Abstract
Background
The relationship between plasma concentrations of betaine and choline metabolism and major cardiovascular disease (CVD) end points remains unclear. We have evaluated the association between metabolites from the choline pathway and risk of incident CVD and the potential modifying effect of Mediterranean diet interventions.
Methods and Results
We designed a case‐cohort study nested within the PREDIMED (Prevention With Mediterranean Diet) trial, including 229 incident CVD cases and 751 randomly selected participants at baseline, followed up for 4.8 years. We used liquid chromatography–tandem mass spectrometry to measure, at baseline and at 1 year of follow‐up, plasma concentrations of 5 metabolites in the choline pathway: trimethylamine N‐oxide, betaine, choline, phosphocholine, and α‐glycerophosphocholine. We have calculated a choline metabolite score using a weighted sum of these 5 metabolites. We used weighted Cox regression models to estimate CVD risk. The multivariable hazard ratios (95% confidence intervals) per 1‐SD increase in choline and α‐glycerophosphocholine metabolites were 1.24 (1.05–1.46) and 1.24 (1.03–1.50), respectively. The baseline betaine/choline ratio was inversely associated with CVD. The baseline choline metabolite score was associated with a 2.21‐fold higher risk of CVD across extreme quartiles (95% confidence interval, 1.36–3.59; P<0.001 for trend) and a 2.27‐fold higher risk of stroke (95% confidence interval, 1.24–4.16; P<0.001 for trend). Participants in the higher quartiles of the score who were randomly assigned to the control group had a higher risk of CVD compared with participants in the lower quartile and assigned to the Mediterranean diet groups (P=0.05 for interaction). No significant associations were observed for 1‐year changes in individual plasma metabolites and CVD.
Conclusions
A metabolite score combining plasma metabolites from the choline pathway was associated with an increased risk of CVD in a Mediterranean population at high cardiovascular risk.
Clinical Trial Registration
URL: http://www.controlled-trials.com. Unique identifier: ISRCTN35739639.
Keywords: cardiovascular disease, choline, gut microbiota, Mediterranean diet, metabolomics
Subject Categories: Cardiovascular Disease, Lifestyle, Diet and Nutrition, Epidemiology, Primary Prevention
Clinical Perspective
What Is New?
Baseline plasma concentrations of choline pathway metabolites (choline, phosphocholine, and α‐glycerophosphocholine) and a metabolite score combining choline pathway metabolites were associated with the risk of major cardiovascular events after 4.8 years of follow‐up in a Mediterranean population at high cardiovascular risk.
What Are the Clinical Implications?
Our findings suggest that metabolites from choline pathways may play a role in the development of cardiovascular disease.
The present work may have important public health implications for focusing on dietary interventions, such as adhering to a Mediterranean diet and reducing the intake of animal products, to improve metabolite profiles and, consequently, the risk of cardiovascular disease.
However, further research is needed to replicate these results in other populations and investigate the potential mechanisms underlying the associations between metabolite profiles and cardiovascular disease.
Introduction
In the past few years, interest has emerged on the role of plasma metabolites in the development of cardiometabolic diseases and on how diet can influence disease risk through inducing changes in the metabolome profile. Lately, metabolites from the choline pathway have received increasing attention for its link with the gut microbiota and the potential mechanistic relevance of them.1 In particular, these studies have focused on metabolites associated with betaine and choline metabolism and the gut microbiota–dependent metabolite, trimethylamine N‐oxide (TMAO), which have been associated with atherosclerosis development and cardiovascular disease (CVD).1, 2 Choline can be oxidized to betaine in humans, and the betaine/choline ratio has been inversely associated with nonalcoholic fatty liver disease,3 but its association with CVD has not been previously described.
Dietary lipid phosphatidylcholine is the primary source of choline, and catabolism of betaine and choline by intestinal microbes leads to TMAO production.4 Foods, such as meat, egg yolks, and high‐fat dairy products, which are rich in phosphatidylcholine, choline, carnitine, and TMA, serve as dietary precursors for TMAO generation in mice and humans.2 Dietary supplementation with these metabolites in mice promoted atherosclerosis, and suppression of the microbiota with antibiotics prevented the effect of dietary choline in enhancing atherosclerosis.4 This evidence suggests that diet could modulate intestinal microbiota composition and, subsequently, the ability to synthesize trimethylamine and TMAO, thus providing a mechanistic link between diet, gut microbiome, and atherosclerosis.1, 2, 5, 6 However, not all human studies have confirmed the association between TMAO and cardiovascular events.7, 8, 9, 10 Indeed, the relation between plasma concentration of choline pathway metabolites and the incidence of hard CVD end points in a population‐based level and the role that diet may play in these associations remain to be elucidated.
We hypothesized that higher concentrations at baseline and 1‐year changes in choline pathway metabolites (TMAO, betaine, choline, phosphocholine, and α‐glycerophosphocholine) would be associated with the risk of cardiovascular events. The Mediterranean diet (MedDiet), which is high in plant‐based foods, has been linked with a reduced risk of cardiovascular risk factors and CVD.11 Because previous evidence suggests that excessive consumption of phosphatidylcholine and choline could be reduced by consuming a plant‐based or high‐fiber diet,4 such as the MedDiet, we hypothesized that the associations between choline metabolites and CVD will be modified by MedDiet interventions. Using a case‐cohort study conducted in participants at high cardiovascular risk from the PREDIMED (Prevention With Mediterranean Diet) Study, we aimed to address the following: (1) whether baseline choline pathway metabolites (and their 1‐year changes) predict future risk of CVD and (2) whether these associations are mitigated by a MedDiet intervention.
Methods
Study Population
We designed a case‐cohort study in the framework of the PREDIMED Study. The design and protocol of the PREDIMED Study (http://www.predimed.es) have been described in detail elsewhere.11, 12 Briefly, the PREDIMED Study was a large, multicenter, parallel‐group, randomized, controlled trial aimed at evaluating the effect of the MedDiet on the primary prevention of CVD. A total of 7447 participants, aged 55 to 80 years and at high cardiovascular risk but free of CVD at baseline, were randomly assigned to receive 1 of the following 3 interventions: MedDiet supplemented with extravirgin olive oil, MedDiet supplemented with mixed nuts, or an equivalent education program with advice to reduce the intake of all types of fat (control group). The primary end point of the PREDIMED trial was a composite of cardiovascular events (myocardial infarction, stroke, or death from cardiovascular causes); 288 incident CVD cases occurred during 4.8 years of follow‐up.
In a case‐cohort study design, all incident cases and a randomly selected sample of the original cohort were selected as participants. In the present study, we included all the incident cases of CVD diagnosed during the follow‐up and a random sample of 10% of the original PREDIMED trial (referred to as the “subcohort,” which by its random selection may include some cases) with available EDTA plasma samples at baseline. With this design, we maintained the original randomization scheme of the trial. The present analysis included a total of 980 participants, 751 were noncases and 229 were cases (there were 37 overlapping cases between the subcohort and the total cases). Of these participants, 923 of the 980 had available samples after 1 year of follow‐up and were included in the 1‐year change analyses (Figure S1). We defined cases as the participants who developed a major cardiovascular event (stroke, myocardial infarction, or cardiovascular death) during follow‐up. We defined subcohort as the random sample selected from the full roster of the PREDIMED Study (including 37 incident CVD cases, which were defined as internal cases). The 37 overlapping cases were treated as cases in the analysis (total CVD cases, 229). We defined external cases as the cases that were not randomly included in the subcohort. All participants provided written informed consent, according to a protocol approved by the institutional review boards before inclusion in the study.
Metabolite Profiling
At baseline and at yearly follow‐up visits, trained nurses collected fasting blood samples from the PREDIMED participants. After an overnight fast, tubes for EDTA plasma were collected and aliquots were coded and kept refrigerated until they were stored at −80°C in freezers. Pairs of samples (baseline and first‐year visit) were randomly ordered and shipped on dry ice to the Broad Institute (Cambridge, MA) for the metabolomics analysis.
The present work was a hypothesis‐driven analysis of 5 metabolites from the choline pathway. Polar plasma metabolites, including TMAO, choline, betaine, phosphocholine, and α‐glycerophosphocholine, were profiled using liquid chromatography–tandem mass spectrometry on a system composed of a Shimadzu Nexera X2 U‐HPLC coupled to a Q Exactive hybrid quadrupole orbitrap mass spectrometer.13 Metabolite extracts were prepared from plasma samples (10 μL) via protein precipitation with the addition of 9 volumes of 74.9:24.9:0.2 v/v/v acetonitrile/methanol/formic acid containing stable isotope‐labelled internal standards (valine‐d8 and phenylalanine‐d8). The samples were centrifuged (10 minutes, 9000g, 4°C), and the supernatants were injected directly onto a 150×2‐mm, 3‐μm Atlantis HILIC column. The column was eluted isocratically at a flow rate of 250 μL/min with 5% mobile phase A (10 mmol/L ammonium formate and 0.1% formic acid in water) for 0.5 minutes, followed by a linear gradient to 40% mobile phase B (acetonitrile with 0.1% formic acid) over 10 minutes. Mass spectrometry analyses were performed using electrospray ionization in the positive ion mode using full‐scan analysis over 70 to 800 m/z at 70 000 resolutions and a 3‐Hz data acquisition rate. Other mass spectrometry settings were as follows: sheath gas, 40; sweep gas, 2; spray voltage, 3.5 kV; capillary temperature, 350°C; S‐lens, RF 40; heater temperature, 300°C; microscans, 1; automatic gain control target, 1e6; and maximum ion time, 250 milliseconds. Metabolite identities were confirmed using authentic reference standards. Raw data were processed using TraceFinder software and Progenesis QI. Internal standard peak areas were monitored for quality control and to ensure system performance throughout analyses. Pooled plasma reference samples were also inserted every 20 samples as an additional quality control.
Ascertainment of CVD Cases
For the present analysis, the primary end point was a composite of cardiovascular events (myocardial infarction, stroke, or death from cardiovascular causes). As a secondary end point, we separately analyzed incident stroke, because this was the most common element included in the definition of the composite primary end point in the PREDIMED Study. Information on major cardiovascular events was updated on a yearly basis and obtained from the continuous contact with participants and their families during the trial, contact with general practitioners, the yearly comprehensive review of medical records, and consultation of the National Death Index. The end point was determined by review of the Endpoint Adjudication Committee that was blinded to the intervention group. Only confirmed cases were included in the analyses.
Covariate Assessment
At baseline and at yearly follow‐up visits, a questionnaire about lifestyle variables, educational achievement, history of diseases, medication use, and family history of disease was administered. Physical activity was assessed using the validated Spanish version of the Minnesota Leisure‐Time Physical Activity questionnaire.14 Participants were considered to have diabetes mellitus, hypercholesterolemia, or hypertension if they had previously been diagnosed and/or they were being treated with antidiabetic, cholesterol‐lowering, or antihypertensive agents, respectively. Trained dietitians completed a 137‐item validated semiquantitative food frequency questionnaire in face‐to‐face interviews with participants.15 We used Spanish food composition tables to estimate energy and nutrient intake.16 Trained personnel took anthropometric and blood pressure measurements.
Statistical Analysis
We applied a rank‐based inverse normal transformation to approximate a normal distribution of metabolite levels.17 Baseline characteristics are presented per case status and for quartiles of the choline score as the mean (SD) for quantitative traits and number (percentage) for categorical variables. Baseline characteristics were compared between cases and noncases using t tests for continuous variables and χ2 tests for categorical variables.
We used Cox proportional hazard models, with Barlow weights (to account for oversampling of cases in the study design18), to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) for the primary combined end point of CVD and, separately, for nonfatal stroke. In all Cox models, Barlow weights were used to account for oversampling of cases in the study design. Noncases in the subcohort were weighted inversely proportional to the sampling fraction (ie, in our study, the weight was 10). For noncases, observation time began on the date of their randomization and ended on their date of death (from noncardiovascular causes), the study end date (December 1, 2010), or the date of the last medical visit (if they had stopped attending scheduled intervention appointments), whichever came first. Cases had a weight of 1 at the instant the individual experienced an end point, and observation time was the time when the participant developed CVD. Incident internal cases were treated as noncases (observation time began on the date that they were randomized and ended the instant the individual died) until they became cases, and at such instant their weight changed.
Cox models were adjusted for age (years), sex (men/women), family history of premature heart disease (yes/no), smoking (never, former, or current), and body mass index (kg/m2), and were stratified by intervention group (both MedDiet interventions and low‐fat control group) (model 1). Model 2 was additionally adjusted for physical activity (metabolic equivalent tasks in min/d), baseline hypertension (yes/no), dyslipidemia (yes/no), and diabetes mellitus (yes/no). Baseline individual plasma metabolites were analyzed as both continuous variables (1‐SD increment in transformed concentration of metabolites) and using quartiles (using cut points defined among noncases). To test for the linear trend across quartiles, the median of each quartile was assigned and analyzed as a continuous variable. To account for multiple testing, we adjusted P values of the multivariable‐adjusted associations between 1‐SD increment in metabolite concentration and CVD risk using the Benjamin‐Hochberg procedure.
In addition, we calculated a choline metabolite score as the weighted sum of concentrations of 5 metabolites from the choline pathway and modeled the score as a main exposure variable in the Cox model. The weight for each metabolite was the regression coefficient for a 1‐SD increment in the plasma concentration estimated from the multivariable Cox regression model. We also calculated the ratio between betaine and choline (by dividing the raw values and then applying inverse normal transformations). We conducted stratified analyses by sex, age (<65 versus ≥65 years), obesity (<30 versus ≥30 kg/m2), smoking status (current/former versus never), family history of premature coronary heart disease, baseline type 2 diabetes mellitus, baseline hypertension, and baseline dyslipidemia. The interactions between these variables of stratification and the choline score were tested by adding multiplicative terms into the multivariable Cox models; a likelihood ratio test was used for testing statistical significance of the interaction term.
We also examined the associations of 1‐year changes in the individual plasma metabolites with CVD. We used the same Cox regression models as in the baseline analyses, but we further adjusted for baseline levels and an interaction term between these metabolites at baseline and 1‐year change (both as continuous variables). We repeated the analysis using incident stroke as the outcome, both including and excluding the nonstroke CVD cases (ie, treating the 113 nonstroke CVD cases as noncases or removing them from analyses), and found similar results. Therefore, we present stroke results only after removing the nonstroke CVD cases.
To examine the combined associations of the MedDiet interventions and choline pathway metabolites with the incidence of CVD, we categorized the participants into joint subgroups, according to the assignment to the intervention group (MedDiet merged versus control group), quartiles of the choline metabolite score, and betaine/choline ratio. We used participants assigned to the control group and the first quartile of the choline score as the reference group; we also used participants in the MedDiet group and the first quartile of the betaine/choline ratio as the reference group. We estimated HRs and their 95% CIs for CVD, according to this joint categorization, using the same models as in the previous analysis. To test the interaction between the MedDiet and the metabolite score and betaine/choline ratio, we added a multiplicative term (1 df) between intervention group (MedDiet merged versus control group) and the continuous score into the multivariable Cox models. In secondary analysis, we further adjusted multivariable model 2 for previous metabolites that predicted CVD in our population, including a weighted score of branched‐chain amino acids (isoleucine, leucine, and valine)19; short‐chain (C2carnitine to C7carnitine), medium‐chain (C8carnitine to C14:2carnitine), and long‐chain (C16carnitine to C26carnitine) acylcarnitines score20; and glutamine/glutamate ratio.21 In addition, we further adjusted the models for medication use (dyslipidemia, antihypertensive, and antidiabetic medications).
All statistical analyses were performed using SAS version 9.4 and R version 2.13.0. P<0.05 was considered statistically significant.
Results
The baseline characteristics of the 980 participants are shown in Table 1, according to whether they had a major CVD event during the 4.8 years of follow‐up and quartiles of the choline metabolite score. Participants in the higher quartile of the score had higher risk profiles at baseline than those in the lower quartile of the score, including older age and higher prevalence of hypertension, dyslipidemia, and diabetes mellitus; they were also more likely to smoke. The characteristics of the metabolites included in the analysis and the pathways in which they are involved are described in Table S1. A diagram of the choline pathway is presented in Figure S2. A heat map of Spearman correlation coefficients of the plasma metabolites analyzed in the present study is shown in Figure S3. Means and SD of plasma metabolite concentrations are presented in Table S2 for the total population and in Table S3 stratified by the intervention group.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | Cases (n=229) | Noncases (n=751) | Quartiles of Choline Metabolite Scorea | |||
|---|---|---|---|---|---|---|
| 1 (n=231) | 2 (n=256) | 3 (n=246) | 4 (n=247) | |||
| Age, mean±SD, y | 69.4±6.5 | 67.0±6 | 66.3±5.5 | 67.7±6.5 | 68.0±5.9 | 68.3±6.2 |
| Female sex, n (%) | 91 (39.7) | 437 (58.1) | 151 (67.1) | 135 (53.3) | 118 (48.1) | 137 (46.6) |
| Body mass index, mean±SD, kg/m2 | 29.6±3.7 | 29.7±3.6 | 29.9±3.7 | 29.5±3.7 | 29.7±3.6 | 29.7±3.6 |
| Intervention group, n (%) | ||||||
| MedDiet+EVOO | 82 (35.8) | 281 (37.4) | 74 (32.9) | 94 (37.1) | 86 (35.1) | 121 (41.1) |
| MedDiet+nuts | 65 (28.4) | 249 (33.1) | 72 (32.0) | 74 (29.2) | 82 (33.4) | 98 (33.3) |
| Control | 82 (35.8) | 221 (29.4) | 79 (35.1) | 85 (33.6) | 77 (31.4) | 75 (27.5) |
| Family history of CHD, n (%) | 44 (19.2) | 193 (25.7) | 66 (29.3) | 60 (23.7) | 53 (21.6) | 63 (21.4) |
| Hypertension, n (%) | 189 (82.5) | 628 (83.7) | 183 (81.3) | 215 (84.9) | 203 (82.8) | 246 (83.6) |
| Dyslipidemia, n (%) | 134 (58.5) | 558 (74.3) | 146 (64.9) | 179 (70.7) | 176 (71.8) | 212 (72.1) |
| Diabetes mellitus, n (%) | 147 (64.2) | 347 (46.2) | 115 (51.1) | 129 (50.9) | 121 (49.4) | 154 (52.4) |
| Antihypertensive medication, n (%) | 167 (72.9) | 564 (75.1) | 173 (76.9) | 193 (76.3) | 173 (70.6) | 217 (73.8) |
| Lipid‐lowering medication, n (%) | 91 (39.7) | 367 (48.8) | 94 (41.8) | 119 (47.0) | 118 (48.2) | 143 (48.6) |
| Oral antidiabetic agents, n (%) | 109 (47.60) | 225 (29.96) | 65 (28.9) | 88 (34.8) | 88 (35.9) | 114 (38.8) |
| Smoking, n (%) | ||||||
| Never | 104 (45.4) | 474 (63.1) | 153 (68.0) | 146 (57.7) | 135 (55.1) | 161 (54.7) |
| Former | 79 (34.5) | 184 (24.5) | 42 (18.6) | 68 (26.8) | 80 (32.6) | 89 (30.2) |
| Current | 46 (20.1) | 93 (12.4) | 30 (13.3) | 39 (12.4) | 30 (12.2) | 44 (14.9) |
Cases have a composite of cardiovascular disease events (myocardial infarction, stroke, and cardiovascular death). CHD indicates coronary heart disease; EVOO, extravirgin olive oil; and MedDiet, Mediterranean diet.
Quartiles were calculated on the basis of the distribution of the choline metabolite score in the subcohort. Inverse normal transformation was applied to raw values of metabolites. To build the score, we applied a weighted sum of concentrations of 5 metabolites in the choline pathway (trimethylamine N‐oxide, betaine, choline, phosphocholine, and α‐glycerophosphocholine).
Plasma Concentrations of Choline Pathway Metabolites and CVD
The associations between individual plasma metabolites and the choline metabolite score with the risk of CVD and stroke are presented in Table 2. Compared with participants in the lowest quartile of choline, those in the highest quartile had a significantly higher risk of CVD after adjusting for cardiovascular risk factors (HR [95% CI], 1.72 [1.05–2.81]; P=0.01 for trend). A 1‐SD increment in baseline concentrations of choline and α‐glycerophosphocholine was associated with higher risk of CVD incidence. Similar, but weakened, associations were observed when stroke was used as the end point (Table 2). In the multivariable adjusted models, the choline score was associated with a 2.21‐fold higher risk of CVD and a 2.27‐fold higher risk of stroke across quartiles (HR [95% CI], 2.21 [1.36–3.59] [P<0.001 for trend] and 2.27 [1.24–4.16] [P<0.001 for trend], respectively). The betaine/choline ratio was inversely associated with the incidence of CVD (HR [95% CI], 0.57 [0.35–0.92]; P=0.04 for trend) but not with the incidence of stroke alone. The HRs associated with a 1‐SD increment in the choline metabolite score and the betaine/choline ratio were 2.27 (95% CI, 1.36–3.80) and 0.80 (95% CI, 0.68–0.94), respectively. Subgroup analysis for the association between the choline score and the risk of CVD is shown in Table 3. We observed similar associations between the score and CVD after adjusting for baseline plasma concentrations of branched chain amino acids, acylcarnitines, and glutamine/glutamate ratio: the HR per SD was 2.27 (95% CI, 1.27–4.08). Similarly, the HR per SD for betaine/choline ratio after adjustment for these metabolites was 0.77 (95% CI, 0.64–0.92). The associations between 1‐SD increment in choline and α‐glycerophosphocholine remained significant after multiple‐comparison adjustment. The adjusted P values were 0.03 and 0.04, respectively. Further adjusting the models for medication use (dyslipidemia, antihypertensive, and antidiabetic medications) did not change the results. For the overall score, the HR per SD was 2.36 (95% CI, 1.39–4.02); and for the betaine/choline ratio, the HR per SD was 0.82 (95% CI, 0.69–0.96).
Table 2.
Risk of CVD and Stroke by Baseline Plasma Concentrations of Choline Metabolites in the PREDIMED Study
| Variable | HR (95% CI) for Quartiles of Plasma Metabolite Concentration | P Trend Value | HR (95% CI) per 1‐SD Increment | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| CVD | ||||||
| TMAO | ||||||
| No. of cases | 43 | 69 | 58 | 59 | … | … |
| Multivariable model 1 | Ref. | 1.44 (0.90–2.28) | 1.13 (0.70–1.80) | 1.25 (0.78–1.99) | 0.60 | 1.04 (0.89–1.21) |
| Multivariable model 2 | Ref. | 1.37 (0.85–2.22) | 1.08 (0.66–1.76) | 1.09 (0.67–1.77) | 0.94 | 1.01 (0.85–1.18) |
| Betaine | ||||||
| No. of cases | 51 | 57 | 60 | 61 | … | … |
| Multivariable model 1 | Ref. | 0.94 (0.59–1.48) | 1.03 (0.66–1.61) | 0.77 (0.49–1.21) | 0.30 | 0.89 (0.77–1.03) |
| Multivariable model 2 | Ref. | 1.00 (0.63–1.60) | 1.23 (0.77–1.97) | 0.91 (0.77–1.49) | 0.87 | 0.95 (0.82–1.12) |
| Choline | ||||||
| No. of cases | 34 | 43 | 68 | 84 | … | … |
| Multivariable model 1 | Ref. | 1.03 (0.62–1.71) | 1.30 (0.78–2.14) | 1.55 (0.96–2.52) | 0.03 | 1.21 (1.02–1.43) |
| Multivariable model 2 | Ref. | 1.08 (0.64–1.81) | 1.48 (0.89–2.46) | 1.72 (1.05–2.81) | 0.01 | 1.24 (1.05–1.46) |
| Phosphocholine | ||||||
| No. of cases | 46 | 58 | 57 | 68 | … | … |
| Multivariable model 1 | Ref. | 1.16 (0.73–1.85) | 1.29 (0.81–2.05) | 1.40 (0.89–2.24) | 0.12 | 1.09 (0.92–1.28) |
| Multivariable model 2 | Ref. | 1.18 (0.73–1.93) | 1.27 (0.77–2.08) | 1.41 (0.87–2.28) | 0.15 | 1.09 (0.92–1.30) |
| α‐Glycerophosphocholine | ||||||
| No. of cases | 51 | 56 | 47 | 75 | … | … |
| Multivariable model 1 | Ref. | 0.89 (0.56–1.42) | 0.79 (0.50–1.26) | 1.26 (0.81–1.97) | 0.30 | 1.20 (1.00–1.44) |
| Multivariable model 2 | Ref. | 0.99 (0.62–1.57) | 0.92 (0.57–1.51) | 1.42 (0.89–2.28) | 0.14 | 1.24 (1.03–1.50) |
| Choline metabolite score | ||||||
| No. of cases | 39 | 64 | 57 | 106 | … | … |
| Multivariable model 1 | Ref. | 1.26 (0.77–2.05) | 1.09 (0.64–1.81) | 1.91 (1.20–3.04) | <0.001 | 2.07 (1.25–3.41) |
| Multivariable model 2 | Ref. | 1.33 (0.81–2.19) | 1.25 (0.74–2.11) | 2.21 (1.36–3.59) | <0.001 | 2.27 (1.36–3.80) |
| Betaine/choline ratio | ||||||
| No. of cases | 74 | 52 | 63 | 40 | … | … |
| Multivariable model 1 | Ref. | 0.70 (0.46–1.08) | 0.86 (0.56–1.30) | 0.50 (0.32–0.78) | <0.001 | 0.77 (0.66–0.90) |
| Multivariable model 2 | Ref. | 0.73 (0.47–1.14) | 0.86 (0.55–1.34) | 0.57 (0.35–0.92) | 0.04 | 0.80 (0.68–0.94) |
| Stroke | ||||||
| TMAO | ||||||
| No. of cases | 24 | 38 | 31 | 25 | … | … |
| Multivariable model 1 | Ref. | 1.32 (0.74–2.35) | 1.06 (0.58–1.94) | 0.91 (0.49–1.69) | 0.55 | 0.93 (0.77–1.12) |
| Multivariable model 2 | Ref. | 1.19 (0.65–2.17) | 0.98 (0.53–1.84) | 0.78 (0.41–1.49) | 0.31 | 0.89 (0.73–1.10) |
| Betaine | ||||||
| No. of cases | 27 | 32 | 32 | 27 | … | … |
| Multivariable model 1 | Ref. | 1.08 (0.61–1.91) | 1.06 (0.60–1.86) | 0.73 (0.39–1.34) | 0.29 | 0.91 (0.75–1.11) |
| Multivariable model 2 | Ref. | 1.15 (0.64–2.04) | 1.32 (0.74–2.36) | 0.89 (0.46–1.71) | 0.85 | 1.00 (0.81–1.23) |
| Choline | ||||||
| No. of cases | 19 | 24 | 31 | 44 | … | … |
| Multivariable model 1 | Ref. | 1.04 (0.54–2.00) | 1.18 (0.59–2.33) | 1.58 (0.84–2.99) | 0.10 | 1.24 (0.98–1.56) |
| Multivariable model 2 | Ref. | 1.08 (0.56–2.09) | 1.33 (0.67–2.62) | 1.73 (0.91–3.29) | 0.06 | 1.27 (1.02–1.59) |
| Phosphocholine | ||||||
| No. of cases | 23 | 26 | 29 | 40 | … | … |
| Multivariable model 1 | Ref. | 0.95 (0.51–1.79) | 1.33 (0.72–2.44) | 1.67 (0.94–2.98) | 0.04 | 1.23 (0.99–1.53) |
| Multivariable model 2 | Ref. | 0.96 (0.50–1.84) | 1.28 (0.66–2.46) | 1.70 (0.93–3.11) | 0.05 | 1.25 (1.00–1.57) |
| α‐Glycerophosphocholine | ||||||
| No. of cases | 31 | 23 | 23 | 41 | … | … |
| Multivariable model 1 | Ref. | 0.63 (0.34–1.18) | 0.63 (0.35–1.15) | 1.15 (0.67–1.98) | 0.52 | 1.17 (0.91–1.50) |
| Multivariable model 2 | Ref. | 0.68 (0.36–1.26) | 0.71 (0.38–1.35) | 1.29 (0.74–2.25) | 0.31 | 1.22 (0.96–1.55) |
| Choline metabolite score | ||||||
| No. of cases | 21 | 25 | 22 | 50 | … | … |
| Multivariable model 1 | Ref. | 1.10 (0.58–2.09) | 0.78 (0.40–1.53) | 1.94 (1.07–3.50) | 0.03 | 1.83 (1.13–2.97) |
| Multivariable model 2 | Ref. | 1.21 (0.63–2.33) | 0.88 (0.44–1.76) | 2.27 (1.24–4.16) | 0.01 | 2.07 (1.25–3.44) |
| Betaine/choline ratio | ||||||
| No. of cases | 38 | 26 | 33 | 21 | … | … |
| Multivariable model 1 | Ref. | 0.68 (0.39–1.20) | 0.88 (0.52–1.52) | 0.54 (0.30–0.97) | 0.08 | 0.78 (0.64–0.95) |
| Multivariable model 2 | Ref. | 0.73 (0.41–1.30) | 0.91 (0.52–1.61) | 0.66 (0.35–1.26) | 0.31 | 0.83 (0.66–1.03) |
Inverse normal transformation was applied to raw values of metabolites. To build the score, we applied a weighted sum of concentrations of 5 metabolites in the choline pathway (TMAO, betaine, choline, phosphocholine, and α‐glycerophosphocholine). The betaine/choline ratio was calculated by dividing the raw values and then applying inverse normal transformations. Model 1 was adjusted for age, sex, body mass index, family history of premature heart disease, and smoking and was stratified by intervention group (only in the overall analyses). Model 2 was adjusted as for model 1 and for physical activity (metabolic equivalent task units in min/d), hypertension, dyslipidemia, and diabetes mellitus. CI indicates confidence interval; CVD, cardiovascular disease; HR, hazard ratio; PREDIMED, Prevention With Mediterranean Diet; Ref., reference; and TMAO, trimethylamine N‐oxide.
Table 3.
Subgroup Analysis for the Associations Between Choline Metabolite Score and Risk of CVD
| Characteristics | HR (95% CI) Per SD Increment | P Value for Interaction |
|---|---|---|
| Sex | ||
| Men (n=452) | 2.31 (1.13–4.71) | 0.83 |
| Women (n=528) | 2.23 (1.03–4.83) | |
| Age, y | ||
| ≤65 (n=338) | 2.67 (1.06–6.69) | 0.49 |
| >65 (n=598) | 2.42 (1.27–4.64) | |
| Obesity, kg/m2 | ||
| ≤30 (n=535) | 2.65 (1.31–5.35) | 0.79 |
| >30 (n=445) | 2.04 (0.92–4.52) | |
| Smoking status | ||
| Current/former smoking (n=402) | 2.45 (1.19–5.06) | 0.63 |
| Ever smoking (n=578) | 2.40 (1.09–5.28) | |
| Family history of CHD | ||
| Yes (n=237) | 2.19 (1.22–3.95) | 0.15 |
| No (n=743) | 3.78 (0.84–5.42) | |
| Baseline type 2 diabetes mellitus | ||
| Yes (n=486) | 2.22 (1.00–4.92) | 0.87 |
| No (n=494) | 2.37 (1.20–4.69) | |
| Baseline hypertension | ||
| Yes (n=817) | 1.94 (1.08–3.46) | 0.36 |
| No (n=163) | 3.12 (0.75–5.65) | |
| Baseline dyslipidemia | ||
| Yes (n=692) | 2.55 (1.31–4.94) | 0.73 |
| No (n=288) | 2.03 (0.86–4.78) | |
Multivariate adjusted HRs (95% CIs) of incident CVD for 1‐SD increment in the baseline choline metabolite score. Data were adjusted for age, sex, body mass index, physical activity (metabolic equivalent task units in min/d), family history of premature heart disease, smoking, hypertension, dyslipidemia, and diabetes mellitus and stratified by intervention group, except for the stratification variable in each model. P values for interaction were derived from Cox models adjusted as above, including an interaction term between the stratification variable and the choline metabolite score. CHD indicates coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; and HR, hazard ratio.
Choline Pathway Metabolites and MedDiet Interventions
As illustrated in the Figure, we observed a higher risk of CVD events for those participants who had a higher choline score (quartiles 2–4) and were assigned to the control group compared with those with lower levels in the score and assigned to the MedDiet intervention groups (HR, 2.37; 95% CI, 1.34–4.18). Participants with a higher betaine/choline ratio and assigned to the MedDiet intervention group had a lower risk of CVD compared with those with a lower ratio and in the control group (HR, 0.36; 95% CI, 0.21–0.63) (Figure). The P values for interaction between the continuous choline score, the betaine/choline ratio, and the intervention group (MedDiet versus control group) and CVD were nonsignificant at P=0.05 and P=0.93, respectively (Figure). Likewise, participants with a higher score and in the control group also had a higher risk of stroke (HR, 2.66; 95% CI, 1.22–5.80) (Table S4).
Figure 1.
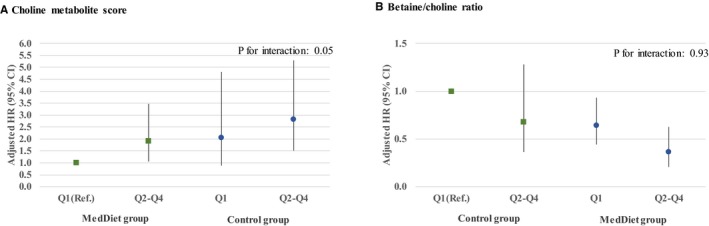
Multivariate adjusted hazard ratios (HRs; 95% confidence intervals [CIs]) of incident cardiovascular disease for quartiles (Q) of choline metabolite score (A) and betaine/choline ratio (B), stratified by intervention group (Mediterranean diet [MedDiet] interventions vs control group). An inverse normal transformation was applied to raw baseline values and a weighted sum of concentrations of 5 metabolites in the choline pathway (trimethylamine N‐oxide, betaine, choline, phosphocholine, and α‐glycerophosphocholine). The betaine/choline ratio was calculated by dividing the raw values and then applying inverse normal transformations. Models adjusted for age, sex, body mass index, smoking, and family history of premature heart disease. P values for interaction were derived from Cox models stratified by intervention group and adjusted as above, including an interaction term between the intervention group and the continuous scores. Ref. indicates reference.
One‐Year Changes in Plasma Concentrations of Metabolites and CVD
No significant associations were observed for quartiles of 1‐year changes in plasma concentrations of individual metabolites and the risk of CVD and stroke. In multivariable model 1, a 1‐SD increment of betaine after 1 year was associated with lower risk of CVD (HR [95% CI], 0.80 [0.66–0.98]), but the association was attenuated in multivariable model 2 and when analyzed as quartiles of the change (Table S5).
Discussion
In this case‐cohort study nested within the PREDIMED Study, we observed that baseline plasma concentrations of choline pathway metabolites (choline, phosphocholine, and α‐glycerophosphocholine) and a metabolite score combining choline pathway metabolites were associated with the risk of major cardiovascular events after 4.8 years of follow‐up. Higher concentrations of a choline metabolite score were associated with higher risk of CVD and stroke, whereas the ratio of betaine/choline was inversely associated with CVD but not with stroke alone. Therefore, our findings suggest that metabolites from choline pathways may play a role in the development of CVD.
Plasma metabolites in the choline pathway, which are derivatives from dietary sources and may also reflect alterations in the gut microbiota, have been previously related to multifactorial diseases, such as obesity, type 2 diabetes mellitus, and CVD1, 22 Several mechanisms are probably involved, including modulation of host energy metabolism, gut epithelial permeability, gut peptide hormone secretion, and an increase in metabolic endotoxemia and inflammatory status.23, 24, 25 One of the mechanisms explaining the potential associations between some of these metabolites and CVD is the potential proatherogenic effects.6 It has been shown that TMAO can inhibit reverse cholesterol transport, alter sterol metabolism,26 and affect platelet activation and thrombosis risk.27 However, the associations between plasma metabolites within the choline pathway, identified using novel high‐throughput metabolomics techniques, and the incidence of CVD at a population‐based level have only recently been reported.
To our knowledge, this is the first case‐cohort study in a clinical trial setting showing that higher concentrations of plasma choline and α‐glycerophosphocholine are associated with an increased risk of CVD; higher concentrations of choline and phosphocholine are associated with a higher risk of stroke. The associations between dietary and plasma choline and other metabolites from this pathway and CVD events in humans are still unclear. Epidemiologic studies have reported inconsistent associations between systemic levels of choline and betaine and cardiovascular events.6, 7, 28, 29 Recently, it has been reported that higher plasma choline and betaine levels were associated with 1.9‐ and 1.4‐fold increased risks, respectively, of major adverse cardiac events after 3 years of follow‐up in patients undergoing elective diagnostic coronary angiography.29 However, these associations were attenuated and no longer significant when TMAO was included in the models.29 In contrast, betaine supplementation attenuated atherosclerotic lesions in apolipoprotein E−/− mice.30 In our study, the betaine/choline ratio was inversely associated with CVD in individuals at high cardiovascular risk, suggesting that the balance between systemic concentrations of betaine and choline could be of importance for the prevention of cardiovascular events. However, we found no significant associations between 1‐year changes in choline pathway metabolites and the incidence of CVD. It is possible that 1 year was a short period to observe these associations, or other mechanisms and pathways are implicated in the pathogenesis of CVD in our population.
Interestingly, in a cross‐sectional subset of the Nutrition, Aging and Memory in Elders cohort,31 higher plasma choline concentrations were associated with an unfavorable cardiometabolic risk factor profile (lower high‐density lipoprotein cholesterol and higher body mass index). They were also associated with greater odds of large‐vessel cerebral vascular disease and history of CVD but lower odds of small‐vessel cerebral vascular disease. On the contrary, plasma betaine concentrations were associated with a favorable cardiometabolic risk factor profile (lower low‐density lipoprotein cholesterol and triglycerides) and lower odds of diabetes mellitus. In the same study, higher plasma phosphatidylcholine was associated with characteristics of both favorable (higher high‐density lipoprotein cholesterol and lower body mass index) and unfavorable (higher low‐density lipoprotein cholesterol and triglycerides) cardiometabolic risk. These findings suggest divergent roles of choline metabolites in the pathogenesis of cardiometabolic risk factors and cerebrovascular disease.31 Similarly, in our study, we have observed that baseline choline concentrations are marginally associated with the prevalence of being overweight and obesity (odds ratios [95% CIs] per 1‐SD increase, 1.13 [1.00–1.29] and 1.15 [1.00–1.34], respectively). Significant positive correlations, although they were weak, were observed for choline and triglycerides (Spearman correlation coefficient=0.08; P=0.04). We have also observed cross‐sectional inverse associations between plasma betaine and prevalence of diabetes mellitus and being overweight (odds ratios [95% CIs] per 1‐SD increase, 0.64 [0.55–0.74] and 0.88 [0.76–1.06], respectively). Plasma betaine was inversely correlated with plasma glucose and body mass index (Spearman correlation coefficient=−0.18 and −0.07 [P<0.01 and P=0.01], respectively). Altogether, these findings suggested a relationship between these metabolites and cardiovascular risk factors.
The associations between dietary choline and betaine on the risk of CVD have been evaluated in several prospective studies.32, 33, 34 Compared with the lowest quartile of intake, incident coronary heart disease after 14 years of follow‐up in participants from the ARIC (Atherosclerosis Risk in Communities) study was nonsignificantly higher in the highest quartile of choline and choline plus betaine (HRs [95% CIs], 1.22 [0.91–1.64] and 1.14 [0.85–1.53], respectively), compared with the lowest quartile.32 In women from the Jackson Heart Study, greater choline intake was associated with lower left ventricular mass (P=0.0006 for trend across choline quartiles) and with abdominal aortic calcium score.34 Similarly, higher dietary intakes of betaine and choline were not associated with CVD risk in postmenopausal Dutch women followed up for 97 months.33 On the other hand, findings from the Nurses' Health Study and Health Professionals Follow‐Up Study, including 80 978 women and 39 434 men, respectively, followed up to 32 years, suggested that higher phosphatidylcholine intake was associated with an increased risk of CVD mortality after adjustment for potential confounders.35
Previous published literature on this topic has mostly focused on the formation of the gut microbiota–dependent metabolite, TMAO.2, 4, 6 One of the most compelling studies linking these metabolites with CVD complications was reported by Wang and collaborators a few years ago.6 The authors identified a novel pathway linking dietary phosphatidylcholine/choline, which can be converted by the intestinal microbiota into trimethylamine and subsequently converted into TMAO by hepatic flavin‐containing mono‐oxygenases, with CVD pathogenesis.13 These findings are consistent with later in vivo and in vitro studies, suggesting that choline metabolites may increase the risk of atherosclerosis and coronary heart disease.4, 29, 36, 37 In addition, the ability of oral broad‐spectrum antibiotics to temporarily suppress the production of TMAO suggests that intestinal microorganisms may play an important role in the production of TMAO from phosphatidylcholine in humans.4 Nevertheless, our data did not support positive significant associations between plasma concentrations of TMAO and the risk of CVD in individuals at high cardiovascular risk after 4.8 years of follow‐up. It is plausible that TMAO may be more relevant for predicting recurrent CVD or CVD survival, being a marker of established disease. Although TMAO was associated with CVD mortality in participants undergoing incident dialysis in a nested case‐control study, when they replicated the findings in an independent study, no differences were found in TMAO concentrations between cases of cardiovascular death and controls.7 One of the potential reasons for these associations is that elevated TMAO levels are strongly related with renal function in patients with chronic kidney disease, and they normalize after renal transplantation.38 Therefore, because kidney disease is a risk factor for CVD, TMAO was probably associated with CVD mortality because individuals undergoing dialysis have more risk of developing CVD.
Findings from a recent study, including 817 participants of the CARDIA (Coronary Artery Risk Development in Young Adults), followed up for 10 years, showed that TMAO was not associated with coronary artery calcium incidence or other measures of CVD risk, including insulin resistance, inflammatory markers, and lipid profile. The authors suggest that it is possible that the adverse effect of TMAO may be more relevant when concentrations of TMAO are higher than in their sample or in later stages of the disease process.10 In another study comparing the concentrations of TMAO, carnitine, and choline in 34 obese individuals undergoing bariatric surgery, TMAO was not elevated in obese patients before the surgery but increased ≈2‐fold after bariatric surgery.39 The authors reported that these results were unexpected and suggested that one explanation could be adaptive shifts in the gut microbiota with increased ability to metabolize dietary choline and carnitine to TMAO precursors.39 In contrast, carnitine and choline, which are abundant in nutrients, such as in red meat and eggs, and not microbiota dependent, were reduced after lifestyle interventions and rebounded after bariatric surgery.39
Along these lines, choline and its metabolites can be obtained from food sources or synthesized de novo. In a randomized, controlled, crossover trial, it was observed that dimethylamine and TMAO were significantly higher after consuming a diet high in fish.40 The authors reported that plasma and urinary concentrations of TMAO were associated with recent fish intake; therefore, high urinary TMAO concentrations can be associated with healthy diets that are rich in fish.40 Our data also showed that plasma TMAO is correlated with the intake of fish and total meat (Spearman correlation coefficients, 0.12 and 0.08, respectively; P<0.01). However, gut bacteria can synthesize TMAO from choline and, hence, high urinary and plasma TMAO concentrations can also originate from red meat consumption, which is generally associated with adverse health outcomes. Thus, the global pattern of metabolites, which reflects the totality of the diet, might be more important in indicating dietary patterns than are individual biomarkers.40 Findings from Tang et al suggest that excessive consumption of phosphatidylcholine and choline should be avoided by consuming a vegetarian or high‐fiber diet,4 which has similar characteristics as the MedDiet intervention in the PREDIMED Study. It is established that foods such as meat, egg yolks, and high‐fat dairy products, which are high in phosphatidylcholine, choline, carnitine, and TMA, can serve as precursors of TMAO production.6
Another point that has not been raised before and deserves consideration is the possible attenuation of detrimental effects of metabolite profiles on CVD risk by adhering to a MedDiet. Multiple lines of evidence could account for the observed benefits of the MedDiet on CVD, including the reduction of low‐grade inflammation,41 enhanced endothelial function,42 lower oxidative stress,43 and lower levels of oxidized low‐density lipoprotein.44 Integrating diet information with metabolomics data holds great potential for further understanding biomarkers and mechanisms that relate diet, health, and disease. The present work may have important public health implications for focusing on dietary interventions, such as adhering to the MedDiet and reducing the intake of animal products, to improve metabolite profiles and, consequently, the risk of CVD.
Several limitations and strengths of the present study warrant mentioning. First, because the participants were elderly Mediterranean individuals at high cardiovascular risk, the results may not be extrapolated to other populations. Notwithstanding, because of their increased risk of CVD, they are an important therapeutic target. In addition, the case‐cohort design maximized the efficiency of the high‐throughput metabolomics profiling, which allowed us to extend the results to all PREDIMED participants. Second, although we adjusted for several potential confounders, residual confounding by other unknown or unmeasured variables cannot be ruled out. Third, liquid chromatography–tandem mass spectrometry–based metabolite measurements may not have a direct clinical translation for each metabolite trait. Finally, in the present analysis, we have only included choline pathway metabolites, as subrogates of diet and gut microbiota–related metabolites, that have been identified in our samples with the use of the metabolomics platform from the present project. However, in the coming years, new metabolites will probably need to be included in this group. The strengths of the present work include the prospective design, the ability to control for potential confounders because of the well‐characterized comprehensive data of the randomized PREDIMED trial, and the accurate and blind assessment of incident CVD cases.
Conclusion
In conclusion, our findings demonstrated, for the first time, that metabolites from the choline pathway were associated with an increased risk of major adverse cardiovascular events, independent of traditional risk factors, in a Mediterranean population at high cardiovascular risk.
Author Contributions
Guasch‐Ferré, Hu, Martínez‐González, and Salas‐Salvadó conceived and designed research; Guasch‐Ferré and Liang performed statistical analysis; Hu, Martínez‐González, and Salas‐Salvadó handled funding and supervision; Guasch‐Ferré, Hu, Ruiz‐Canela, Bulló, Toledo, Wang, Corella, Gómez‐Gracia, Fiol, Estruch, Lapetra, Fitó, Arós, Majem, Ros, Dennis, Liang, Clish, Martínez‐González, and Salas‐Salvadó acquired the data; Guasch‐Ferré and Salas‐Salvadó drafted the article Hu, Ruiz‐Canela, Bulló, Toledo, Wang, Corella, Gómez‐Gracia, Fiol, Estruch, Lapetra, Fitó, Arós, Majem, Ros, Liang, Clish, Martínez‐González, and Salas‐Salvadó critically revised the article for key intellectual content.
Sources of Funding
This work was supported by NIH research grant HL118264. The PREDIMED trial was supported by the official funding agency for biomedical research of the Spanish Government, Instituto de Salud Carlos III, through grants provided to research networks specifically developed for the trial (RTIC G03/140 to Estruch; RTIC RD 06/0045 to Martínez‐González) and through Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y Nutrición; and by grants from Centro Nacional de Investigaciones Cardiovasculares (06/2007), Fondo de Investigación Sanitaria–Fondo Europeo de Desarrollo Regional (PI04–2239, PI05/2584, CP06/00100, PI07/0240, PI07/1138, PI07/0954, PI 07/0473, PI10/01407, PI10/02658, PI11/01647, P11/02505, and PI13/00462), Ministerio de Ciencia e Innovación (AGL‐2009–13906‐C02 and AGL2010–22319‐C03), Fundación Mapfre 2010, Consejería de Salud de la Junta de Andalucía (PI0105/2007), Public Health Division of the Department of Health of the Autonomous Government of Catalonia, Generalitat Valenciana (ACOMP06109, GVA‐COMP2010–181, GVACOMP2011–151, CS2010‐AP‐111, and CS2011‐AP‐042), and Regional Government of Navarra (P27/2011). Guasch‐Ferré was supported by a postdoctoral fellowship granted by the Agency for Administration of University and Research Grants of the Autonomous Government of Catalonia (2014‐BP‐A 00017). None of the funding sources played a role in the design, collection, analysis, or interpretation of the data or in the decision to submit the article for publication.
Disclosures
Hu and Ros received grants from the California Walnut Commission. Martínez‐González and Salas‐Salvadó received grants from the International Nut Council. None of the other authors has a relevant conflict of interest to report related to this research.
Supporting information
Table S1. Characteristics of the Choline Pathway Metabolites
Table S2. Means and SD of Baseline Plasma Concentration of Metabolites (μmol/L) in the Total Population
Table S3. Means and SD of Plasma Concentration of Metabolites (μmol/L) Per Intervention Group at Baseline and 1 Year of Intervention
Table S4. Joint Associations of the Scores and Intervention Group With Risk of Cardiovascular Disease and Stroke
Table S5. Risk of Cardiovascular Disease and Stroke by 1‐Year Changes in Choline Pathway Metabolites in the PREDIMED Study
Figure S1. Flow chart of study participants.
Figure S2. Diagram of choline pathway.
Figure S3. Correlation matrix for plasma metabolite levels.
Acknowledgments
We thank all the participants for their collaboration, all the PREDIMED personnel for their assistance, and all the personnel of affiliated primary care centers for making the study possible.
(J Am Heart Assoc. 2017;6:e006524 DOI: 10.1161/JAHA.117.006524.)29080862
Contributor Information
Marta Guasch‐Ferré, Email: mguasch@hsph.harvard.edu.
Jordi Salas‐Salvadó, Email: jordi.salas@urv.cat.
References
- 1. Griffin JL, Wang X, Stanley E. Does our gut microbiome predict cardiovascular risk? A review of the evidence from metabolomics. Circ Cardiovasc Genet. 2015;8:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian‐Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non‐lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen Y, Liu Y, Zhou R, Chen X, Wang C, Tan X, Wang L, Zheng R, Zhang H, Ling W, Zhu H. Associations of gut‐flora‐dependent metabolite trimethylamine‐N‐oxide, betaine and choline with non‐alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miele L, Giorgio V, Alberelli MA, De Candia E, Gasbarrini A, Grieco A. Impact of gut microbiota on obesity, diabetes, and cardiovascular disease risk. Curr Cardiol Rep. 2015;17:120. [DOI] [PubMed] [Google Scholar]
- 6. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung Y‐M, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalim S, Clish CB, Wenger J, Elmariah S, Yeh RW, Deferio JJ, Pierce K, Deik A, Gerszten RE, Thadhani R, Rhee EP. A plasma long‐chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J Am Heart Assoc. 2013;2:e000542 DOI: 10.1161/JAHA.113.000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaarhorst AAM, Verhoeven A, Weller CM, Böhringer S, Göraler S, Meissner A, Deelder AM, Henneman P, Gorgels APM, van den Brandt PA, Schouten LJ, van Greevenbroek MM, Merry AHH, Verschuren WMM, van den Maagdenberg AMJM, van Dijk KW, Isaacs A, Boomsma D, Oostra BA, van Duijn CM, Jukema JW, Boer JMA, Feskens E, Heijmans BT, Slagboom PE. A metabolomic profile is associated with the risk of incident coronary heart disease. Am Heart J. 2014;168:45–52.e7. [DOI] [PubMed] [Google Scholar]
- 9. Yin J, Liao S‐X, He Y, Wang S, Xia G‐H, Liu F‐T, Zhu J‐J, You C, Chen Q, Zhou L, Pan S‐Y, Zhou H‐W. Dysbiosis of gut microbiota with reduced trimethylamine‐N‐oxide level in patients with large‐artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015;4:e002699 DOI: 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meyer KA, Benton TZ, Bennett BJ, Jacobs DR, Lloyd‐Jones DM, Gross MD, Carr JJ, Gordon‐Larsen P, Zeisel SH. Microbiota‐dependent metabolite trimethylamine N‐oxide and coronary artery calcium in the coronary artery risk development in young adults study (CARDIA). J Am Heart Assoc. 2016;5:e003970 DOI: 10.1161/JAHA.116.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Estruch R, Ros E, Salas‐Salvado J, Covas MI, Corella D, Aros F, Gomez‐Gracia E, Ruiz‐Gutierrez V, Fiol M, Lapetra J, Lamuela‐Raventos RM, Serra‐Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez‐Gonzalez MA; PREDIMED Study Investigators . Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 12. Martinez‐Gonzalez MA, Corella D, Salas‐Salvado J, Ros E, Covas MI, Fiol M, Warnberg J, Aros F, Ruiz‐Gutierrez V, Lamuela‐Raventos RM, Lapetra J, Munoz MA, Martinez JA, Saez G, Serra‐Majem L, Pinto X, Mitjavila MT, Tur JA, Portillo Mdel P, Estruch R; PREDIMED Study Investigators . Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol. 2012;41:377–385. [DOI] [PubMed] [Google Scholar]
- 13. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elosua R, Marrugat J, Molina L, Pons S, Pujol E; MARATHOM Investigators . Validation of the Minnesota leisure time physical activity questionnaire in Spanish men. Am J Epidemiol. 1994;139:1197–1209. [DOI] [PubMed] [Google Scholar]
- 15. Fernandez‐Ballart JD, Pinol JL, Zazpe I, Corella D, Carrasco P, Toledo E, Perez‐Bauer M, Martinez‐Gonzalez MA, Salas‐Salvado J, Martin‐Moreno JM. Relative validity of a semi‐quantitative food‐frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr. 2010;103:1808–1816. [DOI] [PubMed] [Google Scholar]
- 16. Mataix J. Tablas de Composición de Alimentos. 4th ed Granada: Universidad de Granada; 2003. [Google Scholar]
- 17. Beasley TM, Erickson S, Allison DB. Rank‐based inverse normal transformations are increasingly used, but are they merited? Behav Genet. 2009;39:580–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case‐cohort designs. J Clin Epidemiol. 1999;52:1165–1172. [DOI] [PubMed] [Google Scholar]
- 19. Ruiz‐Canela M, Toledo E, Clish CB, Hruby A, Liang L, Salas‐Salvado J, Razquin C, Corella D, Estruch R, Ros E, Fito M, Gomez‐Gracia E, Aros F, Fiol M, Lapetra J, Serra‐Majem L, Martinez‐Gonzalez MA, Hu FB. Plasma branched‐chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin Chem. 2016;62:582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guasch‐Ferré M, Zheng Y, Ruiz‐Canela M, Hruby A, Martínez‐González MA, Clish CB, Corella D, Estruch R, Ros E, Fitó M, Dennis C, Morales‐Gil IM, Arós F, Fiol M, Lapetra J, Serra‐Majem L, Hu FB, Salas‐Salvadó J. Plasma acylcarnitines and risk of cardiovascular disease: effect of Mediterranean diet interventions. Am J Clin Nutr. 2016;103:1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng Y, Hu FB, Ruiz‐Canela M, Clish CB, Dennis C, Salas‐Salvado J, Hruby A, Liang L, Toledo E, Corella D, Ros E, Fitó M, Gómez‐Gracia E, Arós F, Fiol M, Lapetra J, Serra‐Majem L, Estruch R, Martínez‐González MA. Metabolites of glutamate metabolism are associated with incident cardiovascular events in the PREDIMED PREvención con DIeta MEDiterránea (PREDIMED) Trial. J Am Heart Assoc. 2016;5:e003755 DOI: 10.1161/JAHA.116.003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palau‐Rodriguez M, Tulipani S, Isabel Queipo‐Ortuño M, Urpi‐Sarda M, Tinahones FJ, Andres‐Lacueva C. Metabolomic insights into the intricate gut microbial‐host interaction in the development of obesity and type 2 diabetes. Front Microbiol. 2015;6:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host‐bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. [DOI] [PubMed] [Google Scholar]
- 24. Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short‐chain fatty‐acid binding G protein‐coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang WHW, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WHW, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L‐carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang J, Li Z, Chen J, Zhao H, Luo L, Chen C, Xu X, Zhang W, Gao K, Li B, Zhang J, Wang W. Metabolomic identification of diagnostic plasma biomarkers in humans with chronic heart failure. Mol Biosyst. 2013;9:2618–2626. [DOI] [PubMed] [Google Scholar]
- 29. Wang Z, Tang WHW, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota‐generated metabolite trimethylamine‐N‐oxide. Eur Heart J. 2014;35:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lv S, Fan R, Du Y, Hou M, Tang Z, Ling W, Zhu H. Betaine supplementation attenuates atherosclerotic lesion in apolipoprotein E‐deficient mice. Eur J Nutr. 2009;48:205–212. [DOI] [PubMed] [Google Scholar]
- 31. Roe AJ, Zhang S, Bhadelia RA, Johnson EJ, Lichtenstein AH, Rogers GT, Rosenberg IH, Smith CE, Zeisel SH, Scott TM. Choline and its metabolites are differently associated with cardiometabolic risk factors, history of cardiovascular disease, and MRI‐documented cerebrovascular disease in older adults. Am J Clin Nutr. 2017;105:1283–1290. DOI: 10.3945/ajcn.116.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bidulescu A, Chambless LE, Siega‐Riz AM, Zeisel SH, Heiss G. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. 2007;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dalmeijer GW, Olthof MR, Verhoef P, Bots ML, van der Schouw YT. Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur J Clin Nutr. 2008;62:386–394. [DOI] [PubMed] [Google Scholar]
- 34. Millard HR, Musani SK, Dibaba DT, Talegawkar SA, Taylor HA, Tucker KL, Bidulescu A. Dietary choline and betaine; associations with subclinical markers of cardiovascular disease risk and incidence of CVD, coronary heart disease and stroke: the Jackson Heart Study. Eur J Nutr. 2016. Available at: https://jhu.pure.elsevier.com/en/publications/dietary-choline-and-betaine-associations-with-subclinical-markers. Accessed October 6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng Y, Li Y, Rimm EB, Hu FB, Albert CM, Rexrode KM, Manson JE, Qi L. Dietary phosphatidylcholine and risk of all‐cause and cardiovascular‐specific mortality among US women and men. Am J Clin Nutr. 2016;104:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Tang WHW, Hazen SL. Intestinal microbiota‐generated metabolite trimethylamine‐N‐oxide and 5‐year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE‐like patient cohort. J Am Heart Assoc. 2016;5:e002816 DOI: 10.1161/JAHA.115.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Senthong V, Li XS, Hudec T, Coughlin J, Wu Y, Levison B, Wang Z, Hazen SL, Tang WHW. Plasma trimethylamine N‐oxide, a gut microbe‐generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. 2016;67:2620–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Missailidis C, Hällqvist J, Qureshi AR, Barany P, Heimbürger O, Lindholm B, Stenvinkel P, Bergman P. Serum trimethylamine‐N‐oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11:e0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trøseid M, Hov JR, Nestvold TK, Thoresen H, Berge RK, Svardal A, Lappegård KT. Major increase in microbiota‐dependent proatherogenic metabolite TMAO one year after bariatric surgery. Metab Syndr Relat Disord. 2016;14:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garcia‐Perez I, Posma JM, Gibson R, Chambers ES, Hansen TH, Vestergaard H, Hansen T, Beckmann M, Pedersen O, Elliott P, Stamler J, Nicholson JK, Draper J, Mathers JC, Holmes E, Frost G. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 2017;5:184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Estruch R. Anti‐inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proc Nutr Soc. 2010;69:333–340. [DOI] [PubMed] [Google Scholar]
- 42. Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D'Armiento M, D'Andrea F, Giugliano D. Effect of a Mediterranean‐style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. [DOI] [PubMed] [Google Scholar]
- 43. Bulló M, Lamuela‐Raventós R, Salas‐Salvadó J. Mediterranean diet and oxidation: nuts and olive oil as important sources of fat and antioxidants. Curr Top Med Chem. 2011;11:1797–1810. [DOI] [PubMed] [Google Scholar]
- 44. Fitó M, Guxens M, Corella D, Sáez G, Estruch R, de la Torre R, Francés F, Cabezas C, López‐Sabater MDC, Marrugat J, García‐Arellano A, Arós F, Ruiz‐Gutierrez V, Ros E, Salas‐Salvadó J, Fiol M, Solá R, Covas M‐I; PREDIMED Study Investigators . Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med. 2007;167:1195–1203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of the Choline Pathway Metabolites
Table S2. Means and SD of Baseline Plasma Concentration of Metabolites (μmol/L) in the Total Population
Table S3. Means and SD of Plasma Concentration of Metabolites (μmol/L) Per Intervention Group at Baseline and 1 Year of Intervention
Table S4. Joint Associations of the Scores and Intervention Group With Risk of Cardiovascular Disease and Stroke
Table S5. Risk of Cardiovascular Disease and Stroke by 1‐Year Changes in Choline Pathway Metabolites in the PREDIMED Study
Figure S1. Flow chart of study participants.
Figure S2. Diagram of choline pathway.
Figure S3. Correlation matrix for plasma metabolite levels.


