Abstract
Background
The American College of Cardiology (ACC) and European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) have recently published recommendations for the use of proprotein convertase subtilisin/kexin‐9 (PCSK9) inhibitors in situations of very high risk. We aim to assess in the real world the suitability of PCSK9 inhibitors for acute coronary syndromes.
Methods and Results
We analyzed a prospective Swiss cohort of 2023 patients hospitalized for acute coronary syndromes between 2009 and 2014 with available data for low‐density lipoprotein cholesterol and lipid‐lowering therapy at 1 year. Clinical familial hypercholesterolemia was defined using the Dutch Lipid Clinic Network algorithm as unlikely, possible, probable, or definite. We simulated a fixed relative reduction of 24% in low‐density lipoprotein cholesterol levels at 1 year in all patients not treated with ezetimibe, irrespective of the low‐density lipoprotein cholesterol levels and statin regimen. At 1 year, 94.3% of patients were treated with statin, 5.8% with ezetimibe, and 35.8% of patients had on‐target low‐density lipoprotein cholesterol levels (<1.8 mmol/L); 25.6% met criteria for possible or probable/definite familial hypercholesterolemia. After a simulation of the lipid‐lowering effect of ezetimibe, the proportion of patients who would be eligible for PCSK9 inhibitors at 1 year was 13.4% using American College of Cardiology criteria and 2.7% using European Society of Cardiology/European Atherosclerosis Society criteria. Patients with possible or probable/definite familial hypercholesterolemia were more eligible for PCSK9 inhibitors compared with their non–familial hypercholesterolemia counterparts: 27.6% versus 8.8% according to American College of Cardiology criteria and 6.6% versus 1.8% according to European Society of Cardiology/European Atherosclerosis Society criteria (P<0.001).
Conclusions
Recommendations made by the American College of Cardiology guidelines would lead to 5‐fold higher eligibility rates for PCSK9 inhibitors compared to the European Society of Cardiology/European Atherosclerosis Society consensus statement in acute coronary syndrome patients.
Keywords: lipids, PCSK9, secondary prevention
Subject Categories: Secondary Prevention, Myocardial Infarction, Lipids and Cholesterol
Clinical Perspective
What Is New?
-
1
No study has compared the eligibility for proprotein convertase subtilisin/kexin‐9 inhibitors in patients with acute coronary syndromes.
-
2
In this large Swiss cohort we observed that 13% of patients would be eligible for proprotein convertase subtilisin/kexin‐9 inhibitors based on the American College of Cardiology (ACC) criteria, and 3% based on the European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) criteria.
-
3
These differences illustrate the more conservative approach proposed by the ESC/EAS document.
What Are the Clinical Implications?
-
1
The incremental low‐density lipoprotein cholesterol–lowering effect conveyed by pretreatment with ezetimibe would dramatically reduce the indication for proprotein convertase subtilisin/kexin‐9 inhibitors in both algorithms.
-
2
Identifying acute coronary syndrome patients with familial hypercholesterolemia based on the Dutch Lipid Clinic Network clinical score will help clinicians to select patients who would be more likely to formally qualify for treatment with proprotein convertase subtilisin/kexin‐9 inhibitors.
Although long‐term prognosis of patients after acute coronary syndromes (ACS) has considerably improved, the residual risk remains high with a recurrence rate of ischemic events of 20% within 3 years.1 Lipid‐lowering therapies, such as statins or ezetimibe, have shown a reduction of major adverse cardiovascular events in secondary prevention after ACS.2, 3 European Society of Cardiology (ESC) guidelines on the management of ACS recommend decreasing low‐density lipoprotein cholesterol (LDL‐C) to a target level of <1.8 mmol/L using high‐intensity statin therapy in combination with ezetimibe if needed.4, 5
However, real‐life data suggest that less than one third of ACS patients are able to reach the recommended targets, including those treated with high‐intensity statin therapy.6 The reasons for poorly controlled LDL‐C levels are statin resistance, lack of therapy intensification, or statin intolerance (eg, statin‐associated muscle symptoms).7 The proprotein convertase subtilisin/kexin‐9 (PCSK9) inhibitors have emerged as a promising therapy for the treatment of hypercholesterolemia, because monoclonal antibodies against PCSK9 lowered LDL‐C by 50% to 60%.8, 9 Both alirocumab and evolocumab have been approved by regulatory agencies for the treatment of primary hypercholesterolemia in case of poorly controlled LDL‐C with maximal tolerated statin therapy or as a second‐line treatment in case of documented statin intolerance.10 After post hoc analyses suggesting a relative reduction of major adverse cardiovascular events by 50% for both agents,11, 12 the FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) trial including 27 564 patients with atherosclerotic cardiovascular disease (ASCVD) met its primary outcome with a significant reduction of major adverse cardiovascular events by 11.3% (P<0.001) in the evolocumab arm compared with placebo over a median follow‐up of 2.2 years.13 Recently, both the American College of Cardiology (ACC) expert consensus decision pathway on the role of nonstatin therapies for LDL‐C lowering in the management of ASCVD and a joint consensus statement from the ESC and European Atherosclerosis Society (EAS) have provided a practical guidance for the use of PCSK9 inhibitors in patients at very high cardiovascular risk, such as those with manifest ASCVD, familial hypercholesterolemia (FH), and statin intolerance.10, 14 Those statements took an initial position by defining criteria for consideration of PCSK9 inhibition based on current evidence. No real‐world data are available regarding the eligibility of PCSK9 inhibitors according to the suggested European and American criteria and the potential place of those promising agents in secondary prevention after ACS. Against this background, we evaluated the suitability of PCSK9 inhibitors 1 year after the index ACS event in a large, contemporary European population.
Methods
Study Population
The present analysis was performed within the ELIPS (Multidimensional PreventIon Program After Acute Coronary Syndrome) study (NCT01075867). ELIPS is a subproject of the ongoing, prospective, multicenter, and observational SPUM‐ACS (Special Program University Medicine‐Acute Coronary Syndromes) cohort aiming to assess the quality of care and the adherence to the recommended preventive targets in 4 academic centers in Switzerland: Bern, Geneva, Lausanne, and Zurich.6, 7, 15, 16, 17 For this analysis, we considered all ACS patients who were enrolled between 2009 and 2014 and survived 1 year after the index event hospitalization with available data on lipid values and lipid‐lowering therapies as well as data on the occurrence of clinical outcomes at 1‐year follow‐up. Inclusion criteria were age ≥18 years and index diagnosis of ST‐elevation myocardial infarction (MI), non–ST‐elevation MI, or unstable angina. Exclusion criteria were limited to severe physical disability or dementia and life expectancy less than 1 year for noncardiac reasons. The protocol was approved by local ethical committees. All patients provided written informed consent.
Lipid‐Lowering Medications
Medication before hospitalization, at discharge, and at 1‐year follow‐up was systematically collected by trained study nurses. Intensity of statin therapy was classified as low, moderate; or high intensity based on current lipid guidelines (Table S1).5 Physicians were encouraged to prescribe guideline‐recommended statin treatment at discharge, that is, high‐intensity statin except for patients in whom such regimens were not deemed appropriate (eg, because of a history of intolerance, patient characteristics, or concomitant medications that increased the risk for developing statin‐related adverse events, or in elderly patients). Treating general practitioners and cardiologists were encouraged not to discontinue or change statin treatment to lower‐intensity regimens during the follow‐up period unless clinically indicated. Consistently, the present analyses were done with the assumption that patients were on maximally tolerated study treatment at 1 year. Reasons for nonprescription of statins were collected at discharge using medical records, as were reasons for therapy discontinuation at 1 year according to the patients. At the 1‐year follow‐up, we also asked patients to bring the list of medications and the pill boxes to confirm their statement. Nonstatin lipid‐lowering therapies included ezetimibe, fibrates, niacin, and bile acid resins.
Blood Lipid Measurements and Clinical Diagnosis of Familial Hypercholesterolemia
At baseline, levels for total cholesterol, high‐density lipoprotein cholesterol and triglycerides were measured from the first available fasting sample within 24 hours of hospital admission. LDL‐C was calculated using the Friedewald equation when triglycerides were below 4.5 mmol/L.15 One year after hospitalization, blood lipid levels were measured at the scheduled follow‐up visit in a fasting‐state. Clinical diagnosis of FH was based on the recommended Dutch Lipid Clinic Network (DLCN) algorithm (Table S2).15 Variables included personal and family history of premature cardiovascular disease and LDL‐C levels at baseline. Possible FH was defined by a score 3 to 5 and probable/definite FH by a score ≥6. For patients taking lipid‐lowering medications before hospitalization, we estimated untreated baseline LDL‐C levels based on the type and dose of medication, applying appropriate correcting factors corresponding to the reported efficacy of each drug. (Table S3).18 As done previously, only patients with normal triglycerides (<2.3 mmol/L) received scoring for elevated LDL‐C levels as recommended by the DLCN score for FH diagnosis.15
Eligibility for PCSK9 Inhibitors
On the basis of 1‐year LDL‐C levels, lipid‐lowering therapies, and clinical outcomes, the eligibility for the use of PCSK9 inhibitors at follow‐up was defined according to the following ESC/EAS criteria: (1) LDL‐C ≥3.6 mmol/L (140 mg/dL) at 1 year while on statin in combination with ezetimibe; or (2) LDL‐C ≥2.6 mmol/L (100 mg/dL) at 1 year in case of rapid progression of ASCVD. In this study, rapid progression of ASCVD for the ESC/EAS definition included (1) recurrent MI, hospitalization for unstable angina, unplanned coronary revascularization or ischemic stroke within 1 year of the index ACS event; or (2) previous MI, percutaneous coronary intervention, coronary artery bypass surgery, an ischemic cerebrovascular event, or peripheral arterial disease before the index event. The eligibility according ACC criteria was defined: (1) LDL‐C ≥2.6 mmol/L (100 mg/dL) while on statin in combination with ezetimibe and a reduction of LDL‐C levels less than 50% from baseline; or (2) LDL‐C ≥1.8 mmol/L (70 mg/dL) while on statin in combination with ezetimibe and a reduction of LDL‐C levels less than 50% from baseline in case of diabetes mellitus or an ASCVD event 3 months before follow‐up irrespective of statin therapy at discharge, or at any time during follow‐up if on statin at discharge; or (3) baseline LDL‐C ≥4.9 mmol/L (190 mg/dL) and 1‐year LDL‐C ≥1.8 mmol/L (70 mg/dL) while on statin and a reduction of LDL‐C levels less than 50%. Because the use of ezetimibe was infrequent in the present data set, and because a combination of maximally tolerated statin plus ezetimibe is a prerequisite for consideration of PCSK9 inhibitor treatment for both the ACC13 and ESC/EAS algorithms,10 the effect of ezetimibe on top of statin was simulated in all patients not receiving ezetimibe at 1 year. For our main analyses we implemented a fixed relative reduction of 24% in LDL‐C levels at 1 year in all patients not treated with ezetimibe, irrespective of the LDL‐C levels and statin regimen3; in an ancillary analysis, a more conservative approach was applied assuming 18% incremental LDL‐C reduction when modeling the effect of ezetimibe. Clinical end points were adjudicated by a panel of independent experts (3 certified cardiologists) blinded to the results of LDL‐C levels.
Study Objectives and Sensitivity Analyses
The primary objective of this analysis was to evaluate the proportion of patients meeting the criteria for consideration of PCSK9 inhibition at 1 year according to the ESC/EAS and ACC consensus statements. The main analysis compared eligibility for PCSK9 inhibitor treatment according to the ACC versus ESC/EAS algorithms in all patients who were on any statin regimen at 1 year and either received ezetimibe at 1 year or in whom the incremental effect of ezetimibe on 1‐year LDL‐C levels was simulated. Sensitivity analyses were performed to assess eligibility for PCSK9 inhibitors according to the statin intensity at 1 year and in 4 additional different scenarios: in all patients, including those without statin treatment at 1 year (eg, statin intolerance) (scenario 1); including patients on statin treatment at 1 year but without simulating the effect of ezetimibe (scenario 2); including all patients (ie, also those without statin at 1 year) without simulating the effect of ezetimibe (scenario 3); and including patients who were on any statin regimen at 1 year and stimulating an 18% LDL‐C reduction with ezetimibe (scenario 4). In addition, we assessed the proportion of patients reaching the LDL‐C target <1.8 mmol/L according to statin intensity and in different scenarios: (1) based on actual 1‐year LDL‐C levels in the current cohort; (2) after the simulated addition of ezetimibe; (3) after the simulated addition of PCSK9 inhibitors according to the ACC and ESC/EAS eligibility criteria. To model the incremental effect of PCSK9 inhibitors, we simulated a fixed relative reduction of 50% on LDL‐C levels at 1 year in addition to the incremental effect of ezetimibe in treatment‐eligible patient and not in all patients.8
Statistical Analysis
Continuous variables are summarized as mean±standard deviation, categorical variables as actual numbers and percentages. Baseline characteristics, medications, and lipid levels were compared using the Fisher test for binary variables, chi‐squared for more than 2 responses, or unpaired t tests as appropriate. PCSK9 inhibitor eligibility was modeled with mixed‐effects logistic regression models with a random intercept by site. The model included the following predictors: sex, age, body mass index, FH, and attendance to cardiac rehabilitation (at discharge or reported at follow‐up). Odds ratios and 95% confidence intervals from multivariate and univariate models are reported. The analysis was performed for both ESC/EAS and ACC eligibility criteria. All analyses were conducted in Stata version 14.1 (Stata Corporation, College Station, TX) and R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria). The statistical significance was set at the 0.05 level.
Results
Out of 3027 consecutive patients hospitalized for ACS between 2009 and 2014, 65 died within a 1‐year follow‐up, 937 had missing data for LDL‐C levels and 2 for statin therapy, yielding a final sample of 2023 patients (Figure 1). Baseline characteristics for those excluded from the analysis are provided in Table S4. The mean age was 61.7 years, 80.3% were male, 21.8% had a diagnosis of possible and 3.6% of probable/definite FH. The use of statin before hospitalization was 25.9% (Table 1). Most patients were hospitalized for ST‐elevation MI (56.2%), 92.5% had an index percutaneous coronary intervention, and 71.0% attended a cardiac rehabilitation program. At 1 year, 51 patients (2.5%) had presented with MI, 119 (5.9%) unplanned revascularization, 26 (1.3%) ischemic cerebrovascular event, and 40 (2.0%) hospitalization for unstable angina.
Figure 1.

Study flowchart. ACS indicates acute coronary syndromes; LDL, low‐density lipoprotein cholesterol.
Table 1.
Baseline Characteristics of the SPUM‐ACS Cohort (N=2023)
| Variables | Values |
|---|---|
| Age (y, mean±SD) | 61.70±11.86 |
| Sex (female) | 398 (19.7%) |
| BMI (≥30 kg/m²) | 437 (21.6%) |
| Cardiovascular risk factors | |
| Premature CADa | 660 (32.6%) |
| Diabetes mellitus | 307 (15.2%) |
| Hypertension | 1040 (51.4%) |
| Current smoker | 810 (40.0%) |
| Family history of CAD | 560 (27.8%) |
| Previous CVD | 441 (21.8%) |
| MI | 254 (12.6%) |
| PCI | 287 (14.2%) |
| CABG | 68 (3.4%) |
| PAD | 78 (3.9%) |
| Stroke/TIA | 70 (3.5%) |
| CKD (eGFR <60 mL/min) | 223 (11.2%) |
| Familial hypercholesterolemiab | |
| Unlikely | 1333 (74.4%) |
| Possible | 392 (21.9%) |
| Probable | 59 (3.3%) |
| Definite | 8 (0.4%) |
| LDL‐C (mmol/L, mean±SD) | 3.27±1.11 |
| Use of statin | 523 (25.9%) |
| Hospital management | |
| ACS diagnosis | |
| STEMI | 1136 (56.2%) |
| NSTEMI | 806 (39.9%) |
| Unstable angina | 80 (4.0%) |
| Revascularization treatment | |
| PCI | 1871 (92.5%) |
| CABG | 6 (0.3%) |
| Conservative | 146 (7.2%) |
| Attendance to CR after hospital discharge | 1416 (71.0%) |
ACS indicates acute coronary syndromes; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; CR, cardiac rehabilitation; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; NSTEMI, non–ST‐elevation myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SPUM‐ACS, Special Program University Medicine‐Acute Coronary Syndromes; STEMI, ST‐elevation myocardial infarction; TIA, transient ischemic attack.
Premature CAD defined as age <55 years old in male patients and <60 years old in female patients.
231 patients did not receive scoring for LDL‐C levels as recommended by the Dutch Lipid Clinic Network score for FH diagnosis.
The use of a statin was 98.5% at discharge and 94.3% at 1 year. The frequencies of high‐, moderate‐, and low‐intensity statin treatment at discharge and 1 year were, respectively, 65.8% versus 55.3%, 31.9% versus 36.7%, and 0.8% versus 2.2%. (Table 2) Ezetimibe was used in 2.0% of patients at discharge and in 5.8% at 1 year. The patients' reported reasons for discontinuation of statin therapy at 1 year were mainly attributed to physician decision and side effects of treatment (Table S5). Among patients who discontinued statins, 47% reported that treatment was stopped by their physician (documented statin intolerance probable), 30% stopped due to side effects (statin intolerance possible), 18% stopped themselves (probable noncompliance), and 5% stopped for other reasons (eg, costs). Mean LDL‐C levels were 3.27±1.11 mmol/L (126.5±42.9 mg/dL) at baseline and 2.19±0.86 mmol/L (84.7±33.3 mg/dL) at 1 year (P<0.01 using a paired t test). The proportion of patients reaching the LDL‐C <1.8 mmol/L targets at 1 year was 35.8% (Table 3). The main analysis included patients on any statin treatment at 1 year (1908 of 2023 patients). After modeling the effect of ezetimibe in all patients not receiving ezetimibe at 1 year, 13.4% and 2.7% of patients were eligible for PCSK9 inhibitors according to ACC and ESC/EAS guidelines, respectively (Table 4). Among 258 patients eligible for PCSK9 inhibitors according to either of the 2 definitions, 48 (2.3%) were eligible for both ESC/EAS and ACC criteria, 207 (10.2%) only for ACC criteria, and 3 (0.2%) only for ESC/EAS criteria (Figure 2). In 456 patients with FH, the proportion who would be eligible for PCSK9 inhibitors was significantly higher compared with their non‐FH counterparts (27.6% versus 8.8% for ACC criteria and 6.6% versus 1.8% for ESC/EAC criteria, P<0.001, Table 5). The percentage of patients meeting PCSK9 eligibility criteria increased slightly (16.3% versus 4.1%, respectively) when those not treated with statin at 1 year (scenario 1) were included. In regard to actual treatment without modeling the effect of ezetimibe (scenario 2), the proportion of patients who met the respective LDL‐C cutoffs for ACC and ESC/EAS criteria for PCSK9‐inhibitor eligibility were 31.4% and 10.6%, respectively, and 34.2% versus 13.2% (scenario 3) including patients not on statin. Finally, when an 18% LDL reduction with ezetimibe was modeled in patients on any statin treatment at 1 year (scenario 4), the proportions of patients who would be eligible for PCSK9 inhibitors according to ACC and ESC/EAS guidelines were 16.4% and 4.3%, respectively.
Table 2.
Lipid‐Lowering Therapies of the SPUM‐ACS Cohort (N=2023)
| Therapy | At Discharge | At 1‐Year |
|---|---|---|
| Statin | 1992 (98.5%) | 1908 (94.3%) |
| High‐intensity | 1326 (65.8%) | 1107 (55.3%) |
| Moderate‐intensity | 643 (31.9%) | 735 (36.7%) |
| Low‐intensity | 16 (0.8%) | 45 (2.2%) |
| None | 30 (1.5%) | 115 (5.7%) |
| Ezetimibe | 41 (2.0%) | 118 (5.8%) |
| Fibrates | 1 (0.0%) | 9 (0.4%) |
| Niacin | 3 (0.1%) | 3 (0.1%) |
| Resin | 1 (0.0%) | 0 (0.0%) |
SPUM‐ACS indicates Special Program University Medicine‐Acute Coronary Syndromes.
Table 3.
Lipid Values at Baseline and 1 Year
| Baseline Lipids | Values |
|---|---|
| Cholesterol (mmol/L, mean±SD) | 5.07±1.22 |
| HDL‐C (mmol/L, mean±SD) | 1.18±0.35 |
| LDL‐C (mmol/L, mean±SD) | 3.27±1.11 |
| LDL‐C without drug effect (mmol/L, mean±SD) | 3.72±1.34 |
| Triglycerides (mmol/L, mean±SD) | 1.36±0.78 |
| Lipids at 1 Year | |
| Cholesterol (mmol/L, mean±SD) | 4.05±0.99 |
| HDL‐C (mmol/L, mean±SD) | 1.26±0.35 |
| LDL‐C (mmol/L, mean±SD) | 2.19±0.86 |
| LDL‐C <1.8 mmol/L (N, %) | 724 (35.8%) |
| LDL‐C adding ezetimibe (mmol/L, mean±SD) | 1.70±0.69 |
| LDL‐C adding ezetimibe <1.8 mmol/L (N, %) | 1288 (63.7%) |
| LDL‐C adding ezetimibe and PCSK9 inhibitors (ESC/EAS) | 1.63±0.57 |
| LDL‐C <1.8 mmol/L adding ezetimibe and PCSK9 inhibitors (ESC/EAS) (N, %) | 1340 (66.2%) |
| LDL‐C adding ezetimibe and PCSK9 inhibitors (ACC) | 1.48±0.45 |
| LDL‐C <1.8 mmol/L adding ezetimibe and PCSK9 inhibitors (ACC) <1.8 mmol/L (N, %) | 1588 (78.5%) |
| Triglycerides (mmol/L, mean±SD) | 1.32±0.70 |
ACC indicates American College of Cardiology; EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin‐9.
Table 4.
Eligibility for PCSK9 Inhibitors According to ACC and ESC/EAS Guidelines
| Sensitivity Analysis | Total | No Statin (N=115) | Low‐Intensity Statin (N=45)a , b | Moderate‐Intensity Statin (N=735)a , b | High‐Intensity Statin (N=1107)a , b |
|---|---|---|---|---|---|
| Main analysis: patients on statin and with ezetimibe effect (N=1908)c | |||||
| Eligible for ACC | 255 (13.4%) | … | 17 (37.8%) | 120 (16.3%) | 114 (10.3%) |
| Eligible for ESC/EAS | 51 (2.7%) | … | 4 (8.9%) | 25 (3.4%) | 21 (1.9%) |
| Scenario 1: including patients without statin and with ezetimibe effect (N=2023)c | |||||
| Eligible for ACC | 329 (16.3%) | 74 (64.3%) | 17 (37.8%) | 120 (16.3%) | 114 (10.3%) |
| Eligible for ESC/EAS | 83 (4.1%) | 32 (27.8%) | 4 (8.9%) | 25 (3.4%) | 21 (1.9%) |
| Scenario 2: patients on statin and without ezetimibe effect (N=1908)c | |||||
| Eligible for ACC | 599 (31.4%) | … | 31 (68.9%) | 257 (35.0%) | 304 (27.5%) |
| Eligible for ESC/EAS | 202 (10.6%) | … | 10 (22.2%) | 91 (12.4%) | 97 (8.8%) |
| Scenario 3: including patients without statin and without ezetimibe effect (N=2023)c | |||||
| Eligible for ACC | 692 (34.2%) | 93 (80.9%) | 31 (68.9%) | 257 (35.0%) | 304 (27.5%) |
| Eligible for ESC/EAS | 267 (13.2%) | 65 (56.5%) | 10 (22.2%) | 91 (12.4%) | 97 (8.8%) |
| Scenario 4: patients on statin and with modified ezetimibe effect (18% LDL reduction) (N=1908) | |||||
| Eligible for ACC | 313 (16.4%) | … | 20 (44.4%) | 147 (20%) | 141 (12.7%) |
| Eligible for ESC/EAS | 81 (4.3%) | … | 5 (11.1%) | 37 (5%) | 37 (3.3%) |
ACC indicates American College of Cardiology; EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; LDL‐C, low‐density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin‐9.
Missing data for statin potency for 21 patients at 1 year.
Low‐intensity statin was defined as simvastatin 10 mg, pravastatin 10 to 20 mg, fluvastatin 20 to 40 mg. Moderate‐intensity statin was defined as atorvastatin 10 to 20 mg, rosuvastatin 5 to 10 mg, simvastatin 20 to 40 mg, pravastatin 40 to 80 mg, fluvastatin 80 mg. High‐intensity statin was defined as atorvastatin 40 to 80 mg, rosuvatstain 20 to 40 mg.
The ezetimibe effect was defined as a fixed relative reduction of 24% in LDL‐C levels at 1 year in all patients not treated with ezetimibe, irrespective of the LDL‐C levels and statin regimen.3
Figure 2.

Venn diagram showing the number of ACS patients eligible for PCSK9 inhibitors according to the ACC and EAS/ESC consensus documents. The proportions below the absolute numbers pertain to the entire cohort (n=2023). ACC indicates American College of Cardiology; ACS, acute coronary syndromes; EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; PCSK9, proprotein convertase subtilisin/kexin‐9.
Table 5.
Eligibility for PCSK9 Inhibitors According to FH Classification and ACC or ESC/EAS Guidelines
| Sensitivity Analysis | Unlikely FH (N=1333)a | Possible or Probable/Definite FH (N=459)a , b | P Value |
|---|---|---|---|
| Eligible for ACC | 117 (8.8%) | 108 (27.6%) | <0.001 |
| Eligible for ESC/EAS | 24 (1.8%) | 26 (6.6%) | <0.001 |
ACC indicates American College of Cardiology; EAS; European Atherosclerosis Society; ESC, European Society of Cardiology; FH, familial hypercholesterolemia; PCSK9, proprotein convertase subtilisin/kexin‐9.
231 patients did not receive scoring for LDL‐C levels as recommended by the Dutch Lipid Clinic Network score for FH diagnosis.
22.3% vs 2.2% were eligible for possible FH (N=390), 28.8% vs 16.7% for probable/definite FH (N=66).
The simulated addition of ezetimibe to all patients with LDL‐C >1.8 mmol/L increased the proportion of patients who would reach recommended LDL‐C targets (<1.8 mmol/L) to 63.7%. After simulation of the effect of PCSK9 inhibitors in eligible patients, the percentages of patients on target with LDL‐C <1.8 mmol/L in the total cohort were 66.2% using ESC/EAS criteria and 78.5% using ACC criteria (Figure 3). Focusing only on patients who were eligible by either of the 2 criteria (n=258), the proportion of those who would be on target with LDL‐C <1.8 mmol/L after simulation of the PCSK9 inhibitor effect was 93%. Figure 3 also depicts the shift in the distribution of LDL‐C with increasing statin intensity and with the addition of ezetimibe and PCSK9 inhibitors. The mean LDL‐C values would decrease to 1.63±0.57 and 1.48±0.45, respectively, for the ESC/EAS and ACC criteria (Table 3). Among patients with FH, only 20.0% were on target at 1 year, but after simulation of the combined effects of ezetimibe and PCSK9 inhibitors, the proportion of patients who would reach the LDL‐C targets was 50.5% using ESC/EAC criteria and 71.2% using ACC criteria (Table S6).
Figure 3.
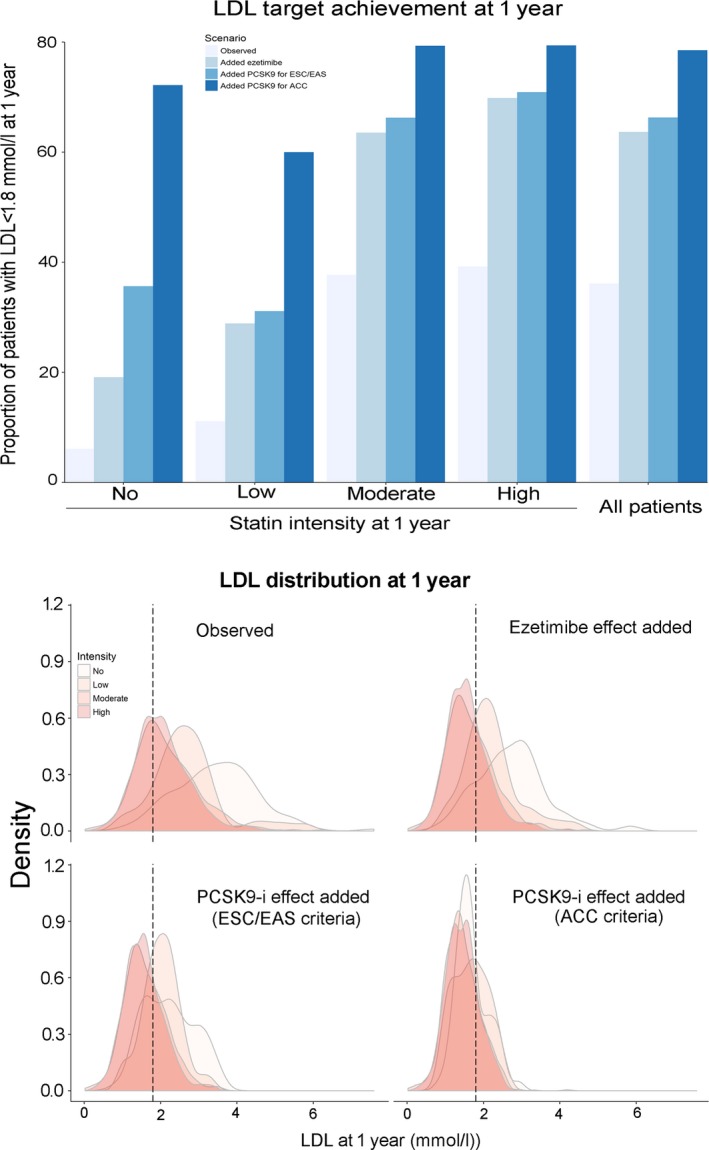
Upper panel, Proportion of patients reaching recommended LDL‐C targets (1.8 mmol/L) 1 year after ACS after adding expected lipid‐lowering effects of ezetimibe and PCSK9 inhibitors. Lower panel, Distribution of LDL‐C levels 1 year after ACS according to statin intensity and after adding expected lipid‐lowering effects of ezetimibe and PCSK9 inhibitors. ACS indicates acute coronary syndromes; LDL‐C, low‐density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin‐9. To model the incremental effect of PCSK9 inhibitors, we simulated a fixed relative reduction of 50% on LDL‐C levels at 1 year in addition to the incremental effect of ezetimibe in eligible patient and not in all patients.
Patients eligible for PCSK9 inhibitors were more likely to have possible and probable/definite FH (odds ratios were 3.99, 95% confidence intervals 2.82 to 5.64 for ACC criteria and 3.38, 95% confidence interval 1.70 to 6.72 for ESC/EAC criteria, Table 6). Attendance at cardiac rehabilitation after hospital discharge was associated with a decrease in PCSK9 eligibility for both algorithms (P<0.001). Additional information is available in Tables S7, S8, and Figure S1 on the use of lipid‐lowering therapies over time.
Table 6.
Associated Factors With Eligibility for PCSK9 Inhibitors at 1 Year
| Variables | Univariate ORs (95% CI, P Values) | Multivariate ORs (95% CI, P Value) |
|---|---|---|
| ESC/EAS criteria for eligibility | ||
| Age (per 10 y) | 0.98 (0.76‐1.26, P=0.88) | 1.09 (0.81‐1.47, P=0.55) |
| Sex (women) | 0.71 (0.31‐1.59, P=0.40) | 0.64 (0.27‐1.48, P=0.30) |
| BMI (per 5 kg/m²) | 1.20 (0.86‐1.67, P=0.29) | 1.17 (0.81‐1.69, P=0.40) |
| Familial hypercholesterolemia | ||
| No | Ref | Ref |
| Possible or probable/definite FH | 2.60 (1.43‐4.71, P<0.001) | 3.38 (1.70‐6.72, P<0.001) |
| Attendance at cardiac rehabilitation | 0.37 (0.20‐0.67, P<0.001) | 0.31 (0.16‐0.60, P<0.001) |
| ACC criteria for eligibility | ||
| Age (per 10 y) | 0.86 (0.76‐0.97, P=0.01) | 1.01 (0.88‐1.17, P=0.86) |
| Sex (women) | 0.85 (0.59‐1.22, P=0.37) | 0.78 (0.53‐1.14, P=0.19) |
| BMI (per 5 kg/m²) | 1.07 (0.90‐1.27, P=0.44) | 1.04 (0.87‐1.24, P=0.65) |
| Familial hypercholesterolemia | ||
| No | Ref | Ref |
| Possible or probable/definite FH | 3.23 (2.41‐4.33, P<0.001) | 3.66 (2.61‐5.14, P<0.001) |
| Attendance at cardiac rehabilitation | 0.57 (0.43‐0.78, P<0.001) | 0.48(0.34‐0.66, P<0.001) |
This model was built as a full‐case analysis based on complete data of 1751 patients. PCSK9 inhibitor eligibility was modeled with mixed‐effects logistic regression models with a random intercept by site. The model included the following predictors: sex, age, BMI, FH, and attendance at cardiac rehabilitation (at discharge or reported at follow‐up). ACC indicates American College of Cardiology; BMI, body mass index; CI, confidence intervals; EAS; European Atherosclerosis Society; ESC, European Society of Cardiology; ORs, odds ratios; PCSK9, proprotein convertase subtilisin/kexin‐9; Ref, reference.
Discussion
In this large prospective cohort of ACS patients with optimal secondary prevention treatment, we found that the eligibility for PCSK9 inhibitors 1 year after the index event varies according to the use of ESC/EAS or ACC eligibility criteria as well as with the simulated pretreatment condition with ezetimibe. The ESC/EAS criteria are more conservative than the ACC criteria, and the use of ezetimibe on top of statin is associated with a significant decrease of the potential use of PCSK9 inhibitors for both criteria. The proportion of patients on LDL‐C target would increase from 36% with actual therapies to 64% after simulated addition of ezetimibe and to 79% after modeling the effect if PCSK9 inhibitors, using ACC criteria for eligibility. Patients with FH based on the DLCN score are more likely to be eligible for PCSK9 inhibitors. Our findings are the first to report the expected clinical impact of these new recommendations on the use of PCSK9 inhibitors in a real‐world ACS population.
In the United States, the 2016 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL‐C lowering in the management of ASCVD defined a less stringent LDL‐C threshold to consider therapy with PCSK9 inhibitors (2.6 mmol/L versus 3.6 mmol/L) compared with the ESC/EAS statement, and an even lower LDL‐C threshold (1.8 mmol/L) among patients with comorbidities or rapidly progressive ASCVD. This explains our finding of a higher proportion of eligible patients when we used ACC compared with ESC/EAS criteria. Both algorithms recommend the use of ezetimibe prior to considering PCSK9 inhibitors and therefore limit a more extended use of PCSK9 inhibitors. In Europe, the aim of the ESC/EAS consensus statement was to ensure appropriate patient pretreatment before consideration of PCSK9 inhibition.10 The algorithms recommend identifying very high‐risk patients who would likely benefit from PCSK9 inhibition via an approach lowering LDL‐C by at least 50% and consequently a significant absolute risk reduction, while also taking into account the costs of these innovative treatments and financial restraints in healthcare budgets. This document defined very high‐risk patients as patients with established ASCVD or with FH without ASCVD, who are likely to have an absolute risk reduction of more than 2% per year. In addition, the LDL‐C threshold for starting therapy is lower in patients with accelerated ASCVD (2.6 mmol/L versus 3.6 mmol/L). These recommendations are currently based on limited data and are likely to be updated once the results of ongoing clinical outcomes trials become available.
We have recently shown that patients with clinical FH had a higher risk of major adverse cardiovascular events after an ACS.17 For the majority of patients, FH is detected only after the acute event. The DLCN score is a simple tool that can be implemented in clinical practice during hospital admission for ACS or in the outpatient setting during a cardiac rehabilitation program to identify FH patients earlier and to discuss with them the potential use of PCSK9 inhibitor in secondary prevention.15 Our analysis suggests that FH patients were more frequently able to reach the recommended criteria for considering PCSK9 inhibition. However, guidelines did not consider systematic screening for FH in this population, and hospital‐based initiation of PCSK9 inhibitors in the acute or subacute phase of ACS has not been tested so far.19 In addition, our data suggest that recommended intervention on lifestyle habits, such as attendance at cardiac rehabilitation, is associated with improved LDL‐C control and a consistent decrease in the need for PCSK9 inhibitors.
The eligibility for PCSK9 inhibitors depends strongly on pretreatment with ezetimibe. Our data suggest that ezetimibe is poorly used at 1 year, probably because the results of IMPROVE‐IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) were published after the time period of our study. It is likely that, in the future, the use of ezetimibe will increase following recent recommendations in guidelines5 to improve the control of LDL‐C. However, none of the PCSK9 clinical trials considered pretreatment with ezetimibe as an inclusion criterion. Moreover, the long‐term feasibility of using 3 different drugs (statin, ezetimibe plus a PCSK9 inhibitor) to lower cholesterol is unclear, and adherence of patients to such intensive regimens remains to be determined. In future guidelines the place of ezetimibe in the context of poorly controlled dyslipidemias with statin alone may need to be updated on the basis of outcome data from the large ongoing clinical trials of PCSK9 inhibitors.
Recently data from the FOURIER trial showed that inhibition of PCSK9 on a background of statin therapy lowered LDL‐C to ≤0.8 mmol/L and resulted in a 15% relative reduction in the risk of CVD events.13 In this trial the use of ezetimibe was low (5%), and the inclusion criterion was an LDL‐C ≥1.8 mmol/L under maximally tolerated statin. These findings added strong evidence for the benefit of an intensive lipid‐lowering strategy below current targets in secondary prevention.13 With these new data, the guidance for the prescription of PCSK9 inhibitors might become even more broadly inclusive (eg, without the need of pretreatment with ezetimibe), and the eligibility rate would become potentially higher, but this remains to be determined in upcoming recommendations and guideline documents. Although our data suggest that a substantial number of patients with ACS would be eligible for PCSK9 inhibitors, there are gaps in current evidence for long‐term safety and efficacy, including neurocognitive and immunogenic effects, lower and upper age limits for treatment, and cost‐effectiveness in patient populations at different levels of cardiovascular risk (eg, coronary disease with comorbidities, such as diabetes mellitus or chronic kidney disease).10.
Limitations
Our study has several limitations. Although the ELIPS program is aimed at optimizing secondary prevention treatments and although our data indeed suggest good adherence to recommended therapies, the possibility that some patients did not receive the maximally tolerated statin doses cannot be excluded. This limitation was partially addressed in sensitivity analyses in patients treated with high‐intensity statins. Second, although statin discontinuation was attributed to medical decision or patient‐reported side effects in the majority (77%) of these patients (Table S4), the diagnosis of statin intolerance cannot be established definitively in those who stopped statin or never used it or used low‐ to moderate‐intensity treatment. Our study has the strength to report reasons for therapy discontinuation, and the presented data strongly suggest that spontaneous discontinuation by the patients was infrequent. In addition, the ESC/EAS consensus statement used the same LDL‐C threshold to consider PCSK9 inhibitors in those with or without statin intolerance, and our results suggest that the addition of patients who discontinued statin only slightly affected eligibility rates, as the majority of these patients would be eligible due to LDL‐C levels above the targets.20
Third, because the majority of patients were not treated with ezetimibe, we had to simulate a fixed effect of ezetimibe based on previous robust evidence.3 The observed effect in real life of ezetimibe might be different and not the same in all individuals. Fourth, rapid ASCVD progression was defined by a recurrent event within a time frame of 5 years in the ESC/EAS document. In the present analysis the exact timing of prior CVD events before hospitalization was not collected. Therefore, we cannot exclude that a proportion of patients were classified as rapid progression of ASCVD although the time frame between the CVD history and the index ACS events might have been longer than 5 years in some cases. Fifth, exclusion of patients who died before 1‐year follow‐up might introduce a selection bias in favor of low‐risk patients as well as those with missing data for LDL‐C. Finally, beyond formal eligibility criteria, the decision to consider treatment with PCSK9 inhibitors should be based on shared decision making between clinicians and individual patients—a critical process that could not be addressed in the present study.
Conclusions
In this large cohort of ACS patients treated with recommended preventive treatment, we observed that 13% of patients would be eligible for PCSK9 inhibitors based on the ACC criteria, and 3% would be eligible based on the ESC/EAS criteria. These differences illustrate the more conservative approach proposed by the ESC/EAS document. The incremental LDL‐C–lowering effect conveyed by pretreatment with ezetimibe would dramatically reduce the indication for PCSK9 inhibitors in both algorithms. Identifying ACS patients with FH based on the DLCN clinical score will help clinicians to select patients who would be more likely to formally qualify for treatment with PCSK9 inhibitors.
Sources of Funding
This work was supported by the Swiss National Science Foundation (SPUM 33CM30‐124112, SPUM 33CM30‐140 336 and SNSF 32473B_163271), Inflammation and ACS‐Novel strategies for prevention and clinical management (SNSF 32473B_163271). Long‐term benefit of the multicenter, multidimensional secondary prevention program in patients with acute coronary syndromes Gencer's research is supported by grants from the Geneva University Hospitals, Swiss Heart Foundation, de Reuter Foundation, Gerbex‐Bourget Foundation, and Schmidheiny Foundation. Rodondi's research is supported by grants from the Swiss National Science Foundation (SNSF 320030‐150025). Auer and Rodondi's research on cardiovascular prevention is supported by grants from the Swiss Heart Foundation. The SPUM consortium was also supported by Roche Diagnostics, Eli Lilly, AstraZeneca, Medtronic, Merck Sharpe & Dome (MSD), Sanofi‐Aventis, and St. Jude Medical. None of the funding institutions had any role in the design or the execution of the study, collection, management, analysis and interpretation of the data, nor had any role in the preparation, review, or approval of the article.
Disclosures
Lüscher reports receiving research grants to the institution from Abbott, Biosensors, Biotronik, Boston Scientific, Daichi Sankyo, Eli Lilly, and Medtronic; and consultant payments from AstraZeneca, Boehringer Ingelheim, Bayer, Merck, and Pfizer, MSD, Roche, and Servier. Matter received grants from MSD, Eli Lilly, AstraZeneca, Roche, and Bayer; received payment for expert testimony by MSD; payment for lectures from MSD, AstraZeneca, and Roche; and patents from Mabimmune, CH. Windecker reports research contracts to the institution from Abbott, Biotronik, Boston Scientific, Biosensors, Cordis, Medtronic, and St. Jude Medical. Mach received honoraria from Amgen, AstraZeneca, BMS, Eli Lilly, MSD, Sanofi, and Pfizer. All other authors report no conflicts of interest.
Supporting information
Table S1. Classification of Intensity of Statin Treatment According to Type and Dose of Statin
Table S2. Dutch Lipid Clinic Network Criteria for Diagnosis of Familial Hypercholesterolemia
Table S3. Correction Factors for Lipid‐Lowering Drugs
Table 4. Baseline Characteristics of the SPUM‐ACS Cohort (N=3027)
Table S5. Reasons for Non–Statin Therapy at Discharge and at 1 Year
Table S6. Lipid Values at Baseline and 1 Year in Non‐FH and FH Patients
Table S7. Number of Patients in Relation to Intensity of Statin Treatment at Discharge and at 1 Year
Table S8. Number of Patients in Relation to Intensity of Statin Treatment at Discharge and at 1 Year
Figure S1. Proportion of patients on no statin and low‐, moderate‐, and high‐intensity statin at baseline (before index event) and at discharge.
Acknowledgments
We acknowledge the work of the clinical event committee for SPUM‐ACS: Matthias Pfisterer, MD, University of Basel (chair), Tiziano Moccetti, MD, CardioCentro Lugano, and Lukas Kappenberger, MD, Lausanne University, Switzerland. We thank the local study nurses, the core lab technicians, the central data monitors, the electronic data conducting system (2mt GmbH Ulm, Jürgen Nagler‐Ihlein, Torsten Illmann), the research coordinator Lambertus J. van Tits, PhD, and the members of the local catheter teams for their invaluable work. Special gratitude is expressed to Aliki Buhayer (Prism Scientific Sarl) for medical writing support.
(J Am Heart Assoc. 2017;6:e006537 DOI: 10.1161/JAHA.117.006537.)29122809
References
- 1. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post‐myocardial infarction patients: Nationwide real world data demonstrate the importance of a long‐term perspective. Eur Heart J. 2015;36:1163–1170. [DOI] [PubMed] [Google Scholar]
- 2. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 4. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol C, Fitzsimons D, Halle M, Hamm C, Hildick‐Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 5. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016;253:281–344. [DOI] [PubMed] [Google Scholar]
- 6. Gencer B, Auer R, Nanchen D, Raber L, Klingenberg R, Carballo D, Blum M, Vogt P, Carballo S, Meyer P, Matter CM, Windecker S, Luscher TF, Mach F, Rodondi N. Expected impact of applying new 2013 AHA/ACC cholesterol guidelines criteria on the recommended lipid target achievement after acute coronary syndromes. Atherosclerosis. 2015;239:118–124. [DOI] [PubMed] [Google Scholar]
- 7. Gencer B, Rodondi N, Auer R, Raber L, Klingenberg R, Nanchen D, Carballo D, Vogt P, Carballo S, Meyer P, Matter CM, Windecker S, Luscher TF, Mach F. Reasons for discontinuation of recommended therapies according to the patients after acute coronary syndromes. Eur J Intern Med. 2015;26:56–62. [DOI] [PubMed] [Google Scholar]
- 8. Navarese EP, Kolodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, Brockmeyer M, Kandzari DE, Kubica JM, D'Agostino RB Sr, Kubica J, Volpe M, Agewall S, Kereiakes DJ, Kelm M. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta‐analysis. Ann Intern Med. 2015;163:40–51. [DOI] [PubMed] [Google Scholar]
- 9. Lipinski MJ, Benedetto U, Escarcega RO, Biondi‐Zoccai G, Lhermusier T, Baker NC, Torguson R, Brewer HB Jr, Waksman R. The impact of proprotein convertase subtilisin‐kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta‐analysis. Eur Heart J. 2016;37:536–545. [DOI] [PubMed] [Google Scholar]
- 10. Landmesser U, John Chapman M, Farnier M, Gencer B, Gielen S, Hovingh GK, Luscher TF, Sinning D, Tokgozoglu L, Wiklund O, Zamorano JL, Pinto FJ, Catapano AL. European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur Heart J. 2017;38:2245–2255. [DOI] [PubMed] [Google Scholar]
- 11. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 12. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 13. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 14. Lloyd‐Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, DePalma SM, Minissian MB, Orringer CE, Smith SC Jr. 2016 ACC expert consensus decision pathway on the role of non‐statin therapies for LDL‐cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68:92–125. [DOI] [PubMed] [Google Scholar]
- 15. Nanchen D, Gencer B, Auer R, Raber L, Stefanini GG, Klingenberg R, Schmied CM, Cornuz J, Muller O, Vogt P, Juni P, Matter CM, Windecker S, Luscher TF, Mach F, Rodondi N. Prevalence and management of familial hypercholesterolaemia in patients with acute coronary syndromes. Eur Heart J. 2015;36:2438–2445. [DOI] [PubMed] [Google Scholar]
- 16. Gencer B, Montecucco F, Nanchen D, Carbone F, Klingenberg R, Vuilleumier N, Aghlmandi S, Heg D, Raber L, Auer R, Juni P, Windecker S, Luscher TF, Matter CM, Rodondi N, Mach F. Prognostic value of PCSK9 levels in patients with acute coronary syndromes. Eur Heart J. 2016;37:546–553. [DOI] [PubMed] [Google Scholar]
- 17. Nanchen D, Gencer B, Muller O, Auer R, Aghlmandi S, Heg D, Klingenberg R, Raber L, Carballo D, Carballo S, Matter CM, Luscher TF, Windecker S, Mach F, Rodondi N. Prognosis of patients with familial hypercholesterolemia after acute coronary syndromes. Circulation. 2016;134:698–709. [DOI] [PubMed] [Google Scholar]
- 18. Besseling J, Kindt I, Hof M, Kastelein JJ, Hutten BA, Hovingh GK. Severe heterozygous familial hypercholesterolemia and risk for cardiovascular disease: a study of a cohort of 14,000 mutation carriers. Atherosclerosis. 2014;233:219–223. [DOI] [PubMed] [Google Scholar]
- 19. Gencer B, Nanchen D. Identifying familial hypercholesterolemia in acute coronary syndrome. Curr Opin Lipidol. 2016;27:375–381. [DOI] [PubMed] [Google Scholar]
- 20. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgozoglu L, Nordestgaard BG, Bruckert E, De Backer G, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, Marz W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN. Statin‐associated muscle symptoms: impact on statin therapy‐European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Classification of Intensity of Statin Treatment According to Type and Dose of Statin
Table S2. Dutch Lipid Clinic Network Criteria for Diagnosis of Familial Hypercholesterolemia
Table S3. Correction Factors for Lipid‐Lowering Drugs
Table 4. Baseline Characteristics of the SPUM‐ACS Cohort (N=3027)
Table S5. Reasons for Non–Statin Therapy at Discharge and at 1 Year
Table S6. Lipid Values at Baseline and 1 Year in Non‐FH and FH Patients
Table S7. Number of Patients in Relation to Intensity of Statin Treatment at Discharge and at 1 Year
Table S8. Number of Patients in Relation to Intensity of Statin Treatment at Discharge and at 1 Year
Figure S1. Proportion of patients on no statin and low‐, moderate‐, and high‐intensity statin at baseline (before index event) and at discharge.


