Abstract
Background
Anthracyclines are associated with cardiotoxic effects. Cardiovascular biomarkers may reflect myocardial injury, dysfunction, inflammation, and fibrosis and may precede and predict the development of left ventricular impairment. The aim of this study was to assess: (1) longitudinal change in circulating cardiovascular biomarkers, (2) the effect of metoprolol succinate and candesartan cilexetil on the biomarker response, and (3) the associations between on‐treatment changes in biomarker concentrations and subsequent left ventricular dysfunction in patients with early breast cancer receiving anthracyclines.
Methods and Results
This report encompasses 121 women included in the 2×2 factorial, placebo‐controlled, double‐blind PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) trial with metoprolol and candesartan given concomitantly with anticancer therapy containing the anthracycline, epirubicin (total cumulative dose, 240–400 mg/m2). Cardiovascular magnetic resonance, echocardiography images, and circulating levels of biomarkers were obtained before and after anthracycline treatment. Cardiac troponins I and T, B‐type natriuretic peptide, N‐terminal pro‐B‐type natriuretic peptide, C‐reactive protein, and galectin‐3 increased during anthracycline therapy (all P<0.05). The troponin response was attenuated by metoprolol (P<0.05), but not candesartan. There was no association between change in biomarker concentrations and change in cardiac function during anthracycline therapy.
Conclusions
Treatment with contemporary anthracycline doses for early breast cancer is associated with increase in circulating cardiovascular biomarkers. This increase is, however, not associated with early decline in ventricular function. Beta‐blockade may attenuate early myocardial injury, but whether this attenuation translates into reduced risk of developing ventricular dysfunction in the long term remains unclear.
Clinical Trial Registration
URL: http://www.clinicaltrial.gov. Unique identifier: NCT01434134.
Keywords: beta‐blocker, brain natriuretic peptide, cardio‐oncology, C‐reactive protein, magnetic resonance imaging, troponin
Subject Categories: ACE/Angiotension Receptors/Renin Angiotensin System, Biomarkers, Clinical Studies, Women, Imaging
Clinical Perspective
What Is New?
Treatment with contemporary doses of anthracycline in early breast cancer is associated with increased cardiovascular biomarker concentrations reflecting myocardial injury (cardiac troponins), dysfunction (natriuretic peptides), inflammation (C‐reactive protein), and fibrosis (galectin‐3).
Baseline or early changes in levels of circulating biomarkers are not diagnostic of early impairment of left ventricular systolic or diastolic function.
Beta‐adrenergic blockade with metoprolol attenuates anthracycline‐induced myocardial injury as expressed by increase of circulating troponin concentrations.
What Are the Clinical Implications?
Preventive beta‐adrenergic blockade may have beneficial early effects on anthracycline‐induced myocardial injury, but longer‐term follow‐up will be necessary to evaluate whether this early attenuation of troponins by metoprolol translates into reduced incidence of late cardiotoxicity.
Angiotensin and beta‐adrenergic blockade may provide complementary cardioprotective effects during anthracycline therapy.
Anthracyclines are frequently used in the treatment of several common malignancies, including breast cancer. However, anthracyclines have well‐known cardiotoxic effects leading to myofibrillar degradation and cardiomyocyte apoptosis and necrosis.1 Different strategies to reduce the cardiotoxicity, including lower peak and cumulative drug doses, have been implemented, but contemporary doses still increase the risk of developing left ventricular (LV) systolic dysfunction.2
Circulating cardiovascular biomarkers may reflect pathophysiological processes that play a crucial role for cardiotoxicity, including cardiomyocyte injury, function, inflammation, and fibrosis. High‐dose anthracycline therapy has been associated with increased concentrations of cardiac troponins3 and B‐type natriuretic peptides (BNPs),4 but in more recent studies with contemporary anthracycline doses, results have been inconsistent.5 Past studies of patients receiving high‐dose anthracyclines have also suggested that the initial response in these biomarkers may predict subsequent decrease in LV function.6 However, in breast cancer patients receiving contemporary doses of anthracyclines, sparse data are available concerning the prognostic value of early changes in cardiovascular biomarkers. Other cardiac biomarkers, such as CRP (C‐reactive protein) and galectin‐3, are thought to reflect systemic inflammation and fibrosis, but the prognostic value in breast cancer patients has been less investigated.
Decline in LV ejection fraction (LVEF) is the established imaging marker for cardiotoxicity.7 Cardiovascular magnetic resonance (CMR) is also an excellent modality to detect focal fibrosis in ischemic and nonischemic cardiomyopathy and edema following acute myocardial injury.8 Although a recent meta‐analysis indicates that intervention with beta‐blockers and angiotensin antagonists prevents or delays the development of LV dysfunction in early‐onset anthracycline‐induced cardiotoxicity,9 there are limited data on how this intervention affects circulating levels of cardiovascular biomarkers. The aim of this substudy of the PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) trial was therefore to (1) longitudinally examine the circulating profile of the biomarkers cardiac troponin I and T (cTnI and cTnT), BNP, and the amino‐terminal fragment of the BNP prohormone (NT‐proBNP), CRP, and galectin‐3, (2) assess the effect of the angiotensin receptor blocker candesartan and the beta blocker, metoprolol, on the biomarker response, and (3) evaluate the association between changes in these biomarkers and subsequent reduction in LV systolic function during anthracycline treatment in patients with early breast cancer.
Methods
Study Design
PRADA was an investigator‐initiated, externally monitored 2×2 factorial, randomized, placebo‐controlled, double‐blind trial conducted at Akershus University Hospital (Lørenskog, Norway).10 Patients were stratified according to planned cumulative anthracycline dose (400 versus <400 mg/m2 epirubicin). They were randomized to 1 of 4 combinations of intervention as following: 29 in the metoprolol succinate and placebo arm; 32 in the candesartan cilexetil and placebo arm; 30 in the metoprolol and candesartan arm; and 30 in the double placebo arm. The target dose for metoprolol was 100 mg q.d. and candesartan 32 mg q.d. The study complied with the Declaration of Helsinki. The study protocol was approved by the Regional Ethics Committee of South‐Eastern Norway (2010/2890) and registered at ClinicalTrials.gov (NCT01434134). All participants provided written informed consent. The rationale and design of the study has been published previously.10, 11
Study Participants
In total, 130 women with early breast cancer scheduled for adjuvant therapy with the anthracycline epirubicin in combination with 5‐fluorouracil and cyclophosphamide were included at Akershus University Hospital, Norway, from September 2011 to September 2014. Main exclusion criteria were pre‐existing cardiovascular disease, previous treatment with chemotherapy or radiation to the chest, and indication or contraindications for the study drugs. Postrandomization 4 patients were excluded; 2 did not receive planned adjuvant treatment, 1 had previously been treated with radiation to the chest, and 1 likely had experienced a cardiovascular complication in the prerandomization phase. In this report, additionally, 5 patients were excluded, 4 did not complete their chemotherapy regimen as planned, and 1 did not have biomarker measurements at completion of anthracycline treatment. Hence, 121 patients constitute the study population of this report. The exact timing of blood sampling and cardiac imaging is shown in Figure 1. Both were performed on the same day postchemotherapy. Data were obtained preanthracycline and postanthracycline treatment, ie before initiation of additional therapy with trastuzumab or radiotherapy.
Figure 1.
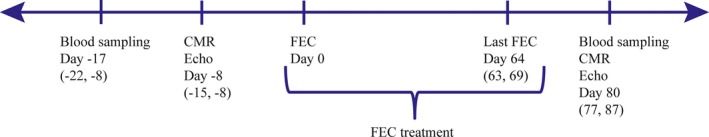
Timing of blood sampling and cardiac imaging. Time of the first FEC cycle defines day 0. Blood sampling, cardiac imaging, and last FEC cycle are shown in relation to the first FEC cycle. Values are given as median (interquartile range). CMR indicates cardiovascular magnetic resonance; Echo, echocardiography; FEC, 5‐fluorouracil, epirubicin, cyclophosphamide.
CMR and Echocardiography
The CMR and echocardiography methodologies have been described in detail previously.10 In brief, CMR examinations were performed on a 1.5‐Tesla scanner (Achieva; Philips Medical Systems, Best, The Netherlands). Steady‐state free precession sequences in contiguous, 8‐mm‐thick, short‐axis slices covering the entire ventricles were used to quantify LVEF. Cardiac edema was assessed by breath‐hold, black‐blood triple inversion recovery T2 imaging in three 15‐mm short‐axis slices of the LV. Repetition time/echo time/flip angle were 2 heartbeats/65 ms/90 degrees and acquired and reconstructed voxel size were 1.5/1.9/15 mm3 and 0.7×0.7×15 mm3, respectively. Late gadolinium enhancement images were acquired 10 minutes after intravenous injection of 0.2 mmol/kg of gadolinium‐DOTA (Dotarem; Guerbet, Villepinte, France). Typically, 2‐dimensional inversion recovery turbo field echo sequence in short axis covering the ventricles, and phase‐sensitive 3‐dimensional inversion recovery turbo field echo sequences in 4‐chamber and left 2‐chamber axis were used. For the 2‐dimensional scans, repetition time/echo time/flip angle were 5.8 ms/2.9 ms/25 degrees, acquired voxel size was 1.5×1.6×8 mm3, and reconstructed voxel size was 0.8×0.8×8 mm3. For the 3‐dimensional scans, repetition time/echo time/flip angle were 4.8 ms/2.3 ms/15 degrees and acquired and reconstructed voxel sizes were 2.0×2.0×10 mm3 and 1.3×1.3×5.5 mm3, respectively. Analyses were performed by a board‐certified radiologist (S.L.H.), who was blinded to treatment assignment and study order.
Transthoracic echocardiography was performed using a Vivid E9 (GE Vingmed, Horten, Norway). Images were digitally stored for offline analysis on custom software (EchoPAC; GE Vingmed). LV 2‐dimensional peak systolic global longitudinal strain was analyzed by a semiautomated speckle‐tracking imaging technique from the 3 standard apical views. LV diastolic function was assessed by the ratio between peak early (E) transmitral velocity by pulsed Doppler and peak early tissue Doppler (E′) by averaging septal and lateral E′ at basal regions. Analyses were performed by a board‐certified physician (G.G.), who was blinded to treatment assignment and study order.
Blood Sampling and Biochemical Analysis
Nonfasting samples of venous blood were drawn, put on ice, processed within 60 minutes, and stored at −80°C pending analysis. Before analysis, thawed specimens were mixed thoroughly by low‐speed vortexing until visibly homogeneity. EDTA‐plasma specimens were centrifuged at 13 500 and serum specimens at 3500 relative centrifugal force for 30 minutes; the clear supernatants were then transferred to the sample cups.
Cardiac Troponins
Serum cTnI was measured with a high‐sensitivity assay (STAT High Sensitive Troponin I) on an Architect i2000SR platform (Abbott Diagnostics, Abbott, IL). The analytic measurement range for this assay is 0 to 50 000 ng/L, the limit of blank 0.8 ng/L, the lower detection limit 1.2 ng/L, and the coefficient of variation (CV) 10% at a concentration of 3.0 ng/L.12 Using control material, we measured a CV of 4.0% in the low‐concentration range (20 ng/L) and 3.6% in the high‐concentration range (15 000 ng/L). Concentrations below or equal to the limit of blank were assigned a value of 0.8 ng/L, whereas levels below or equal to the limit of detection and greater than the limit of blank were assigned a value of 1.2 ng/L.
Serum cTnT was measured by a high‐sensitivity assay (Troponin T hs STAT) on a cobas 8000 e602 analyzer (Roche Diagnostics, Indianapolis, IN). The analytical measurement range is 3 to 10 000 ng/L, limit of blank 3 ng/L, level of detection 5 ng/L, and the CV 10% at a concentration of 13.0 ng/L. Using control material, we measured a CV of 3% in the low‐concentration range (12 pg/mL) and 6% in the high‐concentration range (919 pg/mL). Concentrations below or equal to the limit of blank were assigned a value of 3.0 ng/L, whereas levels below or equal to the limit of detection and greater than the limit of blank were assigned a value of 5 ng/L.
Natriuretic Peptides
BNP in plasma was analyzed by an ARCHITECT BNP assay on an Architect i2000SR platform (Abbott Diagnostics). The analytical measurement range is 10 to 5000 pg/mL with a total CV ≤12%. Using control material, we measured a CV of 5.9% in the low‐concentration range (90 pg/mL) and 4.8% in the high‐concentration range (3500 pg/mL). Samples with levels below 10 pg/mL were assigned a concentration of 5 pg/mL.
NT‐proBNP in serum was measured by the proBNP II assay on a cobas 8000 e602 analyzer (Roche Diagnostics). The analytical measurement range is 5 to 35 000 pg/mL with a total CV 2.9% to 6.1%. Using control material, we measured a CV of 7% in the low‐concentration range (99.2 pg/mL) and 6% in the high‐concentration range (497.5 pg/mL). The level of detection was 5 pg/mL.
Galectin‐3
Galectin‐3 in plasma was measured by an ARCHITECT galectin‐3 assay on an ARCHITECT i2000SR platform (Abbott Diagnostics). The analytical measurement range is 4.0 to 114.0 ng/mL with a total CV ≤10%. The limit of blank is 0.8 ng/mL, and the level of detection 1.0 ng/mL. Using control material, we measured a CV of 4.6% in the low‐concentration range (9.1 ng/mL) and 3.3% in the high‐concentration range (74.1 ng/mL).
C‐Reactive Protein
CRP in serum was measured by a high‐sensitivity assay (CRP Vario) on an ARCHITECT cSystems platform (Abbott Diagnostics). The analytical measurement range is 0.1 to 160 mg/L with a total CV ≤6%. Using control material, we measured a CV of 3.1% in the low‐concentration range (1.42 mg/L) and 1.8% in the high‐concentration range (23.08 mg/L).
Statistical Analysis
Power calculations for the PRADA study were performed for the primary end point (ie, change in LVEF). For this substudy, assuming alpha of 0.05 and an expected correlation coefficient between 0.25 and 0.30, a retrospectively performed power calculation showed that a sample size between 85 and 123 was needed to have a power of 80% to detect an association between change in cardiac troponin and change in LVEF.
Analyses concerning the effect of randomized interventions were conducted according to the intention‐to‐treat principle. The Shapiro–Wilk test was used to test for normality. Normally distributed continuous data are presented as mean±SD, non‐normally distributed continuous data as median and interquartile range and categorical variables as proportions. For normally distributed continuous data, paired‐sample and independent sample Student t tests were used to assess within and between‐group differences; for non‐normally distributed continuous data, Wilcoxon signed‐rank and Mann–Whitney U tests were used. Multivariable linear regression was used to assess the relationship between biomarkers and LV function after adjusting for variables that may affect LV function. We did not correct for multiple comparisons, but a hierarchy of biomarkers was prospectively defined in the PRADA statistical analysis plan, which was signed and locked before data unblinding and analysis. Circulating cardiac troponins were defined as secondary end points and other biomarkers as tertiary end points. All tests were 2‐sided, and a P<0.05 was considered statistically significant. The statistical analyses were carried out with IBM SPSS Statistics for Windows (Version 22.0; IBM Corp, Armonk, NY). Retrospective power calculations were performed by using sample‐size calculators for designing clinical research (www.sample-size.net/correlation-sample-size accessed April 23, 2017).
Results
Baseline Characteristics
All 121 women received the anthracycline epirubicin. In accord with the national guidelines for adjuvant breast cancer treatment in Norway applicable from September 2011 to November 2015, 27 women were treated with a mean cumulative epirubicin dose of 400±0 mg/m2 and 94 with doses between 240 and 360 mg/m2 (mean cumulative dose of 269±52 mg/m2). The baseline characteristics are summarized in Table 1. The following biomarkers were evaluated: cTnI, cTnT, BNP, NT‐proBNP, galectin‐3, and CRP. The number and proportion of patients with detectable levels of troponins are presented in Figure 2.
Table 1.
Baseline Characteristics
| Epirubicin=400 mg/m2 (n=27) | Epirubicin <400 mg/m2 (n=94) | |
|---|---|---|
| Age, y | 51.0 (42.0, 59.0) | 48.5 (43.8, 58.0) |
| Body mass index | 24.5 (22.2, 27.2) | 25.9 (22.9, 29.1) |
| Systolic blood pressure, mm Hg | 135.0 (120.0, 140.0) | 128.5 (120.0, 140.0) |
| Diastolic blood pressure, mm Hg | 80.0 (75.0, 85.0) | 80.0 (74.5, 85.0) |
| Heart rate, beats/min | 71.5±10.3 | 70.9±10.0 |
| Hypertension (n) | 1 (7%) | 7 (7.4%) |
| Diabetes mellitus (n) | 0 (0%) | 2 (2.1%) |
| Current smoking (n) | 6 (22.2%) | 19 (20.2%) |
| Serum creatinine, mg/dL | 0.75 (0.69, 0.81) | 0.72 (0.69, 0.81) |
| Hemoglobin, g/dL | 13.2±0.94 | 13.3±0.81 |
| FEC treatment | ||
| FEC 60 mg/m2×4 (n) | 0 | 71 (75.5%) |
| FEC 60 mg/m2×6 (n) | 0 | 23 (24.5%) |
| FEC 100 mg/m2×4 (n) | 27 (100%) | 0 |
| HER2 status | Positive | Negative |
| Assigned to metoprolol and placebo (n) | 6 | 23 |
| Assigned to candesartan and placebo (n) | 7 | 25 |
| Assigned to metoprolol and candesartan (n) | 7 | 23 |
| Assigned to placebo and placebo (n) | 7 | 23 |
Data are expressed as mean±SD, median (interquartile range) or numbers (percent). FEC indicates 5‐fluorouracil, epirubicin, cyclophosphamide; HER, human epidermal growth factor receptor.
Figure 2.
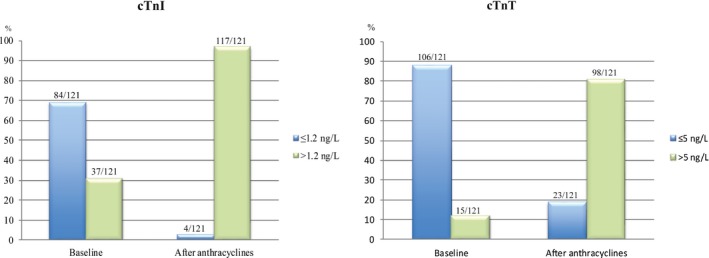
Distribution of cardiac troponin I and T concentrations before and after anthracycline treatment. Blue bars represent values below or equal to the level of detection and green bars values greater than the level of detection. cTn indicates cardiac troponin.
Cardiotoxicity During Anthracycline Treatment
In total, 111 patients had LVEF measured by CMR at baseline and at the completion of anthracycline therapy. LVEF declined from 63.3±4.0% to 60.8±4.5% (P=0.005) in the placebo group. One patient who received 400 mg/m2 of epirubicin fulfilled the criterion for cardiotoxicity as defined by the Cardiac Review and Evaluation Committee Criteria for Cardiotoxicity,13 that is, LVEF declined from 62.7% to 51.0%, without symptoms of heart failure.
Other correlative CMR markers of cardiac injury also increased. There was a dose‐dependent increase of pericardial effusion (Table 2), whereas there was a numerically modest, borderline significant increase in T2 ratio (P=0.053). Late gadolinium enhancement showed no new or increasing areas of focal fibrosis.
Table 2.
Cardiovascular Magnetic Resonance Markers of Cardiac Injury in the Whole Population and in Those Assigned to a Cumulative Anthracycline Dose of <400 mg/m2 and Equal to 400 mg/m2
| n | Baseline | After Anthracyclinesa | P Value | Between‐Group P Value | |
|---|---|---|---|---|---|
| Pericardial effusion, mm | |||||
| All | 111 | 1 (0, 3) | 2 (0, 4) | 0.003 | |
| Epirubicin <400 mg/m2 | 87 | 1 (0, 3) | 2 (0, 3) | 0.175 | 0.001 |
| Epirubicin=400 mg/m2 | 24 | 1 (0, 2) | 3 (2, 4) | 0.001 | |
| T2 (ratio)b | |||||
| All | 109 | 1.86±0.24 | 1.91±0.23 | 0.053 | |
| Epirubicin <400 mg/m2 | 85 | 1.85±0.23 | 1.91±0.23 | 0.101 | 0.953 |
| Epirubicin=400 mg/m2 | 24 | 1.89±0.28 | 1.95±0.24 | 0.311 | |
Anthracycline containing chemotherapy with 5‐fluorouracil, epirubicin, cyclophosphamide (FEC); values are given in median (interquartile range) for non‐normally distributed data, and mean±SD for normally distributed data.
The T2 ratio is between the T2 signal intensity in myocardium and skeletal muscle.
Longitudinal Change of Circulating Biomarkers
Longitudinal values and change of the biomarker levels from baseline to completion of anthracycline therapy are summarized in Figure 3 and Table 3. The median levels of cTnI, cTnT, BNP, NT‐proBNP, galectin‐3, and CRP increased from baseline to completion of anthracycline (all P<0.05; Table 3). The increases in cTnI, cTnT, and CRP concentration were significantly higher in those receiving higher versus lower doses of anthracycline (all P<0.01), whereas no clear dose‐dependency was observed for the increase in BNP, NT‐proBNP, and galectin‐3 levels (Figure 3).
Figure 3.
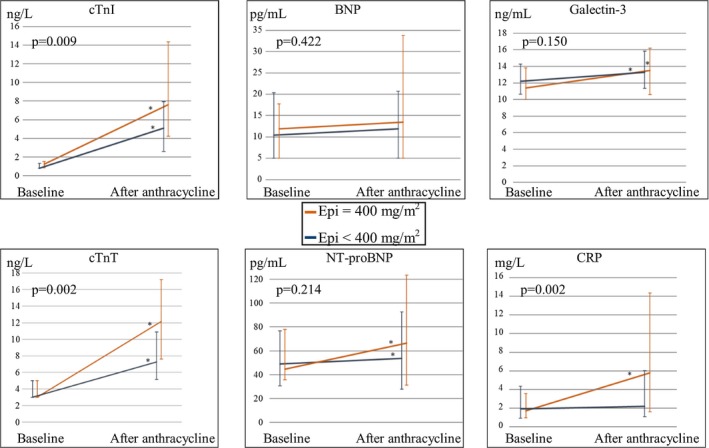
Change in median values of biomarker from baseline to completion of anthracycline therapy. Median biomarker values at baseline and at completion of anthracycline therapy for those treated with a total mean cumulative epirubicin dose of 400 mg/m2 (in orange) and <400 mg/m2 (in gray). Bars represent the interquartile range; *represents significant increase (P<0.05) of the biomarker from baseline to end of anthracycline containing chemotherapy. P value is for median between group differences. BNP indicates B‐type natriuretic peptides; CRP, C‐reactive protein; cTn, cardiac troponin; Epi, epirubicin; NT‐proBNP, amino‐terminal fragment of the BNP prohormone.
Table 3.
Comparison of Change in Biomarkers in All Patients and in Those Assigned to and Not Assigned to Metoprolol
| n | Baseline Median Values (IQR) | After Anthracyclinesa Median Values (IQR) | Median Difference (IQR) | Within‐Group P Value | Between‐Group P Value | |
|---|---|---|---|---|---|---|
| cTnI, ng/L | ||||||
| All | 121 | 0.8 (0.8, 1.4) | 5.6 (3.0, 9.3) | 4.4 (1.8, 7.8) | <0.001 | |
| Metoprolol | 62 | 0.8 (0.8, 1.2) | 4.4 (2.5, 7.6) | 2.9 (1.6, 6.8) | <0.001 | 0.019 |
| No metoprolol | 59 | 1.2 (0.8, 1.5) | 7.2 (3.4, 11.8) | 5.7 (2.3, 9.90) | <0.001 | |
| cTnT, ng/L | ||||||
| All | 121 | 3.0 (3.0, 5.0) | 8.5 (5.6, 12.7) | 4.3 (2.0, 8.0) | <0.001 | |
| Metoprolol | 62 | 3.0 (3.0, 5.0) | 6.8 (5.0, 10.9) | 3.4 (2.0, 7.4) | <0.001 | 0.020 |
| No metoprolol | 59 | 3.0 (3.0, 5.0) | 9.7 (6.5, 13.1) | 5.0 (2.6, 9.8) | <0.001 | |
| BNP, pg/mL | ||||||
| All | 119 | 10.4 (5.0, 19.1) | 12.0 (5.0, 23.0) | 0.0 (−3.2, 10.5) | 0.049 | |
| Metoprolol | 62 | 10.4 (5.0, 21.6) | 15.5 (5.0, 31.5) | 0.4 (−2.0, 13.6) | 0.005 | 0.047 |
| No metoprolol | 57 | 10.5 (5.0, 16.3) | 5.0 (5.0, 18.1) | 0.0 (−5.9, 2.5) | 0.882 | |
| NT‐proBNP, pg/mL | ||||||
| All | 121 | 48.3 (32.0, 76.5) | 55.2 (29.5, 98.1) | 10 (−13.1, 41.2) | 0.002 | |
| Metoprolol | 62 | 52.7 (38.1, 78.2) | 76.8 (41.9, 154.3) | 17.7 (2.0, 59.5) | <0.001 | 0.003 |
| No metoprolol | 59 | 42.7 (29.1, 74.1) | 49.2 (23.0, 68.1) | 1.2 (−22.8, 25.6) | 0.721 | |
| Galectin‐3, ng/mL | ||||||
| All | 120 | 12.1 (10.4, 14.0) | 13.4 (11.2, 16.0) | 1.1 (0.0, 2.4) | <0.001 | |
| Metoprolol | 62 | 11.9 (10.1, 13.9) | 13.5 (11.5, 16.3) | 1.7 (−0.0. 2.9) | <0.001 | 0.119 |
| No metoprolol | 58 | 12.2 (10.6, 14.2) | 13.3 (11.2, 15.7) | 0.9 (−0.0, 2.0) | <0.001 | |
| CRP, mg/L | ||||||
| All | 121 | 1.9 (0.9, 4.3) | 2.9 (1.2, 6.5) | 0.3 (−0.9, 2.7) | 0.019 | |
| Metoprolol | 62 | 1.9 (0.9, 5.0) | 2.1 (1.0, 6.4) | 0.3 (−0.8, 2.3) | 0.081 | 0.979 |
| No metoprolol | 59 | 2.0 (1.0, 4.3) | 3.3 (1.3, 6.8) | 0.3 (−1.3, 3.6) | 0.111 | |
BNP indicates B‐type natriuretic peptides; CRP, C‐reactive protein; cTn, cardiac troponin; IQR, interquartile range; NT‐proBNP, amino‐terminal fragment of the BNP prohormone.
Anthracycline containing chemotherapy with 5‐fluorouracil, epirubicin, cyclophosphamide (FEC).
Effect of Metoprolol and Candesartan on Levels of Circulating Biomarkers
The effect of metoprolol and candesartan on levels of circulating biomarkers is summarized in Tables 3 and 4. The concentrations of cTnI and cTnT increased less in patients assigned to metoprolol than those not assigned metoprolol. Thus, cTnI increased from 0.8 (0.8, 1.2) to 4.4 (2.5, 7.6) ng/L in patients assigned to metoprolol and from 1.2 (0.8, 1.5) to 7.2 (3.4, 11.8) ng/L in those not assigned to metoprolol (between group difference, P=0.019). cTnT increased from 3.0 (3.0, 5.0) to 6.8 (5.0, 10.9) ng/L in patients assigned to metoprolol and from 3.0 (3.0, 5.0) ng/L to 9.7 (6.5, 13.1) ng/L in those not assigned to metoprolol (between group difference, P=0.020). The troponin increase in the no metoprolol group was higher in those treated with a cumulative anthracycline dose of 400 mg/m2 than <400 mg/m2 (Table 5). There was no difference between those assigned to candesartan or not. Because there was no interaction between the effect of metoprolol and candesartan, the 2×2 factorial design permits comparison of the no metoprolol group with the metoprolol group. Similarly, the no candesartan group can validly be compared with the candesartan group. The validity of this approach is supported by data presented in Tables 6 and 7, showing that there is no significant difference in change in cardiac troponin levels between patients assigned to metoprolol compared with the candesartan‐metoprolol combination and those assigned to candesartan compared with the candesartan‐metoprolol combination.
Table 4.
Comparison of Change in Biomarkers in Patients Assigned to and Not Assigned to Candesartan
| n | Baseline Median Values (IQR) | After Anthracyclinesa Median Values (IQR) | Median Difference (IQR) | Within‐Group P Value | Between‐Group P Value | |
|---|---|---|---|---|---|---|
| cTnI, ng/L | ||||||
| Candesartan | 62 | 0.8 (0.8, 1.4) | 5.6 (3.0, 9.1) | 4.2 (1.9, 7.3) | <0.001 | 0.846 |
| No candesartan | 59 | 1.2 (0.8, 1.4) | 5.6 (2.9, 9.9) | 4.8 (1.7, 9.1) | <0.001 | |
| cTnT, ng/L | ||||||
| Candesartan | 62 | 3.0 (3.0, 5.0) | 8.7 (5.8, 12.6) | 4.1 (2.0, 8.3) | <0.001 | 0.942 |
| No candesartan | 59 | 3.0 (3.0, 5.0) | 8.0 (5.6, 13.0) | 4.5 (2.0, 7.9) | <0.001 | |
| BNP, pg/mL | ||||||
| Candesartan | 62 | 5.0 (5.0, 20.8) | 11.8 (5.0, 20.2) | 0.0 (−3.8, 12.9) | 0.048 | 0.453 |
| No candesartan | 57 | 11.9 (5.0, 18.8) | 12.3 (5.0, 23.8) | 0.0 (−3.5, 8.0) | 0.401 | |
| NT‐proBNP, pg/mL | ||||||
| Candesartan | 62 | 48.6 (30.5, 77.8) | 53.3 (27.0, 93.5) | 9.5 (−11.8, 33.9) | 0.054 | 0.717 |
| No candesartan | 59 | 48.2 (35.7, 75.4) | 58.9 (31.3, 99.2) | 10.1 (−15.9, 45.3) | 0.022 | |
| Galectin‐3, ng/mL | ||||||
| Candesartan | 62 | 11.9 (9.9, 14.3) | 13.2 (11.0, 16.2) | 0.9 (−0.2, 2.4) | <0.001 | 0.414 |
| No candesartan | 58 | 12.2 (10.7, 13.9) | 13.5 (11.9, 15.8) | 1.6 (0.2, 2.4) | <0.001 | |
| CRP, mg/L | ||||||
| Candesartan | 62 | 1.8 (1.0, 3.7) | 2.3 (0.9, 6.4) | 0.2 (−0.8, 2.6) | 0.203 | 0.454 |
| No candesartan | 59 | 1.9 (0.9, 5.3) | 3.2 (1.5, 8.0) | 0.8 (−1.4, 3.7) | 0.060 | |
BNP indicates B‐type natriuretic peptides; CRP, C‐reactive protein; cTn, cardiac troponin; IQR, interquartile range; NT‐proBNP, amino‐terminal fragment of the BNP prohormone.
Anthracycline containing chemotherapy with 5‐fluorouracil, epirubicin, cyclophosphamide (FEC).
Table 5.
Cardiac Troponin I and T Concentrations According to the Cumulative Anthracycline Dose in the Whole Cohort and Stratified for Beta Blockade (Metoprolol)
| n | Median Δ Value for Cumulative Epirubicin dose <400 mg/m2 (IQR) | n | Median Δ Value for Cumulative Epirubicin dose=400 mg/m2 (IQR) | P Value | |
|---|---|---|---|---|---|
| cTnI | |||||
| All | 94 | 3.8 (1.6, 6.9) | 27 | 6.4 (2.5, 13.5) | 0.009 |
| Metoprolol | 46 | 2.8 (1.4, 6.3) | 13 | 4.3 (2.4, 11.6) | 0.094 |
| No metoprolol | 48 | 5.1 (1.9, 8.9) | 14 | 9.6 (4.7, 15.1) | 0.036 |
| cTnT | |||||
| All | 94 | 3.6 (2.0, 7.4) | 27 | 7.5 (3.6, 11.7) | 0.002 |
| Metoprolol | 46 | 2.7 (1.5, 7.1) | 13 | 5.8 (3.0, 10.3) | 0.031 |
| No metoprolol | 48 | 4.5 (2.3, 7.7) | 14 | 8.7 (4.9, 13.6) | 0.019 |
cTn indicates cardiac troponin; IQR, interquartile range.
Table 6.
Comparison of Cardiac Troponin Levels in Patients Assigned to Metoprolol Only and Those Assigned to Candesartan and Metoprolol
| n | Baseline Median Values (IQR) | After Anthracyclinesa Median Values (IQR) | Median Difference (IQR) | Between‐Group P Value | |
|---|---|---|---|---|---|
| cTnI | |||||
| Metoprolol only | 29 | 0.8 (0.8, 1.3) | 4.7 (2.2, 8.1) | 2.7 (1.4, 7.1) | 0.96 |
| Candesartan and metoprolol | 30 | 0.8 (0.8, 1.2) | 4.4 (2.8, 7.5) | 3.2 (1.7, 6.4) | |
| cTnT | |||||
| Metoprolol only | 29 | 3.0 (3.0, 5.0) | 6.8 (5.0, 12.0) | 3.0 (1.9, 7.7) | 0.80 |
| Candesartan and metoprolol | 30 | 3.0 (3.0, 5.0) | 6.8 (5.0, 10.5) | 3.6 (2.0, 7.2) | |
cTn indicates cardiac troponin; IQR, interquartile range.
Anthracycline containing chemotherapy in combination with 5‐fluorouracil and cyclophosphamide.
Table 7.
Comparison of Cardiac Troponin Levels in Patients Assigned to Candesartan Only and Those Assigned to Candesartan and Metoprolol
| n | Baseline Median Values (IQR) | After Anthracyclinesa Median Values (IQR) | Median Difference (IQR) | Between Group P Value | |
|---|---|---|---|---|---|
| cTnI | |||||
| Candesartan only | 32 | 0.8 (0.8, 1.5) | 7.3 (3.4, 12.2) | 5.7 (2.2, 10.3) | 0.08 |
| Candesartan and metoprolol | 30 | 0.8 (0.8, 1.2) | 4.4 (2.8, 7.5) | 3.2 (1.7, 6.4) | |
| cTnT | |||||
| Candesartan only | 32 | 3.0 (3.0, 5.0) | 10.2 (6.6, 14.0) | 5.2 (2.1, 10.0) | 0.13 |
| Candesartan and metoprolol | 30 | 3.0 (3.0, 5.0) | 6.8 (5.0, 10.5) | 3.6 (2.0, 7.2) | |
cTn indicates cardiac troponin; IQR, interquartile range.
Anthracycline containing chemotherapy in combination with 5‐fluorouracil and cyclophosphamide.
The levels of BNP increased from 10.4 (5.0, 21.6) to 15.5 (5.0, 31.5) pg/mL and for NT‐proBNP from 52.7 (38.1, 78.2) to 76.8 (41.9, 154.3) pg/mL in patients assigned to metoprolol whereas concentrations did not change significantly in those not assigned to metoprolol (between‐group differences for BNP, P=0.047; for NT‐proBNP, P=0.003). There were no between‐group differences for those assigned to candesartan or not. The interventions did not influence the circulating levels of galecetin‐3 or CRP.
Circulating Biomarkers and LV Systolic and Diastolic Function
There was no association between on‐treatment change in biomarker values and change in LV systolic or diastolic function in multivariable linear regression analysis adjusted for age, body mass index, systolic blood pressure, epirubicin dose, candesartan, and metoprolol (Tables 8, 9 through 10). Established cut‐point values for myocardial infarction for cTnI (Abbott) are levels above 26 ng/L and for cTnT (Roche) levels above 14 ng/L. None of the women had values above these levels at baseline. At completion of anthracycline‐containing chemotherapy, 5 women had values above the cut point for myocardial infarction for cTnI and 24 for cTnT.
Table 8.
Multivariable Linear Regression for Assessing Association Between Change in Circulating Cardiovascular Biomarkers and Change in LVEF as Measured by Cardiovascular Magnetic Resonance Imaging With Adjustment for Variables That Could Affect Change in LVEF
| Variables | Ba | 95% Confidence Interval for B | P Value | Variables | Ba | 95% Confidence Interval for B | P Value |
|---|---|---|---|---|---|---|---|
| ΔcTnI | −0.04 | −0.13, 0.05 | 0.340 | ΔcTnT | −0.09 | −0.24, 0.05 | 0.198 |
| Age, y | 0.04 | −0.05, 0.14 | 0.381 | Age, y | 0.05 | −0.04, 0.15 | 0.283 |
| BMI, kg/m2 | 0.11 | −0.08, 0.30 | 0.257 | BMI, kg/m2 | 0.11 | −0.08, 0.29 | 0.257 |
| SBP, mm Hg | −0.01 | −0.08, 0.06 | 0.872 | SBP | −0.01 | −0.08, 0.06 | 0.848 |
| Epi. doseb | −1.48 | −3.47, 0.52 | 0.146 | Epi. doseb | −1.29 | −3.32, 0.75 | 0.213 |
| Candesartan | 1.44 | −0.18, 3.07 | 0.081 | Candesartan | 1.50 | −0.11, 3.12 | 0.068 |
| Metoprolol | 0.67 | −0.97, 2.31 | 0.419 | Metoprolol | 0.65 | −0.97, 2.27 | 0.428 |
| ΔBNP | 0.01 | −0.04, 0.06 | 0.584 | ΔNT‐proBNP | 0.00 | −0.01, 0.02 | 0.875 |
| Age, y | 0.03 | −0.07, 0.13 | 0.531 | Age, y | 0.04 | −0.06, 0.13 | 0.436 |
| BMI, kg/m2 | 0.11 | −0.08, 0.30 | 0.257 | BMI, kg/m2 | 0.10 | −0.09, 0.29 | 0.304 |
| SBP, mm Hg | −0.01 | −0.08, 0.06 | 0.759 | SBP, mm Hg | −0.01 | −0.08, 0.06 | 0.865 |
| Epi. doseb | −1.77 | −3.79, 0.26 | 0.087 | Epi. doseb | −1.64 | −3.65, 0.36 | 0.107 |
| Candesartan | 1.63 | −0.02, 3.29 | 0.053 | Candesartan | 1.49 | −0.14, 3.12 | 0.073 |
| Metoprolol | 0.55 | −1.12, 2.23 | 0.513 | Metoprolol | 0.80 | −0.91, 2.51 | 0.356 |
| ΔGalectin‐3 | 0.12 | −0.23, 0.46 | 0.499 | ΔCRP | −0.05 | −0.11, 0.01 | 0.120 |
| Age, y | 0.03 | −0.07, 0.13 | 0.530 | Age, y | 0.04 | −0.05, 0.13 | 0.389 |
| BMI, kg/m2 | 0.11 | −0.08, 0.30 | 0.242 | BMI, kg/m2 | 0.08 | −0.10, 0.27 | 0.386 |
| SBP, mm Hg | −0.01 | −0.08, 0.06 | 0.855 | SBP, mm Hg | −0.01 | −0.08, 0.06 | 0.877 |
| Epi. doseb | −1.63 | −3.63, 0.37 | 0.109 | Epi. doseb | −1.35 | −3.34, 0.64 | 0.180 |
| Candesartan | 1.65 | 0.01, 3.29 | 0.049 | Candesartan | 1.53 | −0.08, 3.14 | 0.062 |
| Metoprolol | 0.63 | −1.02, 2.28 | 0.452 | Metoprolol | 0.94 | −0.65, 2.53 | 0.244 |
BMI indicates body mass index; BNP, B‐type natriuretic peptides; CRP, C‐reactive protein; cTnI, cardiac troponin I; cTnT, cardiac troponin T; LVEF, left ventricular ejection fraction; NT‐proBNP, amino‐terminal fragment of the BNP prohormone; SBP, systolic blood pressure at baseline.
B unstandardized regression coefficient.
Dichotomized variable for cumulative epirubicin dose of 400 and <400 mg/m2.
Table 9.
Multivariable Linear Regression for Assessing Association Between Change in Circulating Cardiovascular Biomarkers and Change in Peak Systolic GLS by Echocardiography With Adjustment for Variables That Could Affect Change in GLS
| Variables | Ba | 95% Confidence Interval For B | P Value | Variables | Ba | 95% Confidence Interval for B | P Value |
|---|---|---|---|---|---|---|---|
| ΔcTnI | −0.02 | −0.07, 0.02 | 0.303 | ΔcTnT | −0.03 | −0.11, 0.04 | 0.387 |
| Age, y | −0.04 | −0.09, 0.01 | 0.121 | Age, y | −0.04 | −0.09, 0.02 | 0.162 |
| BMI, kg/m2 | −0.08 | −0.18, 0.02 | 0.115 | BMI, kg/m2 | −0.09 | −0.19, 0.01 | 0.087 |
| SBP, mm Hg | −0.03 | −0.07, 0.01 | 0.111 | SBP, mm Hg | −0.03 | −0.06, 0.01 | 0.120 |
| Epi. doseb | −0.06 | −1.09, 0.96 | 0.904 | Epi. doseb | −0.01 | −1.06, 1.05 | 0.993 |
| Candesartan | −0.07 | −0.92, 0.77 | 0.862 | Candesartan | −0.05 | −0.89, 0.79 | 0.910 |
| Metoprolol | −0.08 | −0.93, 0.76 | 0.845 | Metoprolol | −0.05 | −0.88, 0.79 | 0.914 |
| ΔBNP | 0.02 | −0.01, 0.04 | 0.180 | ΔNT‐proBNP | 0.01 | 0.00, 0.01 | 0.169 |
| Age, y | −0.04 | −0.09, 0.01 | 0.106 | Age, y | −0.04 | −0.09, 0.01 | 0.083 |
| BMI, kg/m2 | −0.07 | −0.18, 0.03 | 0.152 | BMI, kg/m2 | −0.08 | −0.18, 0.02 | 0.126 |
| SBP, mm Hg | −0.04 | −0.07, 0.00 | 0.061 | SBP, mm Hg | −0.03 | −0.07, 0.00 | 0.073 |
| Epi. doseb | −0.05 | −1.07, 0.97 | 0.929 | Epi. doseb | −0.14 | −1.16, 0.88 | 0.783 |
| Candesartan | 0.01 | −0.82, 0.84 | 0.978 | Candesartan | −0.02 | −0.84, 0.80 | 0.961 |
| Metoprolol | −0.20 | −1.06, 0.66 | 0.645 | Metoprolol | −0.17 | −1.02, 0.69 | 0.704 |
| ΔGalectin‐3 | 0.04 | −0.19, 0.27 | 0.726 | ΔCRP | 0.00 | −0.03, 0.03 | 0.993 |
| Age, y | −0.04 | −0.09, 0.01 | 0.102 | Age, y | −0.04 | −0.09, 0.01 | 0.122 |
| BMI, kg/m2 | −0.08 | −0.18, 0.02 | 0.124 | BMI, kg/m2 | −0.09 | −0.19, 0.01 | 0.084 |
| SBP, mm Hg | −0.03 | −0.07, 0.01 | 0.108 | SBP, mm Hg | −0.03 | −0.07, 0.01 | 0.114 |
| Epi. doseb | −0.09 | −1.12, 0.94 | 0.864 | Epi. doseb | −0.11 | −1.16, 0.94 | 0.838 |
| Candesartan | 0.07 | −0.78, 0.91 | 0.875 | Candesartan | 0.01 | −0.83, 0.84 | 0.988 |
| Metoprolol | −0.07 | −0.92, 0.78 | 0.872 | Metoprolol | 0.01 | −0.82, 0.84 | 0.975 |
BMI indicates body mass index; BNP, B‐type natriuretic peptides; CRP, C‐reactive protein; cTnI, cardiac troponin I; cTnT, cardiac troponin T; GLS, global longitudinal strain; NT‐proBNP, amino‐terminal fragment of the BNP prohormone; SBP, systolic blood pressure at baseline.
B unstandardized regression coefficient.
Dichotomized variable for cumulative epirubicin dose of 400 and <400 mg/m2.
Table 10.
Multivariable Linear Regression for Assessing Association Between Change in Circulating Cardiovascular Biomarkers and Change in the Ratio of Peak Early (E) Transmitral Velocity by Pulsed Doppler and Peak Early Tissue Doppler (E′) (E/E′) by Echocardiography With Adjustment for Variables That Could Affect Change in E/E′
| Variables | Ba | 95% Confidence Interval for B | P Value | Variables | Ba | 95% Confidence Interval for B | P Value |
|---|---|---|---|---|---|---|---|
| ΔcTnI | −0.02 | −0.05, 0.02 | 0.297 | ΔcTnT | −0.04 | −0.09, 0.01 | 0.097 |
| Age, y | −0.02 | −0.06, 0.01 | 0.197 | Age, y | −0.02 | −0.06, 0.02 | 0.269 |
| BMI, kg/m2 | −0.03 | −0.09, 0.04 | 0.451 | BMI, kg/m2 | −0.02 | −0.09, 0.04 | 0.495 |
| SBP, mm Hg | 0.02 | −0.01, 0.05 | 0.128 | SBP, mm Hg | 0.02 | −0.01, 0.05 | 0.117 |
| Epi. doseb | 0.65 | −0.08, 1.37 | 0.078 | Epi. doseb | 0.78 | 0.03, 1.52 | 0.041 |
| Candesartan | −0.25 | −0.86, 0.35 | 0.408 | Candesartan | −0.24 | −0.84, 0.36 | 0.423 |
| Metoprolol | 0.57 | −0.04, 1.18 | 0.065 | Metoprolol | 0.55 | −0.05, 1.16 | 0.072 |
| ΔBNP | 0.00 | −0.02, 0.01 | 0.664 | ΔNT‐proBNP | 0.00 | −0.01, 0.00 | 0.286 |
| Age, y | −0.02 | −0.06, 0.02 | 0.259 | Age, y | −0.02 | −0.06, 0.01 | 0.223 |
| BMI, kg/m2 | −0.03 | −0.10, 0.03 | 0.328 | BMI, kg/m2 | −0.03 | −0.10, 0.03 | 0.296 |
| SBP, mm Hg | 0.02 | −0.01, 0.05 | 0.124 | SBP, mm Hg | 0.02 | 0.00, 0.05 | 0.102 |
| Epi. doseb | 0.64 | −0.09, 1.38 | 0.085 | Epi. doseb | 0.65 | −0.08, 1.37 | 0.078 |
| Candesartan | −0.28 | −0.89, 0.33 | 0.369 | Candesartan | −0.24 | −0.84, 0.37 | 0.437 |
| Metoprolol | 0.73 | 0.09, 1.37 | 0.025 | Metoprolol | 0.74 | 0.11, 1.37 | 0.023 |
| ΔGalectin‐3 | −0.03 | −0.16, 0.10 | 0.673 | ΔCRP | 0.00 | −0.02, 0.03 | 0.698 |
| Age, y | −0.02 | −0.06, 0.02 | 0.240 | Age, y | −0.03 | −0.06, 0.01 | 0.187 |
| BMI, kg/m2 | −0.03 | −0.10, 0.03 | 0.330 | BMI, kg/m2 | −0.03 | −0.09, 0.04 | 0.392 |
| SBP, mm Hg | 0.02 | −0.01, 0.05 | 0.144 | SBP, mm Hg | 0.02 | −0.01, 0.05 | 0.148 |
| Epi. doseb | 0.60 | −0.13, 1.33 | 0.108 | Epi. doseb | 0.57 | −0.17, 1.30 | 0.129 |
| Candesartan | −0.27 | −0.89, 0.34 | 0.384 | Candesartan | −0.23 | −0.84, 0.37 | 0.451 |
| Metoprolol | 0.69 | 0.06, 1.31 | 0.031 | Metoprolol | 0.62 | 0.02, 1.23 | 0.044 |
BMI indicates body mass index; BNP, B‐type natriuretic peptides; CRP, C‐reactive protein; cTnI, cardiac troponin I; cTnT, cardiac troponin T; NT‐proBNP, amino‐terminal fragment of the BNP prohormone; SBP, systolic blood pressure at baseline.
B unstandardized regression coefficient.
Dichotomized variable for cumulative epirubicin dose of 400 and <400 mg/m2.
Discussion
The salient findings of the current study of early breast cancer patients are: (1) Circulating cTnI, cTnT, BNP, NT‐proBNP, galectin‐3, and CRP all increase during anthracycline therapy and for cTnI, cTnT, and CRP the increase is dose‐dependent; (2) the cTnI and cTnT responses are attenuated by metoprolol, compatible with a beneficial effect on early cardiotoxic injury; (3) candesartan has no apparent impact on circulating levels of biomarkers of myocardial injury, function, inflammation, or fibrosis; and (4) on‐treatment change in biomarker concentrations are not associated with early change in LV systolic or diastolic function. These findings provide insight in the effects of beta‐adrenergic and angiotensin blockade during anthracycline containing breast cancer therapy and have important implications for the interpretation and use of cardiovascular biomarkers as monitoring and prognostic tools during adjuvant breast cancer therapy.
Cardiotoxicity During Anthracycline Treatment
The decline in LVEF in the placebo group in this substudy was 2.5 percentage points. Even though this may be considered a small effect, the magnitude is comparable to findings in other recent studies.14, 15 Considering that the PRADA study population had a low prevalence of cardiac risk factors and comorbidities and received contemporary anthracycline doses, the observed dose‐dependent biomarker changes are likely to be real and reflect a cardiotoxic signal. This early sign of cardiotoxicity is supported by a significant and dose‐dependent increase in pericardial effusion and borderline significant increase in T2 ratio.
Longitudinal Change of Biomarkers
Different classes of cardiovascular biomarkers are thought to provide information concerning different pathophysiological mechanisms.16 Because anthracycline therapy is associated with both cardiomyocyte injury, loss of cardiac contractile function, inflammation, and development of diffuse fibrosis,17 we selected biomarkers that are believed to reflect these processes in our study.
Cardiac troponins are markers of cardiomyocyte injury and are associated with risk for cardiovascular death and heart failure.18 Moreover, the use of high‐sensitivity assays for cTnI and cTnT also permits detection and monitoring of low‐grade, chronic myocardial injury.19 BNP and NT‐proBNP are associated with cardiac function and provide strong prognostic information across the spectrum of cardiovascular disease.20, 21, 22 CRP is a prototypical inflammatory biomarker that has been associated with the incidence of cardiovascular disease and death, both in the general population, in patients with coronary artery disease, and in heart failure.23 Galectin‐3 is a novel biomarker secreted by activated macrophages, thought to reflect myofibroblast proliferation, macrophage migration, inflammation, cardiac remodeling, and fibrosis.24, 25, 26 Although some past studies have reported increase in 1 or more of all these biomarkers, results have not been consistent.5 The reasons for these inconsistencies may result from heterogeneity in patient populations (eg, different cancer types, cardiovascular disease, and/or risk factors), type and dosage of anthracyclines used, timing of blood samples, and the sensitivity of biomarker assays used. For instance, Cardinale et al reported a high proportion of detectable troponin I values measured with a conventional assay with limited sensitivity (lower limit of detection=350 ng/L) after high‐dose anthracycline therapy.6 More recent, but smaller, studies using higher‐sensitivity assays in patients with breast cancer receiving contemporary doses of anthracyclines have also reported an increase of cTnI and CRP during anthracycline treatment, but found no change in NT‐proBNP and galectin‐3.2 The current study using high‐sensitivity assays confirms and extends information from past studies by demonstrating an increase in all biomarkers investigated.
In accord with earlier findings, these observations suggest that anthracycline therapy at contemporary doses is associated with myocardial injury, inflammation, and fibrosis, whereas the increase in biomarkers of cardiac dysfunction, such as BNP and NT‐proBNP, and reduction in cardiac function evaluated by imaging modalities, seems to be more modest. Moreover, the observation that there was a dose‐dependent increase in cTnI, cTnT, and CRP suggests that these biomarkers may represent the best tools to monitor the immediate cardiotoxic effects of anthracyclines.
Clearly, the analysis and interpretation of the results may be affected by the kinetics of the different biomarkers. The kinetics of the cardiac troponin, natriuretic peptide, CRP, and galectin‐3 response following anthracycline treatment have not been clearly defined, but are likely to vary considerably. In our study, cardiac troponins were defined as the biomarkers of primary interest. Accordingly, 1 important consideration for the timing of blood sampling following anthracycline therapy was to be within a time window where cardiac troponin concentrations could be expected to be elevated. Because we observed a significant increase in all biomarkers, we believe the timing of blood sampling was appropriate. To capture the peak level for all biomarkers, daily blood sampling would have been required, but this was neither logistically feasible nor ethically acceptable.
Effect of Metoprolol and Candesartan on Levels of Circulating Biomarkers
The sympathetic nervous system and the renin‐angiotensin‐aldosterone system exert diverse and complex actions on the myocardium. Blockade of these neuroendocrine systems beneficially modulate the remodeling process that occurs following myocardial injury.27, 28 In the current substudy, candesartan had no effect on the direct cardiotoxic effect of anthracyclines that leads to troponin leakage, whereas in the primary analysis of the PRADA study, angiotensin blockade with candesartan prevented decline in LVEF that occurred after adjuvant breast cancer therapy with anthracycline with or without radiation and/or trastuzumab.10 The reason for this apparent discrepancy is likely the beneficial effect of angiotensin blockade on cardiac remodeling,29, 30 which appears to occur independently of the magnitude of cardiotoxic injury, as assessed by cTnI and cTnT measurements. Conversely, the current study suggests that beta blockade with metoprolol may beneficially impact on the acute toxicity of anthracyclines, reflected in significantly less increase in cTnI and cTnT levels during anthracycline therapy whereas it had no apparent effect on LVEF in the main analysis. Although the current study is not designed to elucidate the exact mechanisms whereby metoprolol reduces myocardial injury and subsequent cardiac troponin release, a potential mechanism mediating this anticardiotoxic effect is the inhibition of beta‐adrenergic–mediated proapoptotic pathways.31, 32, 33, 34 The clinical significance of our observation is unclear because the increase in cardiac troponin levels was not associated with change in ventricular function from baseline to completion of anthracycline therapy. However, until longer‐term follow‐up data are available, a cohesive conclusion cannot be drawn concerning whether the attenuation of troponin increase by metoprolol or the attenuation of decline in LVEF by candesartan is of greater long‐term prognostic importance. Based on the information currently available, it may be argued that combined beta‐adrenergic and angiotensin blockade represents the reasonable approach for prophylactic cardioprotective therapy in these patients, whereas definitive conclusion will await data from longer follow‐up and additional studies.
The observation that metoprolol therapy was associated with higher concentrations of BNP and NT‐proBNP was not unexpected, given that beta blockers have been shown to increase natriuretic peptide concentrations in healthy subjects as well as in a variety of clinical settings.35 One potential mechanism is increased stretch of cardiomyocytes induced by the higher end‐diastolic volume secondary to the reduction in heart rate by metoprolol. Galectin‐3 and CRP were not affected by either of the interventions, suggesting that neuroendocrine blockade with candesartan and metoprolol does not affect the inflammatory and profibrotic response to anthracycline therapy.
Association Between Individual Biomarkers and Cardiac Function
The literature is inconsistent regarding the association between different cardiac biomarkers and the impairment of cardiac function.5 In the current study, blood sampling and cardiac imaging were performed on the same day postchemotherapy. There were no associations between change in biomarkers levels and change in cardiac systolic and diastolic function during contemporary doses of anthracycline treatment. This suggests that circulating biomarkers have limited potential to predict early reduction in ventricular function; however, we cannot rule out a stronger association in populations with pre‐existing cardiovascular disease or with a higher cardiovascular risk factor burden leading to a more‐pronounced decline in cardiac function. Also, there may be a stronger association in patients receiving higher doses of anthracycline or in those reintroduced to anthracyclines because of tumor recurrence. Although the lack of association between change in biomarkers and change in cardiac function was consistent for the biomarkers examined, we recognize that the relatively modest sample size may have contributed to the lack of association. The question whether an early biomarker response may be predictive of late reduction in ventricular function must await long‐term follow‐up.
Strengths and Limitations
Strengths of the current study include its randomized, 2×2 factorial, double‐blind design, permitting a head‐to‐head comparison of 2 different drugs. Also, the study population was well characterized phenotypically and homogeneous with little comorbidity. Importantly, the anthracycline doses used in this study were in accord with contemporary guidelines for breast cancer treatment. Limitations of the current report include the lack of follow‐up information beyond the adjuvant treatment period, but long‐term follow‐up is planned and ongoing. Also, the kinetic profiles of the different biomarkers during and after anthracycline therapy have not been clearly defined, and the optimal timing for biomarker sampling could have been missed.
Conclusions
In patients receiving contemporary treatment for early breast cancer, cTnI, cTnT, and CRP increased during chemotherapy in a dose‐dependent fashion. Long‐term patient follow‐up is required to determine whether the impact of metoprolol on cardiac troponin levels during therapy will translate into clinical benefit. Likewise, the lack of associations between change in biomarker concentrations and early changes in ventricular function suggest that the clinical utility of these biomarkers as prognostic tools is limited, but long‐term studies are warranted.
Sources of Funding
This work was supported by the University of Oslo, The Extra Foundation for Health and Rehabilitation, The Norwegian Cancer Society, and Akershus University Hospital. Study medications and matching placebos were provided free of charge by AstraZeneca (Mölndal, Sweden). Reagents for the analysis of cTnI, BNP, galectin‐3, and CRP were provided by Abbott Diagnostics (Abbott Park, IL). The funders of the study played no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Disclosures
Omland has served on advisory boards for Abbott Diagnostics and Novartis, has received research support from AstraZeneca and Abbott Diagnostics via Akershus University Hospital, and speaker's honoraria from Roche Diagnostics and Novartis. Gulati and Røsjø have received speaker's honoraria from Novartis. No other disclosures were reported.
Acknowledgments
We gratefully acknowledge the important work of the members of Data and Safety Monitoring Board. We are indebted to the staff of the Clinical Research Unit, Division of Medicine, Akershus University Hospital for skillful assistance with all aspects of the trial execution and particularly thank Annika Lorentzen, Vigdis Bakkelund, and Marit Holmefjord Pedersen for the study execution. We also acknowledge the skillful work by Heidi Strand, BSc, Section for Medical Biochemistry, Division for Diagnostics and Technology, Akershus University Hospital for the biomarker analyses.
(J Am Heart Assoc. 2017;6:e006513 DOI: 10.1161/JAHA.117.006513.)29118031
References
- 1. Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010;53:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ky B, Putt M, Sawaya H, French B, Januzzi JL Jr, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, Cipolla CM. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high‐dose chemotherapy. Circulation. 2004;109:2749–2754. [DOI] [PubMed] [Google Scholar]
- 4. Sandri MT, Salvatici M, Cardinale D, Zorzino L, Passerini R, Lentati P, Leon M, Civelli M, Martinelli G, Cipolla CM. N‐terminal pro‐B‐type natriuretic peptide after high‐dose chemotherapy: a marker predictive of cardiac dysfunction? Clin Chem. 2005;51:1405–1410. [DOI] [PubMed] [Google Scholar]
- 5. Tian S, Hirshfield KM, Jabbour SK, Toppmeyer D, Haffty BG, Khan AJ, Goyal S. Serum biomarkers for the detection of cardiac toxicity after chemotherapy and radiation therapy in breast cancer patients. Front Oncol. 2014;4:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, Cinieri S, Martinelli G, Cipolla CM, Fiorentini C. Left ventricular dysfunction predicted by early troponin I release after high‐dose chemotherapy. J Am Coll Cardiol. 2000;36:517–522. [DOI] [PubMed] [Google Scholar]
- 7. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM; Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG) . 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 8. Eitel I, Friedrich MG. T2‐weighted cardiovascular magnetic resonance in acute cardiac disease. J Cardiovasc Magn Reson. 2011;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yun S, Vincelette ND, Abraham I. Cardioprotective role of beta‐blockers and angiotensin antagonists in early‐onset anthracyclines‐induced cardiotoxicity in adult patients: a systematic review and meta‐analysis. Postgrad Med J. 2015;91:627–633. [DOI] [PubMed] [Google Scholar]
- 10. Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz‐Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff‐Brenkenhoff F, Bratland A, Storås TH, Hagve TA, Rosjo H, Steine K, Geisler J, Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo‐controlled, double‐blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heck SL, Gulati G, Ree AH, Schulz‐Menger J, Gravdehaug B, Rosjo H, Steine K, Bratland A, Hoffmann P, Geisler J, Omland T. Rationale and design of the prevention of cardiac dysfunction during an adjuvant breast cancer therapy (PRADA) trial. Cardiology. 2012;123:240–247. [DOI] [PubMed] [Google Scholar]
- 12. Apple FS, Collinson PO; Biomarkers ITFoCAoC . Analytical characteristics of high‐sensitivity cardiac troponin assays. Clin Chem. 2012;58:54–61. [DOI] [PubMed] [Google Scholar]
- 13. Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. [DOI] [PubMed] [Google Scholar]
- 14. Bosch X, Rovira M, Sitges M, Domenech A, Ortiz‐Perez JT, de Caralt TM, Morales‐Ruiz M, Perea RJ, Monzo M, Esteve J. Enalapril and carvedilol for preventing chemotherapy‐induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (prevention of left ventricular dysfunction with enalapril and carvedilol in patients submitted to intensive chemotherapy for the treatment of malignant hemopathies). J Am Coll Cardiol. 2013;61:2355–2362. [DOI] [PubMed] [Google Scholar]
- 15. Drafts BC, Twomley KM, D'Agostino R Jr, Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, Little WC, Hamilton CA, Hundley WG. Low to moderate dose anthracycline‐based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. [DOI] [PubMed] [Google Scholar]
- 17. Zhang S, Liu X, Bawa‐Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin‐induced cardiotoxicity. Nat Med. 2012;18:1639–1642. [DOI] [PubMed] [Google Scholar]
- 18. Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, Maggioni A, Domanski MJ, Rouleau JL, Sabatine MS, Braunwald E; PEACE Investigators . Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61:1240–1249. [DOI] [PubMed] [Google Scholar]
- 19. Omland T. New features of troponin testing in different clinical settings. J Intern Med. 2010;268:207–217. [DOI] [PubMed] [Google Scholar]
- 20. Omland T, Aakvaag A, Bonarjee VV, Caidahl K, Lie RT, Nilsen DW, Sundsfjord JA, Dickstein K. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long‐term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N‐terminal proatrial natriuretic peptide. Circulation. 1996;93:1963–1969. [DOI] [PubMed] [Google Scholar]
- 21. de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E. The prognostic value of B‐type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–1021. [DOI] [PubMed] [Google Scholar]
- 22. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. [DOI] [PubMed] [Google Scholar]
- 23. Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, Hsia J, Gersh BJ, Rifai N, Ridker PM, Pfeffer MA, Braunwald E; PEACE Investigators . Prognostic significance of the Centers for Disease Control/American Heart Association high‐sensitivity C‐reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. [DOI] [PubMed] [Google Scholar]
- 24. Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin‐3 marks activated macrophages in failure‐prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. [DOI] [PubMed] [Google Scholar]
- 25. de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin‐3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11:811–817. [DOI] [PubMed] [Google Scholar]
- 26. Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, van der Harst P, Pitt B, Goldstein IJ, Koerts JA, van Veldhuisen DJ, Bank RA, van Gilst WH, Sillje HH, de Boer RA. Genetic and pharmacological inhibition of galectin‐3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013;6:107–117. [DOI] [PubMed] [Google Scholar]
- 27. Chan V, Fenning A, Hoey A, Brown L. Chronic beta‐adrenoceptor antagonist treatment controls cardiovascular remodeling in heart failure in the aging spontaneously hypertensive rat. J Cardiovasc Pharmacol. 2011;58:424–431. [DOI] [PubMed] [Google Scholar]
- 28. Kim S, Yoshiyama M, Izumi Y, Kawano H, Kimoto M, Zhan Y, Iwao H. Effects of combination of ace inhibitor and angiotensin receptor blocker on cardiac remodeling, cardiac function, and survival in rat heart failure. Circulation. 2001;103:148–154. [DOI] [PubMed] [Google Scholar]
- 29. Schieffer B, Wirger A, Meybrunn M, Seitz S, Holtz J, Riede UN, Drexler H. Comparative effects of chronic angiotensin‐converting enzyme inhibition and angiotensin II type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation. 1994;89:2273–2282. [DOI] [PubMed] [Google Scholar]
- 30. St John Sutton M, Pfeffer MA, Moye L, Plappert T, Rouleau JL, Lamas G, Rouleau J, Parker JO, Arnold MO, Sussex B, Braunwald E. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long‐term use of captopril: information from the survival and ventricular enlargement (SAVE) trial. Circulation. 1997;96:3294–3299. [DOI] [PubMed] [Google Scholar]
- 31. Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–16700. [DOI] [PubMed] [Google Scholar]
- 32. Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)‐ and beta(2)‐adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin‐sensitive G protein. Circulation. 1999;100:2210–2212. [DOI] [PubMed] [Google Scholar]
- 33. Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)‐adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci USA. 2001;98:1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bernstein D, Fajardo G, Zhao M, Urashima T, Powers J, Berry G, Kobilka BK. Differential cardioprotective/cardiotoxic effects mediated by beta‐adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2005;289:H2441–H2449. [DOI] [PubMed] [Google Scholar]
- 35. Davis ME, Richards AM, Nicholls MG, Yandle TG, Frampton CM, Troughton RW. Introduction of metoprolol increases plasma B‐type cardiac natriuretic peptides in mild, stable heart failure. Circulation. 2006;113:977–985. [DOI] [PubMed] [Google Scholar]


