Abstract
Background
Left atrium (LA) enlargement is common in patients with aortic stenosis (AS), yet its prognostic implications are unclear. This study investigates the value of left atrial volume (LAV) and LAV normalized to body size for predicting mortality in AS.
Methods and Results
We included 1351 patients with AS in sinus rhythm at diagnosis and analyzed the occurrence of all‐cause death during follow‐up with medical and surgical management. Five parameters of LA enlargement were tested: nonindexed LAV and normalized LAV by ratiometric (LAV/body surface area [BSA] and LAV/height) and allometric (LAV/BSA 1.7 and LAV/height2.0) scaling. For each parameter, patients in the highest quartile were at high risk of death, whereas outcome was better and similar for the other quartiles. Five‐year survival was lower for patients with LAV >95 mL and LAV/BSA >50 mL/m2 compared with those with no or mild LA enlargement (both P<0.001). After adjustment for established outcome predictors, including surgery, high risk of death was observed with LAV >95 mL (adjusted hazard ratio, 1.40 [95% confidence interval, 1.06–1.88]) and LAV/BSA >50 mL/m2 (adjusted hazard ratio, 1.42 [95% confidence interval, 1.08–1.91]). LAV/BSA and LAV showed good and similar predictive performance, whereas other scaling methods did not show better outcome prediction. In patients with severe AS at baseline, preserved (≥50%) ejection fraction, and no or minimal symptoms, LA enlargement was significantly associated with mortality (adjusted hazard ratio, 1.87 [95% confidence interval, 1.02–3.44] for LAV >95 mL, and adjusted hazard ratio, 1.90 [95% confidence interval, 1.03–3.56] for LAV/BSA >50 mL/m2).
Conclusions
LA enlargement is an important predictor of mortality in AS, incrementally to known predictors of outcome. LAV and LAV/BSA have comparable predictive performance and should be assessed in clinical practice for risk stratification.
Keywords: aortic valve stenosis, echocardiography, left atrial volume index, normalization, outcome
Subject Categories: Valvular Heart Disease, Echocardiography, Mortality/Survival
Clinical Perspective
What Is New?
In patients with aortic stenosis (AS), left atrium enlargement reflects the chronically increased hemodynamic burden and is reliably assessed by the left atrial volume (LAV) using 2‐dimensional echocardiography.
LAV is an independent predictor of all‐cause death under medical and surgical management in patients with severe AS in sinus rhythm, even in those with no or minimal symptoms and preserved left ventricular (LV) ejection fraction.
LAV >95 mL and LAV normalized to body surface area >50 mL/m2 at AS diagnosis have comparable predictive value and are associated with >50% increase in the risk of death during follow‐up.
What Are the Clinical Implications?
LAV could be systematically assessed in all patients with AS and integrated in the decision‐making process for surgery in asymptomatic or minimally symptomatic patients with severe AS.
In the setting of AS, LV remodeling and left atrium enlargement are the results of the progression of the valvular obstacle and often precede the onset of symptoms.
LAV might represent a novel marker of risk, more sensitive than symptoms or LV systolic dysfunction, with the potential to refine the management of patients with AS.
Larger prospective studies are needed to validate the prognostic value of LAV in asymptomatic severe AS.
Introduction
Aortic valve replacement (AVR) for severe aortic stenosis (AS) is recommended in the presence of symptoms or in asymptomatic patients with left ventricular (LV) dysfunction (ejection fraction [EF] <50%).1, 2 However, symptoms and LV dysfunction are insensitive markers of risk observed at advanced stages of the disease. Numerous patients with severe AS remain asymptomatic for several years and show variable hemodynamic progression of the valvular obstruction.3 On the other hand, LV hypertrophy develops in many patients before the onset of symptoms4 and is associated with adverse postoperative outcomes and poor long‐term prognosis.5, 6, 7, 8 With the sustained improvement of early outcomes after surgical AVR and the widespread use of transcatheter AVR for individuals at lower surgical risk, identification of new criteria more sensitive than symptoms or LV systolic dysfunction is warranted and has potential to refine management of patients with AS.
Left atrial (LA) enlargement is common in patients with AS9 and is an established marker of increased LV filling pressures.10 LA enlargement reflects the effect of increased LA pressure over time and is a morphological indicator of chronically increased hemodynamic burden.11, 12 LA enlargement has been reported as a predictor of death,13, 14, 15 incident heart failure,16 atrial fibrillation (AF),17 and stroke14 in the general population. Moreover, there is a close relation between LA enlargement and mortality in patients with dilated cardiomyopathy,18 myocardial infarction,19 and organic mitral regurgitation.20 Data on the prognostic implication of LA enlargement in patients with AS are scant. It has been suggested that LA enlargement, estimated by the anteroposterior diameter, is a marker of increased mortality, even after AVR.5, 21
Although M‐mode LA diameter is easy to acquire and is used in clinical practice, left atrium volume (LAV), estimated by 2‐dimensional echocardiography, is considered superior to LA diameter.22 LAV shows excellent correlation with computed tomography23 and 3‐dimensional echocardiography24 measurements. Data on the prognostic impact of LAV in a large spectrum of patients with AS are lacking.25 Moreover, the role of LAV normalization to body size in refining LAV outcome prediction has never been investigated.
This study analyzes the relationship between LAV measured at AS diagnosis and all‐cause mortality during follow‐up. We enrolled, in 2 tertiary centers (Amiens and Lille, France), patients with AS in sinus rhythm. We evaluated the predictive value of LAV on outcome with medical and surgical management and the impact of LAV normalization to body size on mortality after diagnosis.
Methods
Inclusion Criteria
Between 2002 and 2015, patients ≥18 years of age diagnosed as having ≥ mild AS (aortic leaflet calcification with reduction in systolic movements and peak aortic jet velocity [Vmax] >2.5 m/s) were prospectively identified and included in an electronic database. The following patients were excluded: (1) those with > mild aortic and/or mitral regurgitation; (2) those with prosthetic valves, congenital heart disease, supravalvular or subvalvular AS, or dynamic LV outflow tract obstruction; (3) those with mitral stenosis; and (4) patients who refused to participate in the study. A total of 1620 patients were enrolled. Subsequently, we excluded 225 patients because of AF on the baseline ECG and 44 patients because of missing LAV. Finally, 1351 patients were included and represented the study population. Clinical and demographic baseline characteristics, including cardiovascular risk factors, presence of symptoms, comorbidity status, and presence of coronary artery disease (documented history of acute coronary syndromes and coronary revascularization or significant coronary artery disease confirmed by coronary angiography), were collected. An index summating the patient's individual comorbidities (Charlson comorbidity index) was calculated.26, 27 We obtained institutional review board authorizations before conducting the study. All patients gave an informed consent for the research. The study was conducted in accordance with institutional policies, national legislation, and the revised Declaration of Helsinki.
Echocardiography
All patients underwent a comprehensive Doppler‐echocardiography study, using commercially available ultrasound systems. Aortic valve area was calculated by using the continuity equation. LV outflow tract was measured in the parasternal long axis view with zoom on the aortic valve. The LV outflow tract time‐velocity integral was measured in the apical 5‐chamber view. Vmax was recorded using continuous‐wave Doppler in several acoustic windows (apical 5 chamber, right parasternal, suprasternal, and epigastric).27 The highest aortic velocity was used to calculate aortic time‐velocity integral and mean pressure gradient. Pressure gradients were calculated using the simplified Bernoulli equation. Stroke volume was calculated by multiplying the LV outflow tract area with the LV outflow tract time‐velocity integral. LV EF was measured by the Simpson biplane method. LV internal diameters and wall thickness were measured at end diastole and end systole in the 2‐dimensional parasternal long axis view, and LV mass was calculated using an anatomically validated formula. LA volume was measured in LV end systole by the biplane method of disks (modified Simpson rule) in apical 2‐ and 4‐chamber views (Figure 1).28, 29 LA planimetry was performed at end systole, just before the opening of the mitral valve by tracing the inner border of the LA, excluding the area under the mitral valve annulus, the pulmonary veins, and the LA appendage. Peak Doppler E‐wave/peak mitral annulus velocity (E/e′) ratio was used to estimate the LV filling pressure.10 Because significant calcification of the mitral annulus can confound the E/e′ measurement, this parameter was not assessed in patients with moderate or severe mitral annular calcification.
Figure 1.
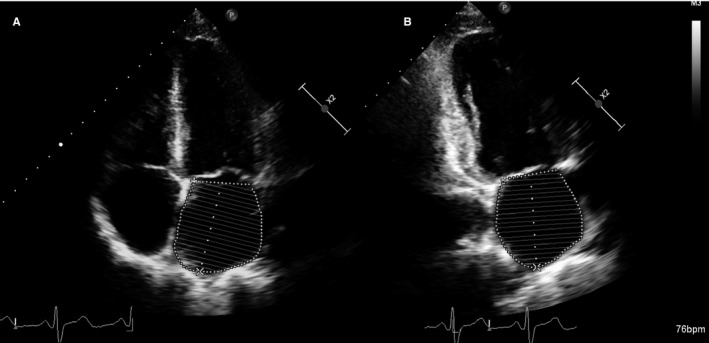
Measurement of left atrial volume from biplane method of disks (modified Simpson rule) using apical 4‐chamber (A) and apical 2‐chamber (B) views at ventricular end systole (maximum volume).
Treatment Decision and Follow‐Up
Therapeutic strategy (conservative, conservative followed by surgery, or surgery) was decided in agreement with the patient's personal physician. Most patients were observed by clinical consultation and echocardiography in the outpatient clinics of the 2 tertiary centers. The others were observed in public hospitals or private practices by referring cardiologists working together with the tertiary centers. Information on follow‐up was obtained by direct patient interview or by repeated follow‐up letters and questionnaires to physicians, patients, and (if necessary) next of kin. Median (25th–75th percentile) follow‐up was 32 (14–74) months. Of patients, 93% were followed up to 3 years or death. Follow‐up was complete up to death or to the end of the study in 1094 patients (81%). The end point was overall survival after diagnosis, with medical and surgical treatment. During follow‐up, patients were monitored by their personal physicians. Clinical decisions about the referral for surgery were made by the heart team with the approval of the patient's referring cardiologist in accordance with practice guidelines.
Statistical Analysis
LA enlargement was analyzed as a nonindexed variable (LAV) and normalized to body size. For normalized LAV, we used ratiometric scaling to body surface area (LAV/BSA) and height (LAV/height) and allometric scaling (LAV/BSA1.7 and LAV/height2.0). The allometric exponents (1.7 for BSA and 2.0 for height) were previously derived on a large group of healthy individuals and best describe the relationship between LAV and body size.30 The study population was divided into groups according to quartiles of LAV parameters (LAV, LAV/BSA, LAV/height, LAV/BSA1.7, and LAV/height2.0). Continuous variables were expressed as mean±1 SD or median (25th and 75th percentiles), and categorical variables were summarized as frequency percentages and counts. The relationship between continuous baseline variables and the groups was explored using 1‐way ANOVA tests (for normally distributed variables) or Kruskal‐Wallis tests (for nonnormally distributed variables). The Pearson χ2 statistic or the Fisher exact test was used to examine the association between the groups and baseline categorical variables. The significance between the lowest quartile and the others was examined if there was a significant difference across quartiles. Individual differences were compared with Mann‐Whitney U tests (with Bonferroni correction for multiple comparisons) and Tukey tests for normally distributed data.
Estimated survival rates ±1 SE were estimated according to the Kaplan‐Meier method and compared with 2‐sided log‐rank tests. Univariate and multivariable analyses of all‐cause mortality were performed using Cox proportional hazards models. We did not use model‐building techniques and entered in the models' covariates considered of potential prognostic impact on an epidemiological basis. These covariates were age, sex, Charlson comorbidity index (not including age), New York Heart Association class, history of hypertension, coronary artery disease, EF, and Vmax. The effect of AVR on outcome was analyzed as a time‐dependent covariate using the entire follow‐up. Age, comorbidity index, EF, and Vmax were used in the multivariable models as continuous variables. The proportional hazards assumption was confirmed using statistics and graphs based on the Schoenfeld residuals. For continuous variables, the assumption of linearity was assessed by plotting residuals against independent variables. Penalized smoothing splines were used to illustrate the association of LAV and LAV/BSA as continuous variables and the risk of death during follow‐up. We conducted subgroup analyses to determine the homogeneity of the association of LAV and LAV/BSA and mortality. First, we estimated the effect of LAV and LAV/BSA on mortality in each subgroup using a Cox univariate model. Second, we formally tested for first‐order interactions in Cox models, entering interaction terms, separately, for each subgroup.
The overall performance of the multivariable models was assessed using the Akaike information criterion, the model with the lowest Akaike information criterion value being the best‐fitting model. The increased discriminative value of LAV parameters was investigated by estimating the C statistics for models with and without LAV parameters. The integrated discrimination improvement (IDI) and the net reclassification improvement (NRI) were determined to further describe the added utility of each LAV parameter when added to the multivariable model.31 The IDI measures the new model's ability to improve integrated sensitivity without compromising integrated specificity. The NRI measures the appropriateness of patient reclassification based on the probability of death at selected time points. In the absence of well‐verified risk categories in AS, we used the continuous NRI method, which does not require a prior definition of strata risk. Thus, we considered the change in the estimation prediction as a continuous variable. NRI and IDI were computed at median follow‐up using the R package survIDINRI developed by Uno et al.32 A significance level of 0.05 was assumed for all tests. All P values are results of 2‐tailed tests. Data were analyzed with SPSS 13.0 (SPSS Inc, Chicago, IL), STATA, version 12 (Statacorp, College Station, TX), and the R software (version 3.2.5, http://www.r-project.org/). The authors had full access to the data and take responsibility for their integrity. All authors have read and agree to the article as written.
Results
Baseline Characteristics
The demographic and clinical characteristics of the 1351 patients, overall and according to LAV and LAV/BSA quartiles, are presented in Table S1 and Table 1. Patients in the higher quartiles of LAV and LAV/BSA were older, were more often symptomatic, and had more comorbid conditions, including hypertension and coronary artery disease.
Table 1.
Baseline Demographic and Clinical Characteristics, Overall and According to LAV/BSA Quartiles
| Variable | All Patients (N=1351) | LAV/BSA <30 mL/m2 (n=338) | LAV/BSA 30–38 mL/m2 (n=338) | LAV/BSA 39–50 mL/m2 (n=337) | LAV/BSA >50 mL/m2 (n=338) | P Value for Trend |
|---|---|---|---|---|---|---|
| Age, y | 73.5±11.6 | 68.9±13.7 | 73.0±11.0a | 74.5±10.0a | 77.0±10.0a | <0.001 |
| Male sex, % (n) | 55.4 (748) | 56.2 (190) | 54.7 (185) | 60.5 (204) | 50 (169)b | 0.051 |
| Body surface area, m2 | 1.89±0.22 | 1.90±0.22 | 1.89±0.21 | 1.90±0.22 | 1.85±0.23b | 0.007 |
| Systolic blood pressure, mm Hg | 136±20 | 137±20 | 136±19 | 138±19 | 135±20 | 0.378 |
| NYHA class, % (n) | <0.001 | |||||
| I–II | 78.4 (1059) | 86.7 (293) | 81.4 (275)a | 76.3 (257)a | 69.2 (234)a | |
| III–IV | 21.6 (292) | 13.3 (45) | 18.6 (63)a | 23.7 (80)a | 30.8 (104)a | |
| Hypertension, % (n) | 73.6 (994) | 69.3 (234) | 74.8 (253)b | 71.7 (242) | 79.8 (270)b | 0.010 |
| Diabetes mellitus, % (n) | 30.6 (413) | 28.0 (95) | 33.5 (113) | 31.5 (106) | 29.7 (100) | 0.442 |
| Coronary artery disease, % (n) | 55.8 (754) | 46.7 (158) | 55.0 (186)b | 58.5 (197)b | 63.0 (213)a | <0.001 |
| Charlson comorbidity index | 2.0±1.7 | 1.9±1.7 | 2.0±1.8 | 2.0±1.8 | 2.1±1.5 | 0.551 |
Continuous normally distributed variables are expressed as mean±1 SD, and categorical variables are expressed as percentages (counts). Continuous variables are compared among groups using 1‐way ANOVA tests (for normally distributed variables); categorical variables are compared among groups with the Pearson χ2 statistic or the Fisher exact test. Individual differences between the lowest quartile and the others are compared with Mann‐Whitney U tests (with Bonferroni correction for multiple comparisons) and Tukey tests for normally distributed data. LAV/BSA indicates left atrial volume indexed to body surface area; and NYHA, New York Heart Association.
P<0.001 individual category vs LAV/BSA <30 mL/m2.
P<0.05 individual category vs LAV/BSA <30 mL/m2.
For echocardiographic variables, patients in the higher quartiles had higher Vmax and mean gradient and lower aortic valve area (Table S2 and Table 2). Patients in the lower quartiles had smaller LV diameters and volumes, greater EF, and lower LV filling pressure and pulmonary pressure (Table S2 and Table 2).
Table 2.
Echocardiographic Parameters, Overall and According to LAV/BSA Quartiles
| Variable | All Patients (N=1351) | LAV/BSA <30 mL/m2 (n=318) | LAV/BSA 30–38 mL/m2 (n=357) | LAV/BSA 39–50 mL/m2 (n=345) | LAV/BSA >50 mL/m2 (n=331) | P Value for Trend |
|---|---|---|---|---|---|---|
| Aortic valve area, cm2 | 0.81 (0.65–1.05) | 0.88 (0.70–1.21) | 0.86 (0.66–1.10) | 0.79 (0.63–1.00)a | 0.78 (0.62–0.91)b | <0.001 |
| Aortic valve area indexed to BSA, cm2/m2 | 0.44 (0.35–0.55) | 0.46 (0.37–0.63) | 0.45 (0.35–0.58) | 0.42 (0.34–0.52)a | 0.42 (0.33–0.5)b | <0.001 |
| Peak aortic jet velocity, m/s | 4.1 (3.3–4.7) | 3.9 (3–4.6) | 4 (3.3–4.7) | 4.2 (3.5–4.7)a | 4.2 (3.6–4.7)a | 0.001 |
| Transaortic mean pressure gradient, mm Hg | 42 (28–55) | 38 (24–53) | 41 (26–56) | 44 (30–55)a | 44 (32–57)b | <0.001 |
| LV outflow tract velocity time integral, cm | 21 (18–24) | 21 (18–24) | 22 (18–24) | 22 (19–24) | 21 (18–24) | 0.253 |
| Cardiac output, mL/min | 5.7 (4.6–6.7) | 5.8 (4.7–6.8) | 5.7 (4.8–6.7) | 5.7 (4.6–6.7) | 5.3 (4.4–6.7) | 0.051 |
| LV end‐diastolic diameter, mm | 49 (45–54) | 49 (43–53) | 49 (45–53) | 50 (45–53) | 51 (46–56)b | <0.001 |
| LV end‐systolic diameter, mm | 31 (27–35) | 30 (26–33) | 30 (26–35)a | 31 (27–35)a | 32 (27–38)b | <0.001 |
| LV end‐diastolic septum thickness, mm | 13 (11–15) | 13 (11–14) | 13 (11–15) | 13 (12–15)a | 13 (12–15)b | <0.001 |
| LV end‐diastolic volume, mL | 119 (86–160) | 111 (80–148) | 123 (90–167)a | 123 (89–154)a | 124 (91–155)b | <0.001 |
| LV end‐systolic volume, mL | 43 (29–61) | 38 (26–54) | 43 (30–62)a | 44 (31–61)a | 47 (31–71)b | <0.001 |
| Ejection fraction, % | 64 (58–69) | 65 (60–70) | 65 (59–70) | 63 (58–69)a | 62 (55–68)b | <0.001 |
| LV mass, g | 233 (185–291) | 213 (173–259) | 227 (184–286) | 244 (195–291)a | 266 (201–323)b | <0.001 |
| Indexed LV mass, g/m2 | 123 (100–152) | 109 (92–135) | 121 (98–147)a | 124 (103–152)b | 142 (110–172)b | <0.001 |
| E/A ratioc | 0.8 (0.6–1.0) | 0.7 (0.6–1.0) | 0.7 (0.6–0.9) | 0.8 (0.6–1.0) | 0.9 (0.7–1.2)b | <0.001 |
| E deceleration time, msc | 233 (187–295) | 240 (193–288) | 242 (197–303) | 240 (200–303) | 210 (163–276)a | <0.001 |
| E/e′ ratiod | 11 (8–15) | 9 (6–13) | 10 (8–14) | 11 (8–15) | 13 (9–18)b | <0.001 |
| Systolic pulmonary artery pressure, mm Hg | 31 (27–28) | 29 (25–35) | 31 (27–36) | 31 (27–38) | 35 (29–45)b | <0.001 |
Nonnormally distributed continuous variables are expressed as median (25th–75th percentile). Continuous variables are compared among groups using Kruskal‐Wallis tests (for nonnormally distributed variables). Individual differences between the lowest quartile and the others are compared with Mann‐Whitney U tests (with Bonferroni correction for multiple comparisons). A indicates late mitral inflow wave; E, early mitral inflow wave; E/e′, mitral peak Doppler E‐ wave/peak mitral annulus velocity ratio; LAV/BSA, left atrial volume indexed to body surface area; and LV, left ventricular.
P<0.05 individual category vs LAV/BSA <30 mL/m2.
P<0.001 individual category vs LAV/BSA <30 mL/m2.
Missing data for 373 patients.
Missing data for 601 patients.
Outcome Impact of LAV
Median LAV was 74 (58–95) mL. A total of 399 deaths were recorded during follow‐up. Five‐year estimated survival was 74±3% for LAV <58 mL, 70±3% for LAV 58 to 74 mL, and 69±3% for LAV 75 to 95 mL (P=0.381 for LAV 58–74 mL versus LAV <58 mL, and P=0.400 for LAV 75–95 mL versus LAV <58 mL; Figure 2A). Patients with LAV >95 mL had significantly lower 5‐year survival compared with patients with LAV <58 mL (57±4% versus 74±3%; P<0.001; Figure 2A).
Figure 2.
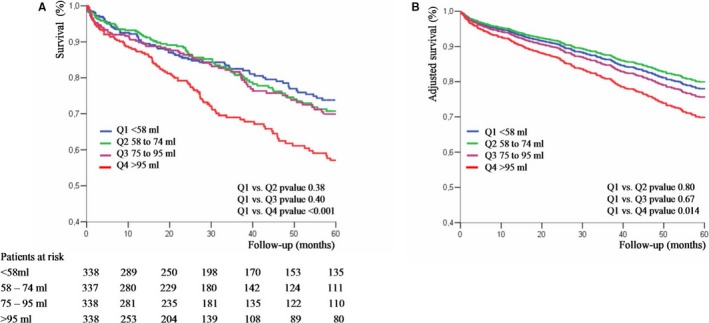
A, Kaplan‐Meier survival curves of patients with aortic stenosis (AS) according to left atrial volume (LAV) quartiles. B, Adjusted survival curves of patients with AS according to LAV quartiles. Curves are adjusted for age, sex, comorbidity, New York Heart Association class, hypertension, coronary artery disease, ejection fraction, and peak aortic jet velocity. Q1, Q2, Q3, and Q4 indicate first, second, third, and fourth quartiles, respectively.
On multivariable analysis, after adjustment for age, sex, comorbidity index, symptoms, hypertension, coronary artery disease, EF, and Vmax, LAV was independently associated with overall mortality (Table 3). The character of the relationship between LAV and the risk of death was estimated using spline functions for LAV (Figure 3). On multivariable analysis, there was no increase in mortality risk with increasing LAV when it remained ≤95 mL (adjusted hazard ratio [HR], 1.01 [95% confidence interval (CI), 0.93–1.10] per 1‐mL LAV increment; P=0.492). With LAV >95 mL, there was a significant increase in mortality risk with increasing LAV (adjusted HR, 1.02 [95% CI, 1.01–1.06] per 1‐mL LAV increment; P=0.031). Patients with LAV 58 to 74 mL and patients with LAV 75 to 95 mL had similar risk of death compared with patients with LAV <58 mL (adjusted HR, 0.96 [95% CI, 0.71–1.31] for LAV 58–74 mL versus <58 mL, and adjusted HR, 1.07 [95% CI, 0.78–1.46] for LAV 75–95 mL versus <58 mL; Table 3; Figure 2B). Patients with LAV >95 mL had significantly greater risk of death (adjusted HR, 1.46 [95% CI, 1.10–1.96]; Table 3; Figure 2B). Results were unchanged after adjustment for AVR (Table 3). In a subgroup of 750 patients in whom the E/e′ ratio was available, the excess risk associated with LAV >95 mL was not altered by further adjustment for E/e′ ratio and indexed LV mass (adjusted HR, 1.49 [95% CI, 1.04–2.06]; P=0.031).
Table 3.
Relative Risk of All‐Cause Death Associated With Left Atrium Enlargement
| Variable | Overall Death | |
|---|---|---|
| HR (95% CI) | P Value | |
| LAV | ||
| Unadjusted | ||
| <58 mL | Referent | |
| 58–74 mL | 1.12 (0.84–1.49) | 0.441 |
| 75–95 mL | 1.10 (0.82–1.48) | 0.517 |
| >95 mL | 1.83 (1.40–2.41) | <0.001 |
| Model 1a | ||
| <58 mL | Referent | |
| 58–74 mL | 0.96 (0.71–1.31) | 0.800 |
| 75–95 mL | 1.07 (0.78–1.46) | 0.665 |
| >95 mL | 1.46 (1.10–1.96) | 0.014 |
| Model 2b | ||
| <58 mL | Referent | |
| 58–74 mL | 0.93 (0.68–1.26) | 0.630 |
| 75–95 mL | 1.10 (0.81–1.51) | 0.542 |
| >95 mL | 1.40 (1.06–1.88) | 0.021 |
| LAV/BSA | ||
| Unadjusted | ||
| <30 mL/m2 | Referent | |
| 30–38 mL/m2 | 1.04 (0.77–1.40) | 0.800 |
| 39–50 mL/m2 | 1.29 (0.97–1.73) | 0.081 |
| >50 mL/m2 | 2.08 (1.58–2.73) | <0.001 |
| Model 1a | ||
| <30 mL/m2 | Referent | |
| 30–38 mL/m2 | 0.96 (0.70–1.32) | 0.819 |
| 39–50 mL/m2 | 1.06 (0.77–1.46) | 0.712 |
| >50 mL/m2 | 1.50 (1.11–2.01) | 0.010 |
| Model 2b | ||
| <30 mL/m2 | Referent | |
| 30–38 mL/m2 | 0.90 (0.66–1.24) | 0.520 |
| 39–50 mL/m2 | 1.12 (0.82–1.53) | 0.474 |
| >50 mL/m2 | 1.42 (1.08–1.91) | 0.035 |
Analyses are univariate and multivariable Cox proportional hazard models. Charlson comorbidity index does not include age. BSA indicates body surface area; HR, hazard ratio; and LAV, left atrial volume.
Model 1 is adjusted for age, sex, hypertension, coronary artery disease, New York Heart Association class, Charlson comorbidity index, peak aortic jet velocity, and left ventricular ejection fraction.
Model 2 is adjusted for covariates included in model 1 and aortic valve surgery as a time‐dependent covariate.
Figure 3.
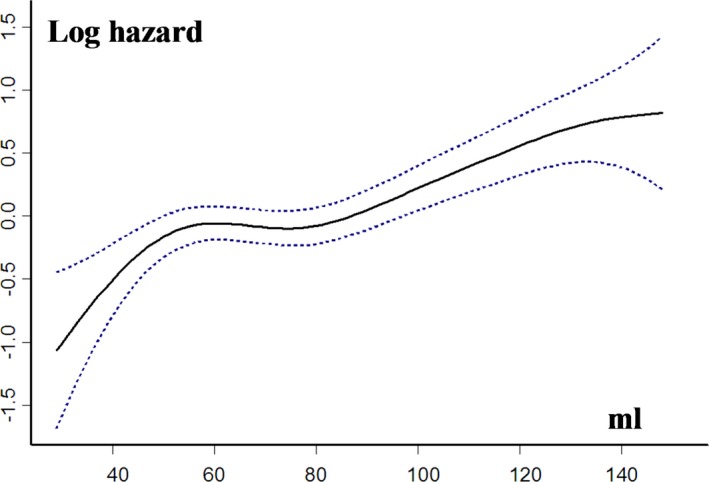
Relationship between left atrial volume (LAV) and the risk of all‐cause death during follow‐up. Hazard ratios and 95% CIs are estimated in Cox models, with LAV represented as a spline function and adjusted for age, sex, comorbidity, New York Heart Association class, hypertension, coronary artery disease, ejection fraction, and peak aortic jet velocity.
Subgroup analysis
There was no interaction between age, sex, BSA, and EF and the outcome impact of LAV >95 mL (all P for interaction, >0.100). Outcome analysis was further stratified by Vmax and LAV. Compared with LAV ≤95 mL, 5‐year survival associated with LAV >95 mL was not significantly different (P=0.110; Figure 4A) in patients with nonsevere AS (Vmax ≤4 m/s), but was considerably lower in patients with severe AS (Vmax >4 m/s) (57±5% versus 78±2%; P<0.001; P for interaction, 0.002; Figure 4B). After adjustment for covariates, including AVR, the risk of death associated with LAV >95 mL was considerable in patients with Vmax >4 m/s (adjusted HR, 1.64 [95% CI, 1.18–2.28]; P=0.003), whereas in patients with Vmax ≤4 m/s, it was not significant (adjusted HR, 1.14 [95% CI, 0.83–1.57]; P=0.431).
Figure 4.
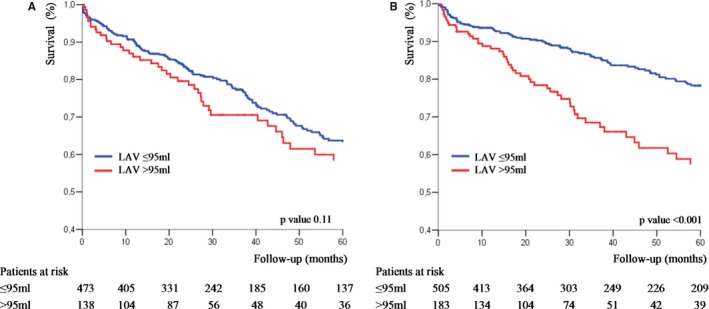
Kaplan‐Meier survival curves of patients with moderate aortic stenosis (AS; peak aortic jet velocity [Vmax], ≤4 m/s; A) and with severe AS (Vmax, >4 m/s; B) according to left atrial volume (LAV; ≤95 and >95 mL).
In the group of 330 patients with severe AS at baseline (Vmax, >4 m/s), EF ≥50%, and no or minimal symptoms, the prognostic impact of LAV >95 mL was still observed. In this subgroup of patients, 5‐year estimated survival was 85±3% for LAV ≤95 mL and 68±9% for LAV >95 mL (P=0.021). After adjustment for covariates, including surgery, LAV >95 mL was independently associated with all‐cause mortality (adjusted HR, 1.87 [95% CI, 1.02–3.44]). The prognostic impact of LAV >95 mL was more pronounced in patients with height ≤1.6 m compared with >1.6 m (P for interaction, 0.031).
Aortic valve surgery and outcome
During the first 3 months after diagnosis, 549 patients underwent AVR, according to the decision of the attending physician based on guideline recommendations. Postoperative survival was greatly affected by LAV (5‐year postoperative survival: 85±2% for LAV ≤95 mL versus 65±5% for LAV >95 mL; P<0.001). On multivariable analysis, the impact of LAV >95 mL on postoperative outcome was significant (adjusted HR, 1.58 [95% CI, 1.02–2.53]; P=0.035).
Outcome Impact of LAV/BSA
Median (25th–75th percentile) LAV/BSA was 39 (30–50) mL/m2. Five‐year estimated survival of LAV/BSA >50 mL/m2 was 55±4%, significantly lower than that of the 3 other quartiles (74±3% for LAV/BSA <30 mL/m2, 76±3% for LAV/BSA 30–38 mL/m2, and 66±3% for LAV/BSA 39–50 mL/m2; P=0.790 for LAV/BSA 30–38 mL/m2 versus LAV/BSA <30 mL/m2, P=0.090 for LAV/BSA 39–50 mL/m2 versus LAV/BSA <30 mL/m2, and P<0.001 for LAV/BSA >50 mL/m2 versus LAV/BSA <30 mL/m2; Figure 5A).
Figure 5.

A, Kaplan‐Meier survival curves of patients with aortic stenosis (AS) according to left atrial volume indexed to body surface area (LAV/BSA) quartiles. B, Adjusted survival curves of patients with AS according to LAV/BSA quartiles. Curves are adjusted for age, sex, comorbidity, New York Heart Association class, hypertension, coronary artery disease, ejection fraction, and peak aortic jet velocity. Q1, Q2, Q3, and Q4 indicate first, second, third, and fourth quartiles, respectively.
On multivariable analysis, after adjustment for age, sex, comorbidity index, symptoms, hypertension, coronary artery disease, EF, and Vmax, LAV/BSA was an independent predictor of all‐cause death (Table 3). The use of spline functions for LAV/BSA (Figure 6) showed that on multivariable analysis, there was no increase in mortality risk with increasing LAV/BSA when it remained ≤50 mL/m2 (adjusted HR, 1.01 [95% CI, 0.92–1.10] per 1‐mL/m2 LAV increment; P=0.371). With LAV >50 mL/m2, there was a significant increase in mortality risk with increasing LAV (adjusted HR, 1.03 [95% CI, 1.01–1.06] per 1‐mL/m2 LAV increment; P=0.022). LAV/BSA >50 mL/m2 was associated with significant risk of all‐cause death during follow‐up (adjusted HR, 1.50 [95% CI, 1.11–2.01]), whereas the mortality risk of the 3 other groups was comparable and significantly lower (adjusted HR, 0.96 [95% CI, 0.70–1.32] for LAV/BSA 30–38 mL/m2 versus <30 mL/m2; and adjusted HR, 1.06 [95% CI, 0.77–1.46] for LAV/BSA 39–50 mL/m2 versus <30 mL/m2; Table 3; Figure 5B). After adjustment for AVR, the strong relationship between LAV/BSA >50 mL/m2 and mortality was still observed (Table 3). In the subgroup of 750 patients in whom the E/e′ ratio was available, the excess risk associated with LAV >95 mL was not altered by further adjustment for E/e′ ratio and indexed LV mass (adjusted HR, 1.62 [95% CI, 1.10–2.38]; P=0.009).
Figure 6.
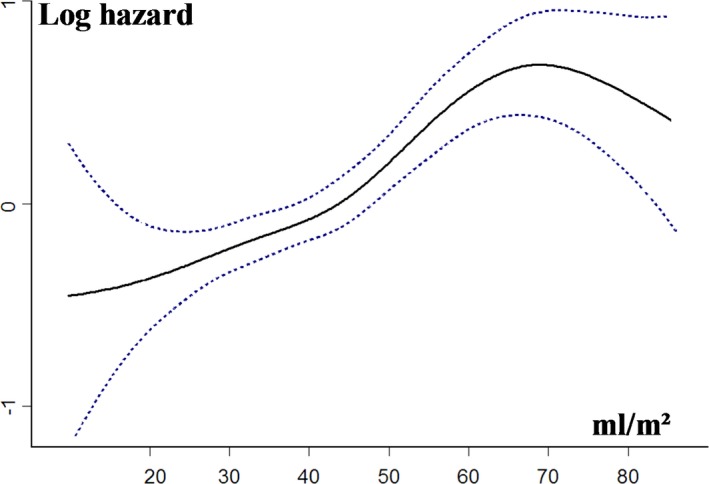
Relationship between left atrial volume indexed to body surface area (LAV/BSA) and the risk of all‐cause death during follow‐up. Hazard ratios and 95% CIs are estimated in Cox models, with LAV/BSA represented as a spline function and adjusted for age, sex, comorbidity, New York Heart Association class, hypertension, coronary artery disease, ejection fraction, and peak aortic jet velocity.
Subgroup analysis
The association of LAV/BSA >50 mL/m2 and all‐cause mortality was consistent in subgroups of patients with AS stratified by age, sex, BSA, and EF (all P for interaction, >0.100). In patients with Vmax ≤4 m/s, 5‐year survival stratified by LAV/BSA was not significantly different (P=0.080; Figure 7A), whereas in patients with Vmax >4 m/s, 5‐year survival was lower with LAV/BSA >50 mL/m2 compared with ≤50 mL/m2 (53±5% versus 80±2%; P<0.001; P for interaction, 0.001; Figure 7B). After adjustment for covariates, including AVR, the risk of death associated with LAV/BSA >50 mL/m2 was considerable in patients with Vmax >4 m/s (adjusted, HR 1.95 [95% CI, 1.41–2.70]; P<0.001) but not in patients with Vmax ≤4 m/s (adjusted HR, 1.24 [95% CI, 0.91–1.69]; P=0.181).
Figure 7.
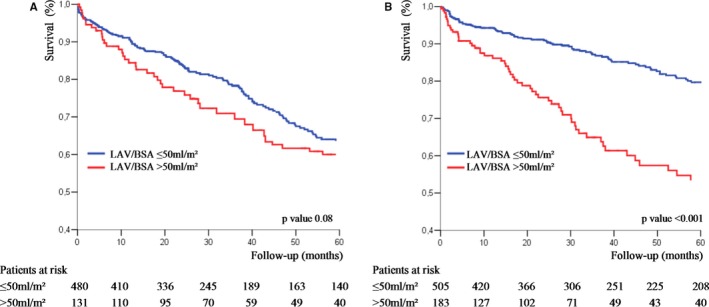
Kaplan‐Meier survival curves of patients with moderate aortic stenosis (AS; peak aortic jet velocity [Vmax], ≤4 m/s; A) and with severe AS (Vmax, >4 m/s; B) according to left atrial volume indexed to body surface area (LAV/BSA; ≤50 and >50 mL/m2).
In patients with severe AS, normal EF at baseline, and no or minimal symptoms, LAV/BSA >50 mL/m2 displayed lower survival compared with LAV/BSA ≤50 mL/m2 (5‐year estimated survival: 87±3% for LAV/BSA ≤50 mL/m2 and 70±10% for LAV/BSA >50 mL/m2; P=0.038). After adjustment for covariates, including surgery, LAV/BSA >50 mL/m2 was independently associated with all‐cause mortality (adjusted HR, 1.90 [95% CI, 1.03–3.56]). There was no interaction between LAV/BSA >50 mL/m2 and height ≤1.6 m (P for interaction, 0.212).
Aortic valve surgery and outcome
Five‐year postoperative survival of patients who underwent AVR during the first 3 months after diagnosis was 85±2% for LAV/BSA ≤50 mL/m2 and 63±5% for LAV/BSA >50 mL/m2 (P<0.001). On multivariable analysis, the impact of LAV >50 mL/m2 on postoperative outcome was considerable (adjusted HR, 2.01 [95% CI, 1.27–3.16]; P=0.003).
Outcome Impact of Other Normalized LAV Parameters
Analysis of the outcome impact of the other normalized LAV parameters (LAV/height, LAV/BSA1.7, and LAV/height2.0) showed similar patterns compared with LAV or LAV/BSA. The highest quartile (LAV/height >57 mL/m, LAV/BSA1.7 >32 mL/[m2]1.7, and LAV/height2.0 >27 mL/[m]2.0) had greater risk of all‐cause death on univariate and multivariable analyses compared with lower quartiles that displayed a more benign and similar outcome (Table S3 and Figures S1 through S3).
Comparison of the Prognostic Value of LAV Parameters
We conducted several analyses to evaluate the added value for event prediction of introducing each LAV parameter (LAV, LAV/BSA, LAV/height, LAV/BSA1.7, and LAV/height2.0) as a covariate in a Cox proportional hazard model, including age, sex, comorbidity, symptoms, hypertension, coronary artery disease, Vmax, and EF. The Akaike information criterion was lowest for the model including LAV/BSA, indicating best model fit (Table 4). The effects on the C statistics when adding normalized aortic valve area indexes to the basic model were overall modest, slightly better for LAV/BSA (Table 4). Reclassification indexes favored slightly LAV/BSA over LAV (NRI: median, 0.15 [25th–75th percentile, 0.008–0.22] [P=0.006] for LAV/BSA; and median, 0.14 [25th–75th percentile, 0.002–0.20] [P=0.019] for LAV; IDI: median, 0.009 [25th–75th percentile, 0.004–0.017] [P=0.017] for LAV/BSA; and median, 0.007 [25th–75th percentile, 0.002–0.016] [P=0.030] for LAV), whereas the other normalized parameters showed lower predictive capacity (Table 4).
Table 4.
Discrimination and Reclassification Associated With LAV and LAV Normalized to Different Indexes of Body Size
| Model | AIC | Harrell C Indexa | P Value | Net Reclassification Improvementa | P Value | Integrated Discrimination Improvementa | P Value |
|---|---|---|---|---|---|---|---|
| Multivariable model | 1760 | 0.721 (0.692–0.750) | Reference | ||||
| Multivariable model with LAVb | 1745 | 0.726 (0.697–0.759) | 0.012 | 0.14 (0.002–0.20) | 0.019 | 0.007 (0.002–0.016) | 0.030 |
| Multivariable model with LAV/BSAb | 1738 | 0.731 (0.699–0.762) | 0.011 | 0.15 (0.008–0.22) | 0.006 | 0.009 (0.004–0.017) | 0.017 |
| Multivariable model with LAV/heightb | 1750 | 0.724 (0.695–0.754) | 0.041 | 0.10 (0.001–0.17) | 0.038 | 0.005 (0.001–0.010) | 0.058 |
| Multivariable model with LAV/BSA1.7 b | 1756 | 0.722 (0.691–0.750) | 0.034 | 0.13 (0.001–0.22) | 0.017 | 0.006 (0.001–0.018) | 0.042 |
| Multivariable model with LAV/height2.0 b | 1752 | 0.723 (0.692–0.751) | 0.031 | 0.12 (0.002–0.20) | 0.026 | 0.004 (0.001–0.016) | 0.050 |
Analyses are multivariable Cox proportional hazard models. Global model fit was assessed by the AIC. Discrimination and reclassification were based on the Harrell C statistic, net reclassification improvement, and integrated discrimination improvement. The Harrell C statistic, net reclassification improvement, and integrated discrimination improvement are calculated for all‐cause death at median follow‐up (36 months). AIC indicates Akaike information criterion; BSA, body surface area; and LAV, left atrial volume.
Data are given as median (25th–75th percentile).
The multivariable model included age, sex, hypertension, coronary artery disease, New York Heart Association class, Charlson comorbidity index, peak aortic jet velocity, and left ventricular ejection fraction.
Discussion
The present study focuses on the influence of LA enlargement on outcome in patients with AS. To the best of our knowledge, we demonstrate for the first time that in AS, LAV is a strong predictor of death under medical and surgical treatment. The effect of LAV on outcome is powerful and remains valid after adjustment for factors known as major determinants of prognosis, such as age, comorbidity, symptoms, and LV function. Thus, severe LA enlargement (LAV >95 mL or LAV/BSA >50 mL/m2) is associated with substantial increase in the risk of all‐cause mortality during follow‐up. LAV and LAV/BSA show comparable prognostic impact, whereas allometric LAV normalization does not prove superior predictive performance in this setting. Severe LA enlargement complicating significant valvular obstruction is a marker of adverse outcome in patients with severe AS, even in asymptomatic and minimally symptomatic patients with preserved EF. On the other hand, when the valvular obstruction is moderate, severe LA enlargement does not significantly affect outcome. Moreover, our results show that severe LA enlargement is an independent determinant of lower survival after AVR, despite the proven beneficial effect of surgery. Therefore, in clinical practice assessment of LA enlargement, measuring LAV should be systematically performed in patients with AS and taken into consideration for decision purposes, even in patients without symptoms or LV dysfunction.
LAV is an accurate and reproducible echocardiographic parameter,24 easily obtainable and relatively preload independent. In this study, we measured LAV by the biplane method of disks (modified Simpson rule), which is routinely used in our echocardiography laboratories. The biplane method of disks and the biplane area‐length method have similar accuracy and reproducibility when compared with computed tomography and magnetic resonance imaging measurements23, 33 and have been both previously recommended for LA size assessment by the American Society of Echocardiography.34 The latest recommendations for cardiac chamber quantification35 favor the biplane method of disks over the area‐length method, which is based on the assumption of an ellipsoidal LA shape and uses several LA linear measurements.
In patients with AS, LA enlargement is the reflection of chronically elevated LV filling pressures necessary to maintain adequate LV filling and cardiac output. In patients with moderate AS, indexed LAV is significantly correlated with aortic valve area, significant mitral regurgitation, hypertension, LV end‐diastolic volume, LV hypertrophy, and restrictive LV filling pattern.9 Recently, Christensen et al suggested that patients with asymptomatic severe AS with greater LAV have a high hemodynamic burden and pulmonary pressure at exercise, especially in the presence of an increased E/e′ ratio.12 Furthermore, the occurrence of symptoms in severe AS is associated with impaired diastolic function, LV hypertrophy, concentric remodeling, and LA dilatation.11 From this perspective, the assessment of LAV appears of great importance because this measure is the consequence of the duration and severity of increased LA pressure because of both the valvular obstacle and the LV remodeling process.
Several studies have linked LA enlargement to death,13, 14, 15 incident heart failure,16 AF,17 and stroke14 in the general population. Moreover, there is a close relation between LAV and mortality in patients with dilated ischemic and nonischemic cardiomyopathy,18 myocardial infarction,19 and organic mitral regurgitation.20 Few studies have investigated the prognostic impact of LA enlargement in AS, and most of them focused on the simple M‐mode echocardiography LA diameter.5, 21, 36 Beach et al reported that increased preoperative LA diameter affects long‐term survival after AVR,5 whereas Rossi et al suggested a link between greater preoperative LA diameter and persistence of symptoms after surgery.36 In a retrospective report of 622 patients with asymptomatic severe AS, LA diameter was reported to predict the occurrence of symptoms and mortality.21 Only 1 study has analyzed the outcome implications of indexed LAV in AS.25 This report from the SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) study included only asymptomatic patients with mild‐to‐moderate AS and failed to demonstrate an association between indexed LAV and the combined end point of AVR, heart failure, and cardiac death.25 We report a strong association between severe LA enlargement and mortality in a large cohort of patients with AS. We observed excess risk of death at the >95‐mL LAV cutoff (50 mL/m2 for LAV/BSA). The predictive performance of nonindexed and indexed LAV was close, and the slight significance of the observed difference is probably not clinically relevant. LAV showed some interaction with very small body size, which was not observed with LAV/BSA. The prognostic value of severe LA enlargement was considerable in patients with severe AS at diagnosis, even in those with no or minimal symptoms and preserved EF. In patients with nonsevere AS, LA enlargement did not significantly affect outcome. Moreover, among operated on patients, severe LA enlargement remained independently predictive of adverse outcome. These finding attest that LAV >95 mL and LAV/BSA >50 mL/m2 are markers of adverse outcome in severe AS and, therefore, should be part of the comprehensive echocardiographic examination.
In normal adults, LAV exhibits considerable variability, and normalization methods might help identify pathologic conditions and distinguish them from normal variants.37 Indexation by BSA appears as a reasonable way to increase the sensitivity and specificity of LAV, assuming that the linear relationship of LAV/BSA is size independent. This assumption fails to account for the nonlinear relationship between LAV and body size.37 Allometric LAV scaling provides an indexed LAV parameter that is independent of age and variability in body size and minimizes any nonconstant variance. In addition to linear normalization, we tested the outcome impact of allometric normalization of LAV to BSA and height. The allometric exponents were 1.7 for BSA and 2.0 for height and have been previously derived on a large cohort of healthy individuals.29 We observed high risk of mortality with LAV/BSA1.7 >32 mL/(m2)1.7 and LAV/height2.0 >27 mL/(m)2.0. However, on reclassification analysis, these parameters did not show superiority over classic linear normalization to BSA.
Strengths and Limitations
Information on follow‐up was retrospectively obtained; therefore, this study has the inherent limitations of such analyses. The specific indications for surgery during follow‐up were not collected in our database. However, diagnosis and follow‐up were performed by cardiologists with expertise in valvular disease, and the surgical decisions were taken by the heart team in accordance with practice guidelines. Objective assessment of symptoms was not systematically performed. For the subgroup analysis, we considered patients with AS and no or minimal symptoms. Indeed, among elderly patients with AS, it is often difficult to differentiate asymptomatic individuals from patients who have minimal subjective manifestations. We included only patients in sinus rhythm at baseline, given the independent effect of AF on LA enlargement. Some patients might have developed AF during follow‐up, which could have affected outcome. This item was not recorded in our database. Brain natriuretic peptide levels were not determined in this study; thus, we could not analyze the influence of this biomarker on the association between LA enlargement and outcome. We acknowledge that a randomized trial of patients with asymptomatic AS who do not meet criteria for AVR could definitively establish whether structural cardiac changes (including LA enlargement) represent more sensitive criteria for early valve replacement in AS.
Conclusions
The analysis of this large cohort shows that severe LA enlargement is independently predictive of mortality in patients with severe AS in sinus rhythm under medical and surgical management. Detection of LAV >95 mL or LAV/BSA >50 mL/m2 at AS diagnosis is associated with substantial increase in the risk of death during follow‐up. Although linear normalization of LAV to BSA is of interest in patients with small body size, allometric scaling of LAV to BSA or height does not refine outcome prediction. The clinical challenge in patients with severe AS is to detect deleterious left‐sided heart remodeling at the subclinical stage to perform AVR before the occurrence of irreversible changes that diminish the long‐term benefit of surgery. Our findings suggest that LAV assessment should be part of the comprehensive echocardiographic evaluation of all patients with severe AS and might be taken into consideration when discussing surgery in asymptomatic or minimally symptomatic patients with severe AS.
Disclosures
None.
Supporting information
Table S1. Baseline Demographic and Clinical Characteristics, Overall and According to LAV Quartiles
Table S2. Echocardiographic Parameters, Overall and According to LAV Quartiles
Table S3. Relative Risk of All‐Cause Death Associated With Left Atrium Enlargement
Figure S1. A, Kaplan‐Meier survival curves of patients with aortic stenosis (AS) according to left atrial volume (LAV)/height quartiles. B, Adjusted survival curves of patients with AS according to LAV/height quartiles. Curves are adjusted for age, sex, comorbidity, New York Heart Association class, hypertension, coronary artery disease, ejection fraction, and peak aortic jet velocity. Q1, Q2, Q3, and Q4 indicate first, second, third, and fourth quartiles, respectively.
Figure S2. A, Kaplan‐Meier survival curves of patients with aortic stenosis (AS) according to left atrial volume indexed to body surface area to 1.7 (LAV/BSA1.7) quartiles. B, Adjusted survival curves of patients with AS according to LAV/BSA1.7 quartiles. Curves are adjusted for age, sex, comorbidity, New York Heart Association class, hypertension, coronary artery disease, ejection fraction, and peak aortic jet velocity. Q1, Q2, Q3, and Q4 indicate first, second, third, and fourth quartiles, respectively.
Figure S3. A, Kaplan‐Meier survival curves of patients with aortic stenosis (AS) according to left atrial volume indexed to height to 2.0 (LAV/height2.0) quartiles. B, Adjusted survival curves of patients with AS according to LAV/height2.0 quartiles. Curves are adjusted for age, sex, comorbidity, New York Heart Association class, hypertension, coronary artery disease, ejection fraction, and peak aortic jet velocity. Q1, Q2, Q3, and Q4 indicate first, second, third, and fourth quartiles, respectively.
(J Am Heart Assoc. 2017;6:e006615 DOI: 10.1161/JAHA.117.006615.)29089338
References
- 1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–2488. [DOI] [PubMed] [Google Scholar]
- 2. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón‐Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schäfers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 3. Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol. 2006;47:2141–2151. [DOI] [PubMed] [Google Scholar]
- 4. Mihaljevic T, Nowicki ER, Rajeswaran J, Blackstone EH, Lagazzi L, Thomas J, Lytle BW, Cosgrove DM. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg. 2008;135:1270–1278. [DOI] [PubMed] [Google Scholar]
- 5. Beach JM, Mihaljevic T, Rajeswaran J, Marwick T, Edwards ST, Nowicki ER, Thomas J, Svensson LG, Griffin B, Gillinov AM, Blackstone EH. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 2014;147:362–369. [DOI] [PubMed] [Google Scholar]
- 6. Orsinelli DA, Aurigemma GP, Battista S, Krendel S, Gaasch WH. Left ventricular hypertrophy and mortality after aortic valve replacement for aortic stenosis: a high risk subgroup identified by preoperative relative wall thickness. J Am Coll Cardiol. 1993;22:1679–1683. [DOI] [PubMed] [Google Scholar]
- 7. Dahl JS, Videbæk L, Poulsen MK, Rudbæk TR, Pellikka PA, Møller JE. Global strain in severe aortic valve stenosis: relation to clinical outcome after aortic valve replacement. Circ Cardiovasc Imaging. 2012;5:613–620. [DOI] [PubMed] [Google Scholar]
- 8. Debry N, Maréchaux S, Rusinaru D, Peltier M, Messika‐Zeitoun D, Menet A, Tribouilloy C. Prognostic significance of left ventricular concentric remodeling in patients with aortic stenosis. Arch Cardiovasc Dis. 2017;110:26–34. [DOI] [PubMed] [Google Scholar]
- 9. Dalsgaard M, Egstrup K, Wachtell K, Gerdts E, Cramariuc D, Kjaergaard J, Hassager C. Left atrial volume in patients with asymptomatic aortic valve stenosis (the Simvastatin and Ezetimibe in Aortic Stenosis study). Am J Cardiol. 2008;101:1030–1034. [DOI] [PubMed] [Google Scholar]
- 10. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert T, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 11. Dahl JS, Christensen NL, Videbæk L, Poulsen MK, Carter‐Storch R, Hey TM, Pellikka PA, Steffensen FH, Moller JE. Left ventricular diastolic function is associated with symptom status in severe aortic valve stenosis. Circ Cardiovasc Imaging. 2014;7:142–148. [DOI] [PubMed] [Google Scholar]
- 12. Christensen NL, Dahl JS, Carter‐Storch R, Bakkestrom R, Jensen K, Steffensen FH, Sondergaard EV, Videbaek L, Moller JE. Association between left atrial dilatation and invasive hemodynamics at rest and during exercise in asymptomatic aortic stenosis. Circ Cardiovasc Imaging. 2016;9:e005156. [DOI] [PubMed] [Google Scholar]
- 13. Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J. M‐mode echocardiographic predictors of six‐ to seven‐year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol. 2001;87:1051–1057. [DOI] [PubMed] [Google Scholar]
- 14. Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death: the Framingham Heart Study. Circulation. 1995;92:835–841. [DOI] [PubMed] [Google Scholar]
- 15. Laukkanen JA, Kurl S, Eränen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle‐aged men. Arch Intern Med. 2005;165:1788–1793. [DOI] [PubMed] [Google Scholar]
- 16. Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TS. Usefulness of left atrial volume in predicting first congestive heart failure in patients ≥65 years of age with well‐preserved left ventricular systolic function. Am J Cardiol. 2005;96:832–836. [DOI] [PubMed] [Google Scholar]
- 17. Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation: the Framingham Heart Study. Circulation. 1994;89:724–730. [DOI] [PubMed] [Google Scholar]
- 18. Rossi A, Cicoira M, Zanolla L, Sandrini R, Golia G, Zardini P, Enriquez‐Sarano M. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1425–1430. [DOI] [PubMed] [Google Scholar]
- 19. Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2212. [DOI] [PubMed] [Google Scholar]
- 20. Le Tourneau T, Messika‐Zeitoun D, Russo A, Detaint D, Topilsky Y, Mahoney DW, Suri R, Enriquez‐Sarano M. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol. 2010;56:570–578. [DOI] [PubMed] [Google Scholar]
- 21. Casaclang‐Verzosa G, Malouf JF, Scott CG, Juracan EM, Nishimura RA, Pellikka PA. Does left atrial size predict mortality in asymptomatic patients with severe aortic stenosis? Echocardiography. 2010;27:105–109. [DOI] [PubMed] [Google Scholar]
- 22. Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–1023. [DOI] [PubMed] [Google Scholar]
- 23. Kircher B, Abbott JA, Pau S, Gould RG, Himelman RB, Higgins CB, Lipton MJ, Schiller NB. Left atrial volume determination by biplane two‐dimensional echocardiography: validation by cine computed tomography. Am Heart J. 1991;121:864–871. [DOI] [PubMed] [Google Scholar]
- 24. Khankirawatana B, Khankirawatana S, Porter T. How should left atrial size be reported? Comparative assessment with use of multiple echocardiographic methods. Am Heart J. 2004;147:369–374. [DOI] [PubMed] [Google Scholar]
- 25. Dalsgaard M, Egstrup K, Wachtell K, Cramariuc D, Kjaergaard J, Gerdts E, Hassager C. Left atrial volume as predictor of valve replacement and cardiovascular events in patients with asymptomatic mild to moderate aortic stenosis. Echocardiography. 2013;30:1008–1014. [DOI] [PubMed] [Google Scholar]
- 26. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classification of prognostic comorbidity for longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 27. Tribouilloy C, Rusinaru D, Maréchaux S, Castel AL, Debry N, Maizel J, Mentaverri R, Kamel S, Slama M, Lévy F. Low‐gradient, low‐flow severe aortic stenosis with preserved left ventricular ejection fraction: characteristics, outcome, and implications for surgery. J Am Coll Cardiol. 2015;65:55–66. [DOI] [PubMed] [Google Scholar]
- 28. Lester SJ, Ryan EW, Schiller NB, Foster E. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–832. [DOI] [PubMed] [Google Scholar]
- 29. Levy F, Rusinaru D, Maréchaux S, Charles V, Peltier M, Tribouilloy C. Determinants and prognosis of atrial fibrillation in patients with aortic stenosis. Am J Cardiol. 2015;116:1541–1546. [DOI] [PubMed] [Google Scholar]
- 30. Kuznetsova T, Haddad F, Tikhonoff V, Kloch‐Badelek M, Ryabikov A, Knez J, Malyutina S, Stolarz‐Skrzypek K, Thijs L, Schnittger I, Wu JC, Casiglia E, Narkiewicz K, Kawecka‐Jaszcz K, Staessen JA. Impact and pitfalls of scaling of left ventricular and atrial structure in population‐based studies. J Hypertens. 2016;34:1186–1194. [DOI] [PubMed] [Google Scholar]
- 31. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013;32:2430–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. [DOI] [PubMed] [Google Scholar]
- 34. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 35. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. [DOI] [PubMed] [Google Scholar]
- 36. Rossi A, Tomaino M, Golia G, Santini F, Pentiricci S, Marino P, Zardini P. Usefulness of left atrial size in predicting postoperative symptomatic improvement in patients with aortic stenosis. Am J Cardiol. 2000;86:567–570. [DOI] [PubMed] [Google Scholar]
- 37. Dewey FE, Rosenthal D, Murphy DJ Jr, Froelicher VF, Ashley EA. Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation. 2008;117:2279–2287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Demographic and Clinical Characteristics, Overall and According to LAV Quartiles
Table S2. Echocardiographic Parameters, Overall and According to LAV Quartiles
Table S3. Relative Risk of All‐Cause Death Associated With Left Atrium Enlargement
Figure S1. A, Kaplan‐Meier survival curves of patients with aortic stenosis (AS) according to left atrial volume (LAV)/height quartiles. B, Adjusted survival curves of patients with AS according to LAV/height quartiles. Curves are adjusted for age, sex, comorbidity, New York Heart Association class, hypertension, coronary artery disease, ejection fraction, and peak aortic jet velocity. Q1, Q2, Q3, and Q4 indicate first, second, third, and fourth quartiles, respectively.
Figure S2. A, Kaplan‐Meier survival curves of patients with aortic stenosis (AS) according to left atrial volume indexed to body surface area to 1.7 (LAV/BSA1.7) quartiles. B, Adjusted survival curves of patients with AS according to LAV/BSA1.7 quartiles. Curves are adjusted for age, sex, comorbidity, New York Heart Association class, hypertension, coronary artery disease, ejection fraction, and peak aortic jet velocity. Q1, Q2, Q3, and Q4 indicate first, second, third, and fourth quartiles, respectively.
Figure S3. A, Kaplan‐Meier survival curves of patients with aortic stenosis (AS) according to left atrial volume indexed to height to 2.0 (LAV/height2.0) quartiles. B, Adjusted survival curves of patients with AS according to LAV/height2.0 quartiles. Curves are adjusted for age, sex, comorbidity, New York Heart Association class, hypertension, coronary artery disease, ejection fraction, and peak aortic jet velocity. Q1, Q2, Q3, and Q4 indicate first, second, third, and fourth quartiles, respectively.


