 15 years (80% boys; incidence rate ratio: 3.5; 95% confidence interval, 2.68–5.67; P<0.0001).
Conclusions
Myocarditis leading to hospital admission is relatively uncommon in children, but occurrence of myocarditis increases with age. There is no sex difference in the risk of myocarditis during the first 6 years of life, but boys have a significantly higher risk at ages 6 to 15 years.
Keywords: epidemiology, infection, inflammation, myocarditis
Subject Categories: Epidemiology, Pediatrics, Cardiovascular Disease
Clinical Perspective
What Is New?
This study is the first to report overall incidence and age‐ and sex‐specific distribution of childhood myocarditis using a nationwide approach.
There is no sex difference in the risk of myocarditis during the first 6 years of life, but boys aged 6 to 15 years have a higher risk than girls, and the risk difference increases with age.
Overall short‐term prognosis of childhood myocarditis seems more favorable than in previous studies with more selected patient populations.
What Are the Clinical Implications?
More precise diagnostic criteria and readily available noninvasive methods are needed to improve myocarditis diagnosis, which is still challenging.
The etiology of myocarditis remains frequently unknown and needs to be more vigorously studied in the future.
The noted male preponderance in the occurrence of myocarditis is largely unknown and warrants further study.
Better assessment of long‐term prognosis of childhood myocarditis is needed.
Myocarditis is a cardiac inflammatory disease that is commonly caused by viral infections, both in children and adults. Additional etiologies include other infections, toxins, hypersensitivity reactions, and a variety of systemic and autoimmune processes, but these etiologies are far less common.1 Virus‐induced direct myocyte injury or innate immunological responses, followed by antigen‐specific immune reaction involving T cells, B cells, and antibody production, are considered the main mechanisms of myocyte injury.2 In some cases, acute myocarditis leads to dilated cardiomyopathy, characterized by chronic inflammation and myocyte injury with or without viral persistence.2
True incidence of myocarditis is unknown. On the one hand, it is probably underdiagnosed because it may be asymptomatic in a considerable number of patients. On the other hand, congestive heart failure, ventricular arrhythmias, and sudden death can be the presenting symptoms. Diagnosis is challenging because symptoms are frequently nonspecific, especially in infants and children, masquerading as respiratory and gastrointestinal infections.2, 3, 4, 5 Lack of readily available sensitive and specific clinical diagnostic tests, wide variation in diagnostic criteria, and selected patient populations among published studies have hampered accurate estimations of epidemiology and natural history of myocarditis. According to experimental and clinical studies, however, young male adolescents and adults are considered most susceptible to viral myocarditis.1, 6, 7 In children, all ages from newborns to adolescents are affected, but 2 incidence peaks have been shown to occur: in infants aged <1 year and in teenagers.8
In the present study, we studied the age‐ and sex‐specific occurrences and features of myocarditis during an 11‐year period in a nationwide study of Finnish children and adolescents.
Methods
Study Patients and Data Collection
Pediatric patients admitted to the hospital with myocarditis during 2004 to 2014 were studied. Data of all pediatric, pediatric surgery, medical and surgical ward admissions in Finland occurring for patients aged ≤15 years (n=882 253) were retrospectively screened for admissions with myocarditis. The Finnish Care Register for Health Care, a nationwide, obligatory, and automatically collected database maintained by the Finnish National Institute for Health and Welfare containing hospital discharge data of all hospital admissions in Finland, was used. Patients with myocarditis as primary, secondary, or tertiary cause of admission (International Classification of Diseases, 10th Revision codes I40, I41, B33.2, I01.2, I09.0, and I51.4) were included. Hospital transfers during the same treatment period were combined. Comorbidities, treatment, and potential etiologies were detected from hospital discharge diagnoses and procedural codes. The study population was mainly white. Incidence rates were estimated using age‐ and sex‐specific population data from Finland during the study period (10 558 824 person‐years), obtained from Statistics Finland. The study was approved by the National Institute for Health and Welfare in Finland (permission nos. THL/143/5.05.00/2015 and THL/1349/5.05.00/2015). The requirement of obtaining informed consent from the study participants was waived.
Statistical Analyses
Sex differences in dichotomous variables (comorbidities and etiology) were analyzed by using the Fisher exact test. Differences in continuous variables (age and admission length) were analyzed with a t test or 1‐way ANOVA with least significant difference post hoc testing, as appropriate. The association of age and sex with the occurrence of myocarditis was studied using Poisson regression modeling. Potential interaction between age and sex was tested with an interaction term. When modeling incidence rate, the logarithm of population was used as an offset parameter. Results of regression modeling are given as incidence rate ratios. Incidence rates were standardized to the 2000 US standard population using a direct method. Continuous variables are presented as mean±SD or median, as appropriate. Categorical variables are presented as counts or percentages. Confidence intervals (CIs) for incidence rates were calculated assuming Poisson distribution. Two‐sided P<0.05 was considered statistically significant. The SAS system version 9.4 (SAS Institute Inc) was used for statistical analyses.
Results
The study period included 213 admissions with myocarditis, representing 0.02% of all admissions. Of those, myocarditis was the primary cause of admission for 86%. The majority of the patients were male (77%, n=163). Sex distribution of the patients with myocarditis, however, was significantly age‐dependent (Figure 1). The proportion of boys with myocarditis started to increase exponentially after 7 years of age. There was similar pattern of increase with advancing age in girls, but a significant proportion of myocarditis in girls also occurred in a population <7 years of age. The median age of a patient with myocarditis was 14 years. Girls were younger than boys (mean: 10.6±4.9 versus 12.8±3.4 years, respectively; P=0.004). Myocarditis‐associated admissions lasted on average for 5.0±4.6 days (range: 1–35 days). There was no difference in admission duration between boys and girls. Admission length was significantly longer for small children (aged ≤5 years) than for 6‐ to 10‐year‐olds (10.2±7.8 versus 4.7±4.2 days, respectively; P<0.0001) but remained similar thereafter (4.5±4.0 days in children aged ≥11 years).
Figure 1.
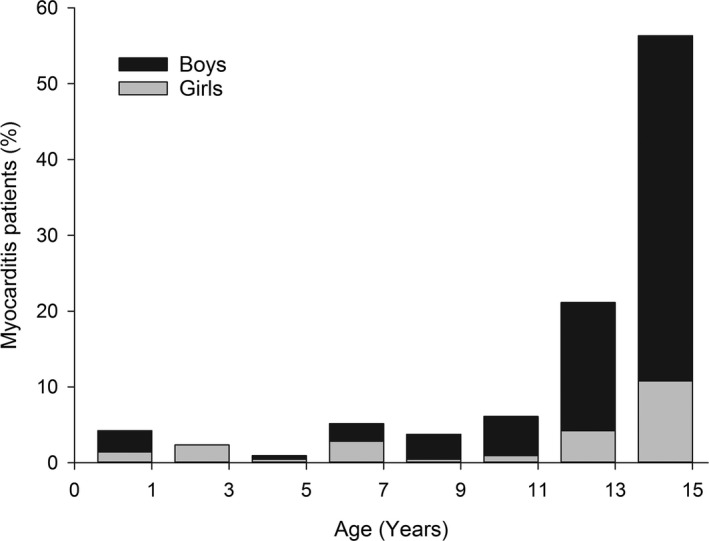
Age distribution of the patients with myocarditis. Sex‐specific distribution is presented as relative proportions within bars.
Comorbidities recorded with myocarditis were rare (Table). Recorded coinfections were most commonly upper respiratory, followed by lower respiratory and gastrointestinal tract infections. Clinical septicemia was present in 1 patient. Heart failure/cardiomyopathy and a previous cardiac transplant were more common among girls, but otherwise, there were no sex differences in comorbidities (Table). Myocarditis was recorded to be of viral etiology in 11% of patients; however, a specific virus was rarely detected during admission, as influenza virus was recorded in 4 patients (1.9%) and Norwalk virus was recorded in 2 (0.9%). Epstein–Barr virus, enterovirus, and mumps virus were recorded in 1 patient each. Acute streptococcus infection was recorded in 2 patients. Rheumatic fever, Kawasaki disease, and mycoplasma infection were considered etiological causes in single cases of myocarditis. Influenza‐associated myocarditis was more common in girls than in boys (6.0% versus 0.6%, respectively; P=0.040), but no other sex differences in confirmed etiologies were present.
Table 1.
Clinical Characteristics and Sex Differences of Patients With Myocarditis
| Codiagnosis | Prevalence n (%) | P Valuea | ||
|---|---|---|---|---|
| All (N=213) | Boys (n=163) | Girls (n=50) | ||
| Upper respiratory tract infection | 14 (6.6) | 9 (5.5) | 5 (10.0) | 0.326 |
| Renal disease | 10 (4.7) | 9 (5.5) | 1 (2.0) | 0.458 |
| Heart failure or cardiomyopathy | 5 (2.4) | 1 (0.6) | 4 (8.0) | 0.011 |
| Previous cardiac transplant | 5 (2.4) | 0 | 5 (10.0) | <0.001 |
| Lower respiratory tract infection | 4 (1.9) | 3 (1.8) | 1 (2.0) | 1.000 |
| Inflammatory bowel disease | 2 (0.9) | 1 (0.6) | 1 (2.0) | 0.415 |
| Juvenile rheumatoid arthritis or SLE | 2 (0.9) | 1 (0.6) | 1 (2.0) | 0.415 |
| Congenital heart disease | 2 (0.9) | 0 | 2 (4.0) | 0.054 |
| Stroke | 2 (0.9) | 1 (0.6) | 1 (2.0) | 0.415 |
| Resuscitation | 2 (0.9) | 0 | 2 (4.0) | 0.054 |
| Gastroenteritis | 2 (0.9) | 0 | 2 (4.0) | 0.054 |
| Diabetes mellitus | 2 (0.9) | 1 (0.6) | 1 (2.0) | 0.415 |
| Down syndrome | 1 (0.5) | 1 (0.6) | 0 | 1.000 |
| Prematurity | 1 (0.5) | 1 (0.6) | 0 | 1.000 |
| Allergic purpura | 1 (0.5) | 1 (0.6) | 0 | 1.000 |
| Grade III atrioventricular block | 1 (0.5) | 1 (0.6) | 0 | 1.000 |
| Sepsis | 1 (0.5) | 1 (0.6) | 0 | 1.000 |
| Asthma | 1 (0.5) | 1 (0.6) | 0 | 1.000 |
| Pulmonary embolism | 1 (0.5) | 1 (0.6) | 0 | 1.000 |
SLE indicates systemic lupus erythematosus.
Between sexes.
There was a pattern of seasonal variation in myocarditis admissions. The lowest rate of myocarditis admissions occurred during late summer, and the highest rates occurred in fall and early winter, with admissions in September to December accounting for >40% of all admissions (Figure 2).
Figure 2.
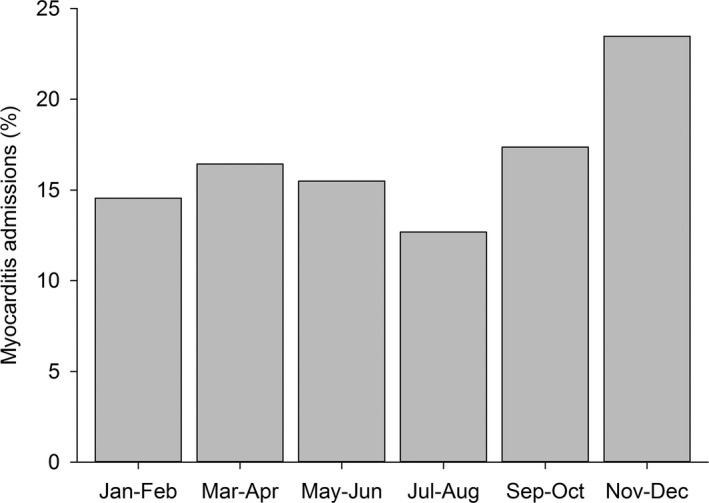
Seasonal variation in the admissions of childhood myocarditis in Finland, 2004–2014.
Incidence Rate
The total standardized incidence rate of myocarditis‐related admissions was 1.95 (95% CI, 1.69–2.24) per 100 000 person‐years (crude rate: 2.02; 95% CI, 1.76–2.31). The overall incidence rate remained roughly stable during the first 11 years of life but increased thereafter (Figure 3A). The standardized incidence rate among girls was 0.94 (95% CI, 0.70–1.25) per 100 000 person‐years. Among boys, the likelihood of myocarditis was notably higher, with an incidence rate of 2.92 (95% CI, 2.49–3.42) per 100 000 person‐years. If only 1 admission per year (n=187) for each patient was included, the standardized incidence rate was 1.71 (95% CI, 1.47–1.98) per 100 000 person‐years (crude rate: 1.77; 95% CI, 1.53–2.04) in the total study population, 0.74 (95% CI, 0.52–1.01) per 100 000 person‐years for girls, and 2.63 (95% CI, 2.22–3.10) per 100 000 person‐years for boys.
Figure 3.
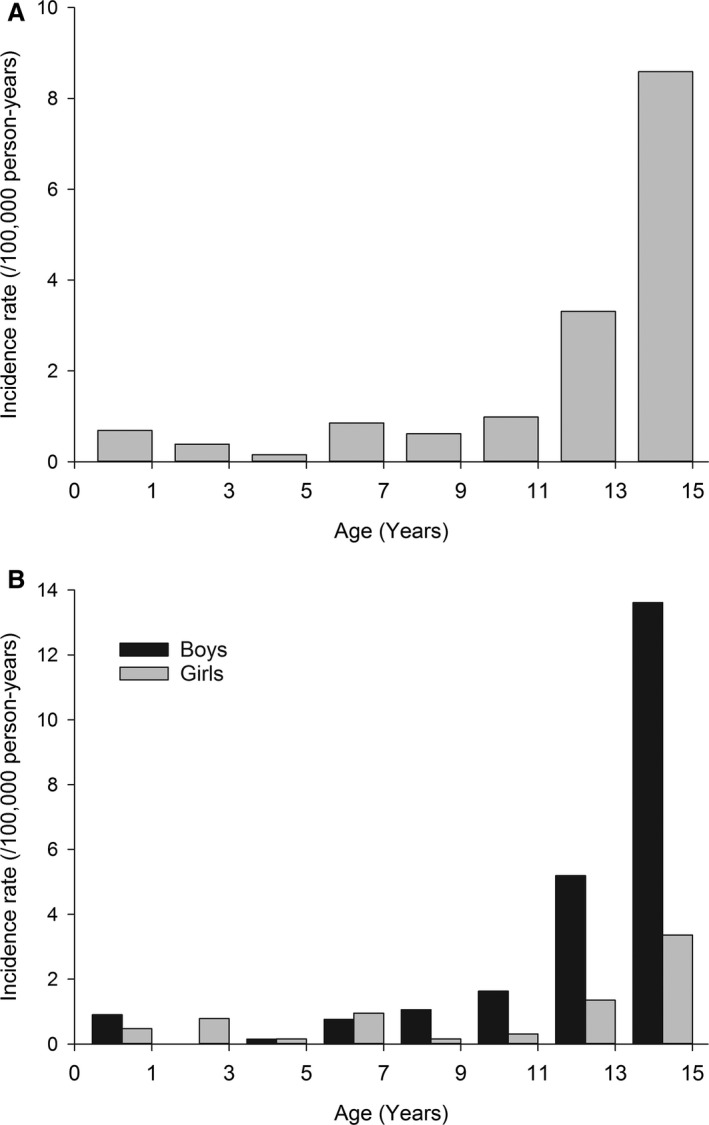
Incidence of myocarditis in a general pediatric population. Total (A) and sex‐specific (B) incidence rates (per 100 000 person‐years) by age.
Sex difference in incidence rate, however, was significantly age‐dependent (interaction P<0.0001; Figure 3B). Few cases of myocarditis occurred during the first year of life among boys (incidence rate: 1.8/100 000 person‐years). No myocarditis admissions occurred in the male population aged 1 to 3 years, but the incidence rate increased exponentially thereafter, with the peak in 15‐year‐old adolescent boys (incidence rate: 18.1/100 000 person‐years; Figure 3B). Incidence rate pattern was more stable among girls, with the rate of myocarditis having a less steeply increasing trend in adolescence (Figure 3B).
There was no sex difference in the incidence rate of myocarditis in children aged 0 to 5 years, but boys aged 6 to 10 years were significantly more likely to acquire myocarditis than girls (incidence rate ratio: 2.46; 95% CI, 1.03–5.89; P=0.043). Sex difference increased further in children and adolescents aged 11 to 15 years (incidence rate ratio: 3.89; 95% CI, 2.68–5.67; P<0.0001).
Adverse Outcomes
The in‐hospital mortality rate of myocarditis admission was 1.4% (n=3), and 1 patient (0.5%) received an urgent cardiac transplant. Three patients (1.4%) were treated with ventricular assist devices. Altogether, 13 patients (6.1%) were treated in intensive care units. Mortality among patients treated with intensive care and ventricular assist devices were 15.4% and 66.7%, respectively. No sex‐ or age‐related differences in outcomes or usage of ventricular assist device or intensive care treatments were noted, although small numbers precluded any statistical analyses.
Discussion
The diagnosis of myocarditis in children is challenging because symptoms and clinical findings are frequently nonspecific.2 Whereas teenagers may suffer from chest pain, palpitations, and rhythm disturbances, smaller children often present with respiratory or gastrointestinal symptoms and newborns with restlessness and poor feeding or symptoms resembling severe bacterial infection.3, 4, 5 Durani et al4 noted that the diagnosis of childhood myocarditis was made on the first presentation in only 17% of cases.
The present study is, to our knowledge, the first to report the overall incidence as well as age‐ and sex‐specific distribution of myocarditis in children and adolescents in a nationwide approach. The incidence rate in children aged 0 to 15 years was 1.95/100 000 person‐years. A Japanese study, based on questionnaires mailed to teaching hospitals, reported a much lower incidence of 0.26 case per 100 000 population for children and adolescents aged 1 month to 17 years,9 but no age‐ or sex‐specific occurrences were reported. The difference in incidences is best explained by study design, but differences in diagnostic criteria and genetic factors might also play roles. Another recent study reported myocarditis representing 0.05% of all pediatric hospital discharges from birth through age 21 years.10 This compares with the rate of 0.02% of all admissions in the present study. The difference is best explained by difference in age groups but probably also by the fact that surgical and adult ward admissions were included in the present study to ensure the completeness of data collection.
In the present study, 9 patients (4.2%) were <1 year of age at diagnosis. After infancy, the incidence remained stable until the early teenage years, with a remarkable increase in early adolescence and even more in 14‐ and 15‐year‐olds, representing almost 50% of all cases. The age distribution is similar to that reported in previous studies,3, 8 except for infants, who represented >20% of all cases in the study by Ghelani et al.8 This difference in incidence may be explained by differences in study design, diagnostic criteria and methods, and genetic background (ie, ethnicity) of the patients. Because the diagnosis of myocarditis is challenging, especially in infants, it is also possible that myocarditis was underdiagnosed in this age group in the present study. Because endomyocardial biopsy was infrequently obtained in infants and children,11 some patients with acute heart failure, poor systolic function, and dilation of the left ventricle were probably diagnosed as having idiopathic dilated cardiomyopathy.12, 13, 14 Moreover, during the study period, cardiac magnetic resonance imaging was used only rarely in the 5 university hospitals in Finland and not at all in central and small regional hospitals. Finally, some infants may have died before hospitalization. A previous retrospective study in Finland reported the incidence of fatal myocarditis being as high as 1.59/100 000 person‐years in infants aged <1 year.15
In both clinical and experimental studies, male sex has been shown to be a risk factor for myocarditis.16, 17, 18, 19 In a recent Finnish study of adult patients (aged ≥16 years) with myocarditis, 77% were men.7 Median age at diagnosis was 33 years, and as much as 47% of all patients were men aged ≤35 years.7 The occurrence of myocarditis was highest in men aged 16 to 20 years, with a linear decline with advancing age. Age distribution of female patients was much more stable, with the highest occurrence in postmenopausal women aged 55 to 60 years.7
In the present study, the incidence rate of myocarditis was higher in boys than in girls in most age groups, and 77% of all patients were boys. From age 12 years onward, the incidence rate increased remarkably, and was as high as 18.1/100 000 person‐years in male 15‐year‐olds. Girls in all age groups showed more stable incidence numbers, the finding being similar to that in a recently reported adult female population,7 although incidence also increased in teenage girls. Some previous studies have shown similar male preponderance,3, 8 but contradictory results have also been reported.4, 10, 20, 21, 22
The exact reason for the noticed sex difference is not known, but exercise and hormonal factors (eg, testosterone) have been shown to be important in experimental studies.6, 16 Conversely, estrogens have been shown to be protective from myocarditis.6, 23 The increase in incidence among teenage boys in the present study may be explained by increasing levels of testosterone. Similarly, the noticed increase in the occurrence of myocarditis in postmenopausal women 7 may be explained by the decreasing levels of estrogens in women aged 55 to 60 years. However, because testosterone levels remain quite stable until 40 to 50 years of age in men, other factors must explain the decrease in occurrence of myocarditis after age 20 years. In addition to sex hormone differences, complex genomic influences of sex chromosomes and additional yet unknown genetic variations might play roles in myocarditis susceptibility.6, 7
In the present study, the specific etiology of myocarditis remained unknown in most cases. Viral myocarditis was assumed to explain most cases, but other etiologies were also included, for example, streptococcal and mycoplasma infections as well as rheumatic fever and Kawasaki disease, the last 2 possibly representing myocardial damage by autoimmune mechanisms. Specific viral infections were recorded in only 11% of patients. The viruses were those most often associated with myocarditis—enterovirus, herpesvirus (Epstein–Barr virus), and influenza A virus2—however, the registry data do not show how extensively viral etiology was studied. Consequently, it is probable that not all viral infections were reported, explaining the low yield. In the study by Weber et al,14 virus was detected in 36% of autopsy cases in whom virological analysis was performed. Two other studies identified a potentially cardiotoxic virus in up to 43% of pediatric patients with myocarditis or new‐onset dilated cardiomyopathy.2, 12 Giant cell myocarditis and cardiac sarcoidosis are rarely detected in children, but they have to be kept in mind, especially in adolescent patients, because their treatment and outcomes differ from those of viral myocarditis.24
The prognosis of childhood myocarditis is variable, ranging from full recovery to death or cardiac transplant. Chronic cardiac dysfunction (ie, dilated cardiomyopathy) has been reported in 15% to 60% of cases.12, 13, 25 In earlier studies, survival rates have been 70% to 100%, with several factors such as age, diagnostic criteria, and patient selection affecting outcome.8, 20, 26 Patients requiring intensive care, extracorporeal membrane oxygenation, or ventricular assist devices have poorer outcomes.27, 28 In the present study, short‐term outcome of acute myocarditis was excellent, with only 1.4% in‐hospital mortality. Major complications, congestive heart failure, and significant arrhythmias were rare, and this was also reflected in the small numbers of patients treated in intensive care units and endomyocardial biopsies performed. The favorable in‐hospital outcome is best explained by the patient population in our study, including all patients with suspected and mild forms of myocarditis.
Our study is limited by its retrospective nature and the absence of detailed clinical and laboratory data. The diagnosis of myocarditis was made by treating physicians, and this may have had an effect on the included patient population and the accuracy of data on comorbidities and etiology. Moreover, the information provided in terms of incidence and outcomes refers to myocarditis that is severe enough to be clinically detectable. It is recommended that asymptomatic and mildly symptomatic pediatric patients with suspected myocarditis should be admitted to the hospital and monitored clinically until a definite diagnosis is established.1 Because even a suspicion of myocarditis results in a hospital admission for Finnish children, the patient population in the present study most probably represents all pediatric patients with clinically suspected myocarditis during the study period. Our study population, however, is not a comprehensive representation of the entire population; for example, those experiencing sudden death outside the hospital or those with asymptomatic myocardial damage were not reached. Conversely, it is impossible to estimate the incidence of asymptomatic myocarditis during common viral infections. Viruses that most often cause myocarditis are highly prevalent in the general population but cause myocarditis only rarely, indicating a yet‐unknown genetic predisposition in some individuals.24 Consequently, screening for myocarditis in children hospitalized for infections does not seem feasible.29
In conclusion, this study shows the age‐ and sex‐specific epidemiology, outcomes, and short‐term prognosis of childhood myocarditis in the general population. A high index of suspicion of myocarditis in a child with an acute illness is the cornerstone of diagnosis but can be challenging. The incidence of myocarditis increases with age, with adolescent boys being most susceptible. There appears to be no sex difference in the risk of myocarditis during the first 6 years of life, but boys aged 6 to 15 years have a higher risk than girls, and the risk difference increases with age.
Sources of Funding
The study was funded by the Finnish Cardiac Society and Clinical Research Foundation of Turku University Hospital.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005306 DOI: 10.1161/JAHA.116.005306.)29151030
References
- 1. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss H‐P, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PE. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- 2. Canter CE, Simpson KE. Diagnosis and treatment of myocarditis in children in the current era. Circulation. 2014;129:115–128. [DOI] [PubMed] [Google Scholar]
- 3. Freedman SB, Haladyn JK, Floh A, Kirsch JA, Taylor G, Thull‐Freedman J. Pediatric myocarditis. Emergency department clinical findings and diagnostic evaluation. Pediatrics. 2007;120:1278–1285. [DOI] [PubMed] [Google Scholar]
- 4. Durani Y, Egan M, Baffa J, Selbst SM, Nagar AL. Pediatric myocarditis: presenting clinical characteristics. Am J Emerg Med. 2009;27:942–947. [DOI] [PubMed] [Google Scholar]
- 5. Verma NA, Zheng XT, Harris MU, Cadichon SB, Melin‐Aldana H, Khetsuriani N, Oberste MS, Shulman ST. Outbreak of coxsackievirus B1 myocarditis in neonates. Clin Infect Dis. 2009;49:759–763. [DOI] [PubMed] [Google Scholar]
- 6. Fairweather D, Cooper LT, Blauwet LA. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol. 2013;38:7–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kytö V, Sipilä J, Rautava P. The effects of gender and age on occurrence of clinically suspected myocarditis in adulthood. Heart. 2013;99:1681–1684. [DOI] [PubMed] [Google Scholar]
- 8. Ghelani SJ, Spaeder MC, Pastor W, Spurney CF, Klugman D. Demographics, trends, and outcomes in pediatric acute myocarditis in the United States, 2006 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:622–627. [DOI] [PubMed] [Google Scholar]
- 9. Saji T, Matsuura H, Hasegawa K, Nishikawa T, Yamamoto E, Ohki H, Yasukochi S, Arakaki Y, Joo K, Nakazawa M. Comparison of the clinical presentation, treatment, and outcome of fulminant and acute myocarditis in children. Circ J. 2012;76:1222–1228. [DOI] [PubMed] [Google Scholar]
- 10. Klugman D, Berger JT, Sable CA, He J, Khandelwal SG, Slonim AD. Pediatric patients hospitalized with myocarditis: a multi‐institutional analysis. Pediatr Cardiol. 2010;31:222–228. [DOI] [PubMed] [Google Scholar]
- 11. Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Straling RC, Towbin J, Virmani R. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology: endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914–1931. [DOI] [PubMed] [Google Scholar]
- 12. Daubeney PE, Nugent AW, Chondros P, Carlin JB, Colan SD, Cheung M, Davis AM, Chow CW, Weintraub RG; National Australian Childhood Cardiomyopathy Study . Clinical features and outcomes of childhood dilated cardiomyopathy: results from a national population‐based study. Circulation. 2006;114:2671–2678. [DOI] [PubMed] [Google Scholar]
- 13. Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. [DOI] [PubMed] [Google Scholar]
- 14. Weber MA, Ashworth MT, Risdon RA, Malone M, Burch M, Sebire NJ. Clinicopathological features of paediatric death due to myocarditis: an autopsy series. Arch Dis Child. 2008;93:594–598. [DOI] [PubMed] [Google Scholar]
- 15. Kytö V, Saraste A, Voipio‐Pulkki L‐M, Saukko P. Incidence of fatal myocarditis. A population‐based study in Finland. Am J Epidemiol. 2007;165:570–574. [DOI] [PubMed] [Google Scholar]
- 16. Huber SA, Job LP, Auld KR. Influence of hormones on Coxsackie B‐3 virus infection in BALB/c mice. Cell Immunol. 1982;67:173–179. [DOI] [PubMed] [Google Scholar]
- 17. Mason JW, O'Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME, Moon TE. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–275. [DOI] [PubMed] [Google Scholar]
- 18. Magnani JW, Danik HJ, Dec GW, DiSalvo TG. Survival in biopsy‐proven myocarditis: a long‐term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J. 2006;151:463–470. [DOI] [PubMed] [Google Scholar]
- 19. Grun S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert E‐M, Hill S, Ong P, Klingel K, Kandolf R, Sechtem U, Mahrholdt H. Long‐term follow‐up of biopsy‐proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–1615. [DOI] [PubMed] [Google Scholar]
- 20. Lee KJ, McGrindle BW, Bohn DJ, Wilson GJ, Taylor GP, Freedom RM, Smallhorn JF, Benson LN. Clinical outcomes of acute myocarditis in childhood. Heart. 1999;82:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foerster SR, Canter CE, Cinar A, Sleeper LA, Webber SA, Pahl E, Kantor PF, Alvarez JA, Colan SD, Jeffries JL, Lamour JM, Margossian R, Messere JE, Rusconi PG, Shaddy RE, Towbin JA, Wilkinson JD, Lipschultz SE. Ventricular remodeling and survival are more favorable for myocarditis than for idiopathic dilated cardiomyopathy in childhood. An outcomes study from the pediatric cardiomyopathy registry. Circ Heart Fail. 2010;3:689–697. [DOI] [PubMed] [Google Scholar]
- 22. Shu‐Ling C, Bautista D, Ching Kit C, Su‐Yin AA. Diagnostic evaluation of pediatric myocarditis in the emergency department. A 10‐year case series in the Asian population. Pediatr Emerg Care. 2013;29:346–351. [DOI] [PubMed] [Google Scholar]
- 23. Li Z, Yue Y, Xiong S. Distinct Th17 inductions contribute to the gender bias in CVB3‐induced myocarditis. Cardiovasc Pathol. 2013;22:373–382. [DOI] [PubMed] [Google Scholar]
- 24. Caforio ALP, Marcolongo R, Basso C, Iliceto S. Clinical presentation and diagnosis of myocarditis. Heart. 2015;101:1332–1344. [DOI] [PubMed] [Google Scholar]
- 25. Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss H‐P, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA. Detection of viruses in myocardial tissues by polymerase chain reaction: evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. [DOI] [PubMed] [Google Scholar]
- 26. Abe T, Tsuda E, Miyazaki A, Ishibashi‐Ueda H, Yamada O. Clinical characteristics and long‐term outcome of acute myocarditis in children. Heart Vessels. 2013;28:632–638. [DOI] [PubMed] [Google Scholar]
- 27. Rajagopal SK, Almond CS, Laussen PC, Rycus PT, Wypij D, Thiagarajan RR. Extracorporeal membrane oxygenation for the support of infants, children, and young adults with acute myocarditis: the review of the Extracorporeal Life Support Organization Registry. Crit Care Med. 2010;38:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Almond CS, Morales DL, Blackstone EH, Turrentine MW, Imamura M, Massicotte P, Jordan LC, Devaney EJ, Ravishankar C, Kanter KR, Holman W, Kroslowitz R, Tjossem C, Thuita L, Cohen GA, Buchholz H, St Louis JD, Nguyen K, Niebler RA, Walters HL III, Reemtsen B, Wearden PD, Reinhartz O, Guleserian KJ, Mitchell MB, Bleiweis MS, Canter CE, Humpl T. Berlin Heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US children. Circulation. 2013; 127:1702–1711. [DOI] [PubMed] [Google Scholar]
- 29. Renko M, Leskinen M, Kontiokari T, Tapiainen T, Uhari M. Cardiac troponin‐I as a screening tool for myocarditis in children hospitalized for viral infection. Acta Paediatr. 2010;99:283–285. [DOI] [PubMed] [Google Scholar]


