Abstract
Background
Advancing age is a prominent risk factor for atrial fibrillation (AF). Shorter telomere length is a biomarker of biological aging, but the link between shorter telomere length and increased risk of AF remains unclear. We examined the association between shorter leukocyte telomere length (LTL) and incident AF.
Methods and Results
We included AF‐free participants from the observational Framingham Heart Study Offspring cohort from 1995 to 1998, who had LTL measurements. We examined the association between baseline LTL and incident AF with multivariable Cox models adjusted for age, sex, current smoking, height, weight, systolic and diastolic blood pressure, use of antihypertensive medication, diabetes mellitus, history of myocardial infarction, and history of heart failure. The study sample comprised 1143 AF‐free participants (52.8% women), with mean age of 60±8 years. The mean LTL at baseline was 6.95±0.57 kb. During 15.1±4.2 years mean follow‐up, 184 participants (64 women) developed AF. Chronological age was associated with increased risk of AF (hazard ratio per 10‐year increase, 2.16; 95% confidence interval, 1.71–2.72). There was no significant association between LTL and incident AF (hazard ratio per 1 SD decrease LTL, 1.01; 95% confidence interval, 0.86–1.19). Our study was observational in nature; hence, we could not exclude residual confounding and we were unable to establish causal pathways.
Conclusions
In our moderate‐sized community‐based cohort, we did not find evidence for a significant association between LTL and risk of incident AF.
Keywords: aging, atrial fibrillation, biomarker, epidemiology, telomere genetics
Subject Categories: Arrhythmias, Aging, Epidemiology
Clinical Perspective
What Is New?
Chronological aging is a strong predictor of incident atrial fibrillation, but leukocyte telomere length shortening is not a major predictor of incident atrial fibrillation.
What Are the Clinical Implications?
There is a potential for using biomarkers as predictors of atrial fibrillation, but the evidence points in a direction that shorter telomere length may not be a suitable biomarker to predict atrial fibrillation.
It is estimated that among persons ≥40 years of age, ≈1 out of 4 will develop atrial fibrillation (AF).1, 2, 3, 4 The prevalence of AF increases with advancing age,5, 6 and with an aging population, the prevalence of AF in United States has been projected to rise from ≈3 to 7 million in 2015 to between 6 and 12 million in 2050.7, 8 AF imposes a burden on the patient and healthcare system, because AF is associated with diminished quality of life and increased risk of chronic kidney disease, dementia, stroke, myocardial infarction, heart failure, sudden cardiac death, and all‐cause mortality.9, 10 Numerous modifiable and nonmodifiable AF risk factors have been identified and incorporated in AF risk prediction models,5, 11 and age has been recognized as the most prominent risk factor for AF.5
Aging is a complicated process involving multiple biological changes related with time; therefore, age may be perceived as chronological and biological. Chronological age is determined by time passed since birth, whereas biological age involves molecular and cellular processes. Telomere length has been proposed as a potential biomarker of biological aging.12 As depicted in Figure 1, telomeres are the cap structure of chromosome ends, which function to protect the chromosome from deterioration. A telomere comprises repetitive DNA sequences. The specific sequence of nucleotides in human telomeres is 5′‐TTAGGG‐3′, which is repeated about 2000 to 2500 times. During cell division, telomere shortening occurs in the process of DNA replication; hence, numerous cell divisions will shorten the telomere length and reflect changes in biological age.13
Figure 1.
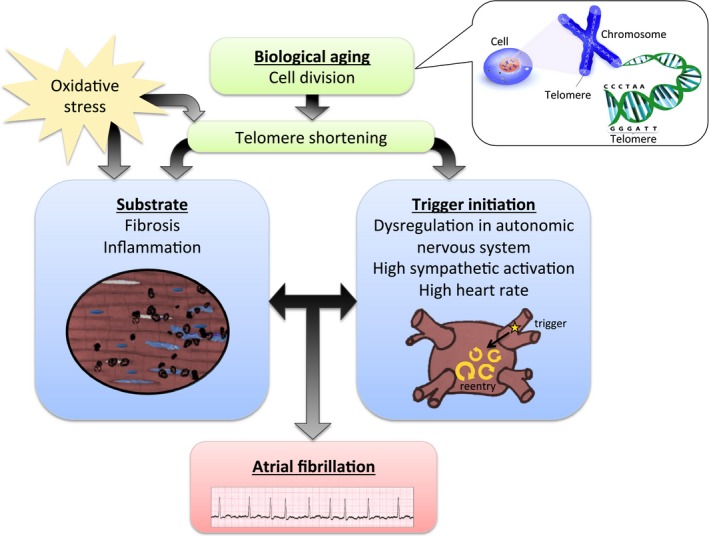
Conceptual figure illustrating possible mechanisms between shortening of telomere length and atrial fibrillation.
Shorter telomere length is associated with a wide range of age‐related chronic diseases, such as hypertension, insulin resistance, vascular dementia, myocardial infarction, and stroke.14, 15, 16, 17, 18 Only a few studies have investigated the association between shorter telomere length and the age‐related disease AF, and the studies reported opposing results with only 1 study supporting the association.19, 20, 21
Theoretically, there may be a possible pathophysiological mechanism linking shorter telomere length and AF, as illustrated in Figure 1. The development of AF may involve a vulnerable atrial substrate, because of fibrosis or inflammation, for example.22 Shorter telomere length has been linked to fibrosis, inflammation, and collagen deposition in other diseases (eg, idiopathic pulmonary fibrosis), and may play a similar role in pathological atrial structure remodeling.23, 24, 25 Oxidative stress not only affects mitochondrial DNA mutations and shortened telomere length,26, 27 but also may play a role for the development of atrial substrate.27, 28 A trigger is required to initiate AF; a highly hypothetical relationship has been drawn between shorter telomere length and autonomic nervous system dysregulation, high sympathetic activation, and high heart rate.23 However, the pathophysiological mechanisms potentially underlying the association between shorter telomere length and AF are unknown.
In the present investigation, we hypothesized that shorter leukocyte telomere length (LTL) is associated with increased risk of incident AF.
Methods
Study Sample
The Framingham Heart Study enrolled the Original cohort in 1948. The Offspring cohort (n=5124) was enrolled in 1971 and comprised adult children of the Original cohort and their spouses. The Offspring cohort was followed up every 4 to 8 years with standardized Framingham Heart Study examinations.29 Participants were eligible for the present study if they attended their routine sixth examination cycle (n=3532), which occurred between 1995 and 1998. The inclusion day was the day the participant attended the sixth examination cycle. The exclusion criteria for the present study were as follows: age <45 years, AF prevalent at inclusion, and missing participant characteristics at inclusion (Figure 2).
Figure 2.
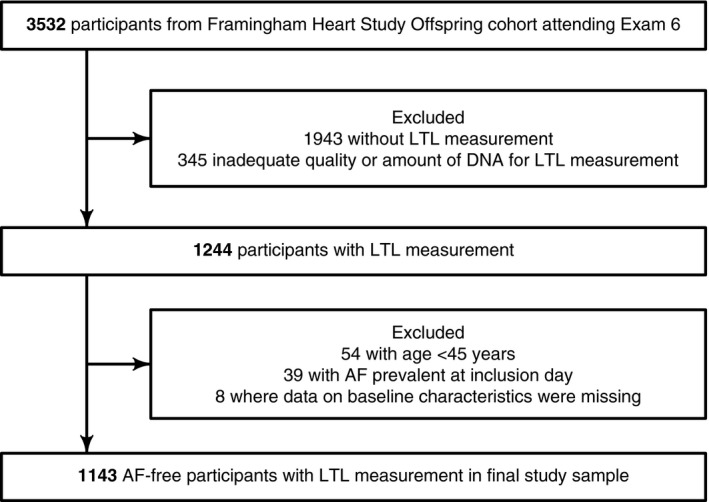
Flow diagram depicting the selection of the study sample. AF indicates atrial fibrillation; LTL, leukocyte telomere length.
Measurement of Leukocyte Telomere Length
All participants provided written informed consent for genetic research. Based on laboratory tests from the sixth examination cycle, DNA was extracted from leukocytes. LTL was measured if participants had sufficient available buffy coat (Data S1 and Figure S1). Southern blotting was performed to obtain the mean length of the terminal restriction fragment, as previously described.30 The mean length of the terminal restriction fragment is referred to as LTL. The coefficient of variation of the LTL measurement for duplicate or triplicate DNA samples was 2.4% on different gels. The laboratory personnel performing the LTL measurement were blinded to clinical characteristics of the Framingham Heart Study participants.
Clinical Evaluation at Baseline
A clinical evaluation was performed at inclusion. We collected variables used for the Cohorts for Heart and Aging Research in Genomic Epidemiology‐AF consortium (CHARGE‐AF) risk model for the prediction of incident AF11: age, sex, height, weight, current smoking (defined as smoking cigarettes regularly during the year before inclusion), systolic blood pressure, diastolic blood pressure, antihypertensive medication, diabetes mellitus (defined as fasting glucose ≥126 mg/dL or use of insulin or hypoglycemic medication), history of myocardial infarction, and history of heart failure.
Assessment of AF and Follow‐Up
Incident AF through December 31, 2014 was the outcome of interest. AF was assessed from Framingham Heart Study examinations, and participants' outside clinical visits or hospitalizations; questions about AF status were included in health history updates. In the Framingham Heart Study, 2 medical doctors evaluated all available records and ECGs of incident AF. AF was defined as atrial flutter or fibrillation. Each included participant was followed from the day of sixth examination cycle until whichever came first: newly diagnosed AF, or last Framingham Heart Study examination or last health history contact, in which the participant was free of AF, death, or end of follow‐up in year 2014.
Association Between Leukocyte Telomere Length and Incident AF
The LTL measurements were normally distributed and therefore logarithmic transformations of the measurements were not applied. The association of LTL with incident AF was assessed using Cox proportional hazards regression models. We analyzed LTL as a continuous variable. We first adjusted the model for age and sex; we fitted a second model further adjusted for smoking, height, weight, systolic blood pressure, diastolic blood pressure, antihypertensive medication, diabetes mellitus, history of myocardial infarction, and history of heart failure. The results were expressed as the hazard ratio (HR) associated with 1 SD unit decrease in LTL. We verified the proportional hazards assumption in the Cox model with respect to LTL, by using a predictor‐by‐time interaction term (P=0.96). To illustrate the results graphically, we estimated multivariable‐adjusted Kaplan–Meier curves to show the adjusted cumulative risk of AF across 3 groups defined according to LTL tertiles (short=5.4–6.7, medium=6.8–7.2, or long LTL=7.3–8.7). The adjustment was achieved with inverse probability weighting and we used the R package IPWsurvival.31
In sensitivity analyses, (1) we excluded participants with history of myocardial infarction or heart failure; (2) age (instead of calendar time) was used as the time scale in the Cox models32; and (3) we calculated the subdistribution HRs using the Fine and Gray model to account for the competing risk of death. Lastly, we explored the functional form (beyond linearity) between LTL and the hazard of incident AF by using fractional polynomials and splines. Analyses involved use of R version 3.3.2 (R Development Core Team, Vienna, Austria).
We conducted post hoc analysis testing for sex and age as effect modifiers of the association between LTL and AF. In the latter, age was analyzed as a continuous variable and we plotted the marginal effect associated with a 1 SD decrement in LTL across the range of age 45 to 85 years; we also reported a stratified analysis with age categorized into age <60 and age ≥60 years.
Statistical Power and Minimal Detectable Effect
The proportion of participants experiencing incident AF in the study sample was 16% (184/1143). For a type I error of 0.05, we estimated that, with power of 80%, the minimally detectable HR was about 1.25 per 1 SD decrease in LTL. We considered a 2‐sided P value of <0.05 as statistically significant.
Ethics
The study was approved by the Framingham Heart Study review committee.
Results
Study Sample Characteristics
The selection of participants is illustrated in Figure 2; after exclusions, we studied 1143 AF‐free participants. The characteristics of the study sample at inclusion (time participant attended sixth examination cycle during 1995–1998) are shown in Table 1. The mean age was 60±9 years, 52.8% were women, and ≈1 out of 3 participants used antihypertensive medications. The mean LTL was 6.95±0.57 kb. The baseline characteristics of the study sample did not differ systematically from participants who did not have LTL measurements (Table S1). Figure S2 depicts a scatterplot of LTL against age, and a linear regression model showed that the mean LTL was associated with ≈0.22‐kb decrease per 10‐year higher mean chronological age.
Table 1.
Characteristics of the Study Population at Baseline
| Characteristic | n=1143 |
|---|---|
| Age, y | 60.0±8.6 |
| Women, % | 604 (52.8) |
| Leukocyte telomere length, kb | 6.95±0.57 |
| Height, cm | 168±9 |
| Weight, kg | 79±17 |
| Current smoker, % | 158 (13.8) |
| Systolic blood pressure, mm Hg | 130±19 |
| Diastolic blood pressure, mm Hg | 76±9 |
| Treatment for hypertension, % | 339 (29.7) |
| Diabetes mellitus, % | 128 (11.2) |
| History of myocardial infarction, % | 32 (2.8) |
| History of heart failure, % | 5 (0.4) |
Data are mean±SD for continuous traits and n (%) for dichotomous traits.
Association Between Leukocyte Telomere Length and Incident AF
The mean follow‐up was 15.1±4.2 years. During follow‐up, a total of 184 participants (35% women) developed incident AF, and 262 participants died free of AF. The main results are shown in Table 2. Chronological age was strongly associated with AF (multivariable HR per 10‐year increment in age, 2.16; 95% confidence interval [CI], 1.71–2.72). With adjustment for sex only, LTL was associated with incident AF (HR associated with a 1 SD decrease in LTL, 1.30; 95% CI, 1.12–1.52). In the multivariable‐adjusted Cox regression, we did not find evidence for an association between LTL and incident AF (HR associated per 1 SD decrease in LTL, 1.01; 95% CI, 0.86–1.19).
Table 2.
Association of LTL Shortening and Incident AF
| Age‐ and Sex‐Adjusted | Multivariable‐Adjusteda | |||
|---|---|---|---|---|
| HR (95% CI)b | P Value | HR (95% CI)b | P Value | |
| LTL | 1.06 (0.90–1.25) | 0.48 | 1.01 (0.86–1.19) | 0.90 |
AF indicates atrial fibrillation; CI, confidence interval; LTL, leukocyte telomere length.
Covariates included age, sex, height, weight, smoking, systolic blood pressure, diastolic blood pressure, antihypertensive medication, diabetes mellitus, history of myocardial infarction, and history of heart failure.
HR=hazard ratio associated with a decrease in LTL of 1 SD (0.57 kb).
In Figure 3, we show the multivariable‐adjusted cumulative risk of incident AF in 3 groups defined according to LTL tertiles. Participants with longer LTL did not have higher probability of being free of AF in follow‐up compared with those with shorter LTL.
Figure 3.
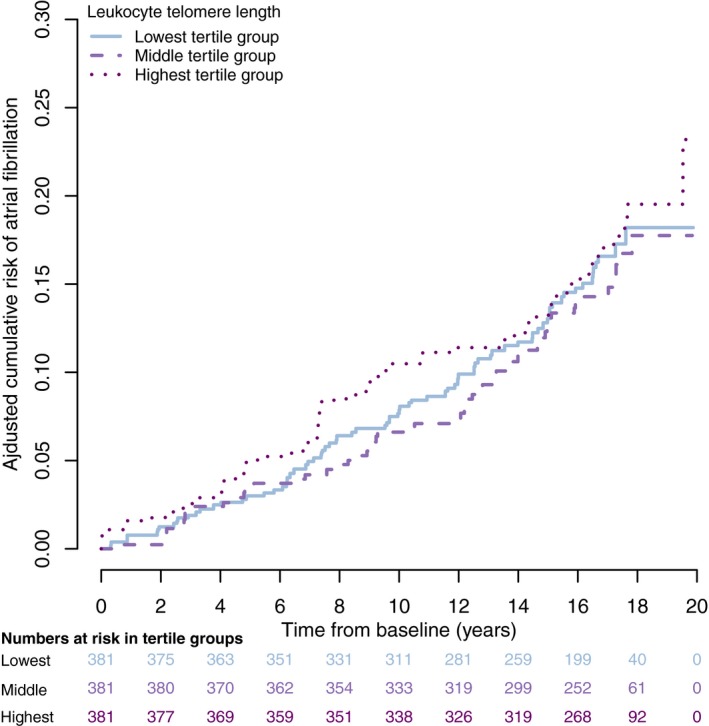
Multivariable‐adjusted Kaplan–Meier curves depict the cumulative risk of atrial fibrillation across 3 groups defined according to LTL tertiles: short LTL (5.42–6.66 kb), medium LTL (6.67–7.2 kb), and long LTL (7.3–8.7 kb). The cumulative risk estimates are adjusted for age, sex, smoking, height, weight, systolic blood pressure, diastolic blood pressure, antihypertensive medication, diabetes mellitus, history of myocardial infarction, and history of heart failure. LTL indicates leukocyte telomere length.
Sensitivity Analyses
Table 3 includes the sensitivity analyses. Excluding participants with history of myocardial infarction or heart failure did not substantially change the results compared with the main models depicted in Table 2. Similarly, using age as a time scale model showed results similar to the main results, indicating that the adjustment for age was acceptable in the main analysis. Analyses taking the competing risk of death into account (Table S2) did not show any statistically significant association between LTL and incident AF either (subdistribution HR, 0.99; 95% CI, 0.84–1.17).
Table 3.
Sensitivity Analyses for the Association of LTL Shortening and Incident AF
| Exclusion of Participants With a History of Myocardial Infarction and Heart Failure | ||||
|---|---|---|---|---|
| Age‐ and Sex‐Adjusted | Multivariable‐Adjusteda | |||
| HR (95% CI)b | P Value | HR (95% CI)b | P Value | |
| LTL | 1.10 (0.93–1.30) | 0.27 | 1.06 (0.89–1.26) | 0.50 |
| Age Used as Time Scale | ||||
|---|---|---|---|---|
| Sex‐Adjusted | Multivariable‐Adjustedc | |||
| HR (95% CI)b | P Value | HR (95% CI)b | P Value | |
| LTL | 1.05 (0.90–1.23) | 0.54 | 0.99 (0.84–1.17) | 0.92 |
AF indicates atrial fibrillation; CI, confidence interval; HR, hazard ratio; LTL, leukocyte telomere length.
Covariates included age, sex, height, weight, smoking, systolic blood pressure, diastolic blood pressure, antihypertensive medication, diabetes mellitus, history of myocardial infarction, and history of heart failure.
Hazard ratio associated with a decrease in LTL of 1 SD (0.57 kb).
Same covariates as above, except for age (controlled for as the time scale).
Post Hoc Analyses
We did not observe significant effect modification by sex (P=0.45), but we found a significant qualitative interaction between LTL and age (P=0.03; Figure S3). In stratified analyses, the HR associated with 1 SD decrease of LTL was 0.80 (95% CI, 0.60–1.08) in participants <60 years and 1.21 (95% CI, 0.67–1.48) in those ≥60 years. Lastly, Figure S4 illustrates a smoothing spline fit for the relative hazard of incident AF as a function of LTL. The graph does not support a nonlinear relationship between hazards of LTL and incident AF (P=0.15 for the nonlinear term).
Discussion
In our prospective study examining the relation of LTL measurements to incident AF in the Framingham Heart Study, we have several principal findings. First, we did not observe a statistically significant association between lower mean or categorical LTL and incident AF. Second, in the multivariable‐adjusted model, the association between chronological age and incident AF was positive, which suggests that chronological age was a stronger predictor of AF than biological age, as assessed by LTL. In Table 4, we have summarized the evidence before our study, the added value of our study, and the implications of the published evidence.
Table 4.
Findings in Context
| Evidence before this study:We searched MEDLINE up to March 10, 2017 for observational studies that assessed the association between telomere length and incident atrial fibrillation (“Telomere Shortening”[Mesh] OR “Telomere”[Mesh] OR telomere[tiab]) AND (“Atrial Fibrillation”[Mesh] OR “atrial fibrillation”[tiab]). Three previous studies have examined the association between shorter telomere length and risk of atrial fibrillation. The 3 studies were designed differently and reported conflicting results, with only 1 study supporting an association between shorter telomere length and atrial fibrillation |
|
Added value of our study: Our observational study based on data from Framingham Heart Study did not support an association between shorter leukocyte telomere length and incident atrial fibrillation. The findings of our study are consistent with the observation that chronological aging is a strong predictor of incident atrial fibrillation, but leukocyte telomere length shortening is not a major predictor of incident atrial fibrillation |
|
Implications of all the available evidence: There is a potential for using biomarkers as predictors of atrial fibrillation, but the evidence points in a direction that shorter telomere length may not be a suitable biomarker to predict atrial fibrillation |
Another longitudinal study by Roberts et al also examined the association between telomere length and incident AF.20 The study was performed with data from the Cardiovascular Health Study, which is a population‐based cohort study with Medicare‐participants from 4 different communities in the United States. In total, 1639 participants were included in the study by Roberts et al, and mean age was 72.2 years and mean LTL measurement 6.33 kb. In our study, the mean age was lower and the mean LTL length longer. As in our study, Roberts et al found no association between LTL shortening and incident AF when LTL shortening was examined as both a continuous variable and across tertiles. Categorization of continuous predictors may create a loss of power and residual confounding33; in our study we explored whether a linear association would not accurately represent the relationship between LTL and the hazard of incident AF, and we did not find support for a nonlinear association (Figure S3). The studies by Roberts et al and our group considered telomere length from circulating leukocytes. However, Roberts et al also had measurements of left atrial cell telomere length from 35 participants, who had undergone cardiac surgery, and in comparison they reported that atrial telomere length was statistically longer than circulating LTL (P=0.03). The atrial tissue observation was found among participants with and without AF, and the finding supported that shorter telomere length and increased risk of AF may not be correlated. Similar to our study, Roberts et al tested the strength of telomere shortening and chronological age, and they also concluded that chronological aging was a stronger predictor of AF than telomere length as a biomarker for biological aging.20
In contrast to the findings in the longitudinal studies by Roberts et al and our group, a cross‐sectional study by Carlquist et al found a stepwise positive association between telomere shortening categorized according to quintiles. Among the 3576 included participants with an available LTL measurement from the Intermountain Heart Collaborative Study, 379 had prevalent AF. The mean age was 69.8, 68.4, and 72.2 years in the paroxysmal, persistent, and permanent groups, respectively. The prevalence of AF was 11.6% in the shortest LTL group to 7.8% in the longest LTL group (P<0.0001). However, this stepwise reduction in LTL and increased risk of AF was less apparent between the 3 middle quintiles. Among AF cases, Carlquist et al were able to classify the subtype of AF in 277 participants; they reported that the presence of paroxysmal AF was more strongly associated with LTL shortening than the presence of persistent and permanent AF. It has long been held that AF progresses from paroxysmal to a more persistent subtype.34 The finding by Carlquist et al was surprising, since theoretically one would anticipate that a stepwise LTL shortening would occur from paroxysmal to persistent or permanent AF. However, diverse measurement methods of telomere length, variations between longitudinal versus cross‐sectional data, and measuring telomere length as a linear or categorical variable (eg, tertiles or quintiles) may influence the results in the different studies. Moreover, the study by Carlquist et al did not include longitudinal follow‐up. Finally, the included participants were all referred for angiography and a large percentage of the participants had coronary heart disease. Coronary heart disease is a risk factor for AF5, 6; hence, the findings in the study by Carlquist et al may have been affected by selection bias.
Shorter telomere length and risk of cardiovascular disease was assessed in a meta‐analysis performed by Haycock et al.18 Based on multivariable‐adjusted studies, the meta‐analysis reported an increased relative risk of coronary heart disease and cerebrovascular disease comparing the shortest third versus the longest third of telomere length. However, the association between cerebrovascular disease and shorter telomere length did not persist in subgroup analyses including only prospective or high‐quality studies. The meta‐analysis did not examine the association between shortening telomere length and risk of AF. As illustrated in Figure 1, a relationship between shorter telomere length and incident AF is theoretically possible, but the relationship was not evident in our primary analyses. We found an interaction between LTL and age in a post hoc analysis. As illustrated in Figure 1, a relationship between shorter telomere length and incident AF is potentially possible in older adults. However, the relationship was not evident in our primary analyses, so we must consider the finding hypothesis generating. Future studies on the interaction between age and telomeres and risk of AF will need larger study samples to clarify whether there is a subtle relationship between shorter telomere length and incident AF in older adults.
Strengths and Limitations
The major strength of our study was the use of prospective data from the Framingham Heart Study, which has previously been used for LTL analyses.15, 30, 35 However, our study also had several limitations. First, this was an observational study; therefore, we cannot rule out residual confounding, establish causality, or provide mechanistic explanations. Second, we were not able to distinguish between atrial flutter or fibrillation and AF subtypes. Third, we acknowledge that we may have missed some AF cases in our study in the absence of continuous cardiac rhythm monitoring. Another possible reason for missing AF cases was that those who developed AF might have stopped attending Framingham Heart Study examinations, though we attempted to obtain their outside records regardless of attendance. Fourth, telomere attrition evolves with time, and we were not able to assess time‐dependent effects of LTL, because we only measured LTL at a single examination. However, telomere attrition over time is an important issue; accumulation of major life stressors predicts telomere shortening, whereas healthy behavior gives protection against major life stressors' effect on telomere attrition.36, 37 Moreover, telomere length was measured in circulating leukocytes. Because the deterioration rate and telomere length differ between cell types, it would have been superior to measure the telomere length in cardiomyocytes instead of circulating leukocytes. However, in an observational community‐based study it is not feasible to measure cardiomyocyte telomere length, which is a limitation. We also acknowledge that the exact amount of sufficient buffy coat was unspecified in the protocol. Another limitation was that the participants in the lowest LTL tertile group were more likely to be lost to follow‐up or to die. It is likely that our post hoc subgroup analysis with participants age ≥60 years lacks statistical power for an association of smaller magnitude. Finally, the Framingham Heart Study participants were largely middle‐aged to older adults of European ancestry, and the generalizability of our findings to individuals of non‐European ancestry and other ages is unknown.
Conclusions
In our observational study based on data from 1143 participants from the Framingham Heart Study with LTL measurements, there was no significant association between shorter telomere length and risk of incident AF.
Sources of Funding
This work was supported by the Boston University School of Medicine and the National Heart, Lung, and Blood Institute's Framingham Heart Study (contract: NIH/NHLBI 1R01HL128914; 2R01 HL092577; HHSN268201500001I; N01‐HC 25195).
Disclosures
Staerk has received funding for research from Boehringer Ingelheim. Ellinor is the PI on a grant from Bayer HealthCare to the Broad Institute focused on the genetics and therapeutics of atrial fibrillation. McManus has received research and/or consulting support from Samsung Electronics, Biotronik, Bristol‐Myers Squibb, Pfizer, Astra Zeneca, Philips, Boston Biomedical Associates, FLEXCon, and is an equity holder in Mobile Sense, Inc. Lubitz has received sponsored research support from Bayer HealthCare, Biotronik, and Boehringer Ingelheim, and has consulted for St. Jude Medical and Quest Diagnostics. The remaining authors have no disclosures to report.
Supporting information
Data S1. Protocol for leukocyte telomere length analysis by the terminal restriction fragment length (TRFL analysis).
Table S1. Characteristics of Participants With and Without Leukocyte Telomere Length Measurements at Examination Cycle 6
Table S2. Association of Leukocyte Telomere Length and Incident Atrial Fibrillation, When Taking the Competing Risk of Death Into Account
Figure S1. Illustration of an autoradiogram showing the terminal restriction fragments (LTLs) (A), the molecular weight ladders (B), and the superimposition of (A and B).
Figure S2. Scatterplot of leukocyte telomere length against age at baseline.
Figure S3. Marginal effect for incident atrial fibrillation associated with a 1 SD decrement in of leukocyte telomere length across the range of age 45 to 85 y.
Figure S4. Smoothing spline fit showing the relationship between relative hazard of incident atrial fibrillation and leukocyte telomere length.
(J Am Heart Assoc. 2017;6:e006541 DOI: 10.1161/JAHA.117.006541.)29138179
This article was handled independently by N.A. Mark Estes III, MD, as a guest editor.
References
- 1. Lloyd‐Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 2. Heeringa J, van der Kuip DAM, Hofman A, Kors JA, van Herpen G, Stricker BHC, Stijnen T, Lip GYH, Witteman JCM. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 3. Guo Y, Tian Y, Wang H, Si Q, Wang Y, Lip GYH. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest. 2015;147:109–119. [DOI] [PubMed] [Google Scholar]
- 4. Mandalenakis Z, Koch LV, Eriksson H, Dellborg M, Caidahl K, Welin L, Rosengren A, Hansson P‐O. The risk of atrial fibrillation in the general male population: a lifetime follow‐up of 50‐year‐old men. Europace. 2015;17:1018–1022. [DOI] [PubMed] [Google Scholar]
- 5. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC Jr, Heckbert SR. Atrial fibrillation incidence and risk factors in relation to race‐ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi‐Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25:71–76.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 8. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TSM. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 9. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta‐analysis. BMJ. 2016;354:i4482. [DOI] [PubMed] [Google Scholar]
- 10. Thrall G, Lane D, Carroll D, Lip GYH. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119:448.e1–19. [DOI] [PubMed] [Google Scholar]
- 11. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens ACJW, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kääb S, Couper D, Harris TB, Soliman EZ, Stricker BHC, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc. 2013;2:e000102 DOI: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang W‐G, Zhu S‐Y, Bai X‐J, Zhao D‐L, Jian S‐M, Li J, Li Z‐X, Fu B, Cai G‐Y, Sun X‐F, Chen X‐M. Select aging biomarkers based on telomere length and chronological age to build a biological age equation. Age (Dordr). 2014;36:9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. [DOI] [PubMed] [Google Scholar]
- 14. von Zglinicki T, Serra V, Lorenz M, Saretzki G, Lenzen‐Grossimlighaus R, Gessner R, Risch A, Steinhagen‐Thiessen E. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab Invest. 2000;80:1739–1747. [DOI] [PubMed] [Google Scholar]
- 15. Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. [DOI] [PubMed] [Google Scholar]
- 16. Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–846. [DOI] [PubMed] [Google Scholar]
- 17. Allende M, Molina E, González‐Porras JR, Toledo E, Lecumberri R, Hermida J. Short leukocyte telomere length is associated with cardioembolic stroke risk in patients with atrial fibrillation. Stroke. 2016;47:863–865. [DOI] [PubMed] [Google Scholar]
- 18. Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta‐analysis. BMJ. 2014;349:g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carlquist JF, Knight S, Cawthon RM, Le VT, Jared Bunch T, Horne BD, Rollo JS, Huntinghouse JA, Brent Muhlestein J, Anderson JL. Shortened telomere length is associated with paroxysmal atrial fibrillation among cardiovascular patients enrolled in the Intermountain Heart Collaborative Study. Heart Rhythm. 2016;13:21–27. [DOI] [PubMed] [Google Scholar]
- 20. Roberts JD, Dewland TA, Longoria J, Fitzpatrick AL, Ziv E, Hu D, Lin J, Glidden DV, Psaty BM, Burchard EG, Blackburn EH, Olgin JE, Heckbert SR, Marcus GM. Telomere length and the risk of atrial fibrillation: insights into the role of biological versus chronological aging. Circ Arrhythm Electrophysiol. 2014;7:1026–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siland JE, Geelhoed B, van Gelder IC, van der Harst P, Rienstra M. Telomere length and incident atrial fibrillation—data of the PREVEND cohort. PLoS One. 2017;12:e0171545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D'Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim Y‐H, Lip GYH, Ma C‐S, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR, Nattel S. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18:1455–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Révész D, Verhoeven JE, Milaneschi Y, de Geus EJCN, Wolkowitz OM, Penninx BWJH. Dysregulated physiological stress systems and accelerated cellular aging. Neurobiol Aging. 2014;35:1422–1430. [DOI] [PubMed] [Google Scholar]
- 24. Stuart BD, Lee JS, Kozlitina J, Noth I, Devine MS, Glazer CS, Torres F, Kaza V, Girod CE, Jones KD, Elicker BM, Ma S‐F, Vij R, Collard HR, Wolters PJ, Garcia CK. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med. 2014;2:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richter T, von Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp Gerontol. 2007;42:1039–1042. [DOI] [PubMed] [Google Scholar]
- 27. Lin PH, Lee SH, Su CP, Wei YH. Oxidative damage to mitochondrial DNA in atrial muscle of patients with atrial fibrillation. Free Radic Biol Med. 2003;35:1310–1318. [DOI] [PubMed] [Google Scholar]
- 28. Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, Tofler GH, Selhub J, Jacques PF, Wolf PA, Magnani JW, Ellinor PT, Wang TJ, Levy D, Vasan RS, Benjamin EJ. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol. 1998;82:2N–9N. [DOI] [PubMed] [Google Scholar]
- 30. Vasan RS, Demissie S, Kimura M, Cupples LA, Rifai N, White C, Wang TJ, Gardner JP, Cao X, Benjamin EJ, Levy D, Aviv A. Association of leukocyte telomere length with circulating biomarkers of the renin‐angiotensin‐aldosterone system: the Framingham Heart Study. Circulation. 2008;117:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xie J, Liu C. Adjusted Kaplan–Meier estimator and log‐rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24:3089–3110. [DOI] [PubMed] [Google Scholar]
- 32. Pencina MJ, Larson MG, D'Agostino RB. Choice of time scale and its effect on significance of predictors in longitudinal studies. Stat Med. 2007;26:1343–1359. [DOI] [PubMed] [Google Scholar]
- 33. Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. [DOI] [PubMed] [Google Scholar]
- 34. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. [DOI] [PubMed] [Google Scholar]
- 35. Vasan RS, Demissie S, Kimura M, Cupples LA, White C, Gardner JP, Cao X, Levy D, Benjamin EJ, Aviv A. Association of leukocyte telomere length with echocardiographic left ventricular mass: the Framingham Heart Study. Circulation. 2009;120:1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Puterman E, Epel E. An intricate dance: life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Soc Personal Psychol Compass. 2012;6:807–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Puterman E, Lin J, Krauss J, Blackburn EH, Epel ES. Determinants of telomere attrition over 1 year in healthy older women: stress and health behaviors matter. Mol Psychiatry. 2015;20:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Protocol for leukocyte telomere length analysis by the terminal restriction fragment length (TRFL analysis).
Table S1. Characteristics of Participants With and Without Leukocyte Telomere Length Measurements at Examination Cycle 6
Table S2. Association of Leukocyte Telomere Length and Incident Atrial Fibrillation, When Taking the Competing Risk of Death Into Account
Figure S1. Illustration of an autoradiogram showing the terminal restriction fragments (LTLs) (A), the molecular weight ladders (B), and the superimposition of (A and B).
Figure S2. Scatterplot of leukocyte telomere length against age at baseline.
Figure S3. Marginal effect for incident atrial fibrillation associated with a 1 SD decrement in of leukocyte telomere length across the range of age 45 to 85 y.
Figure S4. Smoothing spline fit showing the relationship between relative hazard of incident atrial fibrillation and leukocyte telomere length.


