Abstract
Background
Nitrate is a dietary component as well as an endogenously formed metabolite and source of the signaling molecule nitric oxide. Harmful as well as beneficial effects of nitrate have been advocated. Data regarding the prognostic relevance of plasma nitrate are limited. The aim of this study was to evaluate the prospective association of plasma nitrate with cardiovascular disease (CVD) and all‐cause mortality.
Methods and Results
We assayed plasma nitrate in 2855 Framingham Offspring Study participants (mean age 59 years, 54% women) by gas chromatography–mass spectrometry and evaluated its association with all‐cause mortality and incident CVD. On follow‐up (median 17.3 years), 775 participants died and 522 developed new‐onset CVD (of 2546 participants free of CVD at baseline). In multivariable models adjusting for standard risk factors, plasma nitrate was associated with an increased risk of death in participants (hazard ratio per unit increase in log‐nitrate 1.21; 95% confidence interval, 1.04–1.40 [P=0.015]). The strength of the association was attenuated by additional adjustment for estimated glomerular filtration rate (hazard ratio, 1.16; 95% confidence interval, 1.00–1.35 [P=0.057]). In contrast, no evidence was found for an association of plasma nitrate with incident CVD (multivariable‐adjusted hazard ratio per unit increase log‐nitrate 1.08; 95% confidence interval, 0.89–1.31 [P=0.42]).
Conclusions
In our prospective community‐based investigation, a higher plasma nitrate concentration was associated with all‐cause mortality but not with incident CVD. The association of nitrate with mortality may at least in part be attributable to its association with renal function.
Keywords: mortality, myocardial infarction, nitrate, population studies, risk prediction
Subject Categories: Biomarkers, Epidemiology, Cardiovascular Disease, Endothelium/Vascular Type/Nitric Oxide, Mortality/Survival
Clinical Perspective
What Is New?
Major cardiovascular risk factors including smoking, diabetes mellitus, and reduced renal function are correlated with higher plasma nitrate levels.
Higher plasma nitrate is associated with an increased risk of death.
The observed association of nitrate and mortality may at least in part be attributable to the inverse correlation of plasma nitrate with renal function.
Elevated plasma nitrate concentrations are not associated with the incidence of cardiovascular disease events.
What Are the Clinical Implications?
Considering its complex association with other risk factors, nitrate may be a risk marker rather than a cause of mortality.
The clinical setting as well as the major source(s) and cause(s) of elevated plasma nitrate have to be considered when assessing the association of nitrate and clinical outcome.
Associations of fasting plasma nitrate with clinical outcome should not be confused with clinical effects of nitrate supplements.
Only long‐term randomized double‐blind placebo‐controlled nitrate supplementation studies will be able to settle the issue of causality as well as the harms and benefits of nitrate supplementation.
Introduction
Nitrate in human plasma stems from two major sources: endogenous nitric oxide (NO) signaling and diet.1, 2 In vivo NO synthesized from l‐arginine by NO synthases (NOS) is rapidly degraded (via nitrite) to nitrate.3 A simplified overview of the interrelation of NO and nitrate metabolism is provided in Figure 1. Over the past decades, the intensified use of artificial fertilizers and disposal of nitrate‐rich dung from animal farming have been associated with a progressive increase in the dietary intake of nitrate in many parts of the world.4, 5 The generation of nitrite and nitrate from NO has long been viewed as irreversible in mammals, but this view has been challenged by several independent studies demonstrating a significant (re‐) conversion of nitrate to nitrite, and then to NO in animals and in humans.6, 7, 8 Consequently, there has been a long‐standing interest in the potential impact of nitrate on human health. Several reports have linked nitrate both to beneficial and to adverse effects on human health, ranging from the initial report on potentially beneficial cardiovascular effects of nitrate dating back to ancient China9 to subsequent investigations emerging in the 1970s that linked high nitrate consumption with a greater risk of cancer.5, 10 Whereas a causal role for nitrate in cancer remains uncertain,5, 11 recent studies indicating possible beneficial cardiovascular effects rekindled interest in nitrate as a marker and potential mediator of cardiovascular risk and function.7, 12, 13
Figure 1.
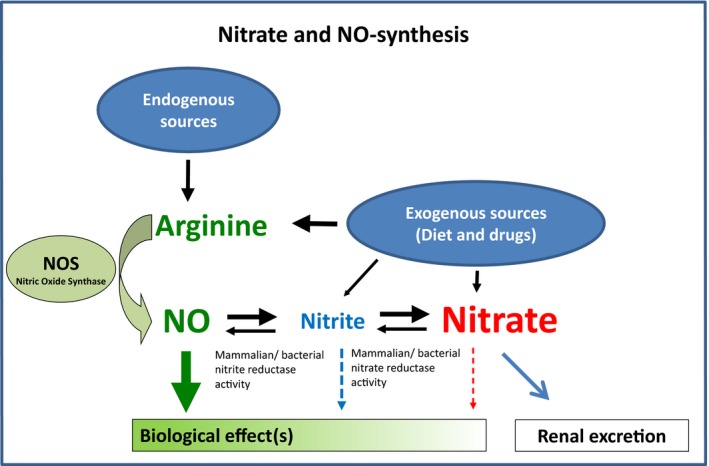
Interrelation of nitrate, nitrite, and the l‐arginine‐nitric oxide (NO) pathway. The plasma nitrate and nitrite concentration depends on dietary uptake as well as endogenous formation of nitrite and nitrate as metabolites of nitric oxide (NO). A significant (re‐) conversion from nitrate to nitrite may occur during the entero‐salivary circulation of nitrate. Nitrite may be endogenously reconverted to NO by tissues nitrite/nitrate reductase activity.
In this regard, more recent reports have underscored a possible cardioprotective effect of nitrate11, 13 and official recommendations to limit nitrate intake have been called into question.1 However, prior reports have largely been based on experimental data or short‐term clinical observations. In humans on a regular diet, plasma nitrate represents a global measure of exposure to nitrate from endogenous and exogenous sources. To our knowledge, there are no prior reports relating circulating nitrate levels to health outcomes in the general population.5, 10, 12, 14
Accordingly, we prospectively investigated the associations of plasma nitrate with incident cardiovascular disease (CVD) and all‐cause mortality in a large community‐based sample. We hypothesized that higher plasma nitrate levels are associated with a decreased risk of CVD and all‐cause mortality.
Methods
Study Sample
The sixth examination cycle of the Framingham Offspring Study (1995–1998) was attended by 3532 participants15 and served as the baseline examination for the present investigation. Overall, 677 individuals were excluded from the survival analysis for the following reasons: serum creatinine >2.0 mg/dL (n=15), unavailable plasma nitrate (n=405), and missing covariates and outliers (n=257). After this, 2855 participants remained eligible and comprised our study sample for mortality analysis. For the analysis of incident CVD, we excluded 309 participants because of prevalent CVD. As previously described,16 all participants provided a medical history and underwent a physical examination and laboratory assessment of cardiovascular risk factors.
The institutional review board of the Boston University Medical Center and the ethics committee of the University of Erlangen‐Nuremberg approved the study protocol. All participants provided written informed consent.
Determination of Nitrate
Nitrate was determined in plasma samples obtained from fasting participants at the sixth examination cycle using a gas chromatography–mass spectrometry based on a method previously described.17, 18, 19
Chemicals and materials
Sodium nitrate, sodium 15N‐nitrate, and pentafluorobenzyl bromide were purchased from Sigma Aldrich (Steinheim) each at the highest purity available. Acetone (GC grade), toluene (GC grade), and water were purchased from Merck (Darmstadt). Bovine serum was purchased from Fiebig Animalblood Products (Idstein‐Niederauroff).
Standard preparation
Stock solution was prepared by dissolving 68 mg of sodium nitrate in 100 mL of water (8 mmol/L). The calibration levels were prepared by spiking of bovine serum with different volumes of the stock solution to achieve final concentrations of 8 to 80 μmol/L. The stock solution of the internal standard was prepared by dissolving 34.4 mg of sodium 15N‐nitrate in 50 mL of water (8 mmol/L). Two‐hundred fifty microliters of the stock solution was diluted in 25 mL of water to obtain a working solution of the internal standard of 80 μmol/L.
Calibration procedure
The calibration was performed using 6 calibration levels. Additionally, identical bovine serum was used as a blank sample and was included in each analytical series. Linear calibration curves were obtained by plotting the quotients of the analyte's peak areas to the peak areas of the labeled internal standards as a function of spiked concentration.
Sample preparation
Plasma samples were thawed, equilibrated to room temperature, and vortex mixed. For analysis, an aliquot of 10 μL of plasma and 10 μL of the internal standard working solution were transferred into a 1.8‐mL glass vial and were vortex mixed. Two‐hundred fifty microliters of acetone were added to the mixture and vortex mixing was performed immediately. Three microliters of pentafluorobenzyl bromide were added to the mixture and the vial was subsequently sealed and vortex mixed. Then the sealed vials were incubated at 50°C for 1 hour. Subsequently, the reaction mixture was concentrated under a gentle stream of nitrogen to dryness. Toluene (600 μL) was added to the residue and resolution was performed by vortex mixing. The samples were kept at a temperature of 4°C and centrifuged for 5 minutes at 400g. Two‐hundred microliters of the supernatant were transferred into a 0.2‐mL GC microvial for gas chromatography–mass spectrometry analysis.
Gas chromatography–mass spectrometry
Analytical determination was performed on gas chromatography–mass spectrometry, consisting of an Agilent 7890A gas chromatograph equipped with a split/splitless injector and an Agilent 5975 C quadrupole mass spectrometer with chemical ionization source installed (Agilent Technologies). A sample volume of 1 μL was injected into the inlet assembly in splitless mode with purge flow to vent after 1 minute and an injector temperature of 200°C. For gas chromatographic separation, a 14% cyanopropylphenyl polysiloxane low bleed capillary column (VF 1701 ms, 60 m×250 μm×0.25 μm) (Varian Inc) was used at a constant flow of 1.2 mL/min helium carrier gas. The initial column temperature of 85°C was held for 12 minutes, then raised at a rate of 25°C/min to 160°C (ramp time 1 minute), then raised at a rate of 10°C/min to 200°C and subsequently raised at a rate of 30°C/min to 270°C, remaining at this level for 1 minute. The temperature of the transfer line was set to 280°C. The ion source was operating at −70 eV, at a temperature of 150°C. The mass selective detector was adjusted to 150°C quadrupole temperature. Ionization was performed in the negative chemical ion using methane as reaction gas (flow rate ratio 40%). For detection, the mass spectrometer was working in the selected ion monitoring mode. For the detection of the nitrate derivative, the ion of 62.1 m/z was detected (retention time: 7.04 minutes). The monitoring of the internal standard was conducted at 63.1 m/z (retention time: 7.05 minutes).
Quality control
Quality‐control materials were prepared by spiking bovine serum with sodium nitrate to achieve a nitrate concentration of 49 μmol/L. The quality‐control material was divided into aliquots of 100 μL and stored at −18°C. Four quality‐control samples and 4 blank values were included in each analytical series. From the quality‐control results of 19 series, a coefficient of variation of 6.23% was estimated for the precision between series.
Clinical Outcomes
All participants were followed for incident major CVD events and all‐cause mortality. CVD was defined as presence or history of coronary heart disease (myocardial infarction, coronary insufficiency, angina pectoris), peripheral vascular disease (intermittent claudication), cerebrovascular disease (stroke or transient ischemic attack), or heart failure. As previously described, major CVD events included fatal or nonfatal coronary heart disease, stroke or transient ischemic attack, intermittent claudication, or heart failure.20
Statistical Analysis
Since the distribution of plasma nitrate values was right skewed, values were natural logarithmically transformed to normalize the distribution. We used multivariable linear regression to evaluate the cross‐sectional clinical correlates of plasma nitrate. The candidate variables included in this analysis were age, sex, body mass index (BMI), systolic and diastolic blood pressure, hypertension treatment, ratio of total to high‐density lipoprotein cholesterol, glomerular filtration rate, serum triglycerides, fasting blood glucose, diabetes mellitus status, smoking status, CRP (C‐reactive protein), and alcohol consumption. We used the Chronic Kidney Disease Epidemiology Collaboration equation to calculate the estimated glomerular filtration rate (eGFR).21
We confirmed that the assumption of proportionality of hazards for both outcomes (major CVD and mortality) was not violated and then used Cox proportional hazards models to relate plasma nitrate concentrations to the incidence of CVD events and to death (both outcomes considered separately) and adjusted for age, sex, BMI, systolic blood pressure, antihypertensive medication, smoking, diabetes mellitus, ratio of total cholesterol to high‐density lipoprotein cholesterol, heart rate, and high alcohol consumption. Analysis of all‐cause mortality additionally adjusted for prevalent CVD, CRP, and eGFR. We also created tertiles of nitrate and used Kaplan–Meier plots to graphically present the survival time for each tertile. Data are given as mean+standard deviation unless otherwise indicated. P<0.05 was considered statistically significant.
Results
The baseline characteristics of our study samples are presented in Table 1. Our analyses included middle‐aged to older participants with a BMI in the overweight range.
Table 1.
Baseline Characteristics of Study Participants—All‐Cause Mortality Analysis Set
| All | Lowest Nitrate Tertile | Intermediate Nitrate Tertile | Highest Nitrate Tertile | No Prevalent CVD Group | |
|---|---|---|---|---|---|
| No. of participants | 2855 | 949 | 952 | 954 | 2546 |
| Nitrate, μmol/L, median (25th–75th percentile) | 38.4 (29.3–53.0) | 25.8 (22.1–29.3) | 38.3 (34.9–42.3) | 60.9 (53.0–76.9) | 37.9 (29.0–52.3) |
| Ln‐nitrate, μmol/L | 3.7±0.47 | 3.2±0.2 | 3.6±0.1 | 4.2±0.3 | 3.7±0.5 |
| Age, y | 59±10 | 58±10 | 59±10 | 60±10 | 58±10 |
| Men, No. (%) | 1315 (46.0) | 437 (46.5) | 446 (46.9) | 432 (45.3) | 1118 (43.9) |
| BMI, kg/m² | 27.9±5.2 | 28.0±5.5 | 28.0±5.0 | 27.7±5.1 | 27.8±5.2 |
| BP, mm Hg | |||||
| Systolic | 128±19 | 127±19 | 129±19 | 130±19 | 128±19 |
| Diastolic | 75±9 | 75±9 | 75±10 | 75±10 | 75±9 |
| Antihypertensive medication, No. (%) | 799 (28.0) | 204 (21.5) | 263 (27.63) | 332 (34.8) | 614 (24.1) |
| Smokers, No. (%) | 406 (14.2) | 91 (9.6) | 153 (16.1) | 162 (17.0) | 358 (14.1) |
| Diabetes mellitus, No. (%) | 319 (11.2) | 72 (7.6) | 107 (11.2) | 140 (14.7) | 235 (9.2) |
| Prevalent CVD, No. (%) | 309 (10.8) | 75 (7.9) | 100 (10.5) | 134 (14.5) | Excluded |
| High alcohol consumptiona | 409 (14.3) | 116 (12.2) | 134 (14.1) | 159 (16.7) | 366 (14.4) |
| C‐reactive protein, mg/dL, median (25th–75th percentile) | 2.01 (0.92–4.62) | 1.83 (0.79–4.26) | 2.11 (0.93–4.69) | 2.13 (1.02–4.85) | 1.95 (0.88–4.48) |
| eGFR, mL/min per 1.73 m2 | 70.9±14.1 | 73.3±13.3 | 71.1±14.4 | 68.4±14.3 | 71.4±13.8 |
Values are expressed as mean+standard deviation unless otherwise indicated. BMI indicates body mass index; BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate.
More than 14 drinks per week for men and >7 drinks per week for women.
Cross‐Sectional Correlates of Plasma Nitrate
As shown in Table 2, plasma nitrate was positively related to smoking, antihypertensive medication, high alcohol consumption, and diabetes mellitus, whereas it was inversely related to eGFR. These clinical variables explained 4.2% of the interindividual variation of plasma nitrate. Of note, BMI and CRP were not statistically significantly associated with plasma nitrate concentrations.
Table 2.
Cross‐Sectional Correlates (Multivariable Linear Regression) of Log‐Plasma Nitrate
| Parameter Estimate | Standard Error | P Value | |
|---|---|---|---|
| eGFR, mL/min | −0.005 | 0.00067 | <0.0001 |
| Smoking | 0.134 | 0.02625 | <0.0001 |
| Antihypertensive drug use | 0.075 | 0.02194 | 0.0006 |
| High alcohol consumptiona | 0.082 | 0.02582 | 0.0016 |
| Diabetes mellitus | 0.079 | 0.03196 | 0.0140 |
Cross‐sectional correlates of natural‐logarithmically transformed nitrate in 2546 individuals free of cardiovascular disease. Reported are variables that were retained as significant in the multivariable linear regression model. Candidate variables were: age, sex, body mass index, systolic and diastolic blood pressure, hypertension treatment, ratio of total to high‐density lipoprotein cholesterol, estimated glomerular filtration rate (eGFR), serum triglycerides, fasting blood glucose, diabetes mellitus, smoking status, C‐reactive protein, and alcohol consumption. All variables are dichotomous except for eGFR (continuous). R 2 of the final model was 0.042.
More than 14 drinks per week for men and >7 drinks per week for women.
Relationship of Plasma Nitrate to All‐Cause Mortality
During a median follow‐up of 17.3 years, 775 of 2855 participants died (including 332 women). The unadjusted cumulative incidence of all‐cause mortality increased by tertile of plasma nitrate (Figure 2). Baseline characteristics for tertiles of plasma nitrate are shown in Table 1. In a Cox regression model adjusting for standard risk factors, elevation of plasma nitrate was associated with an increased risk of death (Table 3). Additional inclusion of eGFR as a continuous variable into the Cox model reduced the association of plasma nitrate and mortality to borderline significance (hazard ratio, 1.16; 95% confidence interval, 1.0–1.35 [P=0.057]).
Figure 2.
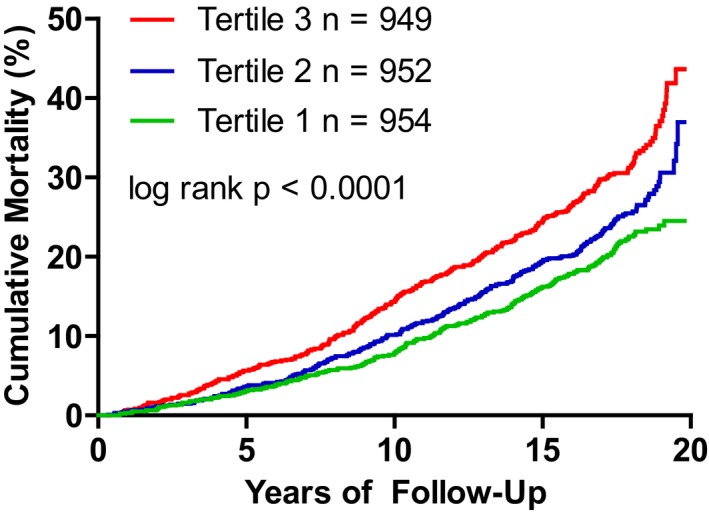
Kaplan–Meier plot for the relationship between tertile of plasma nitrate and survival (tertile 1=low, tertile 2=intermediate, and tertile 3=high plasma nitrate).
Table 3.
Plasma Nitrate and Risk of Death and CVD
| Outcome | No. of Events/No. at Risk | Age and Sex Adjusted Hazards Ratio (95% CI) Per 1‐Unit Increase in Ln‐Nitrate | P Value | Fully Adjusteda Hazards Ratio (95% CI) Per 1‐Unit Increase in Ln‐Nitrate | P Value |
|---|---|---|---|---|---|
| Incident CVD | 522/2546 | 1.13 (0.95–1.36) | 0.18 | 1.08 (0.89–1.31) | 0.42 |
| All‐cause mortality | 775/2855 | 1.32 (1.14–1.52) | 0.0002 | 1.21 (1.04–1.40) | 0.015 |
Models for all‐cause mortality were further adjusted for history of cardiovascular disease (CVD). CI indicates confidence interval.
Adjusted for age, sex, body mass index, systolic blood pressure, antihypertensive medication, smoking, diabetes mellitus, total to high‐density lipoprotein cholesterol ratio, heart rate, and high alcohol consumption (>14 drinks per week for men and >7 drinks per week for women), and C‐reactive protein.
Relationship of Plasma Nitrate to CVD Incidence
Of 2546 participants free of CVD at baseline, 522 developed incident CVD (258 in women) during a median follow‐up of 16.5 years. Adjusting for major cardiovascular risk factors, we did not observe any statistically significant association between plasma nitrate and incident CVD (Table 3).
Discussion
To our knowledge, the present investigation provides the first large‐scale and long‐term prospective analysis relating plasma nitrate (assayed with a highly sensitive method) to CVD incidence and mortality in the community. Our principal findings in a community‐based sample of elderly and overweight participants are 4‐fold:
First, major cardiovascular risk factors including smoking, diabetes mellitus, and reduced renal function are correlated with higher plasma nitrate levels.
Second, higher plasma nitrate is associated with increased risk of death in the general population.
Third, the observed association of nitrate and mortality may at least in part be attributable to the inverse correlation of plasma nitrate with renal function.21
Fourth, elevated plasma nitrate concentrations are not associated with the incidence of CVD events in a statistical model that includes the main risk factors.
Factors Correlating With Plasma Nitrate
Plasma nitrate is frequently considered a measure of the individual overall exposure to nitrate derived from various individual (patho‐) physiological pathways and exogenous (ie, dietary) sources, as well as the individual capacity to eliminate nitrate.2, 22, 23 Dietary intake of nitrate in the United States is commonly estimated to range between 40 and 100 mg/d, but may exceed 1200 mg with specific diets or dietary supplements.24, 25 High doses of nitrate may be required to significantly increase the plasma nitrate concentration. In adults, a low (≈35 mg/d of nitrate for a person of 70 kg) or high (≈133 mg/d of nitrate for a person of 70 kg) nitrate diet induced no clinically relevant fluctuations or differences in the plasma nitrate concentration.25 However, specific nitrate‐enriched diets or intake of dietary supplements corresponding to nitrate doses above 300 mg may lead to significant fluctuations and increases in plasma nitrate, with peak concentrations reached after ≈1 to 3.0 hours.7, 25, 26 Urinary nitrate excretion usually exceeds dietary intake, which indicates a significant endogenous nitrate formation rate.27 NO synthesis has been identified as the major endogenous source of nitrite and nitrate.28, 29, 30, 31, 32 However, NO is highly reactive and has a short half‐life in vivo, which precludes its measurement outside of experimental settings. Free NO reacts within seconds to form nitrite (NO2 −), which, in turn, rapidly reacts to form nitrate (N03 −).19, 28, 31 This may explain why the plasma nitrate concentration is several orders of magnitude above the nitrite concentration.8, 17 Therefore, plasma nitrate and/or nitrite are frequently used as surrogates for endogenous NO synthesis and metabolism in clinical studies,18, 31 although a significant contribution to circulating levels from dietary sources has been acknowledged.1, 2, 8, 22, 23, 27 Nitrate metabolism by the oral and gastrointestinal microbiome also has to be considered.8, 27, 33 In clinical studies, the major part of the oral and gastric microbial nitrate metabolism to nitrite, and possibly NO, was observed shortly after ingestion.8, 27 However, despite a steep rise in plasma nitrite, the effects in absolute terms on plasma nitrate are limited.7, 8 It appears that the nitrite is either rapidly reconverted back to nitrate or only a small fraction of the ingested or salivary nitrate is actually converted to nitrite.
Endothelial NOS, inducible NOS, and neuronal NOS all contribute to endogenous NO synthesis. Under physiological conditions, the major proportion of endogenous NO generation is generally attributed to endothelial NOS. Based on the highly sensitive 18oxygen inhalation method, the rate of total endogenous NO synthesis in men is 0.6 to 0.7 mmol (corresponding to 37–43 mg nitrate)/24 h (≈0.4 μmol/kg per hour).30 The endogenous NO synthesis may increase substantially under several conditions such as exercise or high‐altitude hypoxia.34, 35 Experimental data suggest that the plasma nitrite concentration may provide a more specific measure of acute endogenous NO signaling than nitrate.36 However, with respect to sampling issues, plasma nitrate can be considered much more stable (relative to nitrite) with its longer half‐life and its 20‐ to 100‐fold higher plasma concentration.19
In humans on an unrestricted diet, the dietary intake accounts for about 25% to 50% of fasting plasma nitrate.27, 37 The extent to which the plasma nitrate concentration represents exogenous (diet) versus endogenous (NO synthesis) sources may also depend on the timing of blood sampling. The half‐life of ingested nitrate is estimated to be about 5 hours1, 26; therefore, plasma nitrate levels measured after an overnight fast in individuals without overt renal failure should represent the trough levels of systemic exposure to dietary nitrate.38 Supplementation studies with stable isotope labeled nitrate indicate that renal elimination accounts for 55% to 60% of total nitrate clearance, up to 35% appear to be metabolized, and a small fraction is excreted with the feces.27, 39, 40 An inverse relationship of plasma NOx and nitrate levels and renal function has previously been noted39, 41 and was confirmed in several studies performed under controlled dietary nitrate intake in patients with different degrees of chronic renal impairment.42, 43, 44 In these studies, patients with renal impairment had elevated plasma NOx levels. These data are not unchallenged as some other studies report that NOx remains unchanged or even declines in renal failure.45, 46 Moreover, net endogenous NOx generation may actually be lower in renal failure.44 Taken together, these partly conflicting observations indicate that the relationship of nitrate on renal failure may be complex.
As a small molecule nitrate is freely filtered at the glomeruli, but a large proportion of it appears to be reabsorbed by the renal tubuli,47 resulting in an observed renal clearance of ≈20 mL/min healthy humans.48
Sepsis is frequently cited as an example for a state of enhanced endogenous NO synthesis attributable to induction of iNOS in an inflammatory state.49, 50 Stable isotope tracer studies indicate, however, that the observed rise in plasma NOx in patients with hypotensive sepsis may be largely attributable to an impairment of renal function.51 With respect to the observed cross‐sectional correlations of plasma nitrate and common cardiovascular risk markers, the associations reported in the literature have been inconsistent. Different stages of disease or duration of exposure may offer a simple explanation. The methods that have been used to determine nitrate levels and/or NOx concentrations also have to be considered.52, 53, 54 Frequently, investigators measured NOx based on the Griess method, which may somewhat limit the comparability with results that are based on assaying plasma nitrate by mass spectrometry.
Furthermore, in the literature, correlates of plasma nitrate are frequently provided without further adjustments. In contrast, we present only variables that were retained as significant in the multivariable linear regression model. Cigarette smoke contains NO(x), thus an increase in plasma nitrate would be in line with experimental data,55 yet some previous studies reported no or even an inverse correlation of smoking and plasma nitrate.52
Consistent with our findings, however, previous studies noted an association of higher plasma nitrate levels with a greater prevalence of standard cardiovascular risk factors.56 The present data are also compatible with studies indicating an increased NO production after alcohol consumption.57 In contrast, we observed no significant correlation of plasma nitrate and CRP. This does not preclude an association of nitrate and inflammation in general. The contribution of inflammation and inducible NOS‐mediated NO synthesis to plasma nitrate may simply require more extreme clinical conditions, such as sepsis, to become relevant. However, extreme forms of inflammation may differ in their effects on NO synthesis from inflammation observed in the context of other diseases diverse with chronic but lower level inflammation such as rheumatism, chronic viral infections or inflammatory bowel disease.
Plasma Nitrate and Health Outcomes
Prior studies evaluating long‐term nitrate‐related health outcomes in the general population mainly focused on cancer and typically did not measure circulating plasma nitrate concentrations but rather calculated the individual nitrate exposure based on diet questionnaires.5, 10, 14, 24 Yet, after many years of research, there is no unequivocal evidence of a causal association between dietary nitrate exposure and cancer risk, as a key concern of earlier studies.10, 11, 13 In contrast, based on recent data from animal experiments13, 58 and short‐term clinical studies showing beneficial cardiovascular effects of nitrate supplementation,7, 12, 59, 60 opinions have turned in the opposite direction questioning the official recommendations and efforts to limit oral nitrate intake.11, 58, 61 Based on our data, we would like to underscore the need for caution on both sides of this debate, especially when considering that conclusive data regarding the long‐term impact of pharmacological use of inorganic or organic nitrates (ie, NO donors in general) on health outcomes in the general population are still lacking.62
We looked at the long‐term prognosis associated with endogenous levels of nitrate in relation to hard clinical end points, which is different from benefits of short‐term nitrate supplementation in interventional studies on surrogate outcomes. Still, the observed lack of association of plasma nitrate with CVD outcome in the general population was not necessarily to be expected based on the mostly positive data from the nitrate supplementation studies.1, 7, 12, 13, 58, 63 However, the only other population‐based study that provided data on cardiovascular end points also observed no association of NOx and future cardiovascular events.52 But that study relied on NOx as determined by the Griess method and the authors concede that the analysis was underpowered (995 men with 15 events during follow‐up) to establish or exclude an association of NOx and CVD. The lack of association of plasma nitrate and incident CVD observed in our analysis is actually also compatible with the lack of geographical association that becomes evident when comparing the respective maps of environmental nitrate exposure and CVD mortality in the United States.64, 65 Moreover, considering the absence of a direct association of nitrate with CVD, nitrate is more likely a marker than a mediator of clinical outcomes, including mortality. In this respect, the associations of plasma nitrate with numerous other diseases and functionally active biomarkers or the composition of the individual (gut) microbiome remains to be elucidated. It should also be noted that the association of plasma nitrate and mortality observed in the present analysis might at least in part be attributable to its direct association with eGFR (ie, renal function) because adjustment for the latter attenuated the association with mortality. However, it is conceivable that incorporating eGFR into the multivariable model may represent an overadjustment, given the close correlation of plasma nitrate and eGFR. It is still possible that elevated plasma nitrate may be along the causal path from a lower eGFR to greater mortality hazard. Moreover, if the association of nitrate and mortality is primarily attributable to nitrate acting as a marker of renal function, one would expect to observe a similar association of plasma nitrate and CVD events.
With respect to health outcomes, it is likely that the mechanism leading to an increase of plasma nitrate (septic inflammation, physical exercise, ample ingestion of green vegetables, pharmacological nitrate donors, cigarette smoking, or impaired renal function) is important. In other words, elevation of plasma nitrate as such has to be distinguished from elevation of nitrate by specific supplements or diets. Recent data from 536 969 participants in the NIH‐AARP Diet and Health Study indicate that the specific dietary source of nitrate could indeed be important, as a high dietary intake of nitrate from processed meat was associated with all‐cause mortality.66 However, that study does not permit conclusions regarding other sources of nitrate, as nitrate intake from processed meat made up only a fraction of the total dietary nitrate intake from other sources. Only long‐term randomized double‐blind placebo‐controlled nitrate supplementation studies will be able to settle the issue of causality as well as harms and benefits of nitrate supplementation.
Strengths and Limitations
It is a major strength of our investigation that we measured plasma nitrate concentration using a highly specific mass spectrometric method in a well‐characterized community‐based sample with long longitudinal follow‐up. A sample of elderly participants with an elevated BMI does not represent the whole US population. The associations of nitrate with outcome and risk markers may significantly differ in younger or less overweight persons in the US population, especially in those with different dietary habits such as vegetarians. Different dietary habits represent diverse sources of nitrate and may result in different cardiovascular risk and mortality profiles. Participants with significant impairment of renal function were excluded (based on a serum creatinine threshold of 2 mg/dL) from our investigation because we focused on correlates of plasma nitrate in the general population. While it may be tempting, the present assessment of fasting plasma nitrate does not permit any inference regarding the harms and benefits of nitrate supplementation. Furthermore, by its design, the present study does not allow causal inferences based on the associations observed. Additional investigations are required to differentiate the relative contributions of protective (endothelial NO synthesis) and detrimental (smoking and renal impairment) factors to circulating plasma nitrate levels. In order to eliminate the effect of diet on plasma nitrate, several days of a standardized low nitrate diet prior to blood sampling would be required, which is beyond the scope of large population‐based cohort studies. Alternatively, infusion of stable isotope labeled 15N‐l‐arginine offers the principal possibility to selectively determine endogenous NO synthesis–related generation of nitrate,18 but this is also not feasible in large community‐based cohort studies.
Plasma nitrate represents a combined measure of its dietary intake, endogenous generation, elimination, and availability for (re‐) conversion to the more active metabolites. Depending on the timing of its measurement, the dietary or the signaling/metabolic aspect may dominate. To gauge the impact of diet and/or the microbiome on plasma nitrate and nitrate‐related outcome, it would be of interest to compare the predictive power of fasting and postprandial nitrate as well as individual long‐term average plasma nitrate or 24‐hour urinary nitrate excretion.
Conclusions
Our investigation reports the first community‐based data indicating an association of plasma nitrate with total mortality. This association with mortality appears to be modulated by renal function. While nitrate was positively associated with several cardiovascular risk factors in cross‐sectional analyses, it was, however, not associated with incident CVD. Nevertheless, in the general population, possible benefits from reconversion of elevated nitrate to NO may frequently be outweighed by the adverse clinical conditions causing the elevation of nitrate. Nitrate may simultaneously act as both a marker and a player. Overall, plasma nitrate is more likely a marker of multiple distinct physiological and pathophysiological processes than a direct mediator of mortality. Determination of nitrate across different populations and clinical settings will help to identify these yet unknown pathomechanisms.
Sources of Funding
This work has been supported in part by contract NO1 HC‐25195 (NHLBI) to Vasan, and a grant from the Deutsche Forschungsgemeinschaft (DFG) to Göen (GO782/2‐1) and Maas (MA 3324/4‐1). The sponsoring organizations provided funding but had no role in the study concept and design, data acquisition and analysis, drafting of the article, or decision to publish.
Disclosures
None.
Acknowledgments
We thank Dimitrios Tsikas, Centre of Pharmacology and Toxicology, Hannover Medical School, Germany) for mentoring the nitrate analysis and Eva Hoier for her excellent technical assistance.
(J Am Heart Assoc. 2017;6:e006224 DOI: 10.1161/JAHA.117.006224.)29151027
References
- 1. Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. [DOI] [PubMed] [Google Scholar]
- 2. Baylis C, Vallance P. Measurement of nitrite and nitrate levels in plasma and urine—what does this measure tell us about the activity of the endogenous nitric oxide system? Curr Opin Nephrol Hypertens. 1998;7:59–62. [DOI] [PubMed] [Google Scholar]
- 3. Leaf CD, Wishnok JS, Tannenbaum SR. L‐arginine is a precursor for nitrate biosynthesis in humans. Biochem Biophys Res Commun. 1989;163:1032–1037. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Rolling revision of the WHO guidelines for drinking‐water quality. Geneva, Switzerland; 2004.
- 5. Ward MH. Too much of a good thing? Nitrate from nitrogen fertilizers and cancer. Rev Environ Health. 2009;24:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–417. [DOI] [PubMed] [Google Scholar]
- 7. Kapil V, Milsom AB, Okorie M, Maleki‐Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite‐derived NO. Hypertension. 2010;56:274–281. [DOI] [PubMed] [Google Scholar]
- 8. Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. [DOI] [PubMed] [Google Scholar]
- 9. Butler AR, Moffett J. The Dunhuang medical manuscripts In: Lo V, Cullen C, eds. Needham Research Institute Series. London, England: RoutlegeCurzon; 2005:363–368. [Google Scholar]
- 10. European Food Safety Authority . Nitrate in vegetables scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008;689:1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bryan NS, Alexander DD, Coughlin JR, Milkowski AL, Boffetta P. Ingested nitrate and nitrite and stomach cancer risk: an updated review. Food Chem Toxicol. 2012;50:3646–3665. [DOI] [PubMed] [Google Scholar]
- 12. Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim‐Shapiro DB, Kozlov AV, Lancaster JR Jr, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter‐Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Machha A, Schechter AN. Inorganic nitrate: a major player in the cardiovascular health benefits of vegetables? Nutr Rev. 2012;70:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton‐Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. [DOI] [PubMed] [Google Scholar]
- 16. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 17. Tsikas D. Simultaneous derivatization and quantification of the nitric oxide metabolites nitrite and nitrate in biological fluids by gas chromatography/mass spectrometry. Anal Chem. 2000;72:4064–4072. [DOI] [PubMed] [Google Scholar]
- 18. Maas R, Schwedhelm E, Kahl L, Li H, Benndorf R, Luneburg N, Forstermann U, Boger RH. Simultaneous assessment of endothelial function, nitric oxide synthase activity, nitric oxide‐mediated signaling, and oxidative stress in individuals with and without hypercholesterolemia. Clin Chem. 2008;54:292–300. [DOI] [PubMed] [Google Scholar]
- 19. Tsikas D, Mitschke A, Gutzki FM, Engeli S, Jordan J. Evidence by gas chromatography‐mass spectrometry of ex vivo nitrite and nitrate formation from air nitrogen oxides in human plasma, serum, and urine samples. Anal Biochem. 2010;397:126–128. [DOI] [PubMed] [Google Scholar]
- 20. Kannel WB, Wolf PA, Garrison RJ, eds. Section 34: Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death in Pooled Repeated Biennial Measurements. Framingham Heart Study, 30 Year Follow‐Up. Bethesda, MD: National Institute of Health; 1987. Publication NIH 87‐2703. [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. CKD EPI . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoon Y, Song J, Hong SH, Kim JQ. Plasma nitric oxide concentrations and nitric oxide synthase gene polymorphisms in coronary artery disease. Clin Chem. 2000;46:1626–1630. [PubMed] [Google Scholar]
- 23. Minamino T, Kitakaze M, Sato H, Asanuma H, Funaya H, Koretsune Y, Hori M. Plasma levels of nitrite/nitrate and platelet cGMP levels are decreased in patients with atrial fibrillation. Arterioscler Thromb Vasc Biol. 1997;17:3191–3195. [DOI] [PubMed] [Google Scholar]
- 24. Dellavalle CT, Daniel CR, Aschebrook‐Kilfoy B, Hollenbeck AR, Cross AJ, Sinha R, Ward MH. Dietary intake of nitrate and nitrite and risk of renal cell carcinoma in the NIH‐AARP Diet and Health Study. Br J Cancer. 2013;108:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller GD, Marsh AP, Dove RW, Beavers D, Presley T, Helms C, Bechtold E, King SB, Kim‐Shapiro D. Plasma nitrate and nitrite are increased by a high‐nitrate supplement but not by high‐nitrate foods in older adults. Nutr Res. 2012;32:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Velzen AG, Sips AJ, Schothorst RC, Lambers AC, Meulenbelt J. The oral bioavailability of nitrate from nitrate‐rich vegetables in humans. Toxicol Lett. 2008;181:177–181. [DOI] [PubMed] [Google Scholar]
- 27. Wagner DA, Schultz DS, Deen WM, Young VR, Tannenbaum SR. Metabolic fate of an oral dose of 15N‐labeled nitrate in humans: effect of diet supplementation with ascorbic acid. Cancer Res. 1983;43:1921–1925. [PubMed] [Google Scholar]
- 28. Tsikas D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res. 2005;39:797–815. [DOI] [PubMed] [Google Scholar]
- 29. Mariotti F, Petzke KJ, Bonnet D, Szezepanski I, Bos C, Huneau JF, Fouillet H. Kinetics of the utilization of dietary arginine for nitric oxide and urea synthesis: insight into the arginine‐nitric oxide metabolic system in humans. Am J Clin Nutr. 2013;97:972–979. [DOI] [PubMed] [Google Scholar]
- 30. Sakinis A, Jungersten L, Wennmalm A. An 18oxygen inhalation method for determination of total body formation of nitric oxide in humans. Clin Physiol. 1999;19:504–509. [DOI] [PubMed] [Google Scholar]
- 31. Westfelt UN, Benthin G, Lundin S, Stenqvist O, Wennmalm A. Conversion of inhaled nitric oxide to nitrate in man. Br J Pharmacol. 1995;114:1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Castillo F, Dobao MM, Reyes F, Blasco R, Roldan MD, Gavira M, Caballero FJ, Moreno‐Vivian C, Martinez‐Luque M. Molecular and regulatory properties of the nitrate reducing systems of Rhodobacter. Curr Microbiol. 1996;33:341–346. [DOI] [PubMed] [Google Scholar]
- 33. Tannenbaum SR, Sinskey AJ, Weisman M, Bishop W. Nitrite in human saliva. Its possible relationship to nitrosamine formation. J Natl Cancer Inst. 1974;53:79–84. [PubMed] [Google Scholar]
- 34. Erzurum SC, Ghosh S, Janocha AJ, Xu W, Bauer S, Bryan NS, Tejero J, Hemann C, Hille R, Stuehr DJ, Feelisch M, Beall CM. Higher blood flow and circulating NO products offset high‐altitude hypoxia among Tibetans. Proc Natl Acad Sci USA. 2007;104:17593–17598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jungersten L, Ambring A, Wall B, Wennmalm A. Both physical fitness and acute exercise regulate nitric oxide formation in healthy humans. J Appl Physiol (1985). 1997;82:760–764. [DOI] [PubMed] [Google Scholar]
- 36. Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA. 2001;98:12814–12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang J, Brown MA, Tam SH, Chan MC, Whitworth JA. Effects of diet on measurement of nitric oxide metabolites. Clin Exp Pharmacol Physiol. 1997;24:418–420. [DOI] [PubMed] [Google Scholar]
- 38. Node K, Kitakaze M, Yoshikawa H, Kosaka H, Hori M. Reduced plasma concentrations of nitrogen oxide in individuals with essential hypertension. Hypertension. 1997;30:405–408. [DOI] [PubMed] [Google Scholar]
- 39. Himeno M, Ishibashi T, Nakano S, Furuya K, Yoshida J, Kigoshi T, Uchida K, Nishio M. Implication of steady state concentrations of nitrite and nitrate metabolites of nitric oxide in plasma and whole blood in healthy human subjects. Clin Exp Pharmacol Physiol. 2004;31:591–596. [DOI] [PubMed] [Google Scholar]
- 40. Green LC, Ruiz de Luzuriaga K, Wagner DA, Rand W, Istfan N, Young VR, Tannenbaum SR. Nitrate biosynthesis in man. Proc Natl Acad Sci USA. 1981;78:7764–7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mackenzie IM, Ekangaki A, Young JD, Garrard CS. Effect of renal function on serum nitrogen oxide concentrations. Clin Chem. 1996;42:440–444. [PubMed] [Google Scholar]
- 42. Schmidt RJ, Yokota S, Tracy TS, Sorkin MI, Baylis C. Nitric oxide production is low in end‐stage renal disease patients on peritoneal dialysis. Am J Physiol. 1999;276:F794–F797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmidt RJ, Domico J, Samsell LS, Yokota S, Tracy TS, Sorkin MI, Engels K, Baylis C. Indices of activity of the nitric oxide system in hemodialysis patients. Am J Kidney Dis. 1999;34:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmidt RJ, Baylis C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int. 2000;58:1261–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uzun H, Konukoglu D, Besler M, Erdenen F, Sezgin C, Muderrisoglu C. The effects of renal replacement therapy on plasma, asymmetric dimethylarginine, nitric oxide and C‐reactive protein levels. Clin Invest Med. 2008;31:E1–E7. [DOI] [PubMed] [Google Scholar]
- 46. Tomic M, Galesic K, Markota I. Endothelin‐1 and nitric oxide in patients on chronic hemodialysis. Ren Fail. 2008;30:836–842. [DOI] [PubMed] [Google Scholar]
- 47. Godfrey M, Majid DS. Renal handling of circulating nitrates in anesthetized dogs. Am J Physiol. 1998;275:F68–F73. [DOI] [PubMed] [Google Scholar]
- 48. Wennmalm A, Benthin G, Edlund A, Jungersten L, Kieler‐Jensen N, Lundin S, Westfelt UN, Petersson AS, Waagstein F. Metabolism and excretion of nitric oxide in humans. An experimental and clinical study. Circ Res. 1993;73:1121–1127. [DOI] [PubMed] [Google Scholar]
- 49. Annane D, Sanquer S, Sebille V, Faye A, Djuranovic D, Raphael JC, Gajdos P, Bellissant E. Compartmentalised inducible nitric‐oxide synthase activity in septic shock. Lancet. 2000;355:1143–1148. [DOI] [PubMed] [Google Scholar]
- 50. Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345:588–595. [DOI] [PubMed] [Google Scholar]
- 51. Villalpando S, Gopal J, Balasubramanyam A, Bandi VP, Guntupalli K, Jahoor F. In vivo arginine production and intravascular nitric oxide synthesis in hypotensive sepsis. Am J Clin Nutr. 2006;84:197–203. [DOI] [PubMed] [Google Scholar]
- 52. Jeerooburkhan N, Jones LC, Bujac S, Cooper JA, Miller GJ, Vallance P, Humphries SE, Hingorani AD. Genetic and environmental determinants of plasma nitrogen oxides and risk of ischemic heart disease. Hypertension. 2001;38:1054–1061. [DOI] [PubMed] [Google Scholar]
- 53. Ghasemi A, Zahedi Asl S, Mehrabi Y, Saadat N, Azizi F. Serum nitric oxide metabolite levels in a general healthy population: relation to sex and age. Life Sci. 2008;83:326–331. [DOI] [PubMed] [Google Scholar]
- 54. Tsikas D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the L‐arginine/nitric oxide area of research. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:51–70. [DOI] [PubMed] [Google Scholar]
- 55. Epperlein MM, Nourooz‐Zadeh J, Noronha‐Dutra AA, Woolf N. Nitric oxide in cigarette smoke as a mediator of oxidative damage. Int J Exp Pathol. 1996;77:197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Caimi G, Montana M, Calandrino V, Caruso M, Carollo C, Catania A, Lo Presti R. Nitric oxide metabolites (nitrite and nitrate) in young patients with recent acute myocardial infarction. Clin Hemorheol Microcirc. 2008;40:157–163. [PubMed] [Google Scholar]
- 57. Matsuo S, Nakamura Y, Takahashi M, Ouchi Y, Hosoda K, Nozawa M, Kinoshita M. Effect of red wine and ethanol on production of nitric oxide in healthy subjects. Am J Cardiol. 2001;87:1029–1031. A1026. [DOI] [PubMed] [Google Scholar]
- 58. Hendgen‐Cotta UB, Luedike P, Totzeck M, Kropp M, Schicho A, Stock P, Rammos C, Niessen M, Heiss C, Lundberg JO, Weitzberg E, Kelm M, Rassaf T. Dietary nitrate supplementation improves revascularization in chronic ischemia. Circulation. 2012;126:1983–1992. [DOI] [PubMed] [Google Scholar]
- 59. Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate‐intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1121–R1131. [DOI] [PubMed] [Google Scholar]
- 60. Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim‐Shapiro DB, Schechter AN, Cannon RO III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. [DOI] [PubMed] [Google Scholar]
- 61. Katan MB. Nitrate in foods: harmful or healthy? Am J Clin Nutr. 2009;90:11–12. [DOI] [PubMed] [Google Scholar]
- 62. Bode‐Boger SM, Kojda G. Organic nitrates in cardiovascular disease. Cell Mol Biol (Noisy‐le‐grand). 2005;51:307–320. [PubMed] [Google Scholar]
- 63. Heiss C, Meyer C, Totzeck M, Hendgen‐Cotta UB, Heinen Y, Luedike P, Keymel S, Ayoub N, Lundberg JO, Weitzberg E, Kelm M, Rassaf T. Dietary inorganic nitrate mobilizes circulating angiogenic cells. Free Radic Biol Med. 2012;52:1767–1772. [DOI] [PubMed] [Google Scholar]
- 64. Nolan BT, Hitt KJ, Ruddy BC. Probability of nitrate contamination of recently recharged groundwaters in the conterminous United States. Environ Sci Technol. 2002;36:2138–2145. [DOI] [PubMed] [Google Scholar]
- 65. CDC . Heart disease death rates, total population ages 35+. Available at: https://www.cdc.gov/dhdsp/maps/national_maps/hd_all.htm. Accessed 16‐10‐2017.
- 66. Etemadi A, Sinha R, Ward MH, Graubard BI, Inoue‐Choi M, Dawsey SM, Abnet CC. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH‐AARP Diet and Health Study: population based cohort study. BMJ. 2017;357:j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


