Abstract
Background
We wanted to explore the association of metabolic syndrome (MetS) versus its individual components with 20‐year all‐cause mortality among patients with stable coronary artery disease.
Methods and Results
The cohort comprised 12 403 nondiabetic patients with stable coronary artery disease who were enrolled in the Bezafibrate Infarction Prevention Registry between February 1990 and October 1992 and followed up through December 2014. The study cohort was divided into 4 groups: patients without MetS or impaired fasting glucose (IFG), patients with IFG but without MetS, patients with MetS but without IFG, and patients with both MetS and IFG. Kaplan‐Meier survival analysis showed that at 20 years of follow‐up, the rates of all‐cause mortality were the highest among patients with both MetS and IFG (66%). Patients with IFG without MetS experienced a significantly higher mortality rate compared with those with MetS without IFG (61% versus 56%; log‐rank P<0.001). Multivariable Cox proportional hazard analysis showed that the final Cox model demonstrated that the additive effect of MetS (hazard ratio, 1.13; 95% confidence interval, 1.1–1.16; P=0.02) and IFG (hazard ratio, 1.54; 95% confidence interval, 1.46–1.62; P<0.001) on 20 years mortality was nonsignificant (hazard ratio, 1.01; 95% confidence interval, 0.93–1.11; P=0.69). IFG was associated with the most pronounced increase in mortality risk among the individual components (hazard ratio, 1.22; 95% confidence interval, 1.14–1.3; P<0.001).
Conclusions
Our findings suggest that IFG alone is a major independent predictor of long‐term mortality among patients with stable coronary artery disease versus other components of the MetS.
Keywords: impaired glucose tolerance, metabolic syndrome, mortality
Subject Categories: Diabetes, Type 2; Cardiovascular Disease; Lifestyle; Obesity; Risk Factors
Clinical Perspective
What Is New?
Impaired fasting glucose (IFG) presence is associated with increased mortality risk compared with the reference group without metabolic syndrome (MetS) or IFG, whereas MetS without the IFG component had a less pronounced effect.
Of the different components of MetS, IFG was associated with a 22% independent greater mortality risk compared with absence of IFG after adjustment to other MetS components and to comorbidities.
The independent risk associated with IFG (69% greater adjusted mortality risk) was greater than the combined risk of all other 4 components.
What Are the Clinical Implications?
IFG is a principle determinant of mortality risk associated with MetS in patients with nondiabetic stable coronary artery disease.
Presence of IFG is associated with marked increased mortality risk in the presence or absence of MetS.
Tighter monitoring and treatment with coronary artery disease and IFG can possibly improve important clinical outcomes.
The effect of the metabolic syndrome (MetS) in subjects without established cardiovascular disease is well established, including increased noncardiovascular morbidity and general mortality.1, 2, 3, 4, 5, 6, 7 Moreover, each of the independent components of the MetS has also been associated with an increased risk of cardiovascular events and mortality, with a variation in the magnitude of these relationships among the different individual components.4, 8, 9, 10, 11
In contrast to the clear association of MetS as an important risk factor for cardiovascular disease and mortality in subjects without coronary artery disease (CAD), several studies found no significant association,12, 13, 14, 15 or even a possible protective effect (“the obesity paradox phenomena”), in patients with CAD.16 A recent large meta‐analysis, including 18 457 patients' data, showed that MetS was associated with an increase in all‐cause mortality only in patients with acute coronary syndrome and only after revascularization (not in patients with stable CAD).17
To confound matters further, controversy exists about the assumption that MetS is useful in predicting mortality beyond its components.13, 14, 15 The prognostic importance of MetS compared with that of its individual components has repeatedly been challenged. Several studies argued that not all individual components of MetS contributed to the increased risk of all‐cause mortality.13, 14, 15 This risk was significantly predicted by impaired fasting glucose (IFG) in all subjects, as well as by IFG and low high‐density lipoprotein (HDL) cholesterol in women among an Italian population.18, 19, 20
Furthermore, the follow‐up period in most studies about MetS or its components and mortality prediction in patients with CAD is <3 years.12, 17, 21, 22, 23, 24 Most studies explored cardiovascular mortality rather than all‐cause mortality as the primary outcome.12, 17, 21, 22, 23, 24
Accordingly, in the present study, we aimed to do the following: (1) determine the independent association of the MetS components, as defined by the National Cholesterol Education Program criteria, with long‐term, 20‐year, all‐cause mortality among nondiabetic patients with stable CAD; and (2) compare the mortality risk associated with MetS without the IFG component with risk conferred by IFG alone without MetS.
Methods
Study Population
The present study population was composed of patients who were screened for participation in the Bezafibrate Infarction Prevention (BIP) trial from February 1990 to October 1992 and enrolled in the BIP Registry. The design and rationale of the BIP Registry and study were published previously.25, 26 Of the 15 524 screened patients, only 3090 (20%) were randomized in the prospective interventional 6‐year BIP study that compared bezafibrate with placebo. Because the intervention period ended >15 years ago, we decided to include these subjects in our analysis cohort.
Briefly, the BIP Registry included 15 524 patients, aged 40 to 74 years, with stable CAD fulfilling 1 of the following inclusion criteria: (1) documented myocardial infarction (MI) in the previous 5 years; (2) symptomatic stable angina pectoris and either positive myocardial ischemia by radionuclear scintigraphy or ≥60% stenosis of 1 or more of the major coronary arteries, demonstrated by coronary angiography; (3) documented percutaneous coronary intervention or coronary bypass surgery in the preceding 6 months. Exclusion criteria included the following: insulin‐treated diabetes mellitus (DM), severe heart failure (New York Heart Association functional class, >II), unstable angina, hepatic (glutamate pyruvate transaminase GPT, more than twice the normal value) or renal (creatinine, >1.5 mg/dL) failure, and current use of lipid‐modifying drugs.
All medical examination and biochemical blood test results, historical medical data, and data on drug therapy were prospectively recorded; all vital signs were measured.
Patients were defined as diabetics on the basis of their medical record diagnosis, as prospectively coded at study enrollment, or if they were prescribed hypoglycemic medications. The same method was applied to the definitions of hypertension, smoking status, and other elements of the medical history.
For the purpose of this study, we excluded all the patients diagnosed as having DM (n=2959 [19.2%]) or patients missing laboratory values (n=21[0.13%]); the final data set for the current study comprised 12 403 patients.
The study was approved by our institute's internal review board and was performed according to the principles expressed in the Declaration of Helsinki and the ethics policy of the institute.
The BIP study group was responsible for the enrollment of the patients and the collection of data. The prospective data collection, including mortality status, was performed with the help of the Israeli Association for Cardiovascular Trials.
MetS Definitions
Currently, there are several definitions for MetS. For the purpose of this study, we used the National Cholesterol Education Program—Third Adult Treatment Panel27 definition. Accordingly, patients who were seen with 3 or more of the following 5 risk factors were defined as having MetS:
Central obesity, defined as waist circumference greater than established ethnicity‐specific values. Because the data on waist circumference were not available, for purposes of this analysis, we used the body mass index (BMI) >30 kg/m2 as a criterion for classifying patients as obese.28, 29, 30 Other large studies have previously used this substitution and showed that there is a strong linear correlation between waist circumference and BMI value.30, 31, 32
Low HDL, defined as <50 mg/dL among women and <40 mg/dL among men.
Elevated fasting plasma triglycerides ≥150 mg/dL.
Elevated systolic blood pressure ≥130 mm Hg, diastolic value ≥85 mm Hg, or treatment of previously diagnosed hypertension.
Elevated fasting plasma glucose ≥100 mg/dL.
Group Distribution
To examine the effect of IFG with or without MetS on long‐term mortality outcome, we divided our study cohort into 4 groups: (1) group METS −IFG−, the none group (ie, subjects without MetS or IFG); (2) group METS−IFG+, the IFG group (ie, subjects with IFG but without MetS); (3) group METS+IFG−, MetS without IFG group (ie, subjects with MetS but without the IFG component [IFG not 1 of the 3 components for MetS definition fulfillment]); and (4) group METS+IFG+, MetS with IFG group (ie, subjects with IFG and additional 2 criteria, thus fulfilling the MetS definition).
Laboratory Methods
Detailed data on the laboratory methods were given in a previous report.33 Briefly, blood samples were drawn after at least 12 hours of fasting. Cooled samples, collected in the 18 participating centers using standard equipment and procedures, were transferred to the study's central laboratory. All analyses were performed on a random access analyzer using diagnostic kits.
Primary End Point
The primary end point of this study was all‐cause mortality. Mortality data were obtained by matching the patients' identification numbers with their vital status in the National Population Registry of Israel. Each match record was checked for correct identification by matching the study recorded date of birth during enrollment with the date of birth stored at the national registry.
Statistical Analysis
Variables are expressed as mean±SD or median and interquartile range. Categorical data are summarized as numbers and percentages. The demographic characteristics, clinical characteristics, and laboratory values of patients at baseline, according to the 4 prespecified groups, were compared with the use of the 1‐way ANOVA test for continuous variables and the χ2 test for categorical variables, along with Z‐test with Bonferroni correction. ANOVA post hoc multiple comparisons were performed using the least significant difference or Dunnett T3 test, as appropriate, after equal variance assumption verifications.
Kaplan‐Meier survival analysis was used to graphically present survival estimates of subjects among the 4 different groups. The subsequent long‐term survival probability was compared using the log‐rank test. Additional Kaplan‐Meier survival estimates were generated to compare patients with 1 to 4 components of the MetS, excluding IFG, with the same respective number of components, including IFG (ie, 1 component, excluding IFG, versus IFG alone; any 2 components of the MetS, excluding IFG, versus IFG plus 1 other component). The survival results are presented in the form of a bar graph figure.
Multivariable Cox proportional hazard regression modeling was used to assess the independent risk associated with each component of the MetS (IFG, low HDL, elevated blood pressure [National Cholesterol Education Program definitions], triglycerides >150 mg/dL, or BMI >30 kg/m2). The following covariates were introduced using the best subset method, following a univariable analysis of all relevant variables: age, sex, serum creatinine, hypertension, heart failure, previous MI, or past cerebrovascular accident, together with all the components of MetS. Best subset components were selected using adjusted R 2 and Mallows' Cp.
Finally, a Cox proportional model was run to evaluate the additive contribution of MetS and IFG on 20 years' mortality. The MetS×IFG interaction was added to the previously described model to explore multiplicative effect (ie, whether the presence of 2 factors together has a greater impact). The results were shown as a survival function diagram, presenting the value of the cumulative survival function for a given time (namely, the probability of survival to that time period calculated on the basis of the Cox proportional adjusted hazard model).
The proportionality of hazard assumption was verified using Schoenfeld residuals and the log minus log method. We additionally performed a sensitivity analysis excluding subjects randomized to the BIP randomized study (n=2808).
Statistical significance was accepted for a 2‐sided P<0.05. The statistical analysis was performed with SPSS version 20.0 and SAS version 9.2.
Availability of Data and Material
The data sets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
On the basis of the criteria of the National Cholesterol Education Program, 5245 patients (43%) had MetS, of whom 3234 (27%) also had IFG (group METS+IFG+), and 2011 patients (16%) were considered to have MetS but without IFG (group METS+IFG−). A total of 7158 patients (57%) did not fulfill the criteria for the MetS; among these patients, 1286 (10%) had IFG (group METS−IFG+) and 5872 (47%) had neither MetS nor IFG (group METS−IFG−). Baseline characteristics of the 4 groups are summarized in the Table.
Table 1.
Baseline Characteristics of the Study Population by the 4 Prespecified Groups
| Characteristics | Groups METS−IFG− (N=5872) | METS−IFG+ (N=1286) | METS+IFG− (N=2011) | METS+IFG+ (N=3234) |
|---|---|---|---|---|
| Age, y | 60±7a | 61±7b | 59±7c | 60±7a |
| Male sex | 4837 (83)a | 1136 (89)b | 1569 (78)c | 2657 (82)a |
| Current smoker | 650 (11)a | 122 (10)a | 302 (15)b | 397 (12)a |
| Past smoker | 3079 (52)a | 748 (58)b | 1038 (52)a | 1773 (55)a |
| BMI, kg/m2 | 25.5±3a | 25.7±3a | 28.1±4b | 27.8±4c |
| Hypertension | 1553 (26)a | 337 (26)a | 779 (39)b | 1203 (37)b |
| Prior CVA | 89 (2) | 16 (1) | 26 (1) | 45 (1) |
| COPD | 169 (3) | 43 (3) | 62 (3) | 80 (2) |
| Prior MI | 4226 (72) | 916 (72) | 1422 (71) | 2337 (72) |
| PVD | 172 (3)a | 51 (4)c | 64 (3)c | 113 (4)b |
| NYHA class >2 | 277 (5)a | 75 (6)b | 120 (6)b | 191 (6)b |
| Creatinine, >1.5 mg/dL | 93 (3) | 16 (3) | 37 (3) | 55 (3) |
| LDL, mg/dL | 155±26a | 153±26b | 153±28b | 151±26c |
| Fasting glucose, mg/dL | 89±7a | 114±22b | 90±7a | 120±31c |
| β Blockers | 1829 (31)a | 431 (34)a | 769 (38)b | 1354 (42)b |
| Nitrates | 2761 (47) | 638 (50) | 998 (50) | 1613 (50) |
| CCB | 2811 (48)a | 671 (52)b | 989 (49)b | 1647 (51)b |
| Diuretics | 692 (12)a | 183 (14)b | 303 (15)b | 587 (18)c |
| Antiplatelets | 3664 (62)a | 748 (58)b | 1174 (58)b | 1838 (57)b |
| MetS positive | 0 (0)a | 0 (0)a | 2011 (100)b | 3234 (100)b |
| IFG | 0 (0)a | 1286 (100)b | 0 (0)a | 3234 (100)b |
| Low HDL* | 3418 (63)a | 353 (30)b | 1981 (99)c | 2949 (93)d |
| Elevated BP† | 3312 (56)a | 597 (46)b | 1909 (95)c | 2665 (83)d |
| Triglycerides >150 mg/dL | 1081 (18)a | 58 (5)b | 1765 (88)c | 2042 (63)d |
| BMI >30 kg/m2 | 285 (5)a | 29 (2)b | 673 (33)c | 841 (26)d |
| No. of components | ||||
| 0 | 670 (11)a | 0 (0)b | 0 (0)b | 0 (0)b |
| 1 | 2374 (40)a | 257 (20)b | 0 (0)c | 0 (0)c |
| 2 | 2828 (48)a | 1029 (80)b | 58 (3)c | 13 (0.5)d |
| 3 | 0 (0)a | 0 (0)a | 1667 (83)b | 1575 (49)c |
| 4 | 0 (0)a | 0 (0)a | 286 (14)b | 1297 (40)c |
| 5 | 0 (0)a | 0 (0)a | 0 (0)a | 349 (11)b |
| Mean follow‐up, mo | 208±42 | 197±43 | 203±43 | 189±45 |
Continuous variables are reported as mean±SD if normally distributed; otherwise, they are reported as median (25th–75th percentile range). Categorical variables are reported as number (percentage). + indicates present; −, absent, BMI, body mass index; BP, blood pressure; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; HDL, high‐density lipoprotein; IFG, impaired fasting glucose; LDL, low‐density lipoprotein; MetS, metabolic syndrome; MI, myocardial infarction; NYHA, New York Heart Association; and PVD, peripheral vascular disease.
a,b,c,dEach superscript letter denotes a subset of our study groups whose column proportions do not differ significantly from each other at the P<0.05 level.
*Low HDL is defined as HDL <40 mg/dL in men and HDL <50 mg/dL in women.
†Systolic BP >130 mm Hg or/and diastolic BP >85 mm Hg.
As expected, patients in the METS+IFG− and METS+IFG+ groups (namely, those patients with MetS) had an adverse clinical and biochemical profile, including a higher incidence of hypertension and dyslipidemia than the other 2 groups. However, prevalence of past cerebrovascular accident, chronic obstructive pulmonary disease, low‐density lipoprotein levels, and history of MI were similar to patients without the MetS. Patients with MetS were significantly more likely to receive β blockers, diuretics, calcium channel blockers, and nitrates, yet less likely to receive antiplatelet therapy (Table). Low HDL was the main component among patients with MetS; low HDL was found in 4930 patients (94%) with MetS versus only 3771 patients (53%) without MetS (P<0.001) (Table).
When comparing the METS−IFG+ group with the METS+IFG− group, patients in the METS−IFG+ group (only IFG without MetS) had the lowest rate of comorbidities. These patients were less likely to be seen with other components of the MetS, including lower rates of low HDL (30% in the METS−IFG+ group versus 99% in the METS+IFG− group) and almost no subjects with obesity (2%) or hyperglycemia (5%); however, they were slightly older and included more male patients than the other groups (Table).
Long‐Term Mortality in the 4 Patient Groups
Kaplan‐Meier survival analysis (Figure 1A) showed that at 20 years of follow‐up, the all‐cause mortality rates were the highest among patients with MetS and IFG (66% in the METS+IFG+ group) and the lowest among patients without MetS or IFG (53% in the METS−IFG− group), whereas patients with IFG without MetS and those with MetS but without IFG experienced intermediate mortality rates (log‐rank P<0.001 for the overall difference among the 4 groups during the follow‐up period). However, when mortality rates were directly compared between the 2 latter groups, patients with IFG but without MetS (the METS−IFG+ group) experienced significantly higher unadjusted mortality rates compared with patients with MetS who did not have IFG (the METS+IFG− group; 61% versus 56%; log‐rank P<0.001) (Figure 1B).
Figure 1.
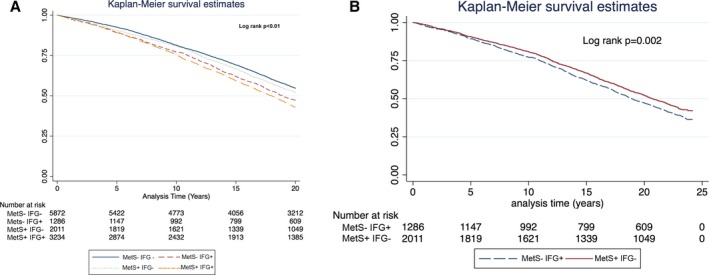
A, Kaplan‐Meier 20‐year survival estimates for the entire cohort, according to the 4 predefined groups. B, Kaplan‐Meier 20‐year survival estimates for the metabolic syndrome absent/impaired fasting glucose present (METS − IFG +) vs the METS + IFG − group.
Notably, separation in event rate curves between patients with and without IFG appeared after 5 years and was sustained thereafter (Figure 1A).
Consistent with the univariate findings seen in the Kaplan‐Meier analysis, multivariate Cox proportional hazards regression modeling, adjusted for age, sex, and comorbidities, demonstrated that patients with IFG without MetS (the MetS−IFG+ group) had 15% greater long‐term mortality risk (hazard ratio [HR], 1.15; 95% confidence interval [CI], 1.01–1.31) compared with patients without MetS or IFG (the MetS−IFG− group, serving as the reference group) (Figure 2). Patients with MetS without IFG did not experience a significant risk increase (HR, 1.08; 95% CI, 0.98–1.20) compared with the reference group. The worst prognosis was observed in subjects with both MetS and IFG (MetS+IFG+ group; HR, 1.31; 95% CI, 1.21–1.42; P<0.001) (Figure 2).
Figure 2.
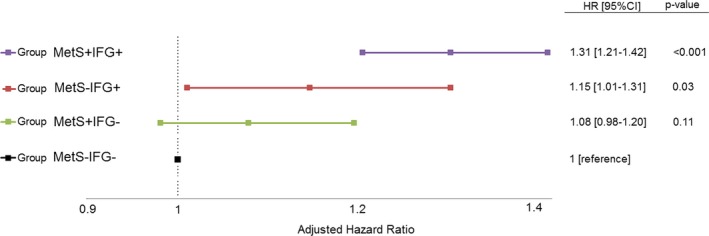
Survival function by the 4 main groups. + indicates present; −, absent; CI, confidence interval; HR, hazard ratio; IFG, impaired fasting glucose; and MetS, metabolic syndrome.
Finally, we performed Cox proportional hazard modeling, including interaction term analysis, by the introduction of the IFG‐by‐MetS product. This analysis demonstrated that the effect of MetS (HR, 1.13; 95% CI, 1.1–1.16; P=0.02) and IFG (HR, 1.54; 95% CI, 1.46–1.62; P<0.001) on 20 years' mortality is nonmultiplicative and independent (P=0.69 for interaction). According to this adjusted Cox model, the worst survival mean was seen among the MetS−IFG+ group. Consistent results were obtained when subjects randomized to the interventional BIP trial (n=2808) were excluded.
Mortality Risk Associated With Individual Component of the MetS
In univariable analysis, the presence of any individual component of the MetS was found to increase risk for long‐term mortality compared with the remaining study population. However, after multivariable adjustment, only IFG and BMI remained significantly associated with increased mortality risk (Figure 3). IFG was associated with the most pronounced increase in mortality risk among the individual components (HR, 1.22; 95% CI, 1.14–1.3; P<0.001). BMI >30 kg/m2 was also significantly associated with increase in long‐term mortality (HR, 1.13; 95% CI, 1.02–1.24; P=0.014), whereas all the remaining components were not significantly associated with all‐cause mortality risk (Figure 3).
Figure 3.
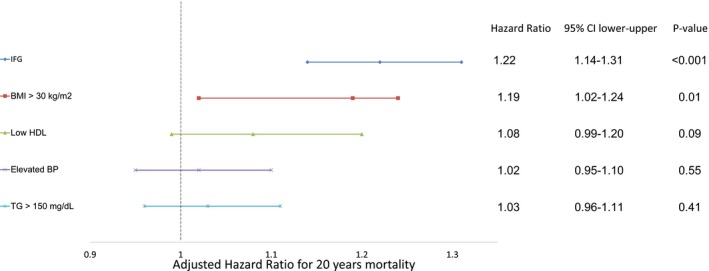
Independent all‐cause mortality risk predictors in nondiabetic patients with stable coronary artery disease with respect to the entire cohort. BMI indicates body mass index; BP, blood pressure; CI, confidence interval; HDL, high‐density lipoprotein; HR, hazard ratio; and IFG, impaired fasting glucose.
Additional independent predictors for long‐term mortality included increased serum creatinine, the presence of advanced heart failure symptoms (New York Heart Association, >2), and a history of MI (Table S1).
Subgroup Analysis Among Patients With MetS
We further performed a subgroup analysis that included only patients with MetS who were enrolled in the BIP Registry. In this patient subset, Kaplan‐Meier survival analysis showed that patients with MetS with the IFG component have a greater mortality risk than patients with MetS without the IFG component but the same number of components (Figure 4). For example, patients with MetS with 2 components other than IFG had lower mortality probability compared with patients with MetS who had 2 components, 1 of them being IFG (52% versus 67%; log‐rank P<0.001).
Figure 4.
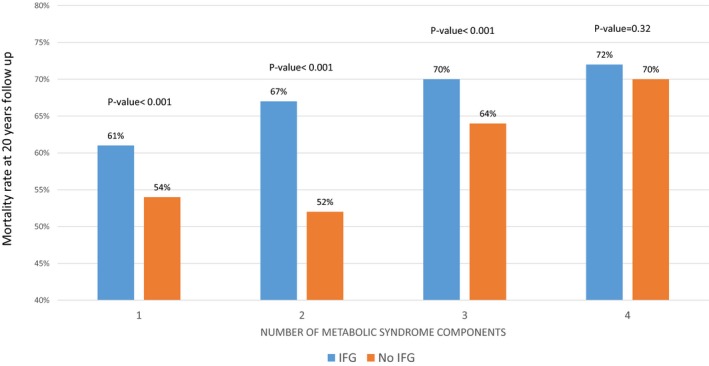
Subgroup analysis of the mortality rates among patients with metabolic syndrome (MetS) by number of MetS components, not including impaired fasting glucose (IFG), compared with the same number of components including IFG (1 of the components is IFG).
Discussion
In the present study, we provide several novel insights into the relationship between MetS and its component association with 20‐year all‐cause mortality among nondiabetic patients with stable CAD. First, we demonstrated that IFG presence is associated with increased mortality risk compared with the reference group without MetS or IFG (METS−IFG−), whereas MetS without the IFG component had a less pronounced effect (8%; P=0.11). Results were consistent in univariable and multivariable analysis. Second, of the different components of MetS, IFG was associated with 22% independent greater mortality risk compared with absence of IFG after adjustment to other MetS components and to comorbidities (Figure 3).
Finally, we have shown that the independent risk associated with IFG (69% greater adjusted mortality risk) was greater than the combined risk of all the other 4 components (52% greater risk) (Figure 3).
Therefore, in the population we studied, the primary risk‐driving factor is the presence of IFG; the addition of risk factors to IFG further increases the long‐term mortality risk, yet in the absence of IFG, the mortality risk is attenuated.
The obligatory requirement of the presence of (at least) 3 risk factors leads to the loss of the significance and the predictive value of some risk factors entirely and from others in part.34, 35, 36 A recent Chinese study, published by Sun et al, found that MetS did not predict all‐cause mortality above and beyond 2 of its individual components (namely, hypertension and IFG).18 However, this study included a relatively small cohort (n=1535) with mostly patients without cardiovascular disease. Our study extends these observations.
Despite the fact that the MetS is often referred to as a uniform entity, it is imperative to admit that no single underlying mechanism has been defined, and one may not exist. Hence, the syndrome could range from a cluster of varied components, unrelated to each other, to a constellation of factors linked through a common underlying mechanism.37 One of the undoubted mechanisms behind the cause of the MetS is insulin resistance. It has been known for decades that insulin resistance plays a central role in the pathophysiological characteristics of MetS, which has led some experts to use the term insulin resistance syndrome.38, 39, 40
It is possible that IFG is a better marker of insulin resistance and, thus, better associated with all‐cause mortality than other components of MetS.30 Several studies have shown that insulin resistance can increase the cardiovascular risk by increasing inflammation and impairing endothelial function.39, 41 Nonetheless, a large study with ≈10 000 patients concluded that fasting plasma glucose >100 mg/dL and/or hypertension were not significantly associated with all‐cause mortality; however, the study was limited to patients with non–ST‐elevation MI and had limited follow‐up.8 Measurement of glucose levels during stress hyperglycemia, such as an acute coronary event or heart failure, is pathophysiologically different; it may possibly serve as a different marker when compared with fasting glucose obtained during a scheduled non–event‐related visit.42, 43, 44
Acute event‐related glucose elevation is driven by a highly complex interplay of counterregulatory hormones, such as catecholamines, growth hormone, cortisol, and cytokines.45, 46, 47 Although the mechanism seems to be well known, the impact on DM development remains scarce.44, 48, 49 Furthermore, the impact on long‐term mortality among patients with stable CAD is unknown. However, values we obtained in the BIP Registry probably better reflect basal metabolic abnormalities and insulin resistance.
Compared with previous studies, the present study has several advantages. First, to the best of our knowledge, this is the first study investigating the relationship between all‐cause mortality and components of the MetS; the study includes long‐term follow‐up in a large cohort and excludes diabetic individuals. The large size of our study, together with the long‐term follow‐up, which incorporated >208 000 person‐years of follow‐up, provided adequate statistical power to assess the associations within the currently accepted definition of MetS and its components.
Second, to our knowledge, this is the first study to compare the MetS with IFG component separately, as well as the relation between the IFG and the number of other MetS components with 20‐year all‐cause mortality outcome in patients with stable CAD.
Attributing the appropriate importance to IFG presence has several practical implications. IFG is easy to detect, and glucose measurements are widely available and inexpensive. Risk stratification as part of secondary prevention efforts should probably better account for IFG and insulin resistance and provide more intensive follow‐up of this high‐risk population. Furthermore, IFG can be reversible, with a regression to normal glucose regulation and the prevention of the development of DM,50 and can serve as an important driving force for lifestyle changes. In addition, it is possible that patients with IFG, and especially MetS with IFG, would benefit from more intensive pharmacotherapy, such as targeting lower low‐density lipoprotein levels, metformin, and possibly lower blood pressure goals.
Limitations
Our study has several limitations. First, it is a retrospective study that enrolled patients during a period in which different treatments were used for controlling blood glucose, hyperlipidemia, and hypertension. Thus, our results warrant validation in more contemporary populations. Second, it is a retrospective analysis and not all confounders can be accounted for; all possible variables were not measured. Third, we have no data on clinical events and management after the screening period, especially on the development of DM through the years among the study groups or the cause of death. Fourth, all patients who were currently using lipid‐modifying drugs were excluded. However, among the components of the MetS, we find hyperglycemia and the HDL level, rather than the low‐density lipoprotein level; therefore, we do not think this exclusion would have affected our results. Finally, our data lack waist circumference assessment, which is important for the definition of central obesity as a component of the MetS. However, we replaced this criterion with the BMI >30 kg/m2 criterion, according to the consensus of the International Diabetes Federation (IDF) criteria and several publications in the field.
Conclusions
IFG is a principle determinant of mortality risk associated with MetS in nondiabetic patients with stable CAD. Presence of IFG is associated with marked increased mortality risk in the presence or absence of MetS. Tighter monitoring and treatment with CAD and IFG can possibly improve important clinical outcomes.
Authors' Contributions
Arwa Y. and Goldkorn designed the study, developed the method, analyzed the data, and wrote the article. Anan Y., Goldenberg, and Tannenbaum analyzed and interpreted the patient data, revised the article, and contributed to the writing. Tzur, Mazu, and Fisman helped with the collection of the data. All authors read and approved the final article.
Disclosures
None.
Supporting information
Table S1. Independent All‐Cause Mortality Risk Predictors in Non‐Diabetic Patients With Stable CAD
Acknowledgments
The study was made possible by the combined efforts of the BIP study group and the Israeli Association for Cardiovascular Trials. The BIP study group was responsible for the enrollment of the patients and the collection of data. The prospective data collection, including mortality status, was performed with the help of the Israeli Association for Cardiovascular Trials.
(J Am Heart Assoc. 2017;6:e006609 DOI: 10.1161/JAHA.117.006609.)29079562
References
- 1. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. [DOI] [PubMed] [Google Scholar]
- 2. Nikolopoulou A, Kadoglou NP. Obesity and metabolic syndrome as related to cardiovascular disease. Expert Rev Cardiovasc Ther. 2012;10:933–939. [DOI] [PubMed] [Google Scholar]
- 3. Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. [DOI] [PubMed] [Google Scholar]
- 4. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA. 2002;288:2709–2716. [DOI] [PubMed] [Google Scholar]
- 5. Ninomiya JK, L'Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. [DOI] [PubMed] [Google Scholar]
- 6. Obunai K, Jani S, Dangas GD. Cardiovascular morbidity and mortality of the metabolic syndrome. Med Clin North Am. 2007;91:1169–1184, x. [DOI] [PubMed] [Google Scholar]
- 7. Thomas GN, Schooling CM, McGhee SM, Ho SY, Cheung BM, Wat NM, Janus ED, Lam KS, Lam TH. Metabolic syndrome increases all‐cause and vascular mortality: the Hong Kong Cardiovascular Risk Factor Study. Clin Endocrinol. 2007;66:666–671. [DOI] [PubMed] [Google Scholar]
- 8. Mehta RH, Westerhout CM, Zheng Y, Giugliano RP, Huber K, Prabhakaran D, Harrington RA, Newby KL, Armstrong PW. Association of metabolic syndrome and its individual components with outcomes among patients with high‐risk non‐ST‐segment elevation acute coronary syndromes. Am Heart J. 2014;168:182–188.e1. [DOI] [PubMed] [Google Scholar]
- 9. Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. [DOI] [PubMed] [Google Scholar]
- 10. Solymoss BC, Bourassa MG, Campeau L, Sniderman A, Marcil M, Lespérance J, Lévesque S, Varga S. Effect of increasing metabolic syndrome score on atherosclerotic risk profile and coronary artery disease angiographic severity. Am J Cardiol. 2004;93:159–164. [DOI] [PubMed] [Google Scholar]
- 11. Mente A, Yusuf S, Islam S, McQueen MJ, Tanomsup S, Onen CL, Rangarajan S, Gerstein HC, Anand SS. Metabolic syndrome and risk of acute myocardial infarction a case‐control study of 26,903 subjects from 52 countries. J Am Coll Cardiol. 2010;55:2390–2398. [DOI] [PubMed] [Google Scholar]
- 12. Iturry‐Yamamoto GR, Zago AC, Moriguchi EH, Manfroi WC, Camargo JL, Gross JL, Zago AJ. Impact of metabolic syndrome and C‐reactive protein on outcome after coronary stenting. J Endocrinol Invest. 2009;32:383–386. [DOI] [PubMed] [Google Scholar]
- 13. Almalla M, Schroder J, Deserno V, Vogt F, Koos R, Koch KC, Marx N, Hoffmann R. Longterm clinical outcome of sirolimus‐eluting stent implantation in metabolic syndrome and diabetes. J Invasive Cardiol. 2010;22:317–321. [PubMed] [Google Scholar]
- 14. Kim JS, Lee HC, Choi BK, Lee HW, Park JS, Lee YH, Ahn MS, Hong TJ, Kim SP, Lee SK, Kim YD. Impact of metabolic syndrome on in‐stent restenosis and clinical outcomes after percutaneous coronary stent implantation. Diabetes Res Clin Pract. 2010;88:e38–e41. [DOI] [PubMed] [Google Scholar]
- 15. Ji MS, Jeong MH, Ahn Y, Kim YJ, Chae SC, Hong TJ, Seong IW, Chae JK, Kim CJ, Cho MC, Rha SW, Bae JH, Seung KB, Park SJ. One‐year clinical outcomes among patients with metabolic syndrome and acute myocardial infarction. Korean Circ J. 2013;43:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patsa C, Toutouzas K, Tsiamis E, Tsioufis C, Spanos A, Karanasos A, Michelongona A, Tousoulis D, Stefanadis C. Impact of metabolic syndrome on clinical outcomes after new generation drug eluting stent implantation: the “obesity paradox” phenomenon is still apparent. Nutr Metab Cardiovasc Dis. 2013;23:307–313. [DOI] [PubMed] [Google Scholar]
- 17. Tie HT, Shi R, Li ZH, Zhang M, Zhang C, Wu QC. Risk of major adverse cardiovascular events in patients with metabolic syndrome after revascularization: a meta‐analysis of eighteen cohorts with 18,457 patients. Metabolism. 2015;64:1224–1234. [DOI] [PubMed] [Google Scholar]
- 18. Sun DL, Wang JH, Jiang B, Li LS, Li LS, Wu L, Wu HY, He Y. Metabolic syndrome vs. its components for prediction of cardiovascular mortality: a cohort study in Chinese elderly adults. J Geriatr Cardiol. 2012;9:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, Ford I, Forouhi NG, Freeman DJ, Jukema JW, Lennon L, Macfarlane PW, Murphy MB, Packard CJ, Stott DJ, Westendorp RG, Whincup PH, Shepherd J, Wannamethee SG. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008;371:1927–1935. [DOI] [PubMed] [Google Scholar]
- 20. Zambon S, Zanoni S, Romanato G, Corti MC, Noale M, Sartori L, Musacchio E, Baggio G, Crepaldi G, Manzato E. Metabolic syndrome and all‐cause and cardiovascular mortality in an Italian elderly population: the Progetto Veneto Anziani (Pro.V.A.) Study. Diabetes Care. 2009;32:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akman T, Binbay M, Erbin A, Tepeler A, Sari E, Kucuktopcu O, Ozgor F, Muslumanoglu A. The impact of metabolic syndrome on long‐term outcomes of percutaneous nephrolithotomy (PCNL). BJU Int. 2012;110:E1079–E1083. [DOI] [PubMed] [Google Scholar]
- 22. Angeloni E, Melina G, Benedetto U, Refice S, Capuano F, Roscitano A, Comito C, Sinatra R. Metabolic syndrome affects midterm outcome after coronary artery bypass grafting. Ann Thorac Surg. 2012;93:537–544. [DOI] [PubMed] [Google Scholar]
- 23. Uchida Y, Ichimiya S, Ishii H, Kanashiro M, Watanabe J, Yoshikawa D, Takeshita K, Sakai S, Amano T, Matsubara T, Murohara T. Impact of metabolic syndrome on various aspects of microcirculation and major adverse cardiac events in patients with ST‐segment elevation myocardial infarction. Circ J. 2012;76:1972–1979. [DOI] [PubMed] [Google Scholar]
- 24. Ivanovic B, Tadic M, Bradic Z, Zivkovic N, Stanisavljevic D, Celic V. The influence of the metabolic syndrome on atrial fibrillation occurrence and outcome after coronary bypass surgery: a 3‐year follow‐up study. Thorac Cardiovasc Surg. 2014;62:561–568. [DOI] [PubMed] [Google Scholar]
- 25. The Bezafibrate Infarction Prevention (BIP) Study Group, Israel. Lipids and lipoproteins in symptomatic coronary heart disease: distribution, intercorrelations, and significance for risk classification in 6,700 men and 1,500 women. Circulation. 1992;86:839–848. [DOI] [PubMed] [Google Scholar]
- 26. Goldbourt U, Behar S, Reicher‐Reiss H, Agmon J, Kaplinsky E, Graff E, Kishon Y, Caspi A, Weisbort J, Mandelzweig L. Rationale and design of a secondary prevention trial of increasing serum high‐density lipoprotein cholesterol and reducing triglycerides in patients with clinically manifest atherosclerotic heart disease (the Bezafibrate Infarction Prevention Trial). Am J Cardiol. 1993;71:909–915. [DOI] [PubMed] [Google Scholar]
- 27. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 28. Zimmet P, M M Alberti KG, Serrano Rios M. A new international diabetes federation worldwide definition of the metabolic syndrome: the rationale and the results [in Spanish]. Rev Esp Cardiol. 2005;58:1371–1376. [PubMed] [Google Scholar]
- 29. Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ. Metabolic syndrome and 10‐year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112:666–673. [DOI] [PubMed] [Google Scholar]
- 30. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 31. Bouguerra R, Alberti H, Smida H, Salem LB, Rayana CB, El Atti J, Achour A, Gaigi S, Slama CB, Zouari B, Alberti KG. Waist circumference cut‐off points for identification of abdominal obesity among the Tunisian adult population. Diabetes Obes Metab. 2007;9:859–868. [DOI] [PubMed] [Google Scholar]
- 32. Gierach M, Gierach J, Ewertowska M, Arndt A, Junik R. Correlation between body mass index and waist circumference in patients with metabolic syndrome. ISRN Endocrinol. 2014;2014:514589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000;102:21–27. [DOI] [PubMed] [Google Scholar]
- 34. Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2005;48:1684–1699. [DOI] [PubMed] [Google Scholar]
- 35. Ding EL, Smit LA, Hu FB. The metabolic syndrome as a cluster of risk factors: is the whole greater than the sum of its parts? Comment on “The metabolic syndrome, its component risk factors, and progression of coronary atherosclerosis.” Arch Intern Med. 2010;170:484–485. [DOI] [PubMed] [Google Scholar]
- 36. Guembe MJ, Toledo E, Barba J, Martínez‐Vila E, González‐Diego P, Irimia P, Díez J, Viñes JJ. Association between metabolic syndrome or its components and asymptomatic cardiovascular disease in the RIVANA‐study. Atherosclerosis. 2010;211:612–617. [DOI] [PubMed] [Google Scholar]
- 37. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: executive summary. Crit Pathw Cardiol. 2005;4:198–203. [DOI] [PubMed] [Google Scholar]
- 38. Reaven GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. [DOI] [PubMed] [Google Scholar]
- 39. Mizuno T, Matsui H, Imamura A, Numaguchi Y, Sakai K, Murohara T, Okumura K. Insulin resistance increases circulating malondialdehyde‐modified LDL and impairs endothelial function in healthy young men. Int J Cardiol. 2004;97:455–461. [DOI] [PubMed] [Google Scholar]
- 40. Roosheroe AG, Setiati S, Istanti R. Insulin resistance as one of indicators for metabolic syndrome and its associated factors in Indonesian elderly. Acta Med Indones. 2012;44:199–206. [PubMed] [Google Scholar]
- 41. Marsland AL, McCaffery JM, Muldoon MF, Manuck SB. Systemic inflammation and the metabolic syndrome among middle‐aged community volunteers. Metabolism. 2010;59:1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kamceva G, Vavlukis M, Kitanoski D, Kedev S. Newly diagnosed diabetes and stress glycaemia and its' association with acute coronary syndrome. Open Access Maced J Med Sci. 2015;3:607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O'Dea H, Stranks SN, Burt MG, Doogue MP. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. 2015;100:4490–4497. [DOI] [PubMed] [Google Scholar]
- 44. Colomo N, Linares F, Rubio‐Martin E, Moreno MJ, de Mora M, García AM, González AM, Rojo‐Martínez G, Valdés S, Ruiz de Adana MS, Olveira G, Soriguer F. Stress hyperglycaemia in hospitalized patients with coronary artery disease and type 2 diabetes risk. Eur J Clin Invest. 2013;43:1060–1068. [DOI] [PubMed] [Google Scholar]
- 45. Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci. 1999;96:513–523. [DOI] [PubMed] [Google Scholar]
- 46. Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, Radermacher P, Calzia E. Glucose metabolism and catecholamines. Crit Care Med. 2007;35:S508–S518. [DOI] [PubMed] [Google Scholar]
- 47. Ishihara M, Kagawa E, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Nakama Y, Maruhashi T, Ookawa K, Dai K, Aokage Y. Impact of admission hyperglycemia and diabetes mellitus on short‐ and long‐term mortality after acute myocardial infarction in the coronary intervention era. Am J Cardiol. 2007;99:1674–1679. [DOI] [PubMed] [Google Scholar]
- 48. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. [DOI] [PubMed] [Google Scholar]
- 49. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373:1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF; Diabetes Prevention Program Research Group . Regression from pre‐diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care. 2009;32:1583–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Independent All‐Cause Mortality Risk Predictors in Non‐Diabetic Patients With Stable CAD


